Abstract
The U.S. National Cancer Institute, in collaboration with the Belarusian Ministry of Health, is conducting a study of thyroid cancer and other thyroid diseases in a cohort of about 12,000 persons who were exposed to fallout from the Chernobyl accident in April 1986. The study subjects were 18 years old or younger at the time of exposure and resided in Belarus in the most contaminated areas of the Gomel and Mogilev Oblasts, as well as in the city of Minsk. All cohort members had at least one direct thyroid measurement made in April–June 1986. Individual data on residential history, consumption of milk, milk products and leafy vegetables as well as administration of stable iodine were collected for all cohort members by means of personal interviews conducted between 1996 and 2007. Based on the estimated 131I activities in the thyroids, which were derived from the direct thyroid measurements, and on the responses to the questionnaires, individual thyroid doses from intakes of 131I were reconstructed for all cohort members. In addition, radiation doses to the thyroid were estimated for the following minor exposure pathways: (a) intake of short-lived 132I, 133I and 132Te by inhalation and ingestion; (b) external irradiation from radionuclides deposited on the ground; and (c) ingestion intake of 134Cs and 137Cs. Intake of 131I was the major pathway for thyroid exposure; its mean contribution to the thyroid dose was 92%. The thyroid doses from 131I intakes varied from 0.5 mGy to almost 33 Gy; the mean was estimated to be 0.58 Gy, while the median was 0.23 Gy. The reconstructed doses are being used to evaluate the risk of thyroid cancer and other thyroid diseases in the cohort.
INTRODUCTION
As a result of the accident on 26 April 1986 at the Chernobyl nuclear power plant located in north eastern Ukraine about 10 km south of the Belarusian border, large amounts of radioactive materials were released into the atmosphere. Radioactive fallout following the accident resulted in substantial radiation exposure to the population of areas in Belarus, Ukraine and Russia located in relative proximity to the damaged nuclear reactor (1). Increases of thyroid cancer in children who were exposed to 131I at the time of the accident were reported a few years later, first in Belarus (2) and Ukraine (3), and later in Russia (4). To evaluate the radiation induced risk of thyroid cancer and other thyroid diseases caused by the Chernobyl accident, a long-term epidemiological study in a cohort of about 25,000 persons in Belarus (~12,000) and Ukraine (~13,000) who were exposed in childhood and adolescence was initiated in the early 1990s by the Ministries of Health of Belarus and Ukraine in collaboration with the U.S. National Cancer Institute (5). The children included in the cohort study were 18 years old or younger at the time of the Chernobyl accident. The cohort members included in the Belarusian-American study resided in the most contaminated areas of Gomel and Mogilev Oblasts3 as well as in the city of Minsk.
To evaluate the radiation-related risk of thyroid cancer and thyroid diseases in the epidemiological study, considerable efforts were made to reconstruct the individual thyroid doses received by all members of the cohort. Every (called “direct thyroid measurement”) performed during 26 April–30 June 1986. An in-depth analysis of these measurements for all cohort members was conducted to subtract from the measured signal the contributions due to the external and internal contamination of the person that was measured. The net signal represents the 131I activity in the thyroid gland at the time of the measurement. The variation with time of the 131I activity in the thyroid, before and after the measurement, was then calculated, using ecological and biokinetic models as well as personal interview data, and the time-integrated activity of 131I in the thyroid was derived from those results. These two components, namely the measured 131I activity in the thyroid and the time-integrated activity of 131I in the thyroid, were used to calculate the individual thyroid doses due to 131I intakes. In addition, radiation doses to the thyroid for the following minor exposure pathways were calculated: (a) intake of short-lived 132I, 133I and 132Te via inhalation and ingestion; (b) external irradiation from radionuclides deposited on the ground and (c) ingestion intake of 134Cs and 137Cs.
This paper describes the methodology, input data and results of reconstruction of individual thyroid doses for all cohort members in the Belarusian-American study. It is recognized that the results of the direct thyroid measurements, the responses given by the respondents during the personal interviews, as well as the parameters of the ecological and biokinetic models include various degrees of uncertainty. The evaluation of uncertainties associated with the thyroid doses reconstructed for the cohort members of the Belarusian-American study is under way.
MATERIALS AND METHODS
Study Population
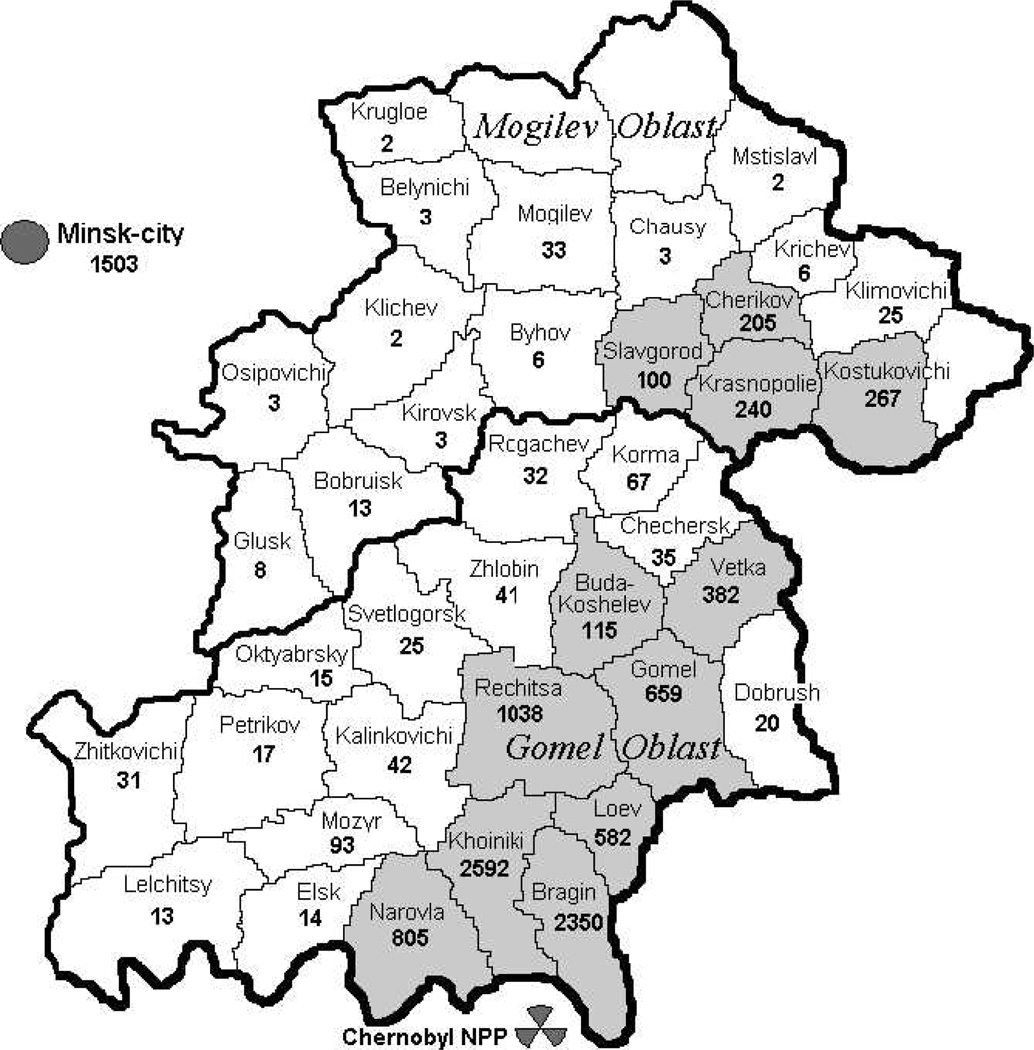
The Belarusian cohort initially included 11,970 individuals who attended screening examinations. In the course of the dose reconstruction process, 238 persons were excluded from the cohort due to ineligible age (n = 114); incorrect identification (n = 20); extremely poor quality of direct thyroid measurements (n = 90) and 14 subjects were not interviewed. The remaining 11,732 cohort members are considered here. The distribution of these cohort members according to the raion of residence at the time of the accident is shown in Fig. 1. Only Gomel and Mogilev4 Oblasts as well as the city of Minsk are shown. An additional 339 individuals resided at the time of the accident in other parts of the country or outside Belarus. Almost half of the cohort (5,747 of 11,732) resided at the time of the accident in Bragin, Khoiniki and Narovla raions, which are the closest in proximity to the Chernobyl nuclear power plant.
FIG. 1.
Distribution of the cohort members by raion of residence at the time of the Chernobyl accident. Raions where 100 and more individuals resided are highlighted in gray.
The study was reviewed and approved by the institutional review boards of the participating organizations in Belarus and the U.S. and all study subjects or their guardians (for subjects who were 16 years or younger at screening) signed informed consent.
Information Used for the Dose Reconstruction
The following information was used to calculate the absorbed doses to the thyroids of the individuals included in the cohort:
Results of the direct thyroid measurements made in April–June 1986.
Results of interviews for all cohort members or their relatives for children who were less than 10 years old at the time of the accident.
Ecological and biokinetic models used to evaluate the variation with time of the 131I activity in the thyroid.
Values of thyroid masses of the Belarusian children close to the time of the Chernobyl accident.
The scheme of thyroid dose calculation for the Belarusian cohort members is shown in Fig. 2. The thyroid doses were estimated using input data specific to each cohort member (direct thyroid measurement and personal interview) and ecological data (131I ground deposition in the settlements). Ecological and biokinetic models were used to reconstruct the transport of 131I from the ground deposition to the child’s thyroid by the activity intake with contaminated air and foodstuffs calculated using data on individual behavior and consumption of foodstuffs reported during the personal interview. These models were used to calculate: (a) the time-integrated activity of 131I in the thyroid, from which the “ecological dose” is derived, and (b) the 131I activity in the thyroid at the time of the direct thyroid measurement, called the “ecological” 131I activity in the thyroid. To calculate the “instrumental” thyroid dose, the ecological dose was calibrated by the 131I activity in the thyroid derived from the direct thyroid measurement. The manner in which the information used in the dose reconstruction was obtained and processed is discussed in the following sections.
