Abstract
Introduction:
Nicotine is a heavily used addictive drug acquired through smoking tobacco. Nicotine in cigarette smoke is deposited and absorbed in the lungs, which results in a rapidly peaked slowly declining arterial concentration. This pattern plays an important role in initiation of nicotine addiction.
Methods:
A method and device were developed for delivering nicotine to rodents with lung alveolar region-targeted aerosol technology. The dose of delivery can be controlled by the nicotine aerosol concentration and duration of exposure.
Results:
Our data showed that, in the breathing zone of the nose-only exposure chamber, the aerosol droplet size distribution was within the respirable diameter range. Rats were exposed to nicotine aerosol for 2min. The arterial blood nicotine concentration reached 43.2±15.7ng/ml (mean ± SD) within 1–4min and declined over the next 20min, closely resembling the magnitude and early pharmacokinetics of a human smoking a cigarette. The acute inhalation toxicity of nicotine: LC50 = 2.3mg/L was determined; it was affected by pH, suggesting that acidification decreases nicotine absorption and/or bioavailability.
Conclusions:
A noninvasive method and toolkit were developed for delivering nicotine to rodents that enable rapid delivery of a controllable amount of nicotine into the systemic circulation and brain-inducing dose-dependent pharmacological effects, even a lethal dose. Aerosol inhalation can produce nicotine kinetics in both arterial and venous blood resembling human smoking. This method can be applied to studies of the effects of chronic intermittent nicotine exposure, nicotine addiction, toxicology, tobacco-related diseases, teratogenicity, and for discovery of pharmacological therapeutics.
INTRODUCTION
Tobacco smoking is the leading preventable cause of disease, disability, and death. Nicotine, a major component of tobacco, is highly addictive, underlying the fact that smokers quickly become addicted—about 1,200 per day in the United States (NIH/NIDA, 2008)—and have great difficulty quitting. To understand the numerous effects of nicotine and to develop more effective and safer medications for smoking cessation as well as for treatment of tobacco-related diseases, animal models relevant to human nicotine exposure are required.
In smokers (and often in those exposed secondhand), exposure to nicotine is chronic and intermittent. Cigarette smoke is an aerosol containing tiny particles within the respirable diameter range (Gowadia, Oldham, & Dunn-Rankin, 2009; Hinds, 1978). Respirable diameter is defined as the aerodynamic diameter of particles capable of reaching the gas exchange region in the lungs (the alveoli) for the organism under study (OECD, 2009). Cigarette smoke particles deposit in the alveoli, where nicotine crosses the pulmonary membrane into the circulation.
Inhalation of cigarette smoke leads to a rapid increase in arterial blood nicotine concentrations. When a human smokes one cigarette (takes ~5min), their arterial blood nicotine concentration reaches its maximum (C max) of 20–60ng/ml in 3–5min (T max), which then declines over the next 20min due to rapid distribution to all body tissues; the venous blood nicotine reaches C max of 15–30ng/ml in T max of 5–8min (Benowitz, Porchet, Sheiner, & Jacob, 1988; Henningfield, Stapleton, Benowitz, Grayson, & London, 1993; Hukkanen, Jacob, & Benowitz, 2005; Rose, Behm, Westman, & Coleman, 1999). Nicotine reaches the brain within ~8–10 s of inhalation (Matta et al., 2007) and it accumulates in the brain to its maximum value within 3–5min (Rose, Mukhin, et al., 2010). Episodic exposure to nicotine in this pharmacokinetic pattern strongly stimulates brain nicotine receptors, inducing numerous psychological and behavioral effects and, eventually, nicotine dependence.
For drug abuse studies with animal models to be relevant to humans, the routes of drug administration as well as the blood pharmacokinetics and target tissue concentrations should be comparable between humans and animals (Brent, 2004; Matta et al., 2007). Commonly used nicotine administration methods for in vivo animal models include the following: (a) Oral application, for example, nicotine dissolved in drinking water. In this case, 70% of nicotine is metabolized in the liver, with only ~30% entering the systemic circulation (Matta et al., 2007). Moreover, oral nicotine absorption is slow compared to that during smoking. (b) Intraperitoneal (i.p.), subcutaneous (s.c.), or intravenous (i.v.) injection of nicotine. These methods are invasive and inconvenient for chronic intermittent exposure. In addition, i.p. nicotine delivery is affected by the first-pass liver metabolism. (c) Subcutaneous osmotic pump delivery (Epping-Jordan, Watkins, Koob, & Markou, 1998; Theeuwes & Yum, 1976). Here, delivery is slow (hours) and continuous. A slowly rising concentration of nicotine results in desensitization without activation of some types of nicotinic receptors (Dani & De Biasi, 2001). In contrast, episodic smoking induces periods of activation followed by a cycle of desensitization/resensitization (Matta et al., 2007). Unfortunately, some nicotine-stimulated neurotransmitter responses, such as increases in dopamine release in the nucleus accumbens (Balfour, 2004; Fu, Matta, Gao, Brower, & Sharp, 2000) or elevated norepinephrine secretion in the paraventricular nucleus (Fu, Matta, Brower, & Sharp, 2001; Sharp & Matta, 1993), depend upon sufficient and rapidly rising brain nicotine concentrations. Moreover, neurobehavioral plasticity and development of addiction are profoundly affected by whether the exposure is continuous or episodic (Rothwell, Gewirtz, & Thomas, 2010; Skjei & Markou, 2003). (d) Intravenous self-administration. This technique is invasive. Surgically implanting the catheter and maintaining i.v. patency for a long period of time are technically challenging (Corrigall & Coen, 1989; DeNoble & Mele, 2006). (e) Cigarette smoking machines (Beven, 1976; Moir et al., 2008), which are most relevant to human smoking. However, the use of cigarette smoke confounds the specific effects of nicotine with the effects of more than 4,000 other compounds (Borgerding & Klus, 2005); also the amount of nicotine delivered is hard to control. (f) Nicotine vapor inhalation (George, Grieder, Cole, & Koob, 2010; Waldum et al., 1996). Nicotine vapor is the gaseous state of nicotine. The concentration of a substance in a vapor form in air depends on its vapor pressure. Nicotine (freebase) is an oily, nonvolatile liquid (its vapor pressure is >600-fold lower than that of water; Meng, Lichtman, Bridgen, & Martin, 1997). Thus, the nicotine concentration in vapor form for inhalation is low. Delivery of nicotine through inhalation of nicotine vapor is slow. Moreover, the amount of nicotine that enters circulation is limited because nicotine vapor mostly deposits and is absorbed in nasal and buccal mucosa, while very little deposits in the lungs (George et al., 2010; Lunell, Molander, Ekberg, & Wahren, 2000; Waldum et al., 1996). Specifically, Bergstrom, Nordberg, Lunell, Antoni, and Langstrom (1995) and Lunell, Bergstrom, Antoni, Langstrom, and Nordberg (1996) used positron emission tomography imaging with radioactive 11C-nicotine for investigating the deposition of nicotine in human subjects. They showed that most nicotine is deposited in the oral cavity and upper airways, while only a minor fraction (5%) of the nicotine is deposited in the lungs when subjects used a nicotine vapor inhaler. In contrast, in subjects who smoked cigarettes, a large fraction of nicotine is deposited in the lungs and a minimal fraction is found in the oral cavity and upper airways. Therefore, animals have to be continuously exposed to the vapor for hours to produce meaningful blood nicotine concentrations comparable to that of human smokers (George et al., 2010). The vapor inhalation method cannot model the kinetic profile of a high and rapid rise followed by a decline in arterial nicotine concentrations seen in cigarette smoking (Henningfield et al., 1993; Lunell et al., 1996, 2000; Rose et al., 1999; Schneider, Olmstead, Franzon, & Lunell, 2001).
