Abstract
Introduction:
N′-nitrosonornicotine (NNN), an esophageal and oral carcinogen present in tobacco products, has a chiral center in its structure. Of its two enantiomers, (S)-NNN exhibits higher tumorigenic potency than (R)-NNN. There is no information available on the levels of (S)-NNN in various tobacco products currently marketed in the United States.
Methods:
We used chiral gas chromatography analysis to determine (S)-NNN levels in a convenience sample of 37 tobacco products currently marketed in the United States: conventional smokeless tobacco, novel smokeless tobacco products, and cigarette tobacco filler.
Results:
Among all products analyzed here, (S)-NNN averaged 62.9±6.3% (SD) of NNN. The absolute amount of (S)-NNN in conventional moist snuff averaged 1.26±0.5 µg/g tobacco; in novel smokeless products 0.70±0.2 µg/g tobacco; and in cigarette filler 1.36±0.6 µg/g tobacco (all values are per wet weight). For each cigarette brand, the enantiomeric composition of NNN in cigarette smoke was similar to that of the corresponding tobacco filler.
Conclusions:
Our results demonstrate that (S)-NNN is the predominant NNN enantiomer in moist snuff, novel smokeless tobacco products, and cigarettes currently marketed in the United States. Efforts toward the reduction of NNN in U.S. tobacco products should take into account its enantiomeric composition, with particular focus on (S)-NNN as a causative agent for esophageal and oral cancers associated with tobacco use.
INTRODUCTION
Existing scientific evidence indicates that the tobacco-specific nitrosamine N′-nitrosonornicotine (NNN) plays an important role in the induction by tobacco products of cancers of the esophagus and oral cavity (Hecht, 1998; International Agency for Research on Cancer [IARC], 2007; Yuan et al., 2011). In laboratory animals, NNN causes esophageal tumors in rats, nasal cavity tumors in rats and mink, and respiratory tumors in mice and hamsters (Hecht, 1998). Oral swabbing with a mixture of NNN and the related tobacco nitrosamine 4-(methylnitrosamino)- 1-(3-pyridyl)-1-butanone (NNK) induces tumors in the oral cavity of rats (Hecht et al., 1986), and our recent study demonstrated that the treatment of rats with NNN in drinking water can cause oral tumors in the absence of NNK (Balbo et al., 2012). IARC (2007) classifies NNN and NNK as human carcinogens (Group I).
The NNN molecule has a chiral center at its 2′ position, leading to the existence of two enantiomers: (S)-NNN and (R)-NNN. The 2′-hydroxylation pathway, which is the dominant metabolic activation pathway for NNN carcinogenicity in rat target tissues, is more favored in (S)-NNN metabolism (McIntee & Hecht, 2000). This, along with the results of studies on the metabolism and carcinogenicity of NNN enantiomers demonstrate that (S)-NNN is more tumorigenic than (R)-NNN to the rat esophagus and oral mucosa (Balbo et al., 2012; Lao, Yu, Kassie, Villalta, & Hecht, 2007; Zhang et al., 2009).
The levels of NNN in various tobacco products sold in the United States and worldwide are substantial and are higher than the levels of nitrosamines found in any other consumer product meant for oral use (Hotchkiss, 1989; IARC, 2004, 2007). Recent studies show that, even though some novel tobacco products sold in the United States contain reduced levels of NNN, the amounts of this carcinogen in tobacco products that are consumed by the majority of U.S. smokers and smokeless tobacco users continue to be substantial (Hecht, Stepanov, & Hatsukami, 2011; Richter, Hodge, Stanfill, Zhang, & Watson, 2008; Stepanov, Jensen, Hatsukami, & Hecht, 2008; Stepanov, Knezevich, et al., 2012). For example, the amount of NNN reaches 8.1 µg/g dry weight in U.S. moist snuff (Hecht et al., 2011) and 4.5 µg/g dry weight in the tobacco filler of U.S. cigarette brands (Stepanov, Knezevich, et al., 2012). The only study that analyzed the enantiomeric composition of NNN was published in 2000 and showed that (S)-NNN was the predominant enantiomer, comprising about 75% of total NNN measured in tobacco products (Carmella, McIntee, Chen, & Hecht, 2000). The tobacco products analyzed in that study included a few unidentified cigarettes and smokeless tobacco products and a set of reference tobacco products.
