Abstract
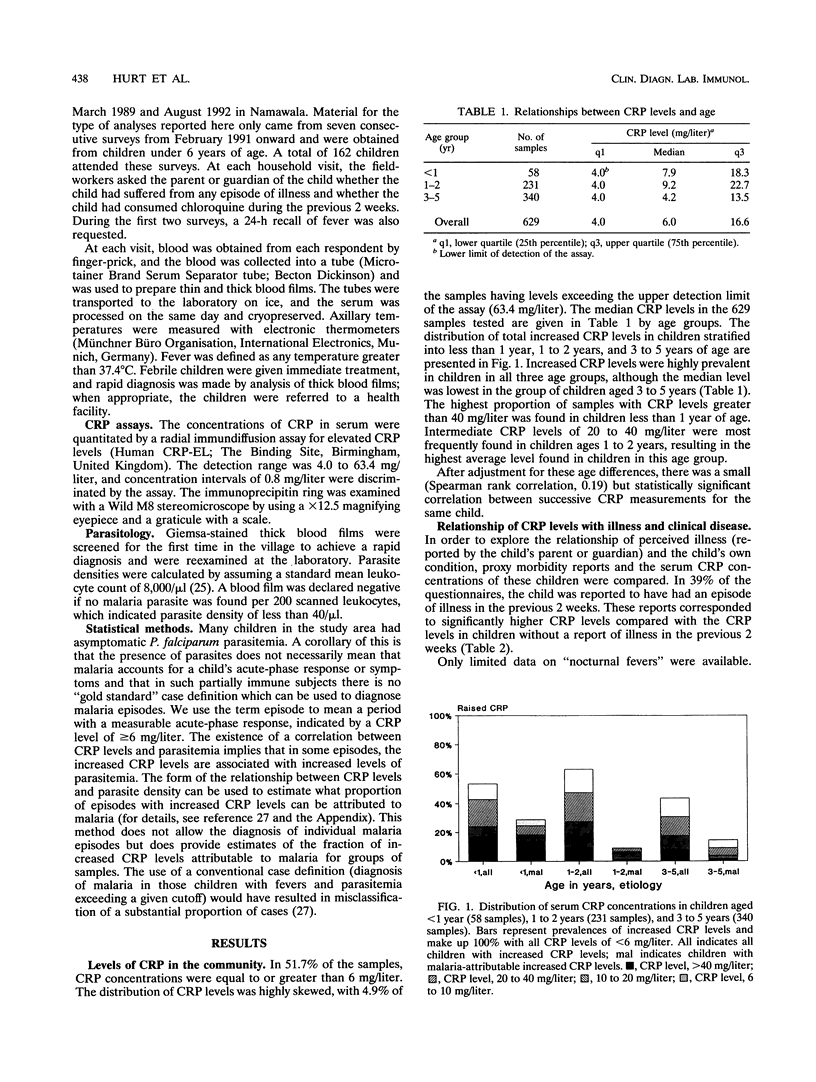
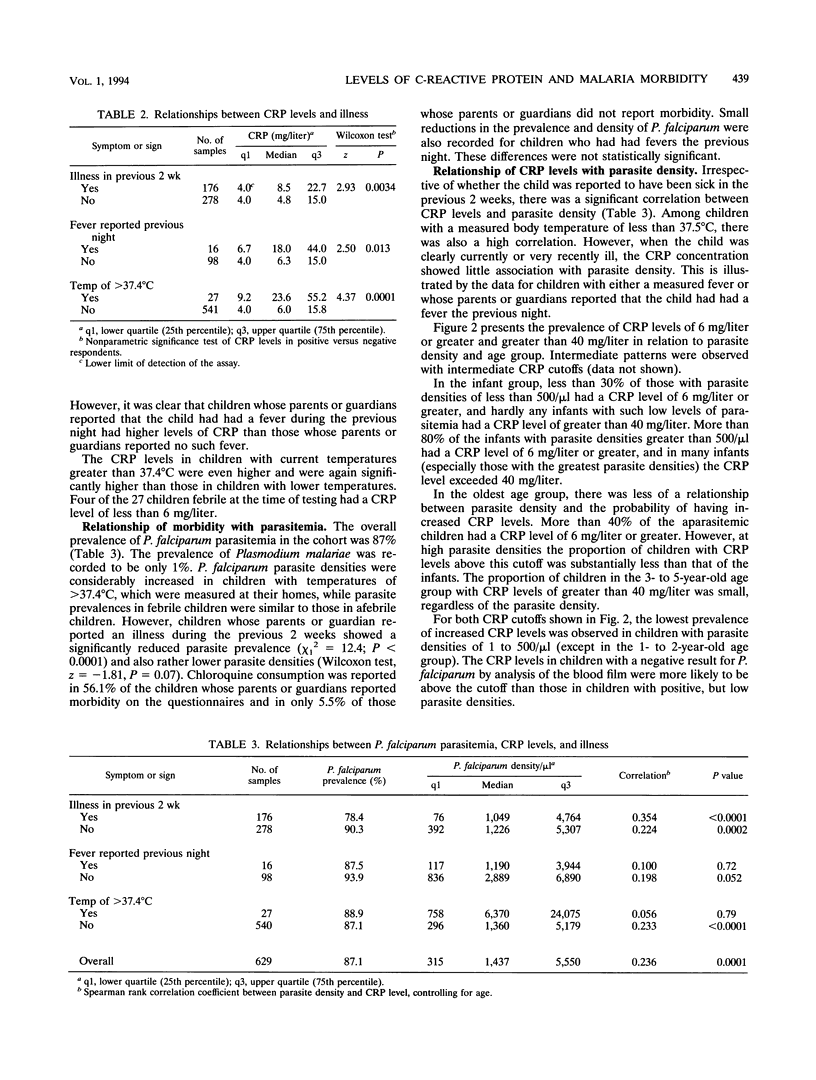
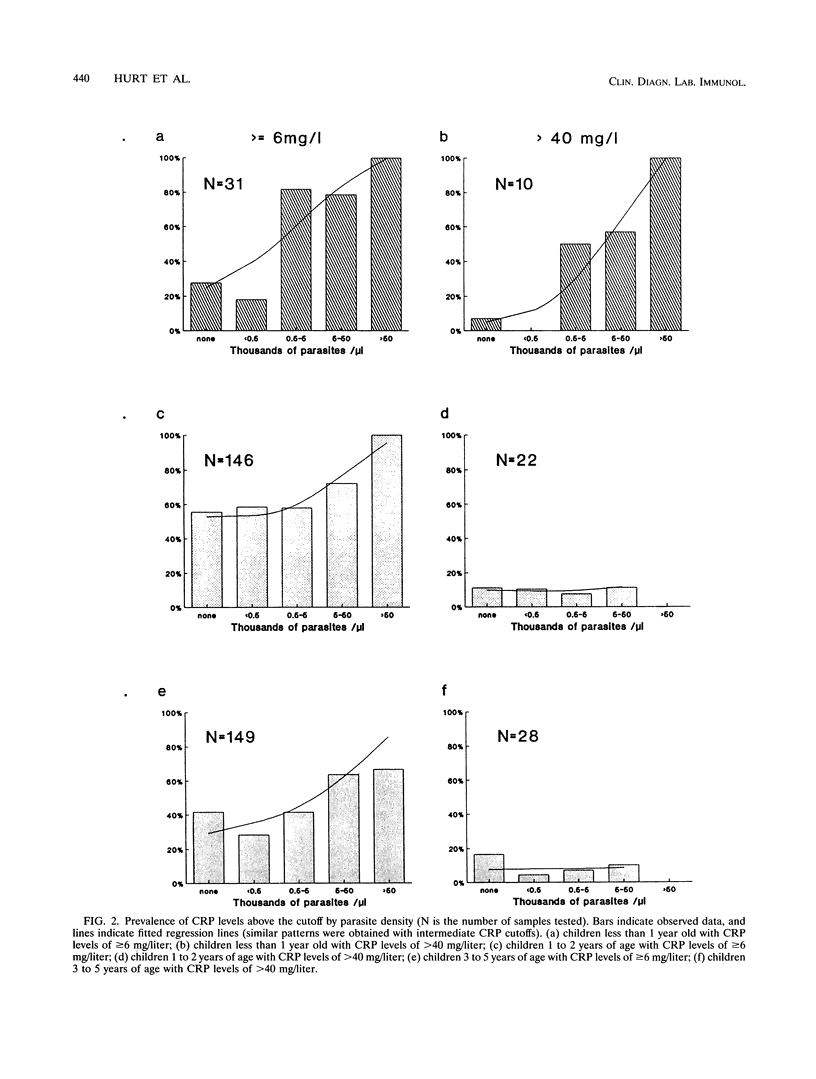
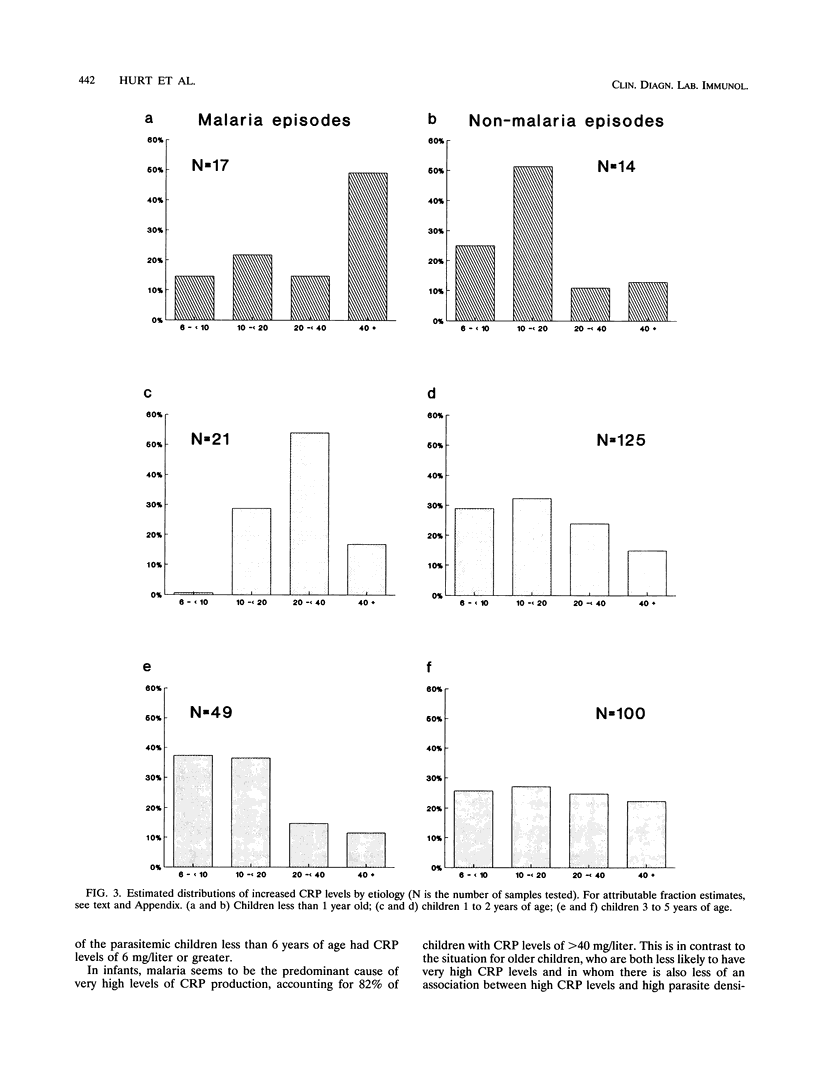
Children under 6 years of age living in an area of Tanzania highly endemic for malaria were tested for C-reactive protein (CRP) in order to determine how the acute-phase response is related to malaria in children of different ages and to investigate whether serum CRP concentrations might be useful in the qualification of morbidity in such children. The median CRP level in the 629 finger-prick blood samples measured, 6.0 mg/liter, was much higher than that reported in the blood of children in Europe. The CRP concentration was correlated with recent illness reported by the parents. High CRP levels were most strongly associated with Plasmodium falciparum parasitemia in children under 1 year of age. In older children, lower levels of CRP were associated with parasitemia, and fewer children had increased CRP levels attributable to parasitemia. The levels of malaria-attributable CRP appear to track the acquisition of parasitological and clinical tolerance in this area with very high levels of P. falciparum transmission. Determination of CRP levels should be useful in the rapid assessment of the overall burden of morbidity, especially in infants. In areas where malaria is endemic, CRP associated with increased parasite densities provides an objective measure of malaria-specific morbidity. This would be an efficient approach to estimating malaria morbidity risks from small-scale serological surveys.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ballou S. P., Kushner I. C-reactive protein and the acute phase response. Adv Intern Med. 1992;37:313–336. [PubMed] [Google Scholar]