Major advances in aerosol technology have led to increasing interest in systemic delivery of drugs by inhalation. Small molecules can be delivered with rapid action, low metabolism, and high bioavailability. Unlike vapor, aerosol is a suspension of small particles in a gas. Brownian diffusion is the dominant mechanism for aerosol particle deposition in the alveolar region. Particle size is critical, as large particles deposit in the airways, mouth, and throat, while particles that are too small can be exhaled, thereby reducing alveolar deposition (OECD, 2009; Patton & Byron, 2007). The purpose of this study was to develop a noninvasive and effective method to deliver nicotine targeting the alveolar region of rodents with advanced aerosol technology. This methodology ensures delivery of adequate and controllable amounts of nicotine into the systemic circulation and brain that mimic the kinetic profile and blood concentration of human cigarette smoking.
METHODS
Animals
All animal use procedures were in accordance with The National Institutes of Health (United States) Guide for the Care and Use of Laboratory Animals and were approved by the UCLA and AfaSci Inc. Institutional Animal Care and Use Committee. Efforts were made to minimize the number of animals used and their pain and suffering. Male Sprague-Dawley rats of 8–11-week-old (body weight 250–400g) were used in this study. They were housed in the vivarium under a 12-hr light/dark cycle and had ad libitum access to food and water.
Nicotine Aerosol Generation and Exposure System
An experimental system was made that included a 3-jet Collison nebulizer (BGI Inc.), an air pressure gauge (Ashcroft@ filled gauge, 0–100 psi, Cole-Parmer), an air flowmeter with a valve (150-mm Direct Reading, 23 LPM, Cole-Parmer) that regulated the airflow rate and the pressure entering the nebulizer (Figure 1), a homemade nose-only exposure chamber, and rat holders (Model #: CHT-250, CH Technologies Inc.). Nicotine aerosols with defined droplet size can be consistently generated using a Collison nebulizer. The outlet of the exposure chamber was opened to a fume hood through a plastic tubing. About 30ml nicotine solution was put in the nebulizer jar. One or two rats can be exposed to nicotine aerosol simultaneously by inserting rat holders into a nose-only chamber. An experimental system where the output of the nebulizer was connected to a sealed cage was made for freely moving rodents. The outlet of the sealed cage was opened to a fume hood. For experimental accuracy, only one rodent was put into the sealed cage. The pressure of the air that enters into the nebulizer for generation of aerosol determines the volume, the droplet size distribution, and the concentration of the output aerosol. The air pressure and flow rate were well controlled and continuously monitored during the nicotine aerosol exposure experiments.
Figure 1.
Diagram of nicotine aerosol generation system. The connections ensure constant pressurized air with constant flow rate applied to the nebulizer and minimize the distance from the nebulizer outlet to the respiratory zone of animals (the components of the diagram are not in proportion).
Note: the air flow indicated on a flowmeter (Q i) under pressure of Pr can be converted to “true” flow rate Q stp under standard conditions, for example, one atmosphere, with the equation:
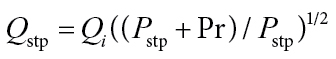 |
(1) |
where P stp is one atmosphere and Pr is pressure in system (Hinds, 1999).
Determination of Nicotine Aerosol Droplet Size Distribution
The experiments were done in a fume hood at room temperature of 22±1 °C. Nicotine (freebase) was dissolved in water or NaCl solution for an osmolality ~300 mOsm/kg. pH was adjusted with HCl to pH 8.0 except those indicated in the Results section.
An Aerodynamic Particle Sizer (APS 3321, TSI Inc.) was used to measure the droplet size distribution of nicotine aerosol generated from nicotine solution at different concentrations in the solution jar of the nebulizer. Since the aerosol was from nebulization of a low vapor pressure liquid (nicotine freebase) dissolved in a relatively volatile solvent (water), the water would evaporate rapidly after droplet formation in general laboratory relative humidity (RH) conditions (Hinds, 1999). To avoid bias in measuring the droplet size, we made a container (volume >11L [~3 gallons]) with a short inlet tubing (5.2cm) from the breathing zone of the nose-only chamber. Water was put into the container and sealed it for >2hr. The RH inside the container was closed to 100% (measured with a Q-Trak Indoor Air Quality Monitor Model 8550, TSI Inc.) (Figure 2). The inlet tubing of the APS sampled the aerosol in the container a short distance away from the breathing zone. In these conditions, evaporation of aerosol droplets entering into the APS was minimized. The container also functioned to dilute the aerosol to avoid saturation of the APS. To further reduce the aerosol droplet concentration to avoid saturation for droplet size distribution measurement, we blocked two of the three holes that supply the liquid to the nozzles. The amount of aerosolized nicotine solution was then reduced to one third of the 3-jets nozzle without changing the airflow. Therefore, the aerosol concentration at the output of the nebulizer would be reduced to one third.
Figure 2.
Diagram for measuring droplet size distribution of nicotine aerosol in the nose-only exposure system (the components of the diagram are not in proportion). The measurement was made in a container where the relative humidity was closed to 100% to minimize droplet evaporation. APS = Aerodynamic Particle Sizer.
Determination of Nicotine Aerosol Mass Concentration in Air
Mass concentrations of the nicotine aerosol were measured with a one-stage cascade impactor (SKC Inc.). The length of the impactor inlet tubing was minimized and directly connected to the breathing zone of the nose-only chamber. The nebulizer worked as 3-jet nebulizer without modification (the above 3-to-1 jet reduction version of the nebulizer was only for droplet size distribution measurement). Before the assay, the filters (PTFE Membrane Disc Filters, Pall Co.) for the impactor were balanced in a temperature (22 °C) and humidity (49%) controlled environment where the electronic balance (Mettler-Toledo MX5 Microbalance, Mettler-Toledo Inc.) for weighing the filters was located. The filter weight difference between pre- and post-nicotine aerosol collection is the mass collected in a preset duration, for example, 1min. To compensate the weight loss due to vaporization of the solvent (water) in the filter, we monitored the weight change during a period equivalent to the time needed from the end of aerosol sampling to the point the filter was weighed on the balance and added this equivalent weight lost to the measured value.
Arterial and Venous Blood Sample Collection and Plasma Nicotine Level Measurement
For arterial blood sample collection, femoral artery precatheterized rats (8–9-week-old) were ordered from Harlan Laboratories. For venous blood collection, local anesthetic cream was applied to the rat tail 30min prior to insertion of the catheter. The rat was put into the rat holder and the tail was warmed with warm water or an infrared light. A temporary catheter (24G) was inserted into the lateral tail vein. Warm saline (2.5ml) was infused into the arterial or venous catheter for blood volume compensation before blood collection. Then 0.1–0.2ml of heparin (100U/ml) was injected.
The first sample as pre-nicotine control was collected. Then nicotine aerosol was generated from the nebulizer and at the same time, a timer was started. The rat was exposed to the nicotine aerosol for 2min. Blood samples (equivalent to 0.2ml plasma) were collected at a series of timepoints (see Results section) with 0.6-ml capillary blood collection tubes containing ethylenediaminetetraacetic acid-K2 (RAM Scientific Inc.). Dropping from the venous catheter was sometimes slow. Bleeding can be greatly facilitated by massaging the tail over the vein.