Given the high carcinogenic potency of (S)-NNN, it is important to provide current data on its contribution to the measured NNN levels in various tobacco products that are being marketed in the United States. The information on (S)-NNN content in smokeless tobacco products is of particular interest due to the recently discovered oral carcinogenicity of this enantiomer, as well as the increasing sales of moist snuff in the United States (Balbo et al., 2012; Nelson et al., 2006; U.S. Federal Trade Commission, 2007), and the introduction of novel smokeless tobacco products that are marketed to smokers as a temporary or permanent substitute for smoking (Hatsukami, Ebbert, Feuer, Stepanov, & Hecht, 2007; Rogers, Biener, & Clark, 2010). Moreover, there is no information available on the enantiomeric composition of NNN in cigarette smoke. In this study, we applied chiral gas chromatography (GC) analysis to determine (S)-NNN levels in three categories of U.S. products: conventional smokeless tobacco, novel tobacco products, and cigarettes.
MATERIALS AND METHODS
Chemicals and Standards
5-Methyl-N′-nitrosonornicotine (5-MeNNN) and NNN enantiomers were synthesized as previously described (Carmella et al., 2000). All other chemicals were obtained from Fisher Scientific.
Tobacco Samples
Smokeless tobacco products were purchased between March 2010 and January 2012: conventional products were obtained from retailers in Minneapolis, and novel products were obtained as a part of the New Product Watch Project (Stepanov, Biener, et al., 2012). Cigarettes were purchased from retail stores in April 2010 (Stepanov, Knezevich, et al., 2012); all samples were king-size filtered cigarettes packaged in hard packs.
Tobacco and Smoke Analyses
NNN was analyzed in conventional and novel smokeless tobacco products by GC with detection by a thermal energy analyzer (TEA) (Thermedics Detection Inc.) as previously described (Stepanov, Biener, et al., 2012). The levels of NNN in the tobacco filler and smoke of cigarettes analyzed here have been previously published (Stepanov, Knezevich, et al., 2012). Sample purification for the analysis of NNN enantiomers was conducted as for the NNN quantitation. The prepared samples were analyzed by GC-TEA using a 30 m × 0.25mm i.d., 0.25-μm film thickness Cyclosil-B chiral column (Agilent) supplied with a 2 m × 0.53mm deactivated silica precolumn, as previously described (Carmella et al., 2000).
The contribution of (S)-NNN to the measured NNN in each sample was calculated based on the peak areas of (S)-NNN and (R)-NNN. The calculated percentage contribution of (S)-NNN and the measured levels of NNN were further used to calculate the amount of (S)-NNN in the analyzed products.
Moisture Content
Conventional and novel smokeless tobacco products were analyzed for moisture content as previously described (Stepanov et al., 2008). The moisture content in the tobacco filler of cigarettes analyzed here was published previously (Stepanov, Knezevich, et al., 2012).
RESULTS
The results of the analyses are summarized in Table 1. The levels of NNN in conventional smokeless products ranged from 1.21 to 4.25 µg/g tobacco, and in the novel smokeless products, NNN levels ranged from 0.72 to 1.79 µg/g product.
Table 1.
