Abstract
Purpose
To investigate whether oxygen stresses experienced in retinopathy of prematurity (ROP) would trigger signaling through reactive oxygen species (ROS) and the Janus kinase-signal transducer and activator of transcription (JAK/STAT) pathways to lead to intravitreous neovascularization (IVNV) in an oxygen-induced retinopathy (OIR) rat model.
Methods
Newborn rat pups exposed to repeated fluctuations in oxygen and rescued in supplemental oxygen (28% O2, 50/10 OIR+SO) were treated with apocynin, an NADPH oxidase and ROS inhibitor (10 mg/kg/day), AG490, a JAK2 inhibitor (5mg/kg/day), or phosphate buffered saline (PBS). Intraperitoneal injections were given from postnatal day (p)12-p17 (apocynin), or from p3-p17 (AG490). Outcomes were intravitreous neovascularization and avascular/total retinal areas, vascular endothelial growth factor (VEGF), phosphorylated JAK2, and phosphorylated STAT3.
Results
Apocynin significantly reduced phosphorylated STAT3 in 50/10 OIR+SO (p=0.04), in association with previously reported inhibition of IVNV area. Inhibition of JAK with AG490 significantly reduced phosphorylated JAK2 (p<0.001), phosphorylated STAT3 (p=0.002), and IVNV area (p=0.033) in the 50/10 OIR+SO model compared to control.
Conclusions
Activation of NADPH oxidase from supplemental oxygen works via activated STAT3 to lead to IVNV. In addition, inhibition of the JAK/STAT pathway reduces IVNV. Further studies are needed to determine the effects and relationships of oxygen stresses on JAK/STAT and NAPDH oxidase signaling.
INTRODUCTION
Retinopathy of prematurity (ROP) is a leading cause of nonreversible childhood blindness world wide. When first described in the 1940’s and 50’s1, ROP likely developed as a result of unregulated high inspired oxygen at birth. Now both inspired oxygen and infant oxygen saturation are monitored, and high oxygen at birth2,3 rarely occurs in preterm infants in the US. However, fluctuations in oxygen and supplemental inspired oxygen later in the course of prematurity have been reported as associated with increased risk of developing severe ROP as it is known today4,5,6,7,8.
In a model of ROP that treats newborn rat pups to repeated fluctuations in oxygen, we found that reactive oxygen species (ROS) were increased in the retina and that NADPH oxidase was activated to trigger apoptosis of endothelial cells, which contributed to avascular retina9. When pups subjected to oxygen fluctuations were placed into supplemental oxygen instead of room air, there was exacerbation of NADPH oxidase activation that contributed to angiogenic blood vessel growth into the vitreous10. This intravitreous neovascularization (IVNV) appeared, in part, to be independent of vascular endothelial growth factor (VEGF)10.
The Janus kinase-signal transducer and activator of transcription (JAK/STAT) signaling pathway can lead to angiogenesis through ROS either by triggering angiogenic factors, such as VEGF11,12, or through alternate pathways13,14. We wished to determine the role of JAK/STAT signaling in IVNV and used models of ROP that expose pups to oxygen stresses similar to those experienced by preterm infants at risk for severe ROP as it is known today (i.e., repeated fluctuations in oxygen and supplemental oxygen). Our hypothesis is that oxygen stresses experienced by preterm infants trigger signaling through JAK/STAT pathways to contribute to IVNV. To address this, we used a JAK2 inhibitor [AG490 (tyrphostin)], which is a chemical compound that potently inhibits JAK2 protein tyrosine kinase (PTK) and blocks the constitutive activation of STAT3 to inhibit DNA synthesis and cell growth, and induce apoptosis15. We measured IVNV area and avascular retina in our models. Since NADPH oxidase can also increase IVNV when supplemental oxygen was an added stress, we studied its potential role in JAK/STAT signaling of angiogenesis.
MATERIALS AND METHODS
All animals were cared for in accordance with the University of North Carolina’s Institute for Laboratory Animal Research (Guide for the Care and Use of Laboratory Animals) and the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
50/10 Oxygen-Induced Fluctuations and Supplemental Oxygen Rat Model
A bioactive gas controller (Oxycycler; BioSpherix, New York, NY), which regulates the atmosphere inside an incubator by injecting either nitrogen or oxygen, was used to induce oxygen-induced retinopathy in newborn Sprague-Dawley rats (Charles River, Wilmington, MA), as previously reported16. Within 4 hours of birth, pups and their mothers were placed into the incubator, which cycled oxygen between 50% and 10% every 24 hours for 14 days. Thereafter, pups were placed in supplemental oxygen (28% O2) for 4 days (50/10 OIR+SO). Carbon dioxide in the chambers was monitored and flushed from the system by maintaining sufficient gas flow and using soda lime. Litter numbers were between 12 and 14 pups for each experiment to assure consistency in outcomes and all animals were weighed and mean body weight of litters found to be within ± 2 g of each other at the time of treatment.