- Ballou S. P., Lozanski G. Induction of inflammatory cytokine release from cultured human monocytes by C-reactive protein. Cytokine. 1992 Sep;4(5):361–368. doi: 10.1016/1043-4666(92)90079-7. [DOI] [PubMed] [Google Scholar]
- Bürger W., Fennert E. M., Pohle M., Wesemeier H. C-reactive protein--a characteristic feature of health control in swine. Zentralbl Veterinarmed A. 1992 Oct;39(8):635–638. doi: 10.1111/j.1439-0442.1992.tb00227.x. [DOI] [PubMed] [Google Scholar]
- Cooper E. H., Forbes M. A., Hambling M. H. Serum beta 2-microglobulin and C reactive protein concentrations in viral infections. J Clin Pathol. 1984 Oct;37(10):1140–1143. doi: 10.1136/jcp.37.10.1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freed B., Walsh A., Pietrocola D., MacDowell R., Laffin R., Lempert N. Early detection of renal allograft rejection by serial monitoring of serum C-reactive protein. Transplantation. 1984 Feb;37(2):215–218. [PubMed] [Google Scholar]
- Gillespie S. H., Dow C., Raynes J. G., Behrens R. H., Chiodini P. L., McAdam K. P. Measurement of acute phase proteins for assessing severity of Plasmodium falciparum malaria. J Clin Pathol. 1991 Mar;44(3):228–231. doi: 10.1136/jcp.44.3.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graninger W., Thalhammer F., Hollenstein U., Zotter G. M., Kremsner P. G. Serum protein concentrations in Plasmodium falciparum malaria. Acta Trop. 1992 Dec;52(2-3):121–128. doi: 10.1016/0001-706x(92)90027-u. [DOI] [PubMed] [Google Scholar]