Levels of NNN and (S)-NNN in the Tobacco of U.S. Products Marketedin 2010–2012
| No. | Product | Moisture,a % | NNN, µg/g wet weighta | (S)-NNN | |
|---|---|---|---|---|---|
| % | µg/g wet weight | ||||
| Conventional moist snuff | |||||
| 1 | Timberwolf Long Cut Wintergreen | 55.2 | 1.56 | 50.2 | 0.78 |
| 2 | Skoal Long Cut Wintergreen | 55.5 | 1.35 | 56.1 | 0.76 |
| 3 | Longhorn Long Cut Wintergreen | 55.4 | 1.71 | 52.6 | 0.90 |
| 4 | Red Man Long Cut Wintergreen | 55.9 | 1.53 | 52.1 | 0.80 |
| 5 | Kodiak Wintergreen | 62.4 | 2.62 | 58.5 | 1.53 |
| 6 | Copenhagen Snuff | 53.0 | 2.32 | 65.5 | 1.52 |
| 7 | Copenhagen Long Cut | 55.8 | 2.15 | 56.8 | 1.22 |
| 8 | Skoal Long Cut Straight | 54.9 | 1.89 | 56.8 | 1.07 |
| 9 | Skoal Bandits Wintergreen Pouches | 56.1 | 1.92 | 58.6 | 1.13 |
| 10 | Grizzly Snuff | 52.3 | 4.25 | 59.0 | 2.50 |
| 11 | Red Seal Fine Cut Natural | 54.0 | 1.55 | 57.5 | 0.89 |
| 12 | Husky Natural | 56.0 | 1.21 | 58.4 | 0.71 |
| 13 | Grizzly Long Cut Straight | 53.2 | 3.28 | 56.4 | 1.85 |
| 14 | Grizzly Long Cut Mint | 54.5 | 3.24 | 59.3 | 1.92 |
| Average for conventional moist snuff SD | 55.3 | 2.18 | 57.0 | 1.26 | |
| 2.4 | 0.9 | 3.7 | 0.5 | ||
| Novel smokeless products | |||||
| 1 | Marlboro Snus Rich | 18.6 | 0.72 | 65.2 | 0.47 |
| 2 | Marlboro Snus Mild | 11.9 | 0.87 | 67.6 | 0.58 |
| 3 | Marlboro Snus Spearmint | 11.8 | 0.80 | 63.8 | 0.51 |
| 4 | Marlboro Snus Peppermint | 12.4 | 0.85 | 64.6 | 0.55 |
| 5 | Camel Snus Robust | 33.8 | 1.79 | 66.6 | 1.19 |
| 6 | Camel Snus Mellow | 32.8 | 1.14 | 63.9 | 0.73 |
| 7 | Camel Snus Frost | 33.7 | 1.04 | 66.4 | 0.69 |
| 8 | Camel Snus Winterchill | 33.1 | 1.20 | 73.5 | 0.88 |
| Average for novel products | 23.5 | 1.05 | 66.4 | 0.70 | |
| SD | 10.7 | 0.3 | 3.1 | 0.2 | |
| Cigarettes | |||||
| 1 | Marlboro Full Flavor | 12.8 | 2.06 | 65.6 | 1.57 |
| 2 | Marlboro Special Blend | 12.5 | 2.40 | 68.0 | 1.63 |
| 3 | Marlboro Blend # 27 | 11.3 | 2.44 | 66.1 | 1.61 |
| 4 | Marlboro Blend # 54 | 11.7 | 3.34 | 69.6 | 2.32 |
| 5 | Marlboro Smooth Menthol | 9.4 | 4.03 | 63.5 | 2.56 |
| 6 | Marlboro Virginia Blend | 11.6 | 0.33 | 52.4 | 0.17 |
| 7 | Basic Full Flavor | 11.9 | 2.35 | 70.7 | 1.66 |
| 8 | Camel Full Flavor | 13.8 | 1.48 | 62.8 | 0.93 |
| 9 | Camel # 9 | 11.8 | 1.78 | 66.2 | 1.18 |
| 10 | Camel # 9 Menthol | 12.8 | 1.56 | 68.3 | 1.06 |
| 11 | Camel Silver | 11.6 | 1.01 | 67.0 | 0.68 |
| 12 | Camel Crush | 12.1 | 1.36 | 62.9 | 0.86 |
| 13 | Winston Full Flavor | 12.0 | 1.35 | 74.8 | 1.01 |
| 14 | Kool Filter Kings | 13.9 | 1.49 | 66.2 | 0.99 |
| 15 | Pall Mall Full Flavor | 11.8 | 1.42 | 67.1 | 0.95 |
| 16 | Doral Full Flavor | 8.8 | 3.17 | 72.8 | 2.31 |
| 17 | Newport Menthol | 14.1 | 1.75 | 68.7 | 1.20 |
| Average for cigarettes | 12.0 | 1.96 | 66.6 | 1.34 | |
| SD | 1.4 | 0.9 | 4.9 | 0.6 | |
Note. NNN = N′-nitrosonornicotine.
aCigarette samples have been previously analyzed for moisture and NNN (Stepanov, Knezevich, et al., 2012).