Treatment with AG490 and Apocynin
AG490 (LC Laboratories, Woburn, MA) is a synthetic PTK inhibitor that inhibits JAK217 and JAK3 activation. It selectively blocks cell survival and proliferation by inducing programmed cell death and was used to inhibit the JAK/STAT pathway in rats18. To test whether JAK2 inhibition affects signaling through STAT to cause apoptosis or angiogenesis and ultimately lead to IVNV in the 50/10 OIR model, we treated rat pups with 5mg/kg AG490 5% dimethyl sulfoxide (DMSO, MP Biomedicals, Solon, OH) in phosphate buffered saline (PBS, Sigma, St. Louis, MI). Intraperitoneal (IP) injections (5μL/g body weight) were administered daily from p3 to p6, p3 to p13, or p3 to p17. Control animals received IP injections of sterile 5% DMSO in PBS. Separate litters received IP injections of apocynin (4-acetovanillione, Sigma, St. Louis, MI) in sterile PBS at 10 mg/kg, (5μl/g body weight) once a day from p12 to p17, as previously reported10. Controls received IP injections of sterile PBS at 5μl/g. Pups were removed from cycling for less than 20 minutes for treatments.
Dissecting Retinal Tissue
Rat pups were anesthetized by IP injection of ketamine (60 mg/kg) and xylazine (18 mg/kg) then perfused with intracardiac paraformaldehyde (PFA, 1.0 mL, 0.5%) prior to euthanasia by intracardiac pentobarbital (50ul, 80mg/kg). Eyes were enucleated and prefixed in 2% PFA for 2 hrs before anterior segments, hyaloidal vessels and vitreous were removed. Retinas with intact ora serratas were dissected, placed into PBS, and flattened onto microscope slides by making 4 right angle incisions. For fresh tissue, animals were euthanized with pentobarbital (80 mg/kg IP), and retinas were isolated without ora serratas. Tissue was frozen in RIPA buffer with protease cocktail inhibitor (1:100, Sigma, St. Louis, MI) and orthovanadate (1mM, Sigma) for protein or RNAlater (Ambion, Austin, TX) for RNA and stored at −80°C until analysis.
Staining Retinal Flat mounts
Flattened retinas were permeabilized in ice cold ethanol (70% v/v) for 20 minutes then in PBS/1% Triton x-100 for 30 minutes. Retinas were incubated with Alexa Fluor 568 conjugated Griffonia simplicifolia (Bandeiraea) isolectin B4 (5 μg/ml, Molecular Probes, OR) in PBS overnight at 4°C, then rinsed three times in PBS and mounted in PBS:glycerol (2:1) with VectaShield (Vector Labs, CA). Slides were secured with coverslips and sealed with nail varnish. Images of the retinal blood vessels were captured using a Nikon TE2000U inverted microscope (Michael-Hooker Microscopy Facility, University of North Carolina, Chapel Hill, NC) and digitally stored for analysis.
Measurement of IVNV and Avascular Areas
To measure IVNV, digitized retinal image sections were assembled with methods that maintain original image dimensions (Tekmate’s PhotoFit Premium v1.44, Tekmate Inc., AK). Total retinal area, summed peripheral avascular retinal and IVNV areas19 were computed in pixels with Image Tool v.3 (University of Texas, San Antonio, TX) and converted to mm2 (using a calibration bar on each image). IVNV has been defined as neovascularization growing into the vitreous at the junction of vascular and avascular retina20. Avascular areas were expressed as a percent of total retinal area for each eye. Measurements were performed by two independent masked reviewers. A third reviewer was used to rectify discrepancies in measurements and a final consensus determined.
REAL-TIME PCR
Samples were removed from RNAlater and total RNA was extracted using the RNeasy Mini kit (Qiagen, Valencia, CA). DNA contamination was removed by using DNA-free (Ambion, Austin, TX), and RNA quantity was determined spectrophotometrically. 1 μg of RNA from each sample was reverse transcribed the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA). Approximately 200 ng of cDNA were analyzed per well by one step real time PCR using the TaqMan MasterMix with reverse transcriptase (3.7 U/reaction, Applied Biosystems, Foster City, CA) and primers specific to rat STAT3 (forward CTAGACAATATCATCATCGACCTTG; reverse TCCCGCTCCTTGCTGATGA, annealing temperature 60°C). Rat β-actin was used as the control gene and was amplified with forward and reverse primers TGCCTGACGGTCAGGTCA and CAGGAAGGAAGGCTGGAAG, respectively. Duplicate reactions in addition to controls were run for each sample in a total volume of 16 μL. Cycle threshold (CT) values were normalized to β-actin and expressed as a fold increase over mRNA expressed at birth (post-natal day 0).