FIG. 2.
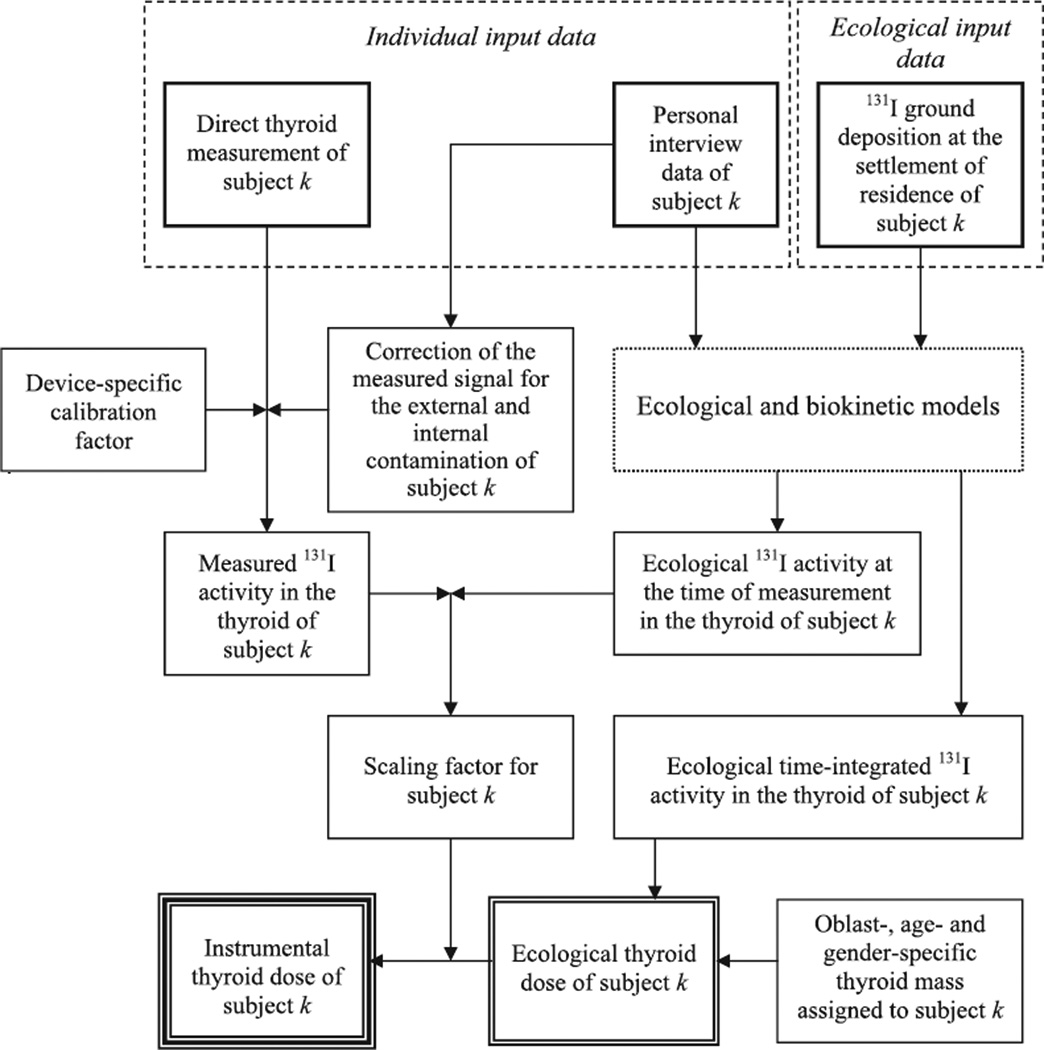
Scheme of thyroid dose calculation for the Belarusian cohort members.
Direct Thyroid Measurements
The direct thyroid measurements were conducted in Belarus during April-June 1986 on about 40,000 individuals aged 18 years or younger at the time of the Chernobyl accident 6. The database created at the Institute of Biophysics (now called Burnasyan Federal Medical Biophysical Center, Moscow, Russia) was used for cohort member selection.
The direct thyroid measurements were performed using different types of radiation monitoring devices, mainly the DP-5 dose-rate meter, the SRP-68-01 survey meter and the DRG3-02 dosimeter (Table 1). The operators recorded the exposure rates measured by the various devices in different units: mR h−1 for the DP-5 device, µR h−1 for the SRP-68-01 device and µR s−1 for the DRG3-02 device. For convenience, the results provided by the three types of devices were converted in this article into a single unit: mR h−1.
TABLE 1.
Distribution of the 11,732 Belarusian Cohort Members by Type of Radiation Monitoring Device
| Number of cohort members measured in |
|||||
|---|---|---|---|---|---|
| Device | Method of measurement | Detector type | Belarus | Russiaa | Total |
| DP-5 | Exposure rate | Geiger-Mueller | 6,740 | – | 6,740 |
| SRP-68-01 | Exposure rate | NaI(Tl) | 4,683 | 93 | 4,776 |
| DRG3-02 | Exposure rate | Plastic scintillator | 133 | 55 | 188 |
| RFT-20046b | Spectrometry | NaI(Tl) | – | 28 | 28 |
| Total | 11,556 | 176 | 11,732 | ||
Measurements done in Saint Petersburg (formerly called Leningrad).
One-channel gamma-spectrometer.
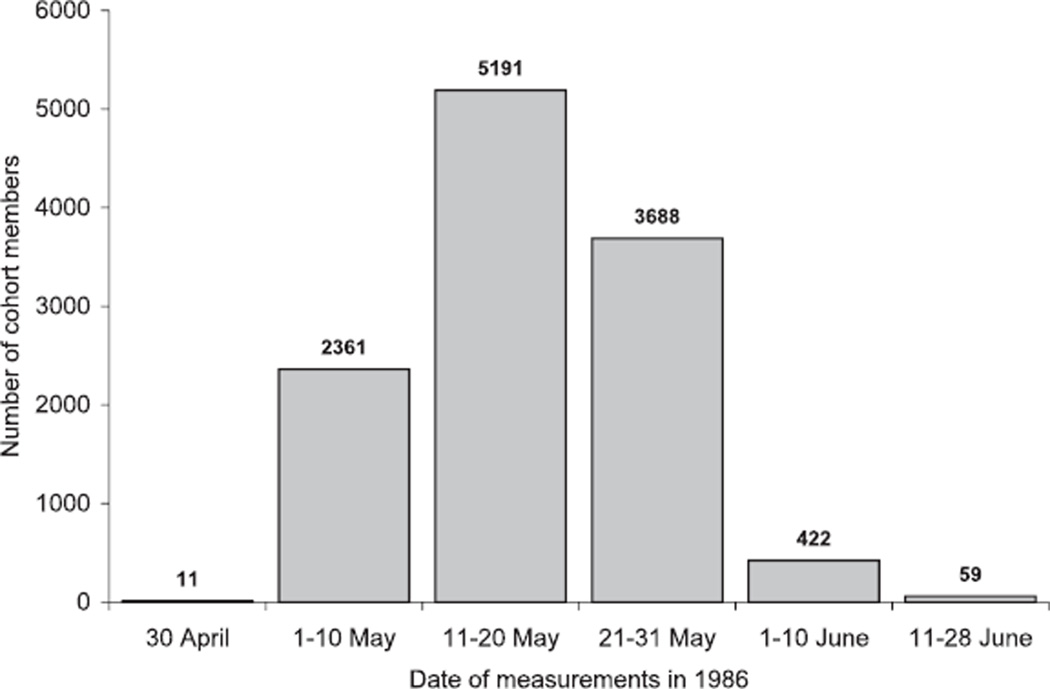
The temporal distribution of the direct thyroid measurements, which were all conducted between 30 April and 28 June 1986, are shown in Fig. 3. The majority of the cohort members (11,251 out of 11,732 or 96%) were measured before 1 June 1986. The measurements started earlier among residents of the city of Minsk (median date of 12 May 1986) and Gomel Oblast (median date of 16 May 1986) than among those of Mogilev Oblast (median date of 27 May 1986), which is relatively far from the Chernobyl nuclear power plant. The median date of the direct thyroid measurements for the entire cohort is 16 May 1986.
FIG. 3.
Temporal distribution of the direct thyroid measurements performed on the cohort members.
The direct thyroid measurements made in Belarus were not performed by professionals, but by people with no experience in radiation measurements. In general, the persons who conducted measurements were given instructions on how to use the measuring devices and to record the results of the measurements; however, there was no quality control of their measuring practices or of the way in which they recorded the measured values. In many instances, the background exposure rate in the presence (or absence) of the subject in the room where the measurements were performed, was not measured or recorded. Therefore, to estimate the device response due only to the 131I content in the thyroid of the subject, it was necessary to investigate whether the measurement results took into account the following contributions to the overall signal registered by the device:
The background radiation in the room where the measurements were taken.
The external surface contamination of the body and the clothes as well as the internal contamination due to 134Cs, 136Cs and 137Cs incorporated in the body.
The manner in which the 131I activities in the thyroids of the subjects were derived from the results of the direct thyroid measurements is described below.
Background Radiation in the Room Where Measurements Were Performed
When the background radiation in the room was measured during the direct thyroid measurements, the net exposure rate was calculated as:
| (1) |
where Pm(tm) is the exposure rate measured near the thyroid gland (mR h−1); Pbg(tm) is the background radiation (mR h −1) in the room where the measurements were performed on day of measurement, tm; and Pnet(tm) is the net exposure rate (mR h−1).
Often, the background radiation in the room where the measurements were performed was not measured during the measurement campaign in Belarus. In those instances, the method used to estimate Pbg(tm) depended upon the availability of outdoor exposure rates.
