Abstract
Introduction:
Little is known about the relationship between cigarette smoking and agonist treatment in opioid-dependent pregnant patients. The objective of this study is to examine the extent to which cigarette smoking profiles differentially changed during the course of pregnancy in opioid-dependent patients receiving either double-blind methadone or buprenorphine. Patients were participants in the international, randomized controlled Maternal Opioid Treatment: Human Experimental Research (MOTHER) study.
Methods:
A sample of opioid-maintained pregnant patients (18–41 years old) with available smoking data who completed a multisite, double-blind, double-dummy, randomized controlled trial of methadone (n = 67) and buprenorphine (n = 57) between 2005 and 2008. Participants were compared on smoking variables based on opioid agonist treatment condition.
Results:
Overall, 95% of the sample reported cigarette smoking at treatment entry. Participants in the two medication conditions were similar on pretreatment characteristics including smoking rates and daily cigarette amounts. Over the course of the pregnancy, no meaningful changes in cigarette smoking were observed for either medication condition. The fitted difference in change in adjusted cigarettes per day between the two conditions was small and nonsignificant (β = −0.08, SE = 0.05, p = .132).
Conclusions:
Results support high rates of smoking with little change during pregnancy among opioid-dependent patients, regardless of the type of agonist medication received. These findings are consistent with evidence that suggests nicotine effects, and interactions may be similar for buprenorphine compared with methadone. The outcomes further highlight that aggressive efforts are needed to reduce/eliminate smoking in opioid-dependent pregnant women.
INTRODUCTION
Cigarette smoking during pregnancy is associated with a number of medical and developmental consequences including low birth weight and stillbirth (Bada et al., 2005; Conter, Cortinovis, Rogari, & Riva, 1995; D’Onofrio et al., 2003; Knopik et al., 2005; McCowan & Horgan, 2009; Salihu et al., 2008; Stroud et al., 2009; Thiriez et al., 2009). Despite the well-known adverse consequences of cigarette smoking, about 21% of reproductive-age women in the United States and Europe smoke cigarettes (Centers for Disease Control and Prevention [CDC], 2008a; World Health Organization, 2010). Although 30%–61% attempt to quit smoking cigarettes when pregnant, about 13% of all U.S. women continue to smoke during pregnancy (Tong et al., 2009).
Over the past 20 years, behavioral treatments have been shown to improve smoking cessation and reduction outcomes for pregnant women who smoke, reducing the incidence of adverse pregnancy outcomes, including low birth weight and preterm birth, in their neonates (Dolan-Mullen, Ramirez, & Groff, 1994; Floyd, Rimer, Giovino, Mullen, & Sullivan, 1993; Heil et al., 2008; Lumley et al., 2009; Tuten, Fitzsimons, Chisolm, Nuzzo, & Jones, 2012). Thus, it is recommended that behavioral treatment for cigarette smokers be a routine part of prenatal care in all maternity care settings (Lumley et al., 2009). Pharmacotherapy (including nicotine replacement therapy) is used clinically to assist pregnant women in quitting; however, there is currently inadequate evidence to evaluate the safety or efficacy of their use (U.S. Preventive Services Task Force, 2009). Despite the availability of effective behavioral treatments for cigarette smoking during pregnancy, only 18%–25% of pregnant women who smoke during pregnancy end up quitting (Office of the Surgeon General (US) & Office on Smoking and Health (US), 2004), and cigarette smoking continues to be the leading cause of preventable pregnancy-related morbidity and mortality (Dietz et al., 2010; Minnes, Lang, & Singer, 2011).
Cigarette smoking is overall the leading preventable cause of death in the United States (Ball, Rounsaville, Tennen, & Kranzler, 2001). Smoking is responsible for more deaths than alcohol and illicit drug use, HIV, motor vehicle injuries, suicides, and homicides combined (Centers for Disease Control and Prevention [CDC], 2008b; Mokdad, Marks, Stroup, & Gerberding, 2004). This deadly behavior is highly prevalent among substance-dependent individuals, with just under half of all cigarettes smoked consumed by those with a substance use or other mental health disorder (Lasser et al., 2000). These individuals are already at higher risk of adverse health effects compared with the general population (Baca & Yahne, 2009; Nahvi, Richter, Li, Modali, & Arnsten, 2006; Richter, Gibson, Ahluwalia, & Schmelzle, 2001), but cigarette smoking compounds this risk (Hurt et al., 1996). Despite the need to address smoking cessation in substance-dependent populations (Fiore & Jaen, 2008; Richter & Arnsten, 2006), 60%–70% of substance abuse treatment programs fail to offer any smoking cessation counseling (Friedmann, Jiang, & Richter, 2008; Fuller et al., 2007).
A group with particularly high rates of smoking is individuals with opioid dependence. In general, more than 90% of methadone-maintained patients smoke cigarettes (Nahvi et al., 2006; Richter et al., 2001). A similar prevalence is found for opioid-dependent pregnant patients (Chisolm, Tuten, Brigham, Strain, & Jones, 2009; Chisolm, Brigham, Tuten, Strain, & Jones, 2010; Jones et al., 2009; Winklbaur et al., 2009). This finding suggests that the prevalence of cigarette smoking in opioid-dependent pregnant women is more than four times higher than the 13% rate reported in the general pregnant population (Tong et al., 2009). In combination with illicit drug use, smoking contributes further to compromised fetal development and birth outcomes (Bada et al., 2005; Bailey & Marien, 2011). Management of opioid dependence per se is insufficient to affect cigarette smoking, and this problem represents a significant but independent challenge that must be addressed. Given pregnancy-specific health risks of cigarette smoking (e.g., premature birth, low birth weight, stillbirth, and sudden infant death syndrome) (Bada et al., 2005; Conter et al., 1995; D’Onofrio et al., 2003; Knopik et al., 2005; McCowan & Horgan, 2009; Salihu et al., 2008; Stroud et al., 2009; Thiriez et al., 2009) and the already high risk of medical complications in opioid-dependent pregnant women (Haug, Stitzer, & Svikis, 2001), there is an even greater need to address smoking cessation in pregnant populations of substance users (Chisolm, Brigham, Lookatch, et al., 2010).
Compared with the general population of pregnant women, little is known about cigarette smoking in opioid-dependent pregnant women. It is known that the prevalence of cigarette smoking and related risks are high among opioid-dependent women (Chisolm et al., 2009; Chisolm, Brigham, Lookatch, et al., 2010; Chisolm, Brigham, Tuten, et al., 2010; Jones et al., 2009). Additionally, heavy maternal nicotine use has been found to potentiate neonatal abstinence syndrome in opioid-exposed infants (Choo, Huestis, Schroeder, Shin, & Jones, 2004). However, the pattern of cigarette smoking over the course of pregnancy in these women, including those treated with opioid agonists, has not been fully examined. Given that pregnancy is a time of high motivation to change for many women, it is possible that those opioid-dependent pregnant patients who smoke may be a group particularly interested and willing to stop smoking.
There are two main medications used to treat opioid dependence: methadone and buprenorphine. Studies of various nonpregnant agonist-treated samples suggest that both methadone and buprenorphine can interact with nicotine. Work conducted over 25 years ago suggests that opioid use, including heroin, methadone, and buprenorphine, is associated with increased smoking (Chait, & Griffiths, 1984; Mello, Lukas, & Mendelson, 1985; Mello, Mendelson, Sellers, & Kuehnle, 1980). More recent research has demonstrated that concurrent nicotine use enhances methadone’s effect on opioid-withdrawal scores in methadone-maintained patients (Elkader, Brands, Selby, & Sproule, 2009), and buprenorphine increases the number of cigarettes smoked per day (CPD) (Mutschler, Stephen, Teoh, Mendelson, & Mello, 2002). Findings of interactions between nicotine and methadone, and nicotine and buprenorphine, suggest that there may be important clinical implications to substance use and its treatment in persons with opioid dependence who smoke cigarettes. However, direct comparisons of smoking among individuals on methadone and buprenorphine have received little attention. One recent study (Pajusco et al., 2012) compared smoking behavior in patients treated with methadone versus buprenorphine and confirmed high rates of smoking and cigarette use patterns among the two groups with no apparent difference between medications. To the authors’ knowledge, no other studies have directly compared cigarette smoking behavior associated with buprenorphine and methadone medication treatment, in either general or pregnant opioid-dependent populations. Given that buprenorphine is increasingly prescribed during pregnancy (Jones et al., 2010; Lacroix et al., 2011) and that the potential public health risks associated with even a small increase in cigarette smoking during pregnancy are great, there is a need to probe for any differences in cigarette smoking behavior among opioid-dependent pregnant patients treated with buprenorphine and methadone.
Because there are signals that suggest methadone and buprenorphine each can interact with smoking and because pregnancy represents a prime opportunity to study the time course of smoking cessation (given women’s motivation to stop), these analyses were conducted to see if there were differential effects by opioid treatment agent—either in overall cessation rates of smoking or in the time course of changes in smoking. The Maternal Opioid Treatment: Human Experimental Research (MOTHER) study (Jones et al., 2010) provided the data for this exploratory comparison. The primary objective of this study was to examine the extent to which cigarette smoking profiles differentially changed during the course of pregnancy among opioid-dependent patients receiving either methadone or buprenorphine.
METHODS
MOTHER Study
The parent study of this secondary data analysis project is the multisite MOTHER study examining the comparative safety and efficacy of methadone and buprenorphine in the treatment of opioid dependence among pregnant women and their neonates. The MOTHER study enrolled participants between May 4, 2005, and October 31, 2008, who were opioid-dependent women between the ages of 18 and 41 years, with a singleton pregnancy between 6 and 30 weeks of gestation. Participants were screened and recruited at eight international sites —six in the United States and one each in Austria and Canada. Seven sites contributed randomized data; the Canadian site screened participants but did not complete randomization. The U.S. sites represented both rural (Burlington, VT; Nashville, TN) and urban (Baltimore, MD; Philadelphia, PA, Detroit, MI; Providence, RI) environments. Treatment programs at the participating sites and community providers served as referral sources. Women were eligible for participation in the study if they had no medical or other conditions contraindicating participation, were not subject to pending legal action that might prevent their participation, had no disorders related to the use of benzodiazepines or alcohol, and did not plan to give birth outside the hospital at the study site. Each site’s local institutional review board approved the parent study. Women were approached by site research staff about possible participation upon determination of eligibility. All participants provided written informed consent at the time of screening and subsequently completed a screening assessment battery, which included the Addiction Severity Index (McLellan, Cacciola, Alterman, Rikoon, & Carise, 2006) and a Tobacco Dependence Screener (Kawakami, Takatsuka, Inaba, & Shimizu, 1999). After meeting screening requirements and completing a morphine washout period, participants were randomized to receive either methadone or buprenorphine. (Randomization assignments were generated by a data coordinating center for all sites.) The study utilized a double-blind, double-dummy design, meaning participants received an active medication and a placebo upon dosing (Martin, Meinert, Breitner, & ADAPT Research Group, 2002). Following induction, participants were maintained on study medication and followed for the duration of pregnancy through 30 days postpartum, so length of study participation varied among participants. Overall study screening details and attrition rates and the primary and key secondary outcomes analyses from the 131 MOTHER study completers have been reported elsewhere (Jones et al., 2010).
Present Study
For this secondary data analysis, smoking data were available from 124 (methadone, n = 67 and buprenorphine, n = 57) of these women (including two participants for each treatment condition who reported no smoking during the study). The two conditions were compared on lifetime and pretreatment smoking characteristics and adjusted smoking rate over the duration of the study. No smoking cessation intervention was implemented as part of this study. Participants were offered site-specific pharmacologic and/or behavioral cessation services as a component of standard care. Data regarding each site’s cessation services and study participant access to these services were not collected.
Measures
Demographic
Age, ethnicity, marital status, current employment, and estimated gestational age were determined for the MOTHER study completers from the initial screening forms that were completed by all potential participants. Ethnicity was defined as Black versus White/other; marital status was defined as not married (never married, widowed, divorced or separated) versus currently married; current employment status was defined as nonemployed versus employed (full or parttime).
Tobacco Dependence Screener
To assess severity of nicotine dependence, all participants completed the Tobacco Dependence Screener (Kawakami et al., 1999) at study intake. An overall score ranging from 0 to 10 can be calculated from this brief, 10-item self-administered questionnaire, with a score of 5 or greater generally indicating more severe dependence. Tobacco Dependence Screener scores appear reliable and correlate well with other self-report smoking characteristics and objective measures including expired carbon monoxide levels (Kawakami et al., 1999).
Addiction Severity Index
All participants completed an initial interview, a modified Addiction Severity Index (ASI) 5.0 (McLellan et al., 2006) semistructured interview, to assess lifetime and pretreatment psychosocial functioning. An abbreviated ASI 5.0 was readministered monthly from study entry through the duration of the study to capture information on multiple domains including drug and cigarette smoking over the 30 days prior. The ASI has been widely studied, and its reliability and validity have been well demonstrated (McLellan et al., 1992).
Cigarette Smoking Outcome Variables
Cigarette Smoking Pretreatment
Lifetime cigarette smoking characteristics for the sample are drawn from the initial ASI interview, including age of first use and lifetime months of use.
Past 30-day cigarette smoking characteristics were also drawn from the initial ASI including current smoking status (reported cigarette smoking yes/no in past 30 days), number of days smoking in past 30 days, and adjusted number of CPD.
The methadone and buprenorphine conditions were also compared on mean overall scores from the Tobacco Dependence Screener to further describe smoking severity at study entry.
Cigarette Smoking During Study Period
The methadone and buprenorphine conditions were also compared on cigarette smoking behavior from study entry through delivery using data from the monthly follow-up ASI interviews. To describe smoking over the course of the study period, the adjusted number of CPD was determined in weekly intervals. The adjusted CPD was derived by taking the number of days smoked in the past 30 days and multiplying it by the number of cigarettes per day; this product was then divided by 30 to arrive at the adjusted CPD.
Statistical Analyses
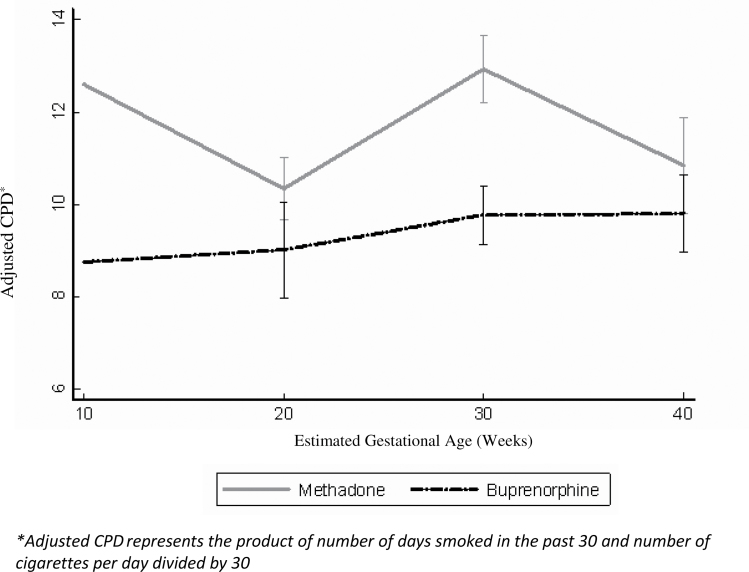
The sample of participants with follow-up ASIs for whom longitudinal smoking data were available (n = 124) was used to characterize the prevalence and severity of smoking in this population of opioid-dependent pregnant women seeking treatment, as well as for comparisons of the smoking profiles between the two treatment conditions. Data were analyzed using independent samples t tests for continuous data and chi-square tests for categorical variables. A longitudinal linear mixed effects model with a random intercept (Dupont & Plummer, 1990; Henderson, Diggle, & Dobson, 2000), which controlled for study site, was used to examine changes in number of CPD by treatment condition. The model that was fit as follows: y ij= β 0 + α 0i + (β 1) time + β 2 treatment + β 3 time * treatment + e ij, where y ij is the adjusted CPD for the ith person at the jth timepoint. This model does not require that all individuals start at the same point in their pregnancy and does not model the change from baseline explicitly. In the context of this study, relying on change from baseline would be inappropriate as participants entered the study at different points in their pregnancies, resulting in CPD being measured at different points in their pregnancies. Instead, the model uses all available data to estimate an “average” rate of change per week in adjusted CPD for each treatment condition (buprenorphine and methadone). This model, thus, enables calculation of a difference in expected rate of change between the two treatment groups, which is the coefficient for the interaction between time and treatment (β 3). Figure 1 shows the fitted trajectories (estimated slopes or changes over time) for each treatment condition.
Figure 1.
Cigarette smoking by treatment condition during study period.
Based on the formulae of Dupont and Plummer (1990), 67 methadone participants and 57 buprenorphine participants would yield 80% power to detect a (moderate) effect size of 0.51 or greater, where effect size is calculated as the difference in means of the two groups divided by the pooled standard error.
All analyses were performed in STATA v. 11.0.
RESULTS
Participant Characteristics
The overall sample characteristics are shown in Table 1. The sample was mostly White, unemployed, and unmarried with a mean (SD) age of 26.4 (5.4) years old. Participants entered the study shortly after the start of the second trimester on average. The characteristics of those randomized to methadone (n = 67) and buprenorphine (n = 57) are also shown in Table 1. These treatment conditions were similar on demographic and other pretreatment characteristics.
Table 1.
Participant Characteristics
| Variable | Overall sample(N = 124) | Methadone condition(n = 67) | Buprenorphine condition(n = 57) | Test statistic | p Value |
|---|---|---|---|---|---|
| Mean age in years (SD) | 26.4 (5.4) | 27.2 (5.4) | 25.4 (5.2) | t = 1.94; df = 122 | p = .054 |
| Black (%) | 8.1 | 10.4 | 5.2 | χ2 = 1.12; df = 1 | p = .29 |
| Not married (%) | 87.1 | 83.6 | 91.2 | χ2 = 4.99; df = 4 | p = .29 |
| Unemployed (%) | 83.1 | 86.6 | 78.9 | χ2 = 2.05; df = 2 | p = .36 |
| Mean EGAa weeks (SD) | 18.26 (5.99) | 18.03 (6.5) | 18.53 (5.4) | t = 0.46; df = 122 | p = .65 |
| Report cigarette smoking past month (%) | 95 | 97 | 93 | χ2 = 1.09; df = 1 | p = .30 |
| Mean age of first cigarette smoking (SD) | 14.4 (2.8) | 14.6 (2.6) | 14.1 (3.0) | t = 0.95; df = 120 | p = .34 |
| Mean months of lifetime cigarette smoking (SD) | 128.1 (75.9) | 137.7 (70.9) | 116.5 (8.06) | t = 1.55; df = 121 | p = .12 |
| Mean days cigarette smoking past month (SD) | 27.8 (7.1) | 28.9 (5.2) | 26.5 (8.8) | t = 1.90; df = 122 | p = .06 |
| Adjusted CPDb past month (SD) | 13.1 (8.09) | 13.99 (8.15) | 12.07 (7.96) | t = 1.32; df = 122 | p = .189 |
| Mean Tobacco Dependence Score (SD) | 7.3 (3.7) | 7.3 (3.8) | 7.2 (3.7) | t = 0.09; df = 115 | p = .93 |
aEGA represents the estimated gestational age of pregnancy measured in weeks.
bAdjusted CPD represents the product of number of days smoked in the past 30 days and number of cigarettes per day divided by 30.
Table 1 shows the lifetime and pretreatment-adjusted cigarette smoking characteristics of the sample as well as by treatment condition. Overall, the women reported first smoking cigarettes at about 14 years of age on average and mean regular use for just over 10 years. Prior to treatment entry, participants reported smoking more than half a pack of cigarettes per day in the past month. The proportion of participants smoking cigarettes and the severity of smoking characteristics was similar among the two medication conditions.
To examine changes over time in cigarette smoking, a longitudinal linear model was fit with a random intercept. Figure 1 shows adjusted CPD over the course