Protein Extraction, Western Blotting
Retinal samples frozen in modified radio immuno precipitation assay (RIPA) buffer (20 mM Tris base, 120 mM NaCl, 1% Triton X-100, 0.5% sodium deoxycholate, 0.1% SDS, 10% glycerol) with protease cocktail inhibitor (1:100, Sigma, St. Louis, MI) and orthovanadate (1mM, Sigma, St. Louis, MI) were homogenized and centrifuged at 13,000 rpm for 10 minutes at 4°C. Total protein in the supernatant was quantified by Bicinchoninic Acid (BCA) Protein Assay (Pierce, Rockford, IL) and 50μg of total protein for each sample was separated by 7.5% or 15% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), transferred to a polyvinylidene difluoride (PVDF) membrane, and incubated with one or more of the following primary antibodies overnight at 4°C: phospho- JAK2, JAK2, phospho-STAT3, STAT3 (all at 1:1000, Cell Signaling Technology Inc., MA). Membranes were washed three times in Tris-Buffered Saline/0.1% Tween-20 (TBST), probed with horseradish peroxidase-linked secondary antibody and visualized by enhanced chemiluminescence (Millipore Corporation, Billerica, MA). After probing with phospho- antibody, membranes were stripped and reprobed to determine the ratio of phospho/total protein. All membranes were reprobed with β-actin (1:20000, Abcam, Cambridge, MA) to ensure equal protein loading. Densitometry analysis was done on exposed films using the software UN-SCAN-IT (ver.6.1; Silk Scientific, Orem, UT).
VEGF ELISA
Aliquots of total protein, previously quantified by BCA protein assay (Pierce, Rockford, IL), were assayed in duplicate without dilution, using a commercially available ELISA kit, raised against rat VEGF (R& D Systems, MN).
Statistical Analyses
Data were analyzed with SPSS software (version 14.0; Chicago, IL). Individual Student’s t-tests were used to analyze parametric data, including avascular area/total retinal area, IVNV area, and normalized densitometric values. For all real-time PCR, and protein analyses, an n ≥5 was used and for measurement of IVNV or areas of avascular/total retina, 14 to 20 retinas were analyzed for each group. In all experiments, at least 2 separate litters were used. For all comparisons, p<0.05 was determined to be significant.
RESULTS
Expression of STAT3 in development and under repeated oxygen fluctuations
We first determined the expression of STAT3 at time points in room air development and under oxygen fluctuations through post-natal day (p)14. We found only a modest increase in expression of STAT3 mRNA under the condition of oxygen fluctuations compared to room air at several time points (Figure 1).
Figure 1.
STAT3 mRNA expression measured at time points in retinas of pups from room air development and under repeated oxygen fluctuations (rep oxy fluc) through post-natal day (p)14. STAT3 mRNA expression was analyzed by real-time PCR in 4 to 5 retinas at each time point and data expressed as fold change over p0. Beta-actin was run as the internal control gene. There was only a modest increase in expression of STAT3 mRNA under the condition of oxygen fluctuations compared to room air at several time points.
AG490 reduces JAK/STAT activation under repeated oxygen fluctuations
AG490 was previously reported to inhibit the JAK/STAT pathway in adult male Sprague-Dawley rats with streptozotocin-induced diabetes mellitus18. To test the effect of AG490 on JAK2 and STAT3 activation, we measured the levels of each of these phosphorylated proteins by western blot, normalized to beta actin, in pups treated with either AG490 or control. We found that JAK2 activation was significantly reduced at p7 (p=0.03) and p14 (p=0.02) in treated compared to controls (Figure 2A,2C). Phospho/total STAT3 was also significantly lower at p7 (p=0.033) and p14 (p=0.007) compared to controls (Figure 2B,2D).
Figure 2.

Densitometry results (A, C) and representative gel images (B, D) of phosphorylated/total JAK2 protein (A, B) and phosphorylated/total STAT3 protein (C, D) in 50/10 OIR retinas from AG490 treated and controls analyzed by western blot at postnatal days (p)7 and p14. 50ug total protein separated by SDS-PAGE and probed with anti- phospho JAK2 and JAK2 antibodies or anti-phospho STAT3 and STAT3 antibodies. Beta-actin was used as a loading control and to normalize values. Odd numbered lanes are from AG490 treated retinas (p7) and even numbered lanes show representative samples of PBS treated retinas (p7). Data represents mean±SE of n= 5 retinas for each analysis. *p=0.04, **p=0.02, ∓p=0.03, and ∓∓p=0.01. There were significant decreases in JAK2 and STAT3 phosphorylation following treatment with AG490 compared to controls.