- Greenwood B. M. Asymptomatic malaria infections--do they matter? Parasitol Today. 1987 Jul;3(7):206–214. doi: 10.1016/0169-4758(87)90061-5. [DOI] [PubMed] [Google Scholar]
- Hind C. R., Savage C. O., Winearls C. G., Pepys M. B. Objective monitoring of disease activity in polyarteritis by measurement of serum C reactive protein concentration. Br Med J (Clin Res Ed) 1984 Apr 7;288(6423):1027–1030. doi: 10.1136/bmj.288.6423.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurt N., Smith T., Tanner M., Mwankusye S., Bordmann G., Weiss N. A., Teuscher T. Evaluation of C-reactive protein and haptoglobin as malaria episode markers in an area of high transmission in Africa. Trans R Soc Trop Med Hyg. 1994 Mar-Apr;88(2):182–186. doi: 10.1016/0035-9203(94)90287-9. [DOI] [PubMed] [Google Scholar]
- Malvy D. J., Povéda J. D., Debruyne M., Montagnon B., Burtschy B., Herbert C., Cacès P., Houot O., Amédée-Manesme O. Laser immunonephelometry reference intervals for eight serum proteins in healthy children. Clin Chem. 1992 Mar;38(3):394–399. [PubMed] [Google Scholar]
- Mayer A. D., McMahon M. J., Bowen M., Cooper E. H. C reactive protein: an aid to assessment and monitoring of acute pancreatitis. J Clin Pathol. 1984 Feb;37(2):207–211. doi: 10.1136/jcp.37.