Measurements of outdoor exposure rate were available
If outdoor exposure rate in the settlement of interest: (a) was measured at the day of thyroid measurement, tm; or (b) was estimated by means of interpolation of measurements performed in that settlement before and after the day tm, the background radiation in the room was calculated as:
| (2) |
where S is the shielding factor that reflects the shielding properties of the building where the direct thyroid measurements were performed (unitless); Pout(tm) is the outdoor exposure rate (mR h−1) on the day tm; and Pnat is the natural background radiation exposure rate of 0.011 mR h−1 in Gomel Oblast and 0.012 mR h−1 in Mogilev Oblast (7). Based on the results of 3,540 measurements performed outdoors and indoors in 1981–1982 in Belarus, the indoor exposure rate due to natural background radiation was taken to be equal to that measured outdoors (8).
The shielding factor was estimated from the measurements of outdoor and indoor exposure rates performed between 3 May and 16 May 1986 in the same settlement during the same day:
| (3) |
where Pin is the exposure rate measured indoors (mR h−1).
The value of the shielding factor varies from 0.05 to 0.5; in this study it was assumed to be equal to 0.28, which is the center of the range.
Measurements of outdoor exposure rate were not available
When outdoor exposure rate was not measured in the settlement of interest, the outdoor exposure rate was calculated as:
| (4) |
where PChern,out(tm) is the exposure rate (mR h−1) outdoors on day tm from radionuclides of Chernobyl origin deposited on the ground and other surfaces, which is calculated for settlements in Gomel and Mogilev Oblasts as PChern,out(tm) = K(tm)GD137, where K(tm) is the oblast-specific value of the exposure rate on day tm normalized to the 137Cs ground deposition density (mR h−1 per kBq m−2) (Table 2); GD137 is the ground deposition density of 137Cs (kBq m−2).
TABLE 2.
Region-Specific Exposure Rate on Day tm Normalized to the 137Cs Ground Deposition DensityK(tm), in Gomel and Mogilev Oblasts from 1 to 31 May 1986
| Date in 1986 |
|||||||
|---|---|---|---|---|---|---|---|
| Region | 1 May | 5 May | 10 May | 15 May | 20 May | 25 May | 31 May |
| K( t) × 103 (mR h−1 kBq−1 m2) | |||||||
| Gomel Oblast | 12.4 | 6.4 | 4.0 | 2.6 | 2.3 | 1.9 | 1.7 |
| Mogilev Oblast | - | - | 1.3 | 0.7 | 0.5 | 0.5 | 0.5 |
In many instances, the handwritten records did not indicate if the background radiation in the room where the measurements were performed was subtracted from the results of measurement of exposure rate near the thyroid. To evaluate if it was subtracted, the exposure rate measured against the neck, Pm(tm), was compared with the background radiation in the room, Pbg(tm), either measured or calculated using Eq. (2). When Pm(tm) < Pbg(tm), the background radiation in the room where the measurements were performed was assumed to have been subtracted from the result of the measurement near the thyroid.
Lower Limits of Exposure Rate
In some cases, the measured exposure rate was recorded in the notebook as zero or “background”, or it was recorded to be less than the background radiation in the room where the measurements were performed. Sometimes, the exposure rate measured by the DP-5 device was recorded in the notebook as less than 0.05 mR h−1, which is the minimal indication of this device. To prevent the use of these values of net exposure rate, Pnet(tm), for the direct thyroid measurements, such values, when they occurred, were replaced with “lower limits of exposure rate” (LLE). The values of LLE depend on the type of device and on the magnitude of the exposure rate due to background radiation in the room. The LLE (mR h−1) were calculated using the following equation (9):
| (5) |
where tp = 1.96 is the Student statistics; k is the scale coefficient for the device from impulse to scale reading and is equal to 3,680 and to 20 (counts s−1 mR−1 h) for the SRP-68-01 and for the DP-5, respectively; T is the duration of measurement recommended in the device’s user manual; it is equal to 30 s and to 45 s for the SRP-68-01 and for the DP-5, respectively; δ = 1 is the relative error of measurement (unitless).
LLE values were assigned to 1,156 cohort members, including 965 of them who were measured with the DP-5 device and 191 persons measured with the SRP-68-01 device.
Contribution to the Measured Signal From The External and Internal Contamination of The Subject
Direct thyroid measurements were made in Belarus on subjects who were internally contaminated with radionuclides other than 131I and whose skin, hair and clothes could also be contaminated. To restrict the device readout to the γ radiation emitted by the thyroid and to eliminate to a large extent the influence of the external and internal contamination of the body, the thyroid detectors can be equipped with lead collimators (10); however, in Belarus, the SRP-68-01 and DRG3-02 devices were not equipped with collimators, while the geometry of the DP-5 detector prevents the use of a collimator. Therefore, when a Belarusian cohort member was measured shortly after fallout, external contamination of his or her body with radionuclides of Chernobyl origin contributed substantially to the response of the radiation device. In June 1986 internal contamination with radiocesium isotopes homogeneously distributed in the body was more important than external contamination. A measurement of background exposure rate performed near the thigh of the subject can be used to account for the contribution of the internal contamination with the radiocesiums and for some of the external contamination (11). However, in Belarus, as a rule, the background against another part of the body of the subject was not measured.
To evaluate the contribution to the measured signal from external and internal contamination of the body it was necessary to estimate (a) the external contamination of every cohort member as well as the activity of cesium isotopes (134Cs, 136Cs and 137Cs) in their bodies at the time of their direct thyroid measurements, and (b) the response of the radiation devices for the radiocesiums in the body as well as for all radionuclides that contributed to the external contamination of the body surfaces. The problem was exacerbated by the facts that (a) the cohort members were children from 0 to 18 years old, so that a range of body sizes had to be considered; (b) the measurements were made using different types of radiation devices; and (c) many cohort members changed places of residence several times between the date of the accident and the date of the direct thyroid measurement.
Models were developed to estimate the levels of external and internal contamination for all cohort members at the time of their direct thyroid measurement. The model of external contamination was used to estimate the activities of 95Zr, 95Nb, 99Mo, 103Ru, 106Ru, 132Te, 131I, 132I, 133I, 134Cs, 136Cs, 137Cs, 140Ba, 140La, 141Ce, 144Ce, 239Np on all surfaces of the body, which was divided into 19 regions, including face, hair, neck, arms, hands, trunk and legs. The activities were calculated for each day between 26 April and 30 June 1986 for individuals of different ages: newborns, children aged 1, 5, 10 and 15 years, and adults. Sixteen areas in Belarus with different scenarios (wet/dry) and radionuclide mix in deposition (12) were considered in the calculations. Twelve different scenarios of residence were considered in the study, including permanent residence at the settlement and relocation from the settlement of residence on 26, 27, 28, 29, 30 April, or 2, 5, 10, 15, 20 or 25 May 1986. The model accounts for indoor and outdoor air and ground surface contamination, natural and man-made resuspension of radioactive particles and washing habits of the population. Calculations were also made assuming that the cohort members at the time of their direct thyroid measurements wore contaminated clothes that either had been washed every week or had not been washed since the time of the accident (26 April 1986). For the purposes of the dose assessment, it was assumed, because the majority of measurements were done two weeks or later after the accident (Fig. 3), that the cohort members at the times of the direct thyroid measurements wore clothes that had been washed every week.
The model of internal contamination was used to estimate the activities of 134Cs, 136Cs and 137Cs in the bodies of the cohort members at the time of their direct thyroid measurements. Two scenarios of cesium intake were considered: inhalation and ingestion of cow’s milk (private milk in rural settlements and commercial milk from trade network in cities) and milk products.
The assessment of the contribution to the measured signal from radionuclides located in the 19 regions representing the surface of body, as well as from radiocesiums into the body, was made by means of Monte Carlo modeling of the human body at various ages and the use of mathematical models of radiation detectors.
The contribution of the external and internal contamination of the subject to the exposure rate measured near the thyroid was taken into account by means of a correction factor as:
| (6) |
where Pnet,corr(tm) is the net exposure rate (mR h−1) measured near the thyroid that was corrected to take into account the external and internal contamination of the body; vcorr(tm) is the device-, age-, region-, scenario of deposition- and behavior-specific correction factor that represents the contribution of the 131I activity in the thyroid to the measured signal (unitless).
To assign a correction factor to a given study subject, his or her personal information from the questionnaire was taken into account. Thirty-five scenarios of relocation between 16 areas in Belarus were considered in the assignment of the correction factors to the study subjects. Only the fact of consumption of milk by the study subject (“yes” or “no”) and the origin of milk and milk products (private cow’s milk or commercial milk) were considered, not his or her individual consumption rates of milk and milk products. Due to the complexity of the task, the administration of stable iodine for the study subject under consideration was not taken into account. However, as is shown below in the section on “Stable Iodine Administration”, the intake of stable iodine would not have had a large effect if it was conducted after 1 May 1986.