AG490 inhibits Intravitreous Neovascularization in Supplemental Oxygen
We then determined whether IVNV would also be reduced by inhibition of JAK2, which is upstream from STAT3. Therefore, we treated pups in the 50/10 OIR+SO model with daily intraperitoneal AG490 injections and measured avascular/total retinal areas, IVNV area, phospho/total JAK2, phospho/total STAT3. At p18, IVNV was significantly reduced by treatment with AG490 compared to control (p=0.033, Figure 3A&B). In addition, there was a significant decrease in retinal phoshorylated JAK2 (p<0.001, Figure 4A) and phosphorylated STAT3 (p=0.002, Figure 4B), both normalized to β-actin, in AG490 treated animals compared to controls. An analysis of VEGF protein showed no significant difference between the groups and there were also no significant differences in total retinal or avascular/total retinal areas (data not shown). These data provide evidence that inhibition of JAK/STAT signaling reduced IVNV.
Figure 3.
Areas of intravitreous neovascularization (IVNV) (indicated by white arrows) at postnatal day (p)18 in retinas from the 50/10 OIR+SO model treated with daily intraperitoneal (IP) injections (5mg/kg) of AG490 or PBS starting at p3 and continuing through p17. (A) Mean±SE pixel density in 10 or more retinas analyzed per group, *p=0.03 (B) Flat mount images representative of each treatment group. AG490 treatment significantly reduced intravitreous neovascularization in the 50/10 OIR+SO model compared to control.
Figure 4.
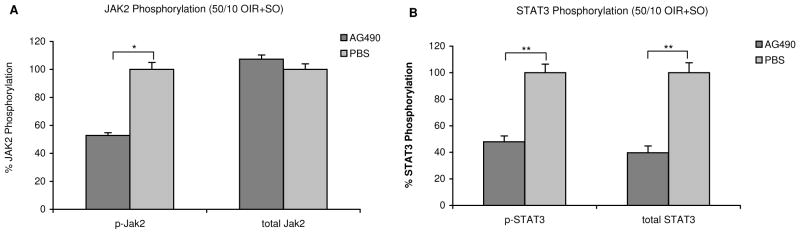
Densitometry results of JAK/STAT activation in 50/10 OIR+SO retinas from AG490 treated and control animals analyzed by western blot at postnatal day (p)18. 50ug total protein separated by SDS-PAGE and probed with respective antibodies. (A) anti- phospho JAK2, and total JAK2, (B) anti-phospho STAT3, and total STAT3. Beta-actin was used as a loading control and to normalize values. Data represents mean±SE of n= 5 retinas each repeated from several litters. *p<0.001, and **p=0.002. Both STAT3 and JAK2 activations were significantly reduced in retinas from AG490 treated animals compared to controls in the 50/10 OIR+SO model.
JAK/STAT Signaling Triggered by Activated NADPH Oxidase from Supplemental Oxygen Contributes to IVNV
JAK/STAT can be triggered by reactive oxygen species (ROS)21,22 and ROS are implicated in ROP23,24,25,26. We previously reported that NADPH oxidase, which when activated releases ROS, contributed to intravitreous neovascularization in the 50/10 OIR+SO model10. To determine whether ROS released from activation of NADPH oxidase triggered signaling through JAK/STAT to contribute to IVNV, pups were exposed to the ROP model (50/10 OIR+SO). All pups were treated with either control or apocynin, a specific inhibitor of NADPH oxidase, at a dose previously shown to reduce IVNV at p18 in the 50/10 OIR+SO model10. Whereas apocynin significantly reduced the level of phospho/total STAT3 in the retina (p=0.04, Figure 5A), there was no difference in JAK2 phosphorylation between apocynin-treated and untreated 50/10 OIR+SO groups (Figure 5B). These data provide evidence that activated NADPH oxidase during supplemental oxygen triggers STAT3 signaling.
Figure 5.

JAK/STAT activation in retinas from 50/10 OIR+SO model. Western blot of (A) phospho/total STAT3 and (B) phospho/total JAK2 after treatment with daily intraperitoneal (IP) injections of apocynin (10 mg/kg) or PBS from postnatal day (p)12 to p17 and analyzed at p18. A total of 6 retinas were analyzed for each group and data represent values following normalization to beta-actin. *p=0.04, n.s. – not significant. In the 50/10 OIR+SO model, apocynin significantly reduced STAT3 but not JAK2 phosphorylaton compared to control.