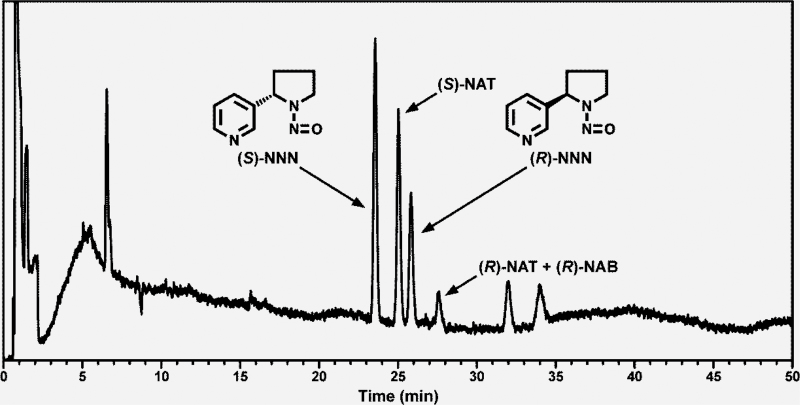
A typical chromatogram obtained upon chiral GC-TEA analysis of NNN enantiomers in a tobacco sample is shown in Figure 1. The retention times of (S)-NNN and (R)-NNN were confirmed by analyzing standard solutions of the enantiomers, both individually and in combination. Enantiomers of the related commonly occurring TSNA N′-nitrosoanatabine (NAT) and N′-nitrosoanabasine (NAB) were also detected, but not quantified because the (R)-NAT peak coelutes with (R)-NAB (Carmella et al., 2000).
Figure 1.
Chromatogram obtained upon chiral GC-TEA analysis of (S)-NNN and (R)-NNN in a tobacco sample (Camel Snus Robust).
In tobacco, the percent contribution of (S)-NNN to NNN was larger than that of (R)-NNN in all product categories, and averaged 62.9±6.3% (SD) among the products analyzed here. The percentage of (S)-NNN was lower in conventional moist snuff than in novel smokeless products or in the cigarette tobacco filler (p < .0001 for both comparisons). The absolute amount of (S)-NNN in conventional moist snuff ranged from 0.71 to 2.5 µg/g tobacco; in novel smokeless products from 0.47 to 1.19 µg/g tobacco; and in cigarette filler from 0.17 to 2.56 µg/g tobacco (all values are per wet weight). The levels of (S)-NNN were lower in novel smokeless products than in conventional moist snuff or in the cigarette tobacco filler (p = .01 for both comparisons). In cigarette smoke, the contribution of (S)-NNN to NNN was similar to that in the corresponding tobacco filler and averaged 64.9% (S)-NNN (range, 51.3%–75.9%) in all brands in Table 1.
DISCUSSION
NNN is a strong carcinogen present in unburned tobacco and cigarette smoke and is believed to play an important role in the esophageal and oral cancers associated with tobacco use. Metabolic and carcinogenicity studies in laboratory animals indicate that its enantiomer (S)-NNN is more tumorigenic than (R)-NNN. Numerous studies documented the levels of NNN in various tobacco products. However, there is no information available on the enantiomeric composition of NNN in products currently marketed in the United States. We report the results of (S)-NNN analysis in a sample of conventional and novel smokeless tobacco products and in cigarettes purchased in the United States in 2010–2012.