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCord F. B., Jenkins J. G., Lim J. H. C reactive protein concentration as screening test for bacterial infection in febrile children. Br Med J (Clin Res Ed) 1985 Dec 14;291(6510):1685–1686. doi: 10.1136/bmj.291.6510.1685-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naik P., Voller A. Serum C-reactive protein levels and falciparum malaria. Trans R Soc Trop Med Hyg. 1984;78(6):812–813. doi: 10.1016/0035-9203(84)90027-0. [DOI] [PubMed] [Google Scholar]
- Ndung'u J. M., Eckersall P. D., Jennings F. W. Elevation of the concentration of acute phase proteins in dogs infected with Trypanosoma brucei. Acta Trop. 1991 Jun;49(2):77–86. doi: 10.1016/0001-706x(91)90055-o. [DOI] [PubMed] [Google Scholar]
- Ramadori G., Van Damme J., Rieder H., Meyer zum Büschenfelde K. H. Interleukin 6, the third mediator of acute-phase reaction, modulates hepatic protein synthesis in human and mouse. Comparison with interleukin 1 beta and tumor necrosis factor-alpha. Eur J Immunol. 1988 Aug;18(8):1259–1264. doi: 10.1002/eji.1830180817. [DOI] [PubMed] [Google Scholar]
- Robey F. A., Ohura K., Futaki S., Fujii N., Yajima H., Goldman N., Jones K. D., Wahl S. Proteolysis of human C-reactive protein produces peptides with potent immunomodulating activity. J Biol Chem. 1987 May 25;262(15):7053–7057. [PubMed] [Google Scholar]
- Rooth I. B., Bjorkman A. Suppression of Plasmodium falciparum infections during concomitant measles or influenza but not during pertussis. Am J Trop Med Hyg. 1992 Nov;47(5):675–681. doi: 10.4269/ajtmh.1992.47.675. [DOI] [PubMed] [Google Scholar]
- Salonen E. M., Vaheri A. C-reactive protein in acute viral infections. J Med Virol. 1981;8(3):161–167. doi: 10.1002/jmv.1890080302. [DOI] [PubMed] [Google Scholar]