Samples were centrifuged within 15min of collection at 1,000g (gravity) for 12min at room temperature. Supernatant plasma was transferred to labeled eppendorf tubes and store below −20 °C. The plasma samples were sent to NMS Labs for measurements of nicotine and its metabolite cotinine. The analysis was performed on a Waters TQD MS with ACQUITY UPLC system equipped with an ACQUITY HSS T3 2.1 × 50mm, 1.8 µm analytical column, with in-line filter and positive-ion electrospray mass spectrometry (LC-MS/MS). Each analytical run was independently calibrated at concentrations of 2.5, 5.0, 15, 50, 150, and 400ng/ml. Two levels of control were run in each analytical batch. This UPLC method had a lower limit of quantification of 2.5ng/ml and between run % CV’s (coefficient of variation) of 7.42% and 6.25% at 10 and 300ng/ml, respectively for nicotine and between run % CV’s of 5.05% and 3.62% at 20 and 600ng/ml, respectively for cotinine.
Statistics; the Up and Down Procedure to Determine Inhalation LC50
For evaluating acute inhalation toxicity, median lethal concentration (LC50), not median lethal dose (LD50), is recommended by the U.S. Environmental Protection Agency (EPA) Guidelines for Acute Inhalation Toxicity (EPA, 1998). LC50 is the concentration of a substance in air that causes death during exposure or within a fixed time after exposure in 50% of animals exposed for a specified duration. LC50 is expressed as weight of test substance per unit volume of air, for example, mg/L.
Nicotine LC50 in rats was examined using the up and down procedure (UDP) recommended by EPA Health Effects Test Guidelines (EPA, 2002). The purpose of the UDP is to minimize the number of animals for toxicity testing. With this method, 6–9 animals could be used to obtain LC50 and its confidence interval (CI) with an accuracy comparable to using 30–50 animals with a conventional LC50 method (also refer to Bruce [1985]).
As conventional LC50 experiments, a range of concentrations was chosen for testing with UDP. Concentration progression factor refers to the multiple by which a concentration is increased or decreased, chosen according to the steepness of the concentration–response curve of the testing substance. A concentration progression factor of antilog 0.5 is commonly used. Animals were exposed to the aerosol in a nose-only system, one at a time. The first animal was exposed to a concentration a step below the level of the best estimate of LC50. If the animal survived, the concentration for the next animal was increased by one step; if it died, the concentration for the next animal was decreased by one step. Dosing was continued until the statistical criteria were satisfied (EPA, 2002). A computer program (AOT425StatPgm) to facilitate animal-by-animal calculations that establish testing sequences has been developed by the USEPA and is available from the EPA Web site. The program gives the estimates of LC50 and its CI from the animal outcomes at test termination. After the experiments, the surviving rats were observed for additional 24hr to observe any delayed deaths.
Data are presented as mean ± SD except those specified as CI or geometric standard deviation (GSD).
Chemicals
Nicotine used in this study was (s)-(-)-nicotine freebase (liquid, 99%) ordered from Alfa Aesar Co.
RESULTS
Nicotine Aerosol Droplet Size Distributions and Mass Concentration in the Breathing Zone of Exposure Chambers
The manufacturer of the Collison nebulizer states that the mass median aerodynamic diameter (MMAD) of aerosol droplets generated in the nebulizer is ~2.5 µm and GSD is 1.8 (http://www.bgiusa.com/agc/output_distribution.htm). These measurements were made using a nonvolatile liquid. However, generation of aerosol from a liquid depends on the later’s physical properties such as viscosity, vapor pressure, and surface tension. After the aerosol is generated, it is a dynamic process whose droplet size distribution and concentration in air vary with time, affected by factors such as coagulation, condensation, and evaporation (Hinds, 1999). Evaporation is a particularly critical process if the solvent, for example, water, is volatile. Therefore, for delivering drugs to the body through the lungs with aerosol technology, one has to establish specific procedures for different drugs based on physical properties of the drugs and their solvents. The characteristics of the aerosol such as droplet size distribution and drug concentration need to be measured at the breathing zone to obtain the information about the aerosol the animal actually inhales. In the Acute Inhalation Toxicity Testing Guidance Document (OECD, 2009) (also refer to EPA [1998]), a respirable droplet size of MMAD between 1–4 µm is recommended.
The aerosol droplet size distribution and mass concentrations in the breathing zones of our nose-only exposure systems were determined with solutions of nicotine at concentrations as they were actually used in experiments (Figure 3, Table 1). The airflow rate and MMAD values obtained in our experiments at 20 and 40 psi are consistent with those of the output data document from BGI Inc. (http://www.bgiusa.com/agc/collison.htm). Table 1 shows that (a) increasing air pressure from 20 to 40 psi led to higher aerosol mass concentration and smaller MMAD, while further increases in pressure did not increase the mass concentration and even decreased it due to the increase in air flow (data not shown). (b) Using a higher nicotine concentration in solution led to an increase in MMAD and in mass concentration, suggesting that physical properties of the solution, for example, solutions with higher nicotine concentration are higher in viscosity and lower in vapor pressure, affect the droplet size distribution and mass concentration of the aerosol.
Figure 3.
Droplet size distribution of the nicotine aerosol in the breathing zone of a nose-only exposure chamber. The nicotine concentration in the nebulizer container was 1% or 32%. Air pressure in the inlet of the nebulizer was 40 psi. Air flow rate was 9.55L/min (standard conditions, adjusted with Eq. 1). Using the 1-jet nebulizer modified from a 3-jet unit (refer to Methods section). The aerosol sampling time was 10 s with the APS.
Table 1.
Nicotine Aerosol Characteristics in Different Experimental Conditions
| Chamber | Nicotine concentration in nebulizer | Air pressure (psi) | MMAD (μm) | GSD | Aerosol mass concentration (mg/L) | Nicotine concentration in air (mg/L) |
|---|---|---|---|---|---|---|
| Nose-only | 1% | 20 | 1.95 | 2.48 | 2.96 | 0.0296 |
| 1% | 40 | 1.69 | 2.59 | 4.22 | 0.0422 | |
| 32% | 40 | 3.55 | 1.83 | 7.17 | 2.3 |
Note. GSD = geometric standard deviation; MMAD = mass median aerodynamic diameter.
Nicotine concentration in air = nicotine concentration in solution in the nebulizer × aerosol mass concentration.
The aerosol sampling time was 10 s for the droplet size distribution with the Aerodynamic Particle Sizer and 1min for the mass concentrations measurement with the impactor.
These results suggest that the nicotine aerosol droplet size in the breathing zone in our nose-only exposure systems was within the range of respirable droplet size recommended by USEPA (EPA, 1998; OECD, 2009). High nicotine mass concentrations for inhalation for animals (3–4 orders of magnitude higher compared to vapor inhalation methods; George et al., 2010) can be achieved in this system.
Nicotine Acute Inhalation Toxicity
Nicotine in water can be in three forms: freebase (Nic), monoprotonated (NicH+), and diprotonated (NicH2 2+). Nic and NicH+ are predominant, with pKa = 8.06 at 20 °C (Pankow, Tavakoli, Luo, & Isabelle, 2003). Therefore, ~50% of nicotine is as Nic at pH 8.0. For inhalation route, the pH of the particles of tobacco smoke or testing aerosol affects nicotine absorption in the lung and its bioavailability (Burch et al., 1993; Pankow et al., 2003).
Rats were exposed to nicotine aerosol for a fixed time (20min) and with a fixed air pressure (40 psi) to the nebulizer. To determine the inhalation LC50 of nicotine for rats using the UDP, we varied the nicotine concentrations in the nebulizer solution container. An ordered concentration progression in a range of 5%–56% nicotine was defined. Since the nicotine dose–response curve is quite steep, a concentration progression factor of antilog 0.25 = 1.78 was chosen. pH was 8.0 in the first experiment.