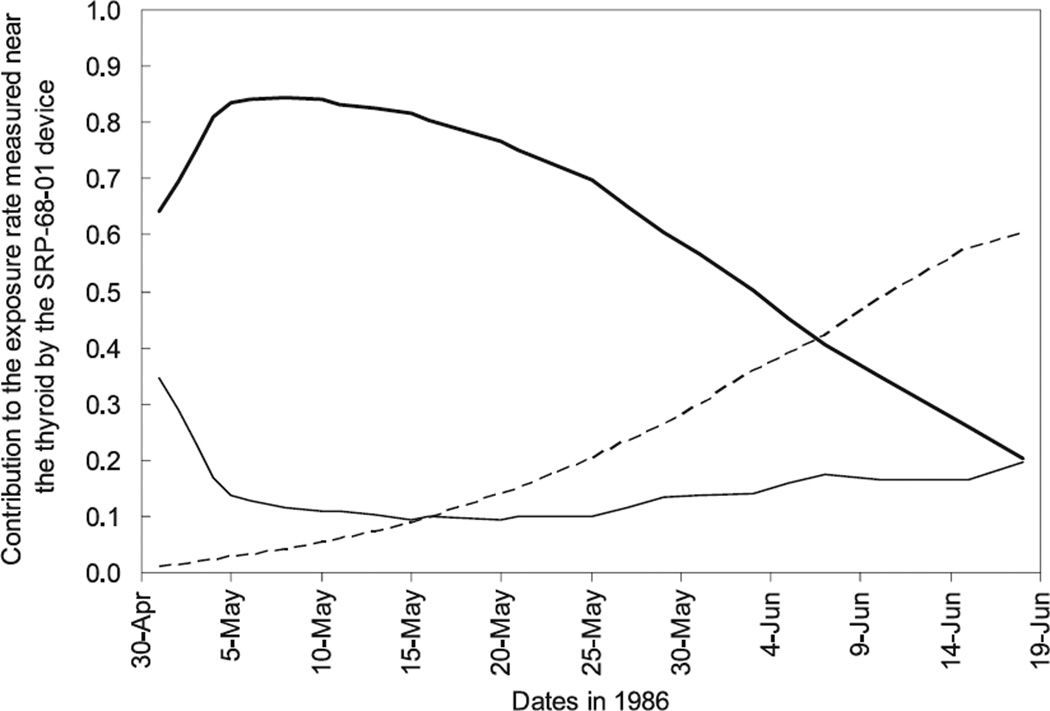
Figure 4 shows, as an example, the variation with time of the relative contributions to the exposure rate measured near the thyroid of the external contamination of the subject, of his or her internal contamination and of the 131I activity in his or her thyroid. The example that is given is for a measurement made by means of a SRP-68-01 device on a 5-year-old child who resided in Khoiniki raion, where the majority of the study subjects resided at the time of the accident (Fig. 1). If the child was measured between 5 May and 20 May 1986, the relative contribution of the 131I activity in the thyroid to the signal measured near the thyroid was around 80% (Fig. 4). For measurements that took place at the beginning of June 1986 and later, 131I in the thyroid contributed less than half to the device response, the remainder of the signal being caused mainly by the radiocesium isotopes incorporated in the body. Similar contributions of cesium activity in the body to the results of the direct thyroid measurements were shown by Ulanovsky et al. 13.
FIG. 4.
Variation with time of the contribution of the external (thin line) and internal (broken line) contamination of the subject, and of the 131I activity in the thyroid (thick line) to the exposure rate measured near the thyroid by the SRP-68-01 device for a 5-year-old child who resided in Khoiniki raion of Gomel Oblast.
Iodine-131 Activity in The Thyroid
The corrected net exposure rate, Pnet,corr(tm), is only due to the 131I activity in the thyroid of the subject at the time of the direct thyroid measurement. The relationship between Pnet,corr(tm) and the 131I activity in thyroid is as follows:
| (7) |
where is the activity of 131I measured in the thyroid of kth study subject (kBq); CFdev is the device-specific calibration factor (kBq mR−1 h).
As direct thyroid measurements were performed using devices that were not designed to measure radioactivity in humans, calibration factors for 131I needed to be estimated for these devices. A Monte Carlo method of numerical simulation of radiation transport was used to calculate the device- and age-specific calibration factors (Table 3) that were used in this study to derive the 131I activities in the thyroids from the results of the direct thyroid measurements. Detailed description of the calculations for the SRP-68-01 device can be found elsewhere 14. Similar work was carried out for the DP-5 and DRG3-02 instruments.
TABLE 3.
Calibration Factors for the Thyroid Detectors That Were Used in Belarus to Derive the 131I Activity in the Thyroid From the Direct Thyroid Measurement
| Calibration factor for the device (kBq mR −1h) |
|||
|---|---|---|---|
| Age group (year) | DP-5 | SPR-68-01 | DRG3-02 |
| Newborn | 190 | 98 | 66 |
| 1 | 185 | 100 | 70 |
| 5 | 200 | 110 | 84 |
| 10 | 285 | 126 | 126 |
| 15 | 390 | 147 | 175 |
| 20 (adult) | 450 | 167 | 233 |
Personal Interviews
Information on person’s whereabouts, diet and administration of stable iodine is required to estimate the thyroid doses that were received by the cohort members. This information was collected by means of personal interviews during two screenings. Dosimetry interviews during the first screening were conducted from 30 December 1996 through 31 March 2001 while the second screening took place between 1 April 2001 and 31 May 2007. Most of the cohort members were interviewed at least 2 times during the first and second screening examinations. The questionnaire used during the second screening was improved by Belarusian, Ukrainian and the U.S. dosimetrists and epidemiologists based on the experience from the first screening. It was used in both Belarusian-American and Ukrainian-American cohort studies. The following types of questionnaire were used to collect the personal information required for dose reconstruction:
Questionnaire for cohort members: administered to 4,419 cohort members during the second screening.
Questionnaire for relatives of the cohort members: for children who were less than 10 years of age at the time of the accident, a close relative, usually the mother, was interviewed: this was done for 6,817 subjects who completed the second screening.
In addition, to assess the dose from mother’s milk, a slightly revised questionnaire was administered to 312 mothers of subjects who were breast-fed during 26 April–30 June 1986. Because 496 cohort members could not be located due to changes of residence that were not documented, they were not interviewed during the second screening, and the questionnaire completed during the first screening was used for their dose reconstruction. For all other subjects, the dose assessment was based on the responses provided during the second screening.
The questionnaire that was used during the second screening includes questions about name, date of birth, address on the date of the interview, and basic and follow-up questions on (a) detailed residential history of the cohort member during the first two months after the accident, that is, between 26 April and 30 June 1986, and less detailed residential history for the time period from 1 July 1986 until the date of the interview, (b) origin and consumption of milk, milk products and leafy vegetables between 26 April and 30 June 1986, and (c) intake of stable iodine to block the uptake of 131I by the thyroid. A positive response to the basic question “Did you drink milk?” provoked follow-up questions: “What kind of milk?”, “How much?”, “How often?”, “When did you stop drinking milk?”, etc. The questionnaires for the cohort members and their relatives included 17 basic and 81 follow-up questions, while the questionnaire for the breast feeding mothers included 7 basic and 39 follow-up questions.
The questionnaires that were completed for the 11,732 respondents (either cohort members or relatives) include about 355,000 answers regarding residential history, dietary habits and stable iodine administration. Some of the respondents experienced difficulties remembering the foodstuff consumption or the dates related to change of residence, modification of dietary habits and stable iodine administration. There were 48,368 answers that were either devoid of information, for example, “I do not remember” or “I do not know” or fuzzy, for example, when respondents were not able to provide the exact date of relocation, stable iodine administration or change of consumption habits. The majority of these imprecise answers (which numbered almost 37,000) related to the dates. A set of logical rules was developed to impute imprecise answers. If a respondent did not recall the exact date of, for example, relocation from one settlement to another, he or she was prompted to estimate the period of time during which the event occurred, such as “end of April (1986)”, “beginning of May”, “middle of May”, “end of May” or “June”. In the dose calculation process, these imprecise answers were replaced by specific dates corresponding to the middle of the selected time interval, i.e., 28 April, 5 May, 15 May, 25 May or 15 June, respectively. For answers that are devoid of information such as “I do not remember how much milk I consumed”, the milk consumption rate that was imputed for the dose calculation was the average value of the age-, gender- and type of settlement-specific milk consumption rate obtained from the cohort members who provided definite answers (see Appendix 1, Table A1.5; see Supplementary Material: http://dx.doi.org/10.1667/RR3153.1.S1).
Ecological and Instrumental Thyroid Doses from 131I Intakes
The ecological and biokinetic models describe processes of 131I transfer starting from deposition of 131I on the ground surface and ending with the accumulation of radioactive iodine in the child’s thyroid. The following processes were taken into account in the dose calculations:
Daily deposition of 131I on ground surface. Iodine-131 and 137Cs daily deposition densities were calculated using the atmospheric transport model developed by Talerko (15, 16) for Ukraine and adapted for Belarus, using meteorological information, such as precipitation, wind speed, wind direction and temperature, that was measured at the time of fallout across the country by the Committee for Hydrometeorology of Belarus (17). The measured 137Cs and 131I cumulative deposition densities were used to calibrate the atmospheric transport model. First, the model calculations of 137Cs deposition densities were scaled to the 137Cs ground deposition densities measured in all 23,325 Belarusian settlements by the Committee for Hydrometeorology of Belarus (18); and, in a second step, the model calculations of 131I deposition densities were scaled to the 131I ground deposition densities measured in 508 Belarusian settlements (19).
Inhalation intake of 131I. The concentration of 131I in ground-level air was calculated from the 131I deposition on the ground, using a generic value for the deposition velocity of iodine on soil and grass surface.
Interception of radioactive iodine by grass.
Consumption of contaminated grass and soil by cows and goats on pastures.
Transfer of radioactive iodine into cow’s and goat’s milk.
Milk and milk products consumption by individuals. The delay between the milking of private cows and the consumption of milk and milk products was considered in the dose assessment. For milk products the factors that define the fraction of radionuclide remaining in the product after culinary preparation were also considered in the model.
Local and regional production of milk for the trade network. After 6 May 1986 the total radioactivity in commercial milk was controlled and was not allowed to exceed 3.7 kBq L 1 (20). This radioactivity concentration level was attributed in this study to the concentration of 131I in commercial milk as it was mainly contaminated with 131I at that time (21).
Consumption of milk and milk products from the trade network. Culinary factors and the delay between the milking of cows and the consumption of milk and milk products were considered.
Consumption of leafy vegetables by individuals. The delay between the harvesting of leafy vegetables and their consumption in urban settlements was considered in the dose assessment. The culinary factor that defines the fraction of radionuclide remaining in the leafy vegetables after washing was also considered.
Stable iodine administration. The intake of stable iodine for prophylactic reasons, when reported by the study subjects (see Table 4), was considered to result in a modification of the thyroid uptake of 131I, which varied with time after the intake of stable iodine. Single and multiple intakes of stable iodine were considered.