DISCUSSION
The pathogenesis of severe ROP is complex and is influenced by several variables, including the effect of changes in oxygen concentration in developing retina and retinal vasculature27,28; the effects of nutrients and growth factors on fragile, newly formed capillaries29,30,31,32,33,34; and the effects of oxidative stress35,36. We are interested in the effects of oxygen stresses, similar to those experienced by preterm infants today, on the development of severe ROP. In contrast to the more commonly used mouse model of extreme hyperoxia-induced capillary obliteration with subsequent relative hypoxia and endothelial budding (which mimicked ROP in the 1940’s prior to more stringent monitoring of oxygen), we use a model of ROP that exposes pups to fluctuations in oxygen and supplemental oxygen, which are current risk factors for severe ROP37,38,39,7,4. In addition, the extremes of the oxygen fluctuations used in this model cause arterial oxygen levels in rats16 to be similar to the transcutaneous oxygen levels measured in human preterm infants with severe ROP 4. Also, the model develops characteristics similar to severe ROP with peripheral avascular retina16,20, followed by central vascular tortuosity40, and subsequently intravitreous neovascularization at the junction of vascular and avascular retina41,20. Finally, the model is highly reproducible in the measurements of characteristic features and the time points of their development16.
We previously reported that with an increasing number of fluctuations in oxygen, there was a trend toward an increase in oxidative compounds9. Oxidative stress can trigger signaling of angiogenic processes through factors including VEGF, and we and others have found that VEGF is important in the development of pathologic IVNV42,43. However, reduction of VEGF alone does not totally prevent IVNV42,43, and VEGF is also important in normal development, which is ongoing in the preterm infant. Therefore, understanding of pathways independent of or downstream of VEGF could permit the development of effective and safer treatments.
We have reported that rescuing pups exposed to oxygen fluctuations in supplemental oxygen (i.e., the 50/10 OIR +SO model) rather than room air, led to a reduction in retinal VEGF levels of room air raised pups at the same post-natal day ages10. We proposed that increased retinal vascular oxygen reduced the stimulus for VEGF overexpression within the retina. However, we also found that retinal hypoxia, determined by conjugated pimonidazole, was not reduced in the 50/10 OIR+SO model compared to the 50/10 OIR model (oxygen fluctuations followed by rescue in room air)10. The overall retinal hypoxia may occur as a result of photoreceptor metabolism which increases during development as shown by other investigators44,45. Therefore, the activation of STAT3 in this model appears to be from increased oxygen in the retinal vasculature; however there may also be an effect from retinal hypoxia. In the current study, we used the same model with rescue in supplemental oxygen (50/10 OIR+SO) to reduce the VEGF effect on IVNV in order to focus on other causative pathways. Since JAK/STAT pathway can lead to angiogenesis through ROS, we wished to study its role in the 50/10 OIR+SO model. We found that inhibiting JAK2 with AG490 significantly reduced IVNV with associated reduced phosphorylated JAK2 and STAT3.
Activation of NAPDH oxidase can lead to release of ROS and, in this manner, has been reported to trigger signaling through STAT3 to increase VEGF expression in models of diabetic retinopathy46. We previously reported that NADPH oxidase activation in the 50/10 OIR+SO model contributed to IVNV through a pathway that appeared independent of VEGF10. In the current study, we found that activation of NADPH oxidase contributed to IVNV through STAT3 signaling in the 50/10 OIR+SO model and that inhibiting NADPH oxidase activation and ROS with apocynin resulted in a reduction in phosphorylated STAT3 but not phosphorylated JAK2 (Figure 6). Previous investigators have provided evidence of direct involvement of ROS in activating STAT3 outside JAK2 activation13,14. Treatment with apocynin10 or AG490 did not significantly affect the VEGF level in the retina, suggesting that other angiogenic factors may be involved in IVNV. Further, since VEGF and STAT3 have been reported to provide neuroprotection in some models47,46, distinction among the contributions of VEGF, JAK2 and STAT3 to IVNV may provide a safer therapeutic strategy to treat severe ROP. Additional study is needed to understand the role of JAK/STAT signaling and pathways downstream of gp130 in OIR models and in the developing preterm infant at risk of ROP.
Figure 6.
Proposed steps in signaling pathways in the 50/10 OIR+SO model that may be triggered to cause intravitreous neonascularization.