To our knowledge, there was only one report in the literature on the levels of (S)-NNN in tobacco (Carmella et al., 2000). In that study, (S)-NNN averaged 75% of total NNN in a set of samples that included a few unidentified cigarettes and conventional smokeless tobacco. In agreement with those results, the (S)-enantiomer of NNN predominated in all products analyzed here (Table 1). These results once again emphasize both the urgent need and the opportunity for the reduction of the levels of this potent carcinogen in tobacco products. It has been shown that NNN can be formed via nitrosation of either nicotine or nornicotine in tobacco (Hecht, Chin, Hirota, et al., 1978; Hecht, Chin, Ornaf, et al., 1978; Mirvish, Sams, & Hecht, 1977). In tobacco, nicotine is >99% (S)-enantiomer (Armstrong, Wang, & Ercal, 1998), and nitrosation of nicotine of such enantiomeric composition produces >99% (S)-NNN (Carmella et al., 2000). On the other hand, nornicotine was shown to contain 70%–96% (S)-nornicotine (Armstrong, Wang, Lee, & Liu, 1999; Carmella et al., 2000), and it has been previously noted that the enantiomeric composition of NNN in tobacco indicates that nornicotine, and not nicotine, is the major precursor of NNN in tobacco (Carmella et al., 2000). Our results further reinforce that hypothesis. Thus, removal of nornicotine from tobacco could be a potential strategy to reduce the levels of NNN in tobacco products (Gavilano et al., 2006).
Even though the variation in percent (S)-NNN among brands and product types was not large, we found statistically significant differences among some product categories. This could be due to the differences in tobacco types and processing methods used. For instance, the % contribution of (S)-NNN to NNN in novel tobacco products was found to be higher than that in conventional moist snuff (Table 1). Previous research showed that, compared with conventional U.S. moist snuff, Marlboro Snus and Camel Snus products are generally low in tobacco-specific nitrosamine content (Stepanov et al., 2008; Stepanov, Biener, et al., 2012). This is most likely due to differences in tobacco processing methods, with the tobacco used for the manufacturing of novel products undergoing pasteurization—a process known to inhibit TSNA formation in processed tobacco. However, due to the higher percentage of (S)-NNN, the absolute amount of this carcinogenic enantiomer in some novel products can be comparable to those found in conventional moist snuff (Table 1).
This is the first study to measure the enantiomeric composition of NNN in cigarette smoke. The measured percent contribution of (S)-NNN in smoke was similar to that in cigarette tobacco, indicating that no significant thermal racemization of NNN occurs during cigarette burning.
The higher carcinogenic potential of (S)-NNN compared to (R)-NNN is indicated in both in vitro and in vivo studies. In vitro, cultured rat esophagus, a target tissue for NNN carcinogenicity, metabolizes (S)-NNN predominantly by 2′-hydroxylation, which is the major bioactivation pathway of NNN in rats (McIntee & Hecht, 2000). In vivo, the urine of rats treated with (S)-NNN contained higher levels of metabolites formed via 2′-hydroxylation than the urine of rats treated with (R)-NNN (McIntee & Hecht, 2000). Furthermore, treatment of rats with (S)-NNN produced two to six times higher levels of DNA adducts in the esophagus and three to five higher levels of DNA adducts in oral tissue, compared with treatment with (R)-NNN (Lao et al., 2007; Zhang et al., 2009). In agreement with these results, the treatment of rats with (S)-NNN in our recent study produced a 100% incidence of oral and esophageal tumors, compared with only 5 and 3 out of 24 rats developing oral and esophageal tumors, respectively, upon treatment with (R)-NNN (Balbo et al., 2012). The relevance of these animal data to humans is supported by the findings of the strong relationship between NNN exposure in smokers and risk of esophageal cancer in a prospective cohort (Yuan et al., 2011), as well as by the epidemiological evidence that smokeless tobacco products contaminated with high levels of NNN cause oral cancer (Gupta, Murti, & Bhonsle, 1996; IARC, 2007; Stepanov, Hecht, Ramakrishnan, & Gupta, 2005).
In summary, our results demonstrate that the carcinogenic (S)-enantiomer of NNN predominates in cigarette tobacco and smoke, moist snuff, and novel smokeless tobacco products currently marketed in the United States. Given the potential role of (S)-NNN as a causative agent for esophageal and oral cancers associated with tobacco use, these results support the importance of reduction, or ideally elimination, of NNN in tobacco products.
FUNDING
This study was supported by grants CA-141631, CA-81301, and CA-135884 from the U.S. National Institutes of Health and by National Cancer Institute Contract HHSN261201000544P.
DECLARATION OF INTERESTS
There are no competing interests.
ACKNOWLEDGMENT
We thank Bob Carlson for editorial assistance.