Biokinetic models of iodine in the human body, after inhalation or ingestion (22,23).
TABLE 4.
Distribution of the Number of Cohort Members Who Took Stable Iodine by Region of Residence at The Time of The Accident and By Dates of Stable Iodine Administration
| Who took stable iodine |
||||||
|---|---|---|---|---|---|---|
| Residence at the time of the accident | Total | Total | 26–30 April | 1–10 May | 11–20 May | 21–31 May |
| Gomel Oblast, including | ||||||
| 30-km zone | 1,464 | 763 | 158 | 432 | 128 | 45 |
| Bragin, Khoiniki, Narovla raionsa | 4,283 | 1,994 | 449 | 1,143 | 320 | 82 |
| Gomel City | 580 | 171 | 15 | 86 | 52 | 18 |
| Remainder of Gomel Oblast | 2,641 | 596 | 41 | 302 | 186 | 67 |
| Mogilev Oblast | 922 | 228 | 12 | 89 | 83 | 44 |
| Minsk City | 1,503 | 513 | 113 | 248 | 120 | 32 |
| Other regions | 339 | 90 | 15 | 39 | 25 | 8 |
| Entire cohort | 11,732 | 4,355 | 803 | 2,342 | 914 | 296 |
Excluding 30-km zone.
The ecological and biokinetic models were used to calculate the “ecological” 131I activity in the thyroid at the time of the direct thyroid measurement and the time integral of 131I in the thyroid, from which the “ecological dose” was derived. To calculate the “instrumental thyroid dose”, which is the dose assigned to the cohort members for the epidemiologic analysis, the ecological dose was calibrated using the 131I activity in the thyroid derived from the direct thyroid measurement: the instrumental dose was obtained as the product of the ecological dose and of the ratio of the measured and calculated 131I activities in the thyroid at the time of the direct thyroid measurement; this ratio is called “scaling factor” in this study (Fig. 2). A detailed description of the models used to calculate the ecological and instrumental thyroid doses is provided in Appendix 1 (see Supplementary Material: http://dx.doi.org/10.1667/RR3153.1.S1).
Thyroid Masses
The thyroid mass is one of the most important parameters in the estimation of the thyroid dose. Data on thyroid masses for Belarusian children who resided in the most contaminated Gomel and Mogilev oblasts at the time of the Chernobyl accident were derived by Skryabin et al. (24) from the ultrasound-based estimates of thyroid volume performed by the Sasakawa Memorial Health Foundation (SMHF) in 1991–1996 (25). The estimated thyroid volumes of about 57,500 Belarusian children and adolescents aged from 3 to 18 years, including about 27,900 individuals from Gomel and 29,600 from Mogilev oblast, were entered in an electronic database. The database was analyzed and cleaned to make it more suitable to the needs of this study. In that process, the individuals aged 3, 4, 17 and 18 years were excluded from the database because of their relatively small numbers and the average thyroid masses were calculated by age and gender for each oblast, using a density of 1.05 g cm−3 for the thyroid gland (26).
Table A1.6 in Appendix 1 shows the average oblast-, age- and gender dependent values of the thyroid mass that were used to calculate the thyroid doses for all cohort members (see Supplementary Material: http://dx.doi.org/10.1667/RR3153.1.S1):
for children aged less than 5 years, the thyroid-mass values were obtained by interpolation between a value for newborns of 1.3 g, as recommended by the International Commission on Radiological Protection (26) and values for children aged 5 years derived by Skryabin et al. (24) from the measurements performed by the SMHF,
for children aged 5–16 years, the values derived by Skryabin et al. (24) from the measurements performed by the SMHF were used, and
for children aged 17 or 18 years, the thyroid-mass values were obtained by extrapolation from the measurements performed on the children aged 10 to 16 years (24).
No measurements of thyroid volume were performed in the city of Minsk. Age- and gender-specific thyroid masses for the subjects from the city of Minsk were taken to be the same as these in Gomel Oblast (as recommended by Professor V. Drozd, personal communication, Minsk, 2008).
Thyroid Doses from Minor Exposure Pathways
In addition to 131I intake, three other contributions to the thyroid dose, which were usually quite small for the majority of individuals, were considered in the dose reconstruction:
-
Intake of short-lived radionuclides (132I, 133I and 132Te) by inhalation and/or ingestion, up to 15 May 1986.
External irradiation from the radionuclides deposited on the ground, doses accrued in each calendar year during the 1986–2006 time period.
134Cs and 137Cs ingestion, doses accrued in each calendar year during the 1986–2006 time period.
The distance of the settlement of residence from the Chernobyl nuclear power plant and the pattern of the radioactive clouds in the first few days after the accident were used for the evaluation of the thyroid doses from short-lived radioiodines and radiotellurium. For each exposure pathway (i.e., inhalation, milk consumption and consumption of leafy vegetables), the age-dependent and region specific ratios of the contributions to the internal thyroid dose due to the intake of short-lived radionuclides and of 131I were estimated. The description of the model used to reconstruct thyroid doses due to intakes of short-lived 132I, 133I and 132Te can be found elsewhere (27).
The approach used to estimate the thyroid doses due to external irradiation from the radionuclides deposited on the ground is the integration of the time-dependent dose rate in air per unit deposition taking into account the shielding properties typical for the residential environment of the settlement where the study subject resided. The variation with time of the dose rate in air was calculated using data on mixture of radionuclides in ground deposition and dose rate in air per unit activity of radionuclide deposited on the ground surface. Calculation of the thyroid doses due to 134Cs and 137Cs ingestion was based on the integration of 137Cs intake function per unit 137Cs deposition and per unit of 137Cs soil-to-milk transfer that were known for the settlement where the study subject resided. The 137Cs intake function was derived from the results of the whole-body measurements of radiocesium body burden that were carried out in regions of Belarus with different contamination levels and in populations of different ages. A detailed description of the models used to calculate the thyroid doses from external irradiation and ingestion of Cs isotopes can be found elsewhere (12).
Only the information on residential history was collected during the personal interview for the time period after 1 July 1986; the study subjects were not asked about their consumption of foodstuffs after 1 July 1986. Therefore, annual thyroid doses due to external irradiation and ingestion of Cs isotopes calculated in this study represent agespecific mean doses in the settlement of residence.
RESULTS
Thyroid Doses from 131I intakes
Table 5 shows the distribution of the thyroid doses from 131I intakes that were reconstructed for the 11,732 Belarusian cohort members. The average thyroid dose was estimated to be 0.58 Gy, while the median was 0.23 Gy. About 5% of the cohort members were estimated to have received doses greater than 2 Gy, with 34 individuals receiving thyroid doses due to 131I intakes of more than 10 Gy. Nineteen of them were evacuees from the 30-km zone around the Chernobyl nuclear power plant; 14 individuals resided in the southern part of Gomel Oblast close to the Chernobyl nuclear power plant; and one person lived in a highly contaminated settlement of Mogilev Oblast with an 131I deposition density of 18 MBq m−2. The highest individual dose from 131I intake was 33 Gy. Table 6 compares the thyroid doses in terms of age at the time of the accident. As can be seen in Table 6, the thyroid dose decreased with increasing age. The mean thyroid dose from 131I intakes received by children aged 1 year is greater than that for 18-years adolescents by a factor of 3.3.
TABLE 5.
Distribution of the Thyroid Doses from 131I Intakes for the 11,732 Belarusian Cohort Members
| Dose interval (Gy) | N | Percentage | Mean dose (Gy) |
|---|---|---|---|
| <0.05 | 2,073 | 17.7 | 0.024 |
| 0.05–0.2 | 3,334 | 28.4 | 0.12 |
| 0.2–0.5 | 2,867 | 24.4 | 0.33 |
| 0.5–2 | 2,812 | 24.0 | 0.96 |
| 2–5 | 515 | 4.4 | 3.0 |
| 5–10 | 97 | 0.8 | 6.7 |
| >10 | 34 | 0.3 | 15 |
| Entire cohort | 11,732 | 100.0 | 0.58 |
TABLE 6.
Distribution of Thyroid Doses from 131I Intakes According to Age of the Belarusian Cohort Members at the Time of the Accident
| Thyroid doses from 131I intakes (Gy) |
|||||
|---|---|---|---|---|---|
| Age, years | N | Mean | Median | Min | Max |
| 0–0.9 | 650 | 1.1 | 0.45 | 0.005 | 17 |
| 1–1.9 | 811 | 1.2 | 0.45 | 0.003 | 27 |
| 2–2.9 | 768 | 1.0 | 0.36 | 0.003 | 33 |
| 3–3.9 | 823 | 0.78 | 0.24 | 0.002 | 20 |
| 4–4.9 | 771 | 0.51 | 0.20 | 0.002 | 7.1 |
| 5–5.9 | 757 | 0.45 | 0.18 | 0.002 | 9.3 |
| 6–6.9 | 694 | 0.44 | 0.16 | 0.002 | 5.8 |
| 7–7.9 | 689 | 0.48 | 0.19 | 0.001 | 8.5 |
| 8–8.9 | 678 | 0.47 | 0.21 | 0.002 | 5.9 |
| 9–9.9 | 662 | 0.54 | 0.21 | 0.002 | 12 |
| 10–10.9 | 658 | 0.46 | 0.20 | 0.001 | 15 |
| 11–11.9 | 644 | 0.50 | 0.22 | 0.001 | 11 |
| 12–12.9 | 629 | 0.45 | 0.19 | 0.001 | 15 |
| 13–13.9 | 550 | 0.47 | 0.24 | 0.001 | 6.3 |
| 14–14.9 | 577 | 0.46 | 0.21 | 0.001 | 8.8 |
| 15–15.9 | 539 | 0.48 | 0.23 | 0.001 | 6.9 |
| 16–16.9 | 468 | 0.42 | 0.20 | 0.001 | 3.3 |
| 17–17.9 | 364 | 0.38 | 0.18 | 0.0005 | 3.6 |
Table 7 shows the thyroid doses from 131I intakes according to the place of residence at the time of the accident, type of the settlement, gender and pathway of exposure. The highest doses (mean of 0.7 Gy and median of 0.32 Gy) were found in Gomel Oblast, the most contaminated area. The higher thyroid doses observed in the rural areas (Table 7) could be explained by higher milk consumption rates there compared to urban populations (0.58 vs. 0.33 L d−1, respectively for mean) and a higher contamination of cow’s milk with 131I in rural settlements compared to urban areas.