Acknowledgments
Grant information: NIH R01 EY015130 (MEH-PI) and Research to Prevent Blindness
The authors thank David Martiniuk for his help with the animal experiments, Marilyn Markowski for her contribution as a masked reviewer and for assistance with compiling the images, and Dr. William David Culp Jr. for critically reviewing the manuscript.
References
- 1.Terry TL. Extreme prematurity and fibroblastic overgrowth of persistent vascular sheath behind each crystalline lens:(1) Preliminary report. Am J Ophthalmol. 1942;25:203–204. doi: 10.1016/j.ajo.2018.05.024. [DOI] [PubMed] [Google Scholar]
- 2.Ashton N, Ward B, Serpell G. Effect of oxygen on developing retinal vessels with particular reference to the problem of retrolental fibroplasia. Br J Ophthalmol. 1954;38:397–430. doi: 10.1136/bjo.38.7.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patz A, Eastham A, Higginbotham DH, Kleh T. Oxygen studies in retrolental fibroplasia. Am J Ophthalmol. 1953;36:1511–1522. [PubMed] [Google Scholar]
- 4.Cunningham S, Fleck BW, Elton RA, Mclntosh N. Transcutaneous oxygen levels in retinopathy of prematurity. Lancet. 1995;346:1464–1465. doi: 10.1016/s0140-6736(95)92475-2. [DOI] [PubMed] [Google Scholar]
- 5.McColm JR, Cunningham S, Wade J, et al. Hypoxic oxygen fluctuations produce less severe retinopathy than hyperoxic fluctuations in a rat model of retinopathy of prematurity. Pediatr Res. 2004;55:107–113. doi: 10.1203/01.PDR.0000099772.66376.02. [DOI] [PubMed] [Google Scholar]
- 6.Saito Y, Omoto T, Cho Y, et al. The progression of retinopathy of prematurity and fluctuation in blood gas tension. Graefes Arch Clin Exp Ophthalmol. 1993;231:151–156. doi: 10.1007/BF00920938. [DOI] [PubMed] [Google Scholar]
- 7.Chow LC, Wright KW, Sola A. Can changes in clinical practice decrease the incidence of severe retinopathy of prematurity in very low birth weight infants? Pediatrics. 2003;111:339–345. doi: 10.1542/peds.111.2.339. [DOI] [PubMed] [Google Scholar]
- 8.Tin W, Milligan DWA, Pennefather PM, Hey E. Pulse oximetry, severe retinopathy, and outcome at one year in babies of less than 28 weeks gestation. Arch Dis Child Fetal Neonatal Ed. 2001;84:106–110. doi: 10.1136/fn.84.2.F106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Saito Y, Geisen P, Uppal A, Hartnett ME. Inhibition of NAD(P)H oxidase reduces apoptosis and avascular retina in an animal model of retinopathy of prematurity. Mol Vis. 2007;13:840–853. [PMC free article] [PubMed] [Google Scholar]
- 10.Saito Y, Uppal A, Byfield G, Budd S, Hartnett ME. Activated NAD(P)H Oxidase from Supplemental Oxygen Induces Neovascularization Independent of VEGF in Retinopathy of Prematurity Model. Invest Ophthalmol Vis Sci. 2008;49:1591–1598. doi: 10.1167/iovs.07-1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ushio-Fukai M, Alexander RW. Reactive oxygen species as mediators of angiogenesis signaling. Role of NAD(P)H oxidase. Mol Cell Biochem. 2004;V264:85–97. doi: 10.1023/b:mcbi.0000044378.09409.b5. [DOI] [PubMed] [Google Scholar]
- 12.Ushio-Fukai M. VEGF Signaling Through NADPH Oxidase-Derived ROS. Antioxid Redox Signal. 2007;9:731–739. doi: 10.1089/ars.2007.1556. [DOI] [PubMed] [Google Scholar]
- 13.Liu T, Castro S, Brasier AR, et al. Reactive Oxygen Species Mediate Virus-induced STAT Activation: ROLE OF TYROSINE PHOSPHATASES. J Biol Chem. 2004;279:2461–2469. doi: 10.1074/jbc.M307251200. [DOI] [PubMed] [Google Scholar]
- 14.Lee YJ, Heo JS, Suh HN, Lee MY, Han HJ. Interleukin-6 stimulates {alpha}-MG uptake in renal proximal tubule cells: involvement of STAT3, PI3K/Akt, MAPKs, and NF-{kappa}B. Am J Physiol Renal Physiol. 2007;293:F1036–F1046. doi: 10.1152/ajprenal.00034.2007. [DOI] [PubMed] [Google Scholar]
- 15.Levitzki A. Tyrphostins--potential antiproliferative agents and novel molecular tools. Biochem Pharmacol. 2008;40:913–918. doi: 10.1016/0006-2952(90)90474-y. [DOI] [PubMed] [Google Scholar]