REFERENCES
- Armstrong D. W., Wang X., Ercal N. (1998). Enantio meric composition of nicotine in smokeless tobacco, medicinal products, and commercial reagents. Chirality, 10, 587–591. 10.1002/(SICI)1520-636X(1998)107<587AID-CHIR6> 3.0.CO;2-# [Google Scholar]
- Armstrong D. W., Wang X., Lee J. T., Liu Y. S. (1999). Enantiomeric composition of nornicotine, anatabine, and anabasine in tobacco. Chirality, 11, 82–84. 10.1002/(SICI)1520-636X(1999)111<82AID-CHIR14>3.0.CO;2-C [Google Scholar]
- Balbo S., James-Yi S., O′Sullivan G., Stepanov I., Wang M., Zhang S, … Hecht S.S. (2012). (S)-N′-Nitrosonornicotine, a constituent of smokeless tobacco, is a potent oral tumorigen in rats. Abstracts, AACR Annual Meeting, March 31–April 4, 2012, Chicago, IL [Google Scholar]
- Carmella S. G., McIntee E. J., Chen M., Hecht S. S. (2000). Enantiomeric composition of N′-nitrosonornicotine and N′-nitrosoanatabine in tobacco. Carcinogenesis, 21, 839–843. 10.1093/carcin/21.4.839 [DOI] [PubMed] [Google Scholar]
- Gavilano L. B., Coleman N. P., Burnley L. E., Bowman M. L., Kalengamaliro N. E., Hayes A, … Siminszky B. (2006). Genetic engineering of Nicotiana tabacum for reduced nornicotine content. Journal of Agricultural and Food Chemistry, 54, 9071–9078. 10.1021/jf0610458 [DOI] [PubMed] [Google Scholar]
- Gupta P. C., Murti P. R., Bhonsle R. B. (1996). Epidemiology of cancer by tobacco products and the significance of TSNA. Critical Reviews in Toxicology, 26, 183–198. 10.3109/10408449609017930 [DOI] [PubMed] [Google Scholar]
- Hatsukami D. K., Ebbert J. O., Feuer R. M., Stepanov I., Hecht S. S. (2007). Changing smokeless tobacco products new tobacco-delivery systems. American Journal of Preventive Medicine, 33,(6Suppl.), S368–S378. 10.1016/j.amepre.2007.09.005 [DOI] [PubMed] [Google Scholar]
- Hecht S. S. (1998). Biochemistry, biology, and carcinogenicity of tobacco-specific N-nitrosamines. Chemical Research in Toxicology, 11, 559–603. 10.1021/tx980005y [DOI] [PubMed] [Google Scholar]
- Hecht S. S., Chen C. B., Hirota N., Ornaf R. M., Tso T. C., Hoffmann D. (1978). Tobacco-specific nitrosamines: Formation from nicotine in vitro and during tobacco curing and carcinogenicity in strain A mice. Journal of the National Cancer Institute, 60, 819–824 [DOI] [PubMed] [Google Scholar]
- Hecht S. S., Chen C. B., Ornaf R. M., Jacobs E., Adams J. D., Hoffmann D. (1978). Reaction of nicotine and sodium nitrite: Formation of nitrosamines and fragmentation of the pyrrolidine ring. The Journal of Organic Chemistry, 43, 72–76 [DOI] [PubMed] [Google Scholar]
- Hecht S. S., Rivenson A., Braley J., DiBello J., Adams J. D., Hoffmann D. (1986). Induction of oral cavity tumors in F344 rats by tobacco-specific nitrosamines and snuff. Cancer Research, 46, 4162–4166 [PubMed] [Google Scholar]
- Hecht S. S., Stepanov I., Hatsukami D. K. (2011). Major tobacco companies have technology to reduce carcinogen levels but do not apply it to popular smokeless tobacco products. Tobacco Control, 20, 443. 10.1136/tc.2010.037648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotchkiss J. H. (1989). Preformed N-nitroso compounds in foods and beverages. Cancer Surveys, 8, 295–321 [PubMed] [Google Scholar]
- International Agency for Research on Cancer [IARC] (2004). Tobacco smoke and involuntary smoking. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, vol. 83, Lyon, France: IARC; [PMC free article] [PubMed] [Google Scholar]
- International Agency for Research on Cancer [IARC] (2007). Smokeless tobacco and tobacco-specific nitrosamines. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, vol. 89, Lyon, France: IARC; [PMC free article] [PubMed] [Google Scholar]