TABLE 7.
Thyroid Doses from 131I Intakes Broken Down According to Various Groupings of the Belarusian Cohort Members
| Thyroid doses (Gy) |
|||
|---|---|---|---|
| Parameter | N | Mean | Median |
| Place of residence at the time of the accident | |||
| Gomel Oblast | 8,968 | 0.70 | 0.32 |
| Mogilev Oblast | 922 | 0.24 | 0.11 |
| Minsk City | 1,503 | 0.11 | 0.028 |
| Others | 339 | 0.20 | 0.038 |
| Type of settlement of residence | |||
| Rural | 6,558 | 0.77 | 0.36 |
| Urban | 5,174 | 0.29 | 0.11 |
| Gender | |||
| Male | 5,693 | 0.63 | 0.26 |
| Female | 6,039 | 0.53 | 0.21 |
| Pathway of exposure | |||
| Inhalation of 131I | 11,730a | 0.072 | 0.010 |
| Intake of 131I in milk | 10,921 | 0.41 | 0.15 |
| Intake of 131I in milk products | 8,504 | 0.087 | 0.027 |
| Intake of 131I in leafy vegetables | 7,946 | 0.081 | 0.018 |
| Entire cohort | 11,732 | 0.58 | 0.23 |
Two cohort members did not have inhalation intake as they resided at non-contaminated from the Chernobyl fallout areas outside Belarus at the time of the accident and returned to Belarus after 131I deposition finished.
Thyroid doses for boys were higher than those for girls, 0.63 vs. 0.53 Gy for mean and 0.26 vs. 0.21 Gy for median (Table 7). There were no gender-specific differences in parameters of the dosimetry model used in this study, except for the thyroid mass for subjects aged 10 years and older. Thyroid mass values are higher among girls compared to boys at age 10–14 years and vice versa for persons aged from 15 to 18 years (Table A1.6: see Supplementary Material: http://dx.doi.org/10.1667/RR3153.1.S1). Higher doses among boys than among girls were due to the larger fraction of milk consumers and higher consumption of milk among boys (0.57 vs. 0.44 L d−1, respectively, for the mean). Intake of 131I in milk was the main pathway for thyroid exposure (Table 7). The mean thyroid dose from intake of 131I in milk among the study subjects was 0.41 Gy (0.15 Gy for the median). Thyroid doses from inhalation of 131I, intake of 131I in milk products and in leafy vegetables were lower, 0.07, 0.09 and 0.08 Gy for the mean, respectively (0.01, 0.03 and 0.02 Gy for the median).
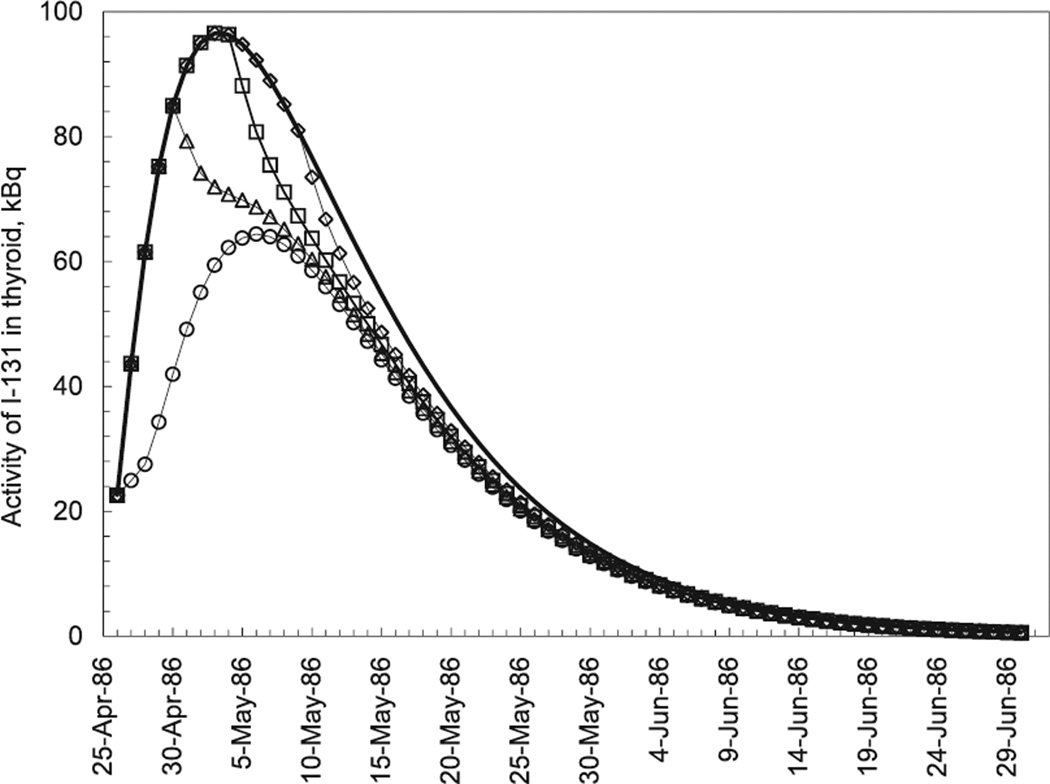
Stable iodine administration led to substantial reduction in thyroid doses from 131I intakes. Figure 5 shows the activity of 131I in thyroid calculated for different behavior scenarios: a subject who did not take stable iodine after the accident, or who took stable iodine on 27 April, 1 May, 5 May or 10 May 1986. Calculations were done assuming that the subject was aged 5, lived in a settlement with a 131I ground deposition density of 1 MBq m−2and consumed 0.6 L d−1 of private cow’s milk. As can be seen from Fig. 5, stable iodine administered on 5 May 1986 and later (i.e., 10 and more days after the accident) did not substantially prevent exposure of the thyroid gland to 131I. About 30% of the thyroid dose due to 131I intake was prevented if stable iodine was administered the day after the accident (on 27 April); around 15, 10 and 5%, if stable iodine was administered 5 days after the accident (on 1 May), 10 days after the accident (on 5 May) or 15 days after the accident (on 10 May), respectively.
FIG. 5.
Activity of 131I in thyroid calculated for different scenarios of stable iodine administration: no intake of stable iodine after the accident (solid line), and intake of stable iodine on 27 April (open circles), 1 May (open triangles), 5 May (open squares), or 10 May 1986 (open diamonds).
Stable iodine was administered before 10 May 1986 to 3,145 (72%) of the 4,355 subjects who reported administration of stable iodine (Table 4). However, only 803 (7%) of the entire cohort of 11,732 study subjects reported intake of stable iodine shortly after the accident, between 26 April and 30 April 1986, when blockade of radioactive iodine uptake was the most effective to prevent thyroid exposure. Indeed, the median thyroid dose among the cohort members who were evacuated from the 30-km zone was 0.45 Gy for 158 persons who took stable iodine between 26 April and 30 April 1986 and 0.57 Gy for those (701 persons) who did not take stable iodine. For non-evacuees from Bragin, Khoiniki and Narovla raions the difference is less marked: the median thyroid dose was 0.30 Gy for 449 persons who took stable iodine between 26 April and 30 April 1986 and 0.33 Gy for those (2,289 persons) who did not take stable iodine.
Thyroid Doses from Minor Exposure Pathways
Table 8 shows the thyroid doses from the minor exposure pathways compared to the thyroid doses from 131I intakes. The estimated doses from short-lived iodine and tellurium isotopes ranged up to 4.9 Gy. The estimated individual thyroid doses from external exposure ranged up to 0.13 Gy, while those from internal exposure due to cesium ingestion did not exceed 0.05 Gy. The mean thyroid dose among the study subjects from all contributions was 0.61 Gy, while the median was 0.25 Gy. Intake of 131I was the major pathway for thyroid exposure; its mean contribution to the thyroid dose was 92%.
TABLE 8.
Thyroid Doses from Different Exposure Pathways Estimated for the Belarusian Cohort Members
| Mean thyroid dose (Gy) due to |
||||||
|---|---|---|---|---|---|---|
| Range of thyroid dose From 131I intakes (Gy) |
N | Intakes of131I |
Intakes of short-lived radionuclidesa |
External exposureb |
134,137Cs ingestionb |
Total thyroid dose (Gy) |
| <0.05 | 2,073 | 0.024 | 0.0003 | 0.004 | 0.002 | 0.03 |
| 0.05–0.2 | 3,334 | 0.12 | 0.003 | 0.009 | 0.003 | 0.13 |
| 0.2–0.5 | 2,867 | 0.33 | 0.010 | 0.011 | 0.003 | 0.35 |
| 0.5–2 | 2,812 | 0.96 | 0.034 | 0.012 | 0.003 | 1.0 |
| 2–5 | 515 | 3.0 | 0.13 | 0.012 | 0.003 | 3.2 |
| 5–10 | 97 | 6.7 | 0.50 | 0.015 | 0.002 | 7.2 |
| >10 | 34 | 15 | 0.85 | 0.020 | 0.002 | 15 |
| Entire cohort | 11,732 | 0.58 | 0.024 | 0.010 | 0.003 | 0.61 |
Short-lived radioiodines 132I, 133I, and 132Te.
Doses accumulated in 1986–2006.