- Selwyn B. J. The epidemiology of acute respiratory tract infection in young children: comparison of findings from several developing countries. Coordinated Data Group of BOSTID Researchers. Rev Infect Dis. 1990 Nov-Dec;12 (Suppl 8):S870–S888. doi: 10.1093/clinids/12.supplement_s870. [DOI] [PubMed] [Google Scholar]
- Smith T., Charlwood J. D., Kihonda J., Mwankusye S., Billingsley P., Meuwissen J., Lyimo E., Takken W., Teuscher T., Tanner M. Absence of seasonal variation in malaria parasitaemia in an area of intense seasonal transmission. Acta Trop. 1993 Jun;54(1):55–72. doi: 10.1016/0001-706x(93)90068-m. [DOI] [PubMed] [Google Scholar]
- Tanner M., Degrémont A., de Savigny D., Freyvogel T. A., Mayombana C., Tayari S. Longitudinal study on the health status of children in Kikwawila village, Tanzania: study area and design. Acta Trop. 1987 Jun;44(2):119–136. [PubMed] [Google Scholar]
- Teuscher T. Household-based malaria control in a highly endemic area of Africa (Tanzania): determinants of transmission and disease and indicators for monitoring--Kilombero Malaria Project. Mem Inst Oswaldo Cruz. 1992;87 (Suppl 3):121–130. doi: 10.1590/s0074-02761992000700018. [DOI] [PubMed] [Google Scholar]
- Tilg H., Vannier E., Vachino G., Dinarello C. A., Mier J. W. Antiinflammatory properties of hepatic acute phase proteins: preferential induction of interleukin 1 (IL-1) receptor antagonist over IL-1 beta synthesis by human peripheral blood mononuclear cells. J Exp Med. 1993 Nov 1;178(5):1629–1636. doi: 10.1084/jem.178.5.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]