- Lao Y., Yu N., Kassie F., Villalta P. W., Hecht S. S. (2007). Analysis of pyridyloxobutyl DNA adducts in F344 rats chronically treated with (R)- and (S)-N′-nitrosonornicotine. Chemical Research in Toxicology, 20, 246–256. 10.1021/tx060208j [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntee E. J., Hecht S. S. (2000). Metabolism of N′-nitrosonornicotine enantiomers by cultured rat esophagus and in vivo in rats. Chemical Research in Toxicology, 13, 192–199. 10.1021/tx990171l [DOI] [PubMed] [Google Scholar]
- Mirvish S. S., Sams J., Hecht S. S. (1977). Kinetics of nornicotine and anabasine nitrosation in relation to N′-nitrosonornicotine occurrence in tobacco and to tobacco-induced cancer. Journal of the National Cancer Institute, 59, 1211–1213 [DOI] [PubMed] [Google Scholar]
- Nelson D. E., Mowery P., Tomar S., Marcus S., Giovino G., Zhao L. (2006). Trends in smokeless tobacco use among adults and adolescents in the United States. American Journal of Public Health, 96, 897–905. 10.2105/AJPH.2004.061580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter P., Hodge K., Stanfill S., Zhang L., Watson C. (2008). Surveillance of moist snuff: Total nicotine, moisture, pH, un-ionized nicotine, and tobacco-specific nitrosamines. Nicotine & Tobacco Research, 10, 1645–1652. 10.1080/14622200802412937 [DOI] [PubMed] [Google Scholar]
- Rogers J. D., Biener L., Clark P. I. (2010). Test marketing of new smokeless tobacco products in four U.S. cities. Nicotine & Tobacco Research, 12, 69–72. 10.1093/ntr/ntp166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepanov I., Biener L., Knezevich A., Nyman A. L., Bliss R., Jensen J, … Hatsukami D. K. (2012). Monitoring tobacco-specific N-nitrosamines and nicotine in novel Marlboro and Camel smokeless tobacco products: Findings from Round 1 of the New Product Watch. Nicotine & Tobacco Research, 14, 274–281. 10.1093/ntr/ntr209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepanov I., Hecht S. S., Ramakrishnan S., Gupta P. C. (2005). Tobacco-specific nitrosamines in smokeless tobacco products marketed in India. International Journal of Cancer, 116, 16–19. 10.1002/ijc.20966 [DOI] [PubMed] [Google Scholar]
- Stepanov I., Jensen J., Hatsukami D., Hecht S. S. (2008). New and traditional smokeless tobacco: Comparison of toxicant and carcinogen levels. Nicotine & Tobacco Research, 10, 1773–1782. 10.1080/14622200802443544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepanov I., Knezevich A., Zhang L., Watson C. H., Hatsukami D. K., Hecht S. S. (2012). Carcinogenic tobacco-specific N-nitrosamines in US cigarettes: Three decades of remarkable neglect by the tobacco industry. Tobacco Control, 21, 44–48. 10.1136/tc.2010.042192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Federal Trade Commission (2007). Smokeless tobacco reports for the years 2002–2005, Washington, DC: Federal Trade Commission [Google Scholar]
- Yuan J. M., Knezevich A. D., Wang R., Gao Y. T., Hecht S. S., Stepanov I. (2011). Urinary levels of the tobacco-specific carcinogen N′-nitrosonornicotine and its glucuronide are strongly associated with esophageal cancer risk in smokers. Carcinogenesis, 32, 1366–1371. 10.1093/carcin/bgr125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S., Wang M., Villalta P. W., Lindgren B. R., Lao Y., Hecht S. S. (2009). Quantitation of pyridyloxobutyl DNA adducts in nasal and oral mucosa of rats treated chronically with enantiomers of N′-nitrosonornicotine. Chemical Research in Toxicology, 22, 949–956. 10.1021/tx900040j [DOI] [PMC free article] [PubMed] [Google Scholar]