- 16.Penn JS, Henry MM, Tolman BL. Exposure to alternating hypoxia and hyperoxia causes severe proliferative retinopathy in the newborn rat. Pediatr Res. 1994;36:724–731. doi: 10.1203/00006450-199412000-00007. [DOI] [PubMed] [Google Scholar]
- 17.Meydan N, Grunberger T, Dadi H, et al. Inhibition of acute lymphoblastic leukaemia by a Jak-2 inhibitor. Nature. 1996;379:645–648. doi: 10.1038/379645a0. [DOI] [PubMed] [Google Scholar]
- 18.Banes AK, Shaw S, Jenkins JA, et al. Angiotensin II blockade prevents hyperglycemia-induced activation of JAK and STAT proteins in diabetic rat kidney glomeruli. Am J Physiol Renal Physiol. 2004;286:F653–F659. doi: 10.1152/ajprenal.00163.2003. [DOI] [PubMed] [Google Scholar]
- 19.Penn JS, McCollum GW, Barnett JM, et al. Angiostatic Effect of Penetrating Ocular Injury: Role of Pigment Epithelium-Derived Factor. Invest Ophthalmol Vis Sci. 2006;47:405–414. doi: 10.1167/iovs.05-0673. [DOI] [PubMed] [Google Scholar]
- 20.Hartnett ME, Martiniuk DJ, Saito Y, et al. Triamcinolone Reduces Neovascularization, Capillary Density and IGF-1 Receptor Phosphorylation in a Model of Oxygen-Induced Retinopathy. Invest Ophthalmol Vis Sci. 2006;47:4975–4982. doi: 10.1167/iovs.06-0450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mascareno E, El Shafei M, Maulik N, et al. JAK/STAT signaling is associated with cardiac dysfunction during ischemia and reperfusion. Circulation. 2001;104:325–329. doi: 10.1161/01.cir.104.3.325. [DOI] [PubMed] [Google Scholar]
- 22.Simon AR, Rai U, Fanburg BL, Cochran BH. Activation of the JAK-STAT pathway by reactive oxygen species. Am J Physiol Cell Physiol. 1998;275:C1640–C1652. doi: 10.1152/ajpcell.1998.275.6.C1640. [DOI] [PubMed] [Google Scholar]
- 23.Katz ML, Robison WG., Jr Autoxidative damage to the retina: Potential role in retinopathy of prematurity. Birth Defects. 1988;24:237–248. [PubMed] [Google Scholar]
- 24.Penn JS. Oxygen-induced retinopathy in the rat: possible contribution of peroxidation reactions. Doc Ophthalmol. 1990;74:179–186. doi: 10.1007/BF02482607. [DOI] [PubMed] [Google Scholar]
- 25.Phelps DL, Rosenbaum AL. Vitamin E in kitten oxygen-induced retinopathy. II Blockage of vitreal neovascularization. Arch Ophthalmol. 1979;97:1522–1526. doi: 10.1001/archopht.1979.01020020184021. [DOI] [PubMed] [Google Scholar]
- 26.Penn JS, Tolman BL, Lowery LA. Variable oxygen exposure causes preretinal neovascularisation in the newborn rat. Invest Ophthalmol Vis Sci. 1993;34:576–585. [PubMed] [Google Scholar]
- 27.McLeod DS, Brownstein R, Lutty GA. Vaso-obliteration in the canine model of oxygen-induced retinopathy. Invest Ophthalmol Vis Sci. 1996;37:300–311. [PubMed] [Google Scholar]
- 28.Chen J, Smith LE. Retinopathy of Prematurity. Angiogenesis. 2007;10:133–140. doi: 10.1007/s10456-007-9066-0. [DOI] [PubMed] [Google Scholar]
- 29.Connor KM, SanGiovanni JP, Lofqvist C, et al. Increased dietary intake of [omega]-3-polyunsaturated fatty acids reduces pathological retinal angiogenesis. Nat Med. 2007;13:868–873. doi: 10.1038/nm1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen J, Connor KM, Aderman CM, Smith LE. Erythropoietin deficiency decreases vascular stability in mice. J Clin Invest. 2008;118:526–533. doi: 10.1172/JCI33813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alon T, Hemo I, Itin A, et al. Vascular endothelial growth factor acts as a survival factor for newly formed retinal vessels and has implications for retinopathy of prematurity. Nat Med. 1995;1:1024–1028. doi: 10.1038/nm1095-1024. [DOI] [PubMed] [Google Scholar]
- 32.Chang KH, Chan-Ling T, McFarland EL, et al. IGF binding protein-3 regulates hematopoietic stem cell and endothelial precursor cell function during vascular development. Proc Natl Acad Sci USA. 2007;104:10595–10600. doi: 10.1073/pnas.0702072104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lofqvist C, Chen J, Connor KM, et al. From the Cover: IGFBP3 suppresses retinopathy through suppression of oxygen-induced vessel loss and promotion of vascular regrowth. Proc Natl Acad Sci USA. 2007;104:10589–10594. doi: 10.1073/pnas.0702031104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hellstrom A, Carlsson B, Niklasson A, et al. IGF-I is critical for normal vascularization of the human retina. J Clin Endocrinol Metab. 2002;87:3413–3416. doi: 10.1210/jcem.87.7.8629. [DOI] [PubMed] [Google Scholar]
- 35.Brooks SE, Gu X, Samuel S, et al. Reduced severity of oxygen-induced retinopathy in eNOS-deficient mice. Invest Ophthalmol Vis Sci. 2001;42:222–228. [PubMed] [Google Scholar]
- 36.Al Shabrawey M, Bartoli M, El Remessy AB, et al. Inhibition of NAD(P)H Oxidase activity blocks vascular endothelial growth factor overexpression and neovascularization during ischemic retinopathy. Am J Pathol. 2005;167:599–607. doi: 10.1016/S0002-9440(10)63001-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hong PH, Wright KW, Fillafer S, Sola A, Chow LC. Strict oxygen management is associated with decreased incidence of severe of retinopathy of prematurity. 2002 Annual Meeting Abstract and Program Planner. accessed at www.arvo.org 20024011-
- 38.Vanderveen DK, Mansfield TA, Eichenwald EC. Lower Oxygen Saturation Alarm Limits Decrease the Severity of Retinopathy of Prematurity. J AAPOS. 2006;10:445–448. doi: 10.1016/j.jaapos.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 39.Wallace DK, Veness-Meehan KA, Miller WC. Incidence of severe retinopathy of prematurity before and after a modest reduction in target oxygen saturation levels. J AAPOS. 2007;11:170–174. doi: 10.1016/j.jaapos.2006.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu K, Akula JD, Falk C, Hansen RM, Fulton AB. The Retinal Vasculature and Function of the Neural Retina in a Rat Model of Retinopathy of Prematurity. Invest Ophthalmol Vis Sci. 2006;47:2639–2647. doi: 10.1167/iovs.06-0016. [DOI] [PubMed] [Google Scholar]
- 41.Penn JS, Tolman BL, Henry MM. Oxygen-induced retinopathy in the rat: Relationship of retinal nonperfusion to subsequent neovascularization. Invest Ophthalmol Vis Sci. 1994;35:3429–3435. [PubMed] [Google Scholar]
- 42.Werdich XQ, Penn JS. Specific Involvement of Src Family Kinase Activation in the Pathogenesis of Retinal Neovascularization. Invest Ophthalmol Vis Sci. 2006;47:5047–5056. doi: 10.1167/iovs.05-1343. [DOI] [PubMed] [Google Scholar]
- 43.Geisen P, Peterson L, Martiniuk D, et al. Neutralizing antibody to VEGF reduces intravitreous neovascularization and does not interfere with vascularization of avascular retina in an ROP model. Mol Vis. 2008;14:345–357. [PMC free article] [PubMed] [Google Scholar]
- 44.Berkowitz BA, Roberts R, Penn JS, Gradianu M. High-Resolution Manganese-Enhanced MRI of Experimental Retinopathy of Prematurity. Invest Ophthalmol Vis Sci. 2007;48:4733–4740. doi: 10.1167/iovs.06-1516. [DOI] [PubMed] [Google Scholar]
- 45.Akula JD, Hansen RM, Martinez-Perez ME, Fulton AB. Rod Photoreceptor Function Predicts Blood Vessel Abnormality in Retinopathy of Prematurity. Invest Ophthalmol Vis Sci. 2007;48:4351–4359. doi: 10.1167/iovs.07-0204. [DOI] [PubMed] [Google Scholar]
- 46.Al Shabrawey M, Bartoli M, El Remessy AB, et al. Role of NADPH Oxidase and Stat3 in Statin-Mediated Protection against Diabetic Retinopathy. Invest Ophthalmol Vis Sci. 2008;49:3231–3238. doi: 10.1167/iovs.08-1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang C, Li H, Liu MG, et al. STAT3 activation protects retinal ganglion cell layer neurons in response to stress. Exp Eye Res. 2008;86:991–997. doi: 10.1016/j.exer.2008.03.020. [DOI] [PubMed] [Google Scholar]