DISCUSSION
This article is our first publication on large-scale dose reconstruction done in a cohort of Belarusian persons exposed to radiation in childhood and adolescence following the Chernobyl accident. Overall, individual thyroid doses due to the main pathways of exposure were estimated for 11,732 study subjects. Intake of 131I was the major pathway of thyroid exposure. Among all Belarusian study subjects, the mean contributions to total thyroid dose from sources of exposure other than 131I intake were estimated to be 2% for intake of short-lived radioiodine isotopes and 132Te, 4.5% for external exposure and 1.5% for ingestion of cesium isotopes. This confirms earlier findings on the predominant role of 131I in the radiation exposure of the thyroid as a result of the Chernobyl accident (12, 28).
We found that thyroid doses from 131I intakes for boys were higher than those for girls due to the larger fraction of milk consumers and higher consumption of milk among boys than that among girls. The same gender-specific differences in thyroid doses from 131I intakes were observed previously in a group of 1,615 children from Belarus and Russia exposed as a result of the Chernobyl accident (29).
The thyroid doses from 131I intakes that are calculated in this study range from 0.54 mGy to 33 Gy, i.e., almost five orders of magnitude. The wide variability in dose reflects the variability in 131I deposition across the country, different consumption habits among study subjects, difference in thyroid mass in persons of different ages and other factors. The scaling factor, which is defined as the ratio of the “measured” 131I activity in the thyroid to the “ecological” 131I activity at the time of measurement (Fig. 2), integrates all steps of the thyroid dose estimation: results of direct thyroid measurement, modeling, and personal interview data. The scaling factor is an indicator of the agreement between the dose estimated using the model and the questionnaire data and the dose derived from the direct thyroid measurement. The closer the scaling factor is to one, the closer the ecological dose is to the instrumental dose. To understand fully the dose assessment, we analyzed the outliers with very low and very high scaling factors. In summary (for details, see Supplementary Material Appendix 2: http://dx.doi.org/10.1667/RR3153.1.S2) the possible reasons for obtaining widely different values for the instrumental and the ecological thyroid dose appear to be: (a) incorrect answers provided during the interviews, (b) the assignment of the direct thyroid measurement to a wrong subject, and/or (c) clerical errors in the recording of the result of the direct thyroid measurement. It is worth pointing out that the assumptions were made in the determination of the point dose estimates that the recorded results of the direct thyroid measurements were correct and had always been assigned to the right subjects, and that the answers provided during the interviews were also correct.
Uncertainties in Doses
The wide inter-individual variability of the scaling factors obtained in this study shows that there are large uncertainties in the estimated thyroid doses. The analysis of the very low and of the very large scaling factors (for details, Supplementary Material Appendix 2: http://dx.doi.org/10.1667/RR3153.1.S2) indicates that clerical errors may have been made, either in the assignment of the direct thyroid measurement or when recording the result of that direct thyroid measurement. In addition to those errors, which cannot be easily quantified, there are many sources of uncertainty that affect the estimation of the thyroid doses of all subjects. The major sources of uncertainty that are currently under investigation include:
Errors in the 131I activities in thyroids that were derived from direct thyroid measurements. These measured errors arose from device error itself, uncertainties in the estimates of the device’s calibration factors, and uncertainties in evaluation of correction factor that takes into account influence of external and internal contamination of human body on measured exposure rate near the thyroid. These sources of unshared errors are important as measured activity defines the individual dose.
The uncertainties attached to the parameters of the ecological model. Although there are variabilities in the 131I deposition in a given location, the same value of deposition needs to be applied for all persons who resided in that location. The majority of the parameters involved in the ecological model are considered to be shared or subject independent.
The uncertainties attached to the biokinetic models. Obviously, there are variabilities in the thyroid mass and metabolic parameters between individuals. These sources of unshared errors are important because the endpoint of the study is the estimation of individual doses.
The uncertainties attached to the information obtained in 2001–2007 during personal interviews regarding relocation history and individual diet. The reliability of this information is not high as it was collected more than 15 years ago after the accident.
Reliability of The Thyroid Dose Estimates
The thyroid dose estimates due to 131I intakes that have been obtained in this study can be compared to those of a similar study that was conducted in an Ukrainian cohort of 13,215 subjects using the same methodology (29). Although the populations were different, the results were very similar in terms of arithmetic mean thyroid dose (0.68 Gy in Ukraine vs. 0.58 Gy in this study), geometric mean thyroid dose (0.23 Gy in both countries), and ranges of thyroid doses (from 0.0006 to 42 Gy in Ukraine vs. 0.0005 to 33 Gy in this study). The average thyroid doses also are in qualitative agreement with the results presented by UNSCEAR (1) for the entire Gomel Oblast of Belarus, where approximately 80% of the study subjects were exposed to 131I fallout: 0.48 Gy for preschool children, 0.25 Gy for school children and 0.15 Gy for adolescents. The UNSCEAR values are somewhat smaller than the average value obtained for the study subjects (0.58 Gy), probably because UNSCEAR refers to the entire population of the Gomel Oblast and not to the population of its most contaminated raions, as in this study.
The two Chernobyl cohort studies in Belarus and Ukraine make use of the best dose estimation methodology that is currently available and are the only ones in which a doserelated measurement (that is, a direct thyroid measurement) was performed on all subjects. In all other studies, the methodology of dose estimation was based on the direct thyroid measurements that were available for only a fraction of the subjects, while the thyroid doses received by the other subjects are derived from relationships with the 137Cs or 131I deposition densities [e.g., (27, 28, 30)]. The strong reliance on the direct thyroid measurements is due to the fact that these measurements can be considered as the most reliable information for dose assessment purposes, despite associated uncertainties arising from errors in the estimation of the 131I thyroidal content and in the evaluation of the 131I intake function (10).
In summary, although a point estimate of dose was provided for each study subject according to the best methodology currently available, there are obvious uncer uncertainties tainties associated with reconstructed doses arising from errors in estimates of 131I activity in thyroid; errors attached to the parameters of the ecological and the biokinetic models; and reliability of the information obtained during interviews regarding personal behavior more than 15 years ago. Calculation for each study subject of a set of 1,000 stochastic thyroid dose estimates which takes into account classification of errors as classical/Berkson and shared/ unshared is underway. For each subject, the resulting database will include, in addition to the 1,000 stochastic thyroid dose estimates, the value of the scaling factor to provide an indication on the quality of the dose. These sets of dose estimates are being used to evaluate radiation risk that takes into account the structure of the errors in the dose estimates.
Supplementary Material
ACKNOWLEDGMENTS
This work has been funded by the National Institutes of Health, National Cancer Institute (U.S.) within the framework of the Belarus-U.S. Study of Thyroid Cancer and Other Diseases Following the Chernobyl Accident; the ISTC Project no. B-488p; and the Intra-Agency Agreement between the National Institute of Allergy and Infectious Diseases (USA) and the National Cancer Institute, NIAID agreement no. Y2-Al-5077 and NCI agreement no. Y3-CO-5117. We would like to thank our colleagues who contributed to the development of the dose reconstruction methods at different stages of the study: Drs L. Anspaugh (U.S.), A. Ulanovsky (Germany), Yu. Konstantinov (Russia), V. Averin (Belarus) and A. Mirkhaidarov (Belarus). The authors are grateful to all of the subjects who participated in the study. Special thanks are to the staff that conducted personal interviews and processed and verified the information collected during personal interviews into databases: Mss. T. Avramenko, N. Garbuzova, T. Lazuko, L. Sentebova, E. Shemiakina, and Mr. S. Stelmashok (Belarusian Medical Academy of Post-Graduate Education, Minsk, Belarus); Mss. N. Aprishko, E. Drozd, G. Evtushkova, T. Shitikova, A. Silina, and Mr. V. Tolstoy (Republican Research Center for Radiation Medicine and Human Ecology, Gomel, Belarus). The authors would like to thank the NIH Library Writing Center for manuscript editing assistance.
Footnotes
The online version of this article (DOI: 10.1667/ RR3153.1) contains supplementary information that is available to all authorized users.
An oblast is the largest administrative unit in Belarus. The typical size of an oblast is 30,000–40,000 km2 with a population of 1.1–1.5 million persons. There are six oblasts in Belarus. Oblasts are subdivided into raions; there are typically about 20 raions of similar size and population in one oblast.
For one study subject there was no information on raion of residence in Mogilev Oblast at the time of the accident and, therefore, this subject is not counted in numbers shown in Fig. 1 for Mogilev Oblast.
Supplementary Information
Appendix 1 includes description of the model used to calculate the ecological and instrumental thyroid doses (http://dx.doi.org/10.1667/RR3153.1.S1). Appendix 2 provides assessment of outliers of scaling factor (http://dx.doi.org/10.1667/RR3153.1.S2).