Rats were exposed to nicotine aerosol in a nose-only system, one at a time. Starting with a nicotine concentration of 10% in the nebulizer container, the first rat survived. A concentration of 18% (increase of one progression factor) was used for the next rat. According to the UDP, if the animal survives, the concentration for the next animal is increased by one step; if it dies, the concentration for the next animal is decreased by one step. The test result (either death or survival) for each rat was entered one by one into the spreadsheet of the program AOT425StatPgm from the USEPA.
When rats were exposed to nicotine aerosol in this high concentration range, they showed a Straub tail within 20–60 s. Within 1–2.5min, the rats became apneic, interspersed with gasps. This lasted 2–5min, and then respiration recovered, but was weak in some rats. Some rats had clonic and/or tonic convulsions and died at 6–20min. Some of them died within 1–3min after the end of nicotine aerosol exposure period of 20min. This experiment proceeded until the result for the seventh rat, which met the “stopping criteria” of the program, with an estimated LC50 = 32% nicotine concentration in the nebulizer container with a 95% CI of 17.3%–56.7%. To convert percentage concentration in the nebulizer into nicotine concentration in air, we calculated: LC50 (20min) = 0.32 × 7.17 = 2.3mg/L (nicotine per liter of the breathing air containing the aerosol); CI was 1.24–4.07mg/L, based on the mass concentration of aerosol at the breathing zone 7.17mg/L at 40 psi (Table 1, the density of nicotine liquid is 1.01 at 20 °C).
Further, we tested if pH affected inhalation LC50 using nicotine solutions of pH 6.8 and 7.4, as the effective pH of smoke particles from commercial cigarettes is in a range of 6–7.8 (Pankow et al., 2003).
LC50 values were not significantly different between experimental groups of nicotine solutions at pH 7.4 and at pH 8 (Table 2). Note that the CI values of LC50 at pH 8 were slightly lower than those at pH 7.4. However, the LC50 of nicotine solution at pH 6.8 was >4mg/L (>56% nicotine concentration in the nebulizer). The factor that prevented us going higher in nicotine concentration was that pure nicotine freebase is liquid and very alkaline (pH ~10). The amount of HCl required to adjust pH to 6.8 significantly diluted the solution; therefore, 68% was the maximum concentration we could achieve. Although the exact value of LC50 cannot be determined, our complete result (see legend of Table 2) in this experiment with pH 6.8 suggests that LC50 at pH 6.8 is much higher than those at pH 7.4 and pH 8.
Table 2.
The Effects of pH of Nicotine Solution on LC50
| pH | Nicotine concentration in nebulizer (%) | 95% CI | LC50 in air (20min) (mg/L)a | 95% CI of LC50 (mg/L)a | n |
|---|---|---|---|---|---|
| 6.8 | >56b | > 4.1 | 6 | ||
| 7.4 | 32 | 20.4–69.2 | 2.3 | 1.46–4.96 | 7 |
| 8.0 | 32 | 17.3–56.7 | 2.3 | 1.24–4.07 | 7 |
Note. CI represents confidence interval. Air pressure for generating nicotine aerosol was 40 psi in LC50 experiments.
aLC50 in air and its CI are calculated as nicotine concentration in solution × aerosol mass concentration in air (Table 1).
bThe value is at least 56%. Only one rat died at 56% nicotine solution during the up and down procedure. No other rats died at 32%, 56%, or 68% in multiple trials.
These results suggest that the method of delivering nicotine through aerosol inhalation is very efficient. Exposure to 2.3mg/L nicotine in air for 20min causes death in 50% of rats. This method can deliver controllable amount of nicotine rapidly into the circulation and brain-inducing dose-dependent pharmacological effects, even enough to cause death. In addition, we showed that pH affects nicotine actions. Acidification, but not basification, of the nicotine solution in the nebulizer minimizes the effects of nicotine, probably due to a reduction in nicotine absorption and/or bioavailability in the lungs.
Nicotine Pharmacokinetics in Arterial and Venous Blood
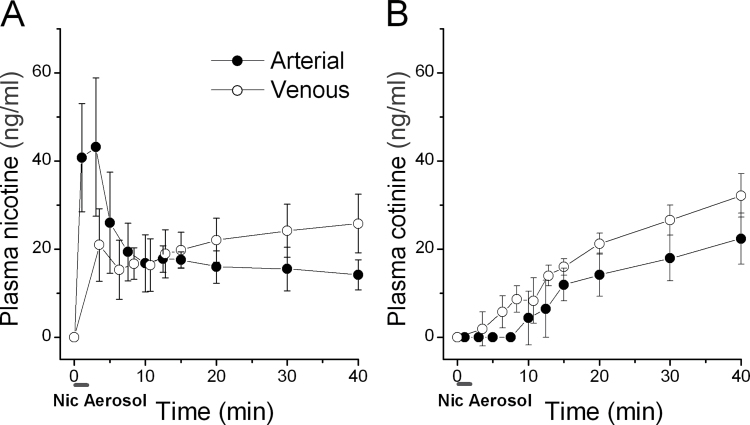
Rats were exposed to nicotine aerosol generated from 1% nicotine solution in the nebulizer in a nose-only system for 2min. The time course and magnitude of plasma nicotine and the nicotine metabolite cotinine in arterial or venous blood (separate rats) were measured from the start of nicotine aerosol delivery until 40min later. The arterial blood nicotine concentration reached a maximum of 43.2±15.7ng/ml (n = 5) within 1–4min and declined over the next 20min to 16.0±3.7ng/ml (Figure 4A). Plasma nicotine levels in venous blood increased slowly to 21.0±8.2ng/ml at 3.5min and then varied between 15 and 25ng/ml in the following 36min (Figure 4A). By comparing our results with those from human subjects smoking a cigarette (Fig. 1B in Lunell et al. [2000]), we demonstrate that the magnitude and early pharmacokinetic pattern of nicotine in arterial and venous blood in rats closely resembled that seen in human smoking (also see Hukkanen et al., 2005). The duration of the peak nicotine concentration was slightly shorter in rat with our experimental conditions compared to that of human smoking a cigarette likely because smokers took 5min to smoke a cigarette in Lunell et al.’s experimental conditions. We noticed that, unlike in human where the arterial blood nicotine level varies widely depending on the smoking pattern (Henningfield et al., 1993; Lunell et al., 2000; Rose et al., 1999), the arterial blood nicotine kinetic patterns were quite consistent among rats in our experimental conditions (note that the error bars in Figure 1A and 1B are SD, not SE). Plasma cotinine levels in both arterial and venous blood started to be detectable after the peak arterial nicotine levels and grew slowly (Figure 4B) consistent with the metabolism of nicotine and the slow metabolism rate of cotinine in naïve rats (Hukkanen et al., 2005).
Figure 4.
Nicotine aerosol inhalation in rats produces blood pharmacokinetics resembling human smoking. (A) Plasma nicotine levels (mean ± SD). (B) Plasma cotinine (a major nicotine metabolite) levels. Rats were exposed to nicotine aerosol in a nose-only exposure chamber for 2min (indicated as a short bar below the time axis in (A) and (B)). Nic = nicotine. Nicotine solution in the nebulizer contained 1% nicotine in 0.7% NaCl (pH 8, osmolality ~300 mOsm/kg). Air pressure for generating nicotine aerosol was 20 psi in these pharmacokinetics experiments. We collected first sample as pre-nicotine control, then collected arterial blood samples at timepoints (take starting of nicotine aerosol generation as 0min): 1, 3, 5, 7.5, 10, 12.5, 15, 20, 30, 40min (n = 5). We collected venous blood (separate rats, n = 5) at timepoints: 3.5, 6.3, 8.4, 10.7, 12.8, 15, 20, 30, 40min (since venous blood dropped slowly, the timepoints are averages of the mid points of collection durations from every rat).
These results suggest that the nicotine aerosol/inhalation method can deliver nicotine to rats with a pharmacokinetic pattern in both arterial and venous blood quantitatively resembling that of a human smoking a cigarette. The magnitude and rapid rise in arterial nicotine concentration are consistent with the hypothesis that nicotine aerosol reaches and deposits in the alveolar region where it is quickly absorbed into the pulmonary circulation during aerosol inhalation.
DISCUSSION
Through inhalation of tobacco smoke, nicotine is deposited and absorbed in the lungs leading to a rapid increase in arterial blood nicotine concentration, entering the systemic circulation and the brain quickly (Matta et al., 2007). Nicotine in this rapid arterial peak/decline concentration pattern stimulates brain nicotinic acetylcholine receptors, activating the dopaminergic reward system that plays an important role in initiation of addiction (De Biasi & Dani, 2011; Henningfield & Keenan, 1993). In addition, nicotine induces diverse effects in the central nervous system (CNS), such as improving cognitive function (Herman & Sofuoglu, 2010; Levin, McClernon, & Rezvani, 2006; Sarter, Parikh, & Howe, 2009), affecting anxiety-like behavior (File, Cheeta, & Kenny, 2000), and analgesia (Marubio et al., 1999; Semenova, Contet, Roberts, & Markou, 2012). In this study, we developed a noninvasive method of nicotine aerosol inhalation. We, for the first time, actually measured nicotine pharmacokinetics in both arterial and venous blood in rodents during and after aerosol inhalation and are able to achieves a rapid nicotine peak/decline pharmacokinetic pattern in the arterial blood that quantitatively simulates that of a human smoking a cigarette. The peak declines due to extensive distribution to body tissues (steady state volume of distribution = 2.2–3.3L/kg) (Hukkanen et al., 2005). On the other hand, nicotine concentrations in venous blood increased slower and after the initial 2-min aerosol exposure it kept increasing slowly consistent with nicotine release from tissues (Hukkanen et al., 2005). We do not exclude the possibility that residuals of certain percentage of relatively large aerosol droplets deposit in the nasal or buccal mucosa that are absorbed slower and last longer than 2min. Nicotine can be administered with this aerosol/inhalation method in vivo under various experimental conditions including freely behaving, sleeping, restrained, anesthetized, or artificially ventilated rodents. This method can be readily used for chronic intermittent nicotine exposure. The devices and method represents a powerful tool for studies of nicotine addiction, nicotine toxicology, tobacco-related diseases affecting, for example, the CNS, the lungs, the cardiovascular system, and, if applied to pregnant rodents, nicotine teratogenicity. This method could have important implications in nicotine replacement therapy (NRT) for smoking cessation and ultimately, for discovery of new pharmacological therapeutics.
In tobacco smoke, nicotine is present in protonated or unprotonated free-base forms. The ratio of protonated and free-base forms is pH dependent. The amount of free-base form of nicotine delivered to the smoker, in addition to the total amount, is an important factor in continuing use of tobacco products, that is, addiction, following an initial exposure (Ashley, Pankow, Tavakoli, & Watson, 2009). pH is hypothesized to dramatically affect nicotine bioavailability because the protonated form is hydrophilic, while the unprotonated free-base form is lipophilic and thus readily diffuses across membranes (Armitage & Turner, 1970; Schievelbein, Eberhardt, Loschenkohl, Rahlfs, & Bedall, 1973). Increasing pH in aerosolized nicotine produces a higher peak plasma nicotine concentration in humans (Burch et al., 1993). As drug delivery rate correlates to addiction potential (Henningfield & Keenan, 1993), increased free-base levels leads to increases in delivery rate, affecting the addiction potential (Ashley et al., 2009). However, this issue is controversial due to the difficulty and uncertainty of pH measurements in cigarette smoke (Pankow, 2001; Seeman, 2007). Some authors argue that because of the buffering capacity of the large surface area of the lungs and the relatively small amount of nicotine in cigarette smoke particles, the pH of nicotine aerosol in the range of effective pH of smoke of commercial cigarettes would not affect the absorption rate and bioavailability of nicotine in the lung (Seeman & Carchman, 2008). To test the hypothesis that pH of nicotine aerosol affects the absorption and bioavailability of nicotine, we examined the inhalation LC50 using nicotine aerosol generated from solutions with known pH. At pH 7.4 and at an alkaline pH 8.0, within the range of smoke effective pH of commercial cigarettes (Pankow et al., 2003), LC50s were equivalent (Table 2), while at pH 6.8, LC50 dramatically increased. These results suggest that a mild increase of pH from the physiological pH has no effect on nicotine absorption and/or bioavailability. While acidification in our conditions of lethal amounts of nicotine application may exceed the buffering capacity in the fluid lining the lungs, leading to reduction of free-base nicotine and thus a decrease in the delivery rate and/or the bioavailability of nicotine.
The nicotine concentrations in the mainstream smoke particulate matter of reference cigarettes are 7%–8% (Chambers, 2003). Our data show that exposure to nicotine in concentration of 32% in aerosol droplets (4–5 times higher than that in cigarette smoke particles) continuously for 20min kills 50% of laboratory rats. LD50 of i.p. injection of nicotine is 23.5mg/kg body weight for rats (Hicks & Sinclair, 1947). LD50 for orally delivered nicotine is 70mg/kg for Sprague-Dawley rats (van den Heuvel et al., 1990). With a simple calculation, Inhaled dose = nicotine concentration in air × minute ventilation × exposure time (Pauluhn, 2003), we can roughly estimate LD50 from LC50. According to Strohl et al. (1997), minute ventilation/100g body weight of Sprague-Dawley rats is 28.17±1.37ml. We get LD50 = 13mg/kg body weight for inhalation for rats. This value is lower than those for oral or i.p. administration, and is consistent with the first-pass metabolism of nicotine in the liver before it enters the systemic circulation when nicotine is administered orally or i.p. (Matta et al., 2007). We are aware of a limitation in this estimation of LD50. Respiratory parameters, for example, minute ventilation, vary during nicotine intoxication. This estimate is only for comparison purposes. Nicotine inhalation toxicity is formally reported as LC50 and its CI as well as exposure time, as recommended by USEPA and OECD (EPA, 1998; OECD, 2009), in this study. Our LC50 experiment results also provide reference data for establishing inhalation dosage regimen for nicotine research.
Nicotine inhalers that generate nicotine vapor for inhalation for human use are commercially available (such as Nicotrol®) and are used as NRT for smoking cessation (Lunell et al., 2000; Schneider et al., 2001). Nicotine vapor is primarily deposited in the mouth, nose, and throat leading to a slow increase in arterial blood nicotine concentration (Bergstrom et al., 1995; Lunell et al., 1996, 2000; Schneider et al., 2001). The pharmacokinetics is not comparable to that of smoking a cigarette. There are reports of nicotine administration to humans through aerosol inhalation. Their clinical significance has yet to be demonstrated (Burch, Erbland, Gann, & Hiller, 1989; Rose, Turner, Murugesan, Behm, & Laugesen, 2010; Simis et al., 2008). Chronic nicotine administration to rats by aerosol inhalation was suggested in an abstract (Littleton & Umney, 1977), but this study has not resulted in further publication. The aerosol droplet size distribution and nicotine pharmacokinetics in rodents was not determined. Without this information, nicotine aerosol inhalation methodology was not established (Chowdhury, Rayford, & Chang, 1992). We developed methods for nicotine delivery to rodent models through an aerosol inhalation route for research of nicotine addiction, tobacco-related diseases, etc., importantly research projects that cannot be done in humans. The potential of nicotine aerosol for therapeutic uses, for example, the efficacy and safety of NRT for smoking cessation, will need to be tested thoroughly in animal models.
During experiments with nicotine aerosol delivery, the nicotine solution concentration in the nebulizer container gradually increases due to evaporative losses of water (Niven & Hickey, 2007). In this study, there would not be a problem for single use of 2min (nicotine pharmacokinetic experiments) or 20min (LC50 experiments). However, for experiments on chronic intermittent exposure of nicotine aerosol, for example, 2min each multiple times a day, it would be important to replace the nicotine solution frequently, for example, at least once a day.
Recently, electronic cigarettes (E-cigarettes) were introduced into the U.S. market as an alternative to (ordinary) cigarettes (Cobb, Byron, Abrams, & Shields, 2010; Etter & Bullen, 2011). People also use E-cigarettes to treat nicotine withdrawal symptoms and to aid smoking cessation (Bullen et al., 2010; Siegel, Tanwar, & Wood, 2011). Public health experts and organizations including the WHO, the CDC, and the American Cancer Society have expressed concerns about the safety of these products (http://www.fda.gov/forconsumers/consumerupdates/ucm173401.htm). In fact, little research has been done on the health risk of E-cigarettes, and in general, on the health risk of inhalation of nicotine. As commercially available nicotine inhalation products like E-cigarettes become more popular, it becomes necessary to further study the health risk of inhalation of pure nicotine with animal models. The method developed in this study provides an effective and highly relevant tool for this line of research.
CONCLUSIONS
(a) A noninvasive method delivering nicotine to rodents through aerosol inhalation is developed. This method, through the same route as smoking tobacco, induces dose-dependent pharmacological effects. (b) In the breathing zone of this aerosol exposure system, the droplet size distribution of the nicotine aerosol was determined to be in the range of respirable size suggesting reaching the alveolar region, enabling rapid delivery of nicotine into the pulmonary circulation and then through the left heart and the arteries to all body systems including the brain. (c) The aerosol inhalation methodology can produce early pharmacokinetics in both the arterial and venous blood closely resembling those of a human smoking a cigarette. (d) This method is able to deliver substantial quantities of nicotine, high enough to rapidly reach fatal blood concentrations. The nicotine dose can be easily controlled by varying the nicotine concentration in solution in the nebulizer container or by varying the duration of aerosol exposure. We report, for the first time, that the acute inhalation toxicity of nicotine, LC50 is 2.3mg/L in air with a CI of 1.24–4.07mg/L (20min) for rats. (e) pH of nicotine aerosol in the range of 7.4–8.0 had similar effects, while lower pH reduced these effects, suggesting acidification decreases nicotine absorption and/or bioavailability.
FUNDING
This work was supported by the California Tobacco-Related Disease Research Program (TRDRP) Grant (18XT-0183) and the National Institutes of Health National Institute on Drug Abuse Grant (1R43DA031578).
DECLARATION OF INTERESTS
There are no conflicts of interests for XMS, BX, JL, YZ, and JLF. XSX is the founder and owns stocks of AfaSci Inc.
ACKNOWLEDGMENTS
We thank Dr. Donatello Telesca in the Department of Biostatistics, UCLA School of Public Health for his help with the up and down procedure statistical data analysis.
REFERENCES
- Armitage A. K., Turner D. M. (1970). Absorption of nicotine in cigarette and cigar smoke through the oral mucosa. Nature, 226, 1231–1232. 10.1038/2261231a0 [DOI] [PubMed] [Google Scholar]
- Ashley D. L., Pankow J. F., Tavakoli A. D., Watson C. H. (2009). Approaches, challenges, and experience in assessing free nicotine. In Henningfield J. E. (Ed.), Nicotine psychopharmacology, (pp. 437–456). Berlin, Heidelberg: Springer-Verlag; [DOI] [PubMed] [Google Scholar]
- Balfour D. J. (2004). The neurobiology of tobacco dependence: A preclinical perspective on the role of the dopamine projections to the nucleus accumbens [corrected]. Nicotine and Tobacco Research, 6, 899–912. 10.1080/14622200412331324965 [DOI] [PubMed] [Google Scholar]
- Benowitz N. L., Porchet H., Sheiner L., Jacob P., 3rd. (1988). Nicotine absorption and cardiovascular effects with smokeless tobacco use: Comparison with cigarettes and nicotine gum. Clinical Pharmacology and Therapeutics, 44, 23–28. 10.1038/clpt.1988.107 [DOI] [PubMed] [Google Scholar]
- Bergstrom M., Nordberg A., Lunell E., Antoni G., Langstrom B. (1995). Regional deposition of inhaled 11C-nicotine vapor in the human airway as visualized by positron emission tomography. Clinical Pharmacology and Therapeutics, 57, 309–317 [DOI] [PubMed] [Google Scholar]
- Beven J. L. (1976). Inhalation toxicity studies on cigarette smoke. I. A versatile exposure system for inhalation toxicity studies on cigarette smoke. Toxicology, 6, 189–196. 10.1016/0300-483X(76)90020–2 [DOI] [PubMed] [Google Scholar]
- Borgerding M., Klus H. (2005). Analysis of complex mixtures--cigarette smoke. Experimental and Toxicologic Pathology, 57(Suppl. 1), 43–73. 10.1016/j.etp.2005.05.010 [DOI] [PubMed] [Google Scholar]
- Brent R. L. (2004). Utilization of animal studies to determine the effects and human risks of environmental toxicants (drugs, chemicals, and physical agents). Pediatrics, 113(4 Suppl.), 984–995. 10.1002/bdrb.20020 [PubMed] [Google Scholar]
- Bruce R. D. (1985). An up-and-down procedure for acute toxicity testing. Fundamental and Applied Toxicology, 5, 151–157. 10.1016/0272-0590(85)90059-4 [DOI] [PubMed] [Google Scholar]
- Bullen C., McRobbie H., Thornley S., Glover M., Lin R., Laugesen M. (2010). Effect of an electronic nicotine delivery device (e cigarette) on desire to smoke and withdrawal, user preferences and nicotine delivery: Randomised cross-over trial. Tobacco Control, 19, 98–103. 10.1136/tc.2009.031567 [DOI] [PubMed] [Google Scholar]
- Burch S. G., Erbland M. L., Gann L. P., Hiller F. C. (1989). Plasma nicotine levels after inhalation of aerosolized nicotine. The American Review of Respiratory Disease, 140, 955–957 Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=2802381 [DOI] [PubMed] [Google Scholar]
- Burch S. G., Gann L. P., Olsen K. M., Anderson P. J., Hiller F. C., Erbland M. L. (1993). Effect of Ph on nicotine absorption and side-effects produced by aerosolized nicotine. Journal of Aerosol Medicine, 6, 45–52 Retrieved from http://online.liebertpub.com/toc/jam/6/1 [Google Scholar]
- Chambers O. (2003). The reference cigarette, Lexington: Reference Cigarette Program, College of Agriculture, University of Kentucky; [Google Scholar]
- Chowdhury P., Rayford P. L., Chang L. W. (1992). Induction of pancreatic acinar pathology via inhalation of nicotine. Proceedings of the Society for Experimental Biology and Medicine, 201, 159–164 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/1409731?dopt=Citation [DOI] [PubMed] [Google Scholar]
- Cobb N. K., Byron M. J., Abrams D. B., Shields P. G. (2010). Novel nicotine delivery systems and public health: The rise of the “e-cigarette”. American Journal of Public Health, 100, 2340–2342. 10.2105/AJPH.2010.199281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrigall W. A., Coen K. M. (1989). Nicotine maintains robust self-administration in rats on a limited-access schedule. Psychopharmacology, 99, 473–478. 10.1007/BF00589894 [DOI] [PubMed] [Google Scholar]
- Dani J. A., De Biasi M. (2001). Cellular mechanisms of nicotine addiction. Pharmacology, Biochemistry, and Behavior, 70, 439–446. 10.1016/S0091-3057(01)00652-9 [DOI] [PubMed] [Google Scholar]
- De Biasi M., Dani J. A. (2011). Reward, addiction, withdrawal to nicotine. Annual Review of Neuroscience, 34, 105–130. 10.1146/annurev-neuro-061010-113734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeNoble V. J., Mele P. C. (2006). Intravenous nicotine self-administration in rats: Effects of mecamylamine, hexamethonium and naloxone. Psychopharmacology, 184, 266–272. 10.1007/s00213-005-0054-z [DOI] [PubMed] [Google Scholar]
- EPA (1998). Health effects test guidelines: Acute inhalation toxicity, The Office of Prevention, Pesticides and Toxic Substances, United States Environmental Protection Agency. Washington, DC: Government Printing Office; [Google Scholar]
- EPA (2002). Health effects test guidelines: Acute oral toxicity, The Office of Prevention, Pesticides and Toxic Substances, United States Environmental Protection Agency. Washington, DC: Government Printing Office [Google Scholar]
- Epping-Jordan M. P., Watkins S. S., Koob G. F., Markou A. (1998). Dramatic decreases in brain reward function during nicotine withdrawal. Nature, 393, 76–79. 10.1038/30001 [DOI] [PubMed] [Google Scholar]
- Etter J. F., Bullen C. (2011). Electronic cigarette: Users profile, utilization, satisfaction and perceived efficacy. Addiction (Abingdon, England), 106, 2017–2028. 10.1111/j.1360-0443.2011.03505.x [DOI] [PubMed] [Google Scholar]
- File S. E., Cheeta S., Kenny P. J. (2000). Neurobiological mechanisms by which nicotine mediates different types of anxiety. European Journal of Pharmacology, 393, 231–236. 10.1016/S0014-2999(99)00889-4 [DOI] [PubMed] [Google Scholar]
- Fu Y., Matta S. G., Brower V. G., Sharp B. M. (2001). Norepinephrine secretion in the hypothalamic paraventricular nucleus of rats during unlimited access to self-administered nicotine: An in vivo microdialysis study. Journal of Neuroscience, 21, 8979–8989. 21/22/8979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y., Matta S. G., Gao W., Brower V. G., Sharp B. M. (2000). Systemic nicotine stimulates dopamine release in nucleus accumbens: Re-evaluation of the role of N-methyl-D-aspartate receptors in the ventral tegmental area. The Journal of Pharmacology and Experimental Therapeutics, 294, 458–465 Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=10900219 [PubMed] [Google Scholar]
- George O., Grieder T. E., Cole M., Koob G. F. (2010). Exposure to chronic intermittent nicotine vapor induces nicotine dependence. Pharmacology, Biochemistry, and Behavior, 96, 104–107. 10.1016/j.pbb.2010.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowadia N., Oldham M. J., Dunn-Rankin D. (2009). Particle size distribution of nicotine in mainstream smoke from 2R4F, Marlboro Medium, and Quest1 cigarettes under different puffing regimens. Inhalation Toxicology, 21, 435–446. 10.1080/08958370802512535 [DOI] [PubMed] [Google Scholar]
- Henningfield J. E., Keenan R. M. (1993). Nicotine delivery kinetics and abuse liability. Journal of Consulting and Clinical Psychology, 61, 743–750. 10.1037//0022-006X.61.5.743 [DOI] [PubMed] [Google Scholar]
- Henningfield J. E., Stapleton J. M., Benowitz N. L., Grayson R. F., London E. D. (1993). Higher levels of nicotine in arterial than in venous blood after cigarette smoking. Drug and Alcohol Dependence, 33, 23–29. 10.1016/0376-8716(93)90030-T [DOI] [PubMed] [Google Scholar]
- Herman A. I., Sofuoglu M. (2010). Cognitive effects of nicotine: Genetic moderators. Addiction Biology, 15, 250–265. 10.1111/j.1369-1600.2010.00213.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks C. S., Sinclair D. A. (1947). Toxicities of the optical isomers of nicotine and nornicotine. The Australian Journal of Experimental Biology and Medical Science, 25, 83–86. 10.1038/icb.1947.12 [DOI] [PubMed] [Google Scholar]
- Hinds W. C. (1978). Size characteristics of cigarette smoke. American Industrial Hygiene Association Journal, 39, 48–54. 10.1080/0002889778507712 [DOI] [PubMed] [Google Scholar]
- Hinds W. C. (1999). Aerosol technology: Properties, behavior, and measurement of airborne particles, (2nd ed.). New York, NY: John Wiley & Sons; [Google Scholar]
- Hukkanen J., Jacob P., 3rd, Benowitz N. L. (2005). Metabolism and disposition kinetics of nicotine. Pharmacological Reviews, 57, 79–115. 10.1124/pr.57.1.3 [DOI] [PubMed] [Google Scholar]
- Levin E. D., McClernon F. J., Rezvani A. H. (2006). Nicotinic effects on cognitive function: Behavioral characterization, pharmacological specification, and anatomic localization. Psychopharmacology, 184, 523–539. 10.1007/s00213-005-0164-7 [DOI] [PubMed] [Google Scholar]
- Littleton J. M., Umney N. D. (1977). The chronic administration of L-nicotine by aerosol to rats [proceedings]. The Journal of Physiology, 266, 11P–12P Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=853385 [PubMed] [Google Scholar]
- Lunell E., Bergstrom M., Antoni G., Langstrom B., Nordberg A. (1996). Nicotine deposition and body distribution from a nicotine inhaler and a cigarette studied with positron emission tomography. Clinical Pharmacology and Therapeutics, 59, 593–594. 10.1016/S0009-9236(96)90188-5 [DOI] [PubMed] [Google Scholar]
- Lunell E., Molander L., Ekberg K., Wahren J. (2000). Site of nicotine absorption from a vapour inhaler--comparison with cigarette smoking. European Journal of Clinical Pharmacology, 55, 737–741. 10.1007/s002280050007 [DOI] [PubMed] [Google Scholar]
- Marubio L. M., del Mar Arroyo-Jimenez M., Cordero-Erausquin M., Lena C., Le Novere N., de Kerchove d’Exaerde A, … Changeux J. P. (1999). Reduced antinociception in mice lacking neuronal nicotinic receptor subunits. Nature, 398, 805–810. 10.1038/19756 [DOI] [PubMed] [Google Scholar]
- Matta S. G., Balfour D. J., Benowitz N. L., Boyd R. T., Buccafusco J. J., Caggiula A. R, … Zirger J. M. (2007). Guidelines on nicotine dose selection for in vivo research. Psychopharmacology, 190, 269–319. 10.1007/s00213-006-0441-0 [DOI] [PubMed] [Google Scholar]
- Meng Y., Lichtman A. H., Bridgen D. T., Martin B. R. (1997). Inhalation studies with drugs of abuse. In Rapaka R. S., Chiang N., Martin B. R. (Eds.), Pharmacokinetics, metabolism, and pharmaceutics of drugs of abuse. NIDA Research Monograph, #173, (pp. 201–224). NIDA/NIH; Washington, DC: Government Printing Office. [PubMed] [Google Scholar]
- Moir D., Rickert W. S., Levasseur G., Larose Y., Maertens R., White P., Desjardins S. (2008). A comparison of mainstream and sidestream marijuana and tobacco cigarette smoke produced under two machine smoking conditions. Chemical Research in Toxicology, 21, 494–502. 10.1021/tx700275p [DOI] [PubMed] [Google Scholar]
- NIH/NIDA (2008). Tobacco and nicotine research: An update from the National Institute on Drug Abuse, National Institute of Health. Washington, DC: Government Printing Office [Google Scholar]
- Niven R. W., Hickey A. J. (2007). Atomization and nebulizers. In Hickey A. J. (Ed.), Inhalation aerosols, (2nd ed., pp. 253–280). New York, London: : Informa Healthcare; [Google Scholar]
- OECD (2009). OECD Guidance Document on Acute Inhalation Toxicity Testing Vol. Series on Testing and Assessment, #39 (pp. 1–71). Retrieved from http://www.oecd.org/officialdocuments/displaydocumentpdf?cote=ENV/JM/MONO(2009)28&doclanguage=en
- Pankow J. F. (2001). A consideration of the role of gas/particle partitioning in the deposition of nicotine and other tobacco smoke compounds in the respiratory tract. Chemical Research in Toxicology, 14, 1465–1481. 10.1021/tx0100901 [DOI] [PubMed] [Google Scholar]
- Pankow J. F., Tavakoli A. D., Luo W., Isabelle L. M. (2003). Percent free base nicotine in the tobacco smoke particulate matter of selected commercial and reference cigarettes. Chemical Research in Toxicology, 16, 1014–1018. 10.1021/tx0340596 [DOI] [PubMed] [Google Scholar]
- Patton J. S., Byron P. R. (2007). Inhaling medicines: Delivering drugs to the body through the lungs. Nature Reviews, 6, 67–74. 10.1038/nrd2153 [DOI] [PubMed] [Google Scholar]
- Pauluhn J. (2003). Issues of dosimetry in inhalation toxicity. Toxicology Letters, 140–141, 229–238. 10.1016/S0378-4274(03)00077-8 [DOI] [PubMed] [Google Scholar]
- Rose J. E., Behm F. M., Westman E. C., Coleman R. E. (1999). Arterial nicotine kinetics during cigarette smoking and intravenous nicotine administration: Implications for addiction. Drug and Alcohol Dependence, 56, 99–107. 10.1016/S0376-8716(99)00025-3 [DOI] [PubMed] [Google Scholar]
- Rose J. E., Mukhin A. G., Lokitz S. J., Turkington T. G., Herskovic J., Behm F. M, … Garg P. K. (2010). Kinetics of brain nicotine accumulation in dependent and nondependent smokers assessed with PET and cigarettes containing 11C-nicotine. Proceedings of the National Academy of Sciences of the United States of America, 107, 5190–5195. 10.1073/pnas.0909184107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose J. E., Turner J. E., Murugesan T., Behm F. M., Laugesen M. (2010). Pulmonary delivery of nicotine pyruvate: Sensory and pharmacokinetic characteristics. Experimental and Clinical Psychopharmacology, 18, 385–394. 10.1037/a0020834 [DOI] [PubMed] [Google Scholar]
- Rothwell P. E., Gewirtz J. C., Thomas M. J. (2010). Episodic withdrawal promotes psychomotor sensitization to morphine. Neuropsychopharmacology, 35, 2579–2589. 10.1038/npp.2010.134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarter M., Parikh V., Howe W. M. (2009). nAChR agonist-induced cognition enhancement: Integration of cognitive and neuronal mechanisms. Biochemical Pharmacology, 78, 658–667. 10.1016/j.bcp.2009.04.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schievelbein H., Eberhardt R., Loschenkohl K., Rahlfs V., Bedall F. K. (1973). Absorption of nicotine through the oral mucosa. I. Measurement of nicotine concentration in the blood after application of nicotine and total particulate matter. Agents and Actions, 3, 254–258. 10.1007/BF01968551 [DOI] [PubMed] [Google Scholar]
- Schneider N. G., Olmstead R. E., Franzon M. A., Lunell E. (2001). The nicotine inhaler: Clinical pharmacokinetics and comparison with other nicotine treatments. Clinical Pharmacokinetics, 40, 661–684. 10.2165/00003088-200140090-00003 [DOI] [PubMed] [Google Scholar]
- Seeman J. I. (2007). Possible role of ammonia on the deposition, retention, and absorption of nicotine in humans while smoking. Chemical Research in Toxicology, 20, 326–343. 10.1021/tx600290v [DOI] [PubMed] [Google Scholar]
- Seeman J. I., Carchman R. A. (2008). The possible role of ammonia toxicity on the exposure, deposition, retention, and the bioavailability of nicotine during smoking. Food and Chemical Toxicology, 46, 1863–1881. 10.1016/j.fct.2008.02.021 [DOI] [PubMed] [Google Scholar]
- Semenova S., Contet C., Roberts A. J., Markou A. (2012). Mice lacking the beta4 subunit of the nicotinic acetylcholine receptor show memory deficits, altered anxiety- and depression-like behavior, and diminished nicotine-induced analgesia. Nicotine and Tobacco Research, [Epub ahead of print]. 10.1093/ntr/nts107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp B. M., Matta S. G. (1993). Detection by in vivo microdialysis of nicotine-induced norepinephrine secretion from the hypothalamic paraventricular nucleus of freely moving rats: Dose-dependency and desensitization. Endocrinology, 133, 11–19. 10.1210/en.133.1.11 [DOI] [PubMed] [Google Scholar]
- Siegel M. B., Tanwar K. L., Wood K. S. (2011). Electronic cigarettes as a smoking-cessation: Tool results from an online survey. American Journal of Preventive Medicine, 40, 472–475. 10.1016/j.amepre.2010.12.006 [DOI] [PubMed] [Google Scholar]
- Simis K., Lei M., Lu A. T., Sharma K. C., Hale R. L., Timmons R., Cassella J. (2008). Nicotine aerosol generation from thermally reversible zinc halide complexes using the Staccato system. Drug Development and Industrial Pharmacy, 34, 936–942. 10.1080/03639040802149061 [DOI] [PubMed] [Google Scholar]
- Skjei K. L., Markou A. (2003). Effects of repeated withdrawal episodes, nicotine dose, and duration of nicotine exposure on the severity and duration of nicotine withdrawal in rats. Psychopharmacology, 168, 280–292. 10.1007/s00213-003-1414-1 [DOI] [PubMed] [Google Scholar]
- Strohl K. P., Thomas A. J., St Jean P., Schlenker E. H., Koletsky R. J., Schork N. J. (1997). Ventilation and metabolism among rat strains. Journal of Applied Physiology, 82, 317–323 Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=9029232 [DOI] [PubMed] [Google Scholar]
- Theeuwes F., Yum S. I. (1976). Principles of the design and operation of generic osmotic pumps for the delivery of semisolid or liquid drug formulations. Annals of Biomedical Engineering, 4, 343–353. 10.1007/BF02584524 [DOI] [PubMed] [Google Scholar]
- van den Heuvel M. J., Clark D. G., Fielder R. J., Koundakjian P. P., Oliver G. J., Pelling D, … Walker A. P. (1990). The international validation of a fixed-dose procedure as an alternative to the classical LD50 test. Food and Chemical Toxicology, 28, 469–482. 10.1016/0278-6915(90)90117-6 [DOI] [PubMed] [Google Scholar]
- Waldum H. L., Nilsen O. G., Nilsen T., Rorvik H., Syversen V., Sanvik A. K, … Brenna E. (1996). Long-term effects of inhaled nicotine. Life Sciences, 58, 1339–1346. 10.1016/0024-3205(96)00100-2 [DOI] [PubMed] [Google Scholar]