REFERENCES
- 1.Annex D: Health effects due to radiation from the Chernobyl accident. New York: United Nations Scientific Committee on the Effects of Atomic Radiation; United Nations; 2011. Sources and Effects of Ionizing Radiation, UNSCEAR 2008 Report. Sales No. E.11.IX.3. [Google Scholar]
- 2.Kazakov VS, Demidchik EP, Astakhova LN. Thyroid cancer after Chernobyl. Nature. 1992;359:321. doi: 10.1038/359021a0. [DOI] [PubMed] [Google Scholar]
- 3.Prisyazhiuk A, Pjatak OA, Buzanov VA, Reeves GK, Beral V. Cancer in the Ukraine, post Chernobyl. Lancet. 1991;338:1334–1335. doi: 10.1016/0140-6736(91)92632-c. [DOI] [PubMed] [Google Scholar]
- 4.Ivanov VK, Tsyb AF, Matveenko YG, Parshkov YM, Maksyutov MA, Gorskiy AI, et al. Radiation epidemiology of cancer- and non-cancer thyroid diseases in Russia after the ChNPP accident: Prognostication and risk estimation. Radiation and Risk. 1995;1:3–29. (in Russian) [Google Scholar]
- 5.Stezhko VA, Buglova EE, Danilova LI, Drozd VM, Krysenko NA, Lesnikova NR, et al. Chornobyl Thyroid Diseases Study Group of Belarus, Ukraine, the USA, 2004. A cohort study of thyroid cancer and other thyroid diseases following the Chornobyl accident: objectives, design, and methods. Radiat Res. 2004;161:481–492. doi: 10.1667/3148. [DOI] [PubMed] [Google Scholar]
- 6.Gavrilin YuI, Khrouch VT, Shinkarev SM, Krysenko NA, Skryabin AM, Bouville A, et al. Chernobyl accident: reconstruction of thyroid dose for inhabitants of the Republic of Belarus. Health Phys. 1999;76:105–119. doi: 10.1097/00004032-199902000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Evaluation of the formation of external and internal exposure of the population living in contaminated areas, in order to refine methods for determining the annual effective dose: RCIRME Report #1996507. Minsk: Research and Clinical Institute of Radiation Medicine and Endocrinology; 1997. (in Russian) [Google Scholar]
- 8.Ternov VI, Kondratiev AG. Exposure rate in Belorussian SSR in 1981–1982. Healthcare of Belorussia. 1990;6:61–62. (in Russian) [Google Scholar]
- 9.ISO 11929-1: 2000 (E) Geneva, Switzerland: International Organization for Standardization; 2000. Determination of the detection limit and decision threshold for ionizing radiation measurements - Part I: Fundamentals and application to counting measurements without the influence of sample treatment. [Google Scholar]
- 10.Likhtarev I, Minenko V, Khrouch V, Bouville A. Uncertainties in thyroid dose reconstruction after Chernobyl. Radiat Prot Dosim. 2003;105:601–608. doi: 10.1093/oxfordjournals.rpd.a006310. [DOI] [PubMed] [Google Scholar]
- 11.Zvonova IA, Balonov MI, Bratilova AA. Thyroid dose reconstruction for population of Russia suffered after the Chernobyl accident. Radiat Prot Dosim. 1998;79:175–178. [Google Scholar]
- 12.Minenko VF, Ulanovsky AV, Drozdovitch VV, Shemiakina EV, Gavrilin YI, Khrouch VT, et al. Individual thyroid dose estimates for a case-control study of Chernobyl-related thyroid cancer among children of Belarus. Part II. Contributions from long-lived radionuclides and external radiation. Health Phys. 2006;90:312–327. doi: 10.1097/01.HP.0000183761.30158.c1. [DOI] [PubMed] [Google Scholar]
- 13.Ulanovsky A, Drozdovitch V, Bouville A. Influence of radionuclides distributed in the whole body on the thyroid dose estimates obtained from direct thyroid measurements made in Belarus after the Chernobyl accident. Radiat Prot Dosim. 2004;112:405–418. doi: 10.1093/rpd/nch410. [DOI] [PubMed] [Google Scholar]
- 14.Khrutchinsky A, Drozdovitch V, Kutsen S, Minenko V, Khrouch V, Luckyanov N, et al. Mathematical modeling of a survey-meter used to measure radioactivity in human thyroids: Monte Carlo calculations of device response and uncertainties. Applied Radiation Isotopes. 2012;70:743–751. doi: 10.1016/j.apradiso.2011.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Talerko N. Mesoscale modelling of radioactive contamination formation in Ukraine caused by the Chernobyl accident. J Environ Radioact. 2005;78:311–329. doi: 10.1016/j.jenvrad.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 16.Talerko N. Reconstruction of (131)I radioactive contamination in Ukraine caused by the Chernobyl accident using atmospheric transport modelling. J Environ Radioact. 2005;84:343–362. doi: 10.1016/j.jenvrad.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 17.Drozdovitch V, Zhukova O, Germenchuk M, Khrutchinsky A, Kukhta T, Luckyanov N, et al. Database of meteorological and radiation measurements made in Belarus during the first three months following the Chernobyl accident. J Environ Radioact. 2013;116:84–92. doi: 10.1016/j.jenvrad.2012.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deposition density of 137Cs over the territory of Belarus. Minsk: Committee for Hydrometeorology of Belarus; Belgeodezy; 2001. [Google Scholar]
- 19.Zhukova OM, Germenchuk MG, Podgaiskaya MA, Golikov YN, Bakarykava ZV, Khrutchinsky AA, et al. Reconstruction of 131I deposition after the Chernobyl accident in the territory of Gomel and Mogilev regions of Belarus. Nature Resources. 2011;2:114–121. (in Russian) [Google Scholar]
- 20.Resolution of Chief Medical Officer # 4104-86, 6 May 1986. Ministry of Health of the USSR; 1986. Temporary permissible levels of radionuclides in food and drinking water. (in Russian) [Google Scholar]
- 21.Drozdovitch V, Germenchuk M, Bouville A. Using of total betaactivity measurements in milk to derive thyroid doses from Chernobyl fallout. Radiat Prot Dosim. 2006;118:402–411. doi: 10.1093/rpd/nci360. [DOI] [PubMed] [Google Scholar]
- 22.Age-dependent doses to members of the public from intake of radionuclides: Part 4. Inhalation dose coefficients. ICRP Publication 71; Ann ICRP. 1995;25(3/4) [PubMed] [Google Scholar]
- 23.Age-dependent doses to members of the public from intake of radionuclides: Part 2. Ingestion dose coefficients. ICRP Publication 67; Ann ICRP. 1993;23(3/4) [PubMed] [Google Scholar]
- 24.Skryabin AM, Drozdovitch V, Belsky Yu, Leshcheva SV, Mirkhaidarov AK, Voilleque P, et al. Thyroid mass in children and adolescents living in the most exposed areas to Chernobyl fallout in Belarus. Radiat Prot Dosim. 2010;142:292–299. doi: 10.1093/rpd/ncq209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ashizawa K, Shibata Y, Yamashita S, Namba H, Hoshi M, Yokoyama N, et al. Prevalence of goiter and urinary iodine excretion levels in children around Chernobyl. J Clin Endocrinol Metab. 1997;82:3430–3433. doi: 10.1210/jcem.82.10.4285. [DOI] [PubMed] [Google Scholar]
- 26.Basic Anatomical and Physiological Data for Use in Radiological Protection: Reference Values. ICRP Publication 89. Ann ICRP. 2002;32:3–4. [PubMed] [Google Scholar]
- 27.Gavrilin Y, Khrouch V, Shinkarev S, Drozdovitch V, Minenko V, Shemiakhina E, et al. Individual thyroid dose estimation for a casecontrol study of Chernobyl-related thyroid cancer among children of Belarus Part 1: 131I, short-lived radioiodines (132I, 133I, 135I), and short-lived radiotelluriums (131mTe and 132Te) Health Phys. 2004;86:565–585. doi: 10.1097/00004032-200406000-00002. [DOI] [PubMed] [Google Scholar]
- 28.Drozdovitch V, Khrouch V, Maceika E, Zvonova I, Vlasov O, Bratilova A, et al. Reconstruction of radiation doses in a casecontrol study of thyroid cancer following the Chernobyl accident. Health Phys. 2010;99:1–16. doi: 10.1097/HP.0b013e3181c910dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Likhtarev I, Bouville A, Kovgan L, Luckyanov N, Voillequé P, Chepurny M. Questionnaire- and measurement-based individual thyroid doses in Ukraine resulting from the Chornobyl nuclear reactor accident. Radiat Res. 2006;166:271–286. doi: 10.1667/RR3545.1. [DOI] [PubMed] [Google Scholar]
- 30.Stepanenko VF, Voillequé PG, Gavrilin YuI, Khrouch VT, Shinkarev SM, Orlov MYu, et al. Estimating individual thyroid doses for a case-control study of childhood thyroid cancer in Bryansk oblast, Russia. Radiat Prot Dosim. 2004;108:143–160. doi: 10.1093/rpd/nch017. [DOI] [PubMed] [Google Scholar]
- 31.Arefieva ZS, Badyin VI, Gavrilin YuI, Gordeev KI, Ilyin LA, Kruchkov VP, et al. Guidance on thyroid dose assessment for a man due to his radioiodine intake. Ilyin LA, Edt. Moscow: Energoatomizdat Publishing House; 1988. (in Russian) [Google Scholar]
- 32.Pro¨hl G. Interception of dry and wet deposited radionuclides by vegetation. J Environ Radioact. 2009;100:675–682. doi: 10.1016/j.jenvrad.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 33.Korobova E, Anoshko Y, Kesminiene A, Kouvyline A, Romanov S, Tenet V, et al. Evaluation of stable iodine status of the areas affected by the Chernobyl accident in an epidemiological study in Belarus and the Russian Federation. Journal of Geochemical Exploration. 2010;107:124–135. [Google Scholar]
- 34.Generic assessment procedures for determining protective actions during a reactor accident. Vienna: International Atomic Energy Agency; 1997. IAEA-TECDOC-955. [Google Scholar]
- 35.Müller H, Pröhl G. ECOSYS-87: A dynamic model for assessing radiological consequences of nuclear accidents. Health Phys. 1993;64:232–252. doi: 10.1097/00004032-199303000-00002. [DOI] [PubMed] [Google Scholar]
- 36.Drozdovitch VV, Goulko GM, Minenko VF, Paretzke HG, Voigt G, Kenigsberg YaI. Thyroid dose reconstruction for the population of Belarus after the Chernobyl accident. Radiat Environ Biophys. 1997;36:17–23. doi: 10.1007/s004110050050. [DOI] [PubMed] [Google Scholar]
- 37.Ilyin LA, Arkhangel’skaya GV, Konstantinov YO, Likhtarev IA. Radioactive iodine in the problem of radiation safety. Moscow: Atomizdat; 1972. [Google Scholar]
- 38.Simon SL, Luckyanov N, Bouville A, VanMiddlesworth L, Weinstock RM. Transfer of 131I into human breast milk and transfer coefficients for radiological dose assessments. Health Phys. 2002;82:796–806. doi: 10.1097/00004032-200206000-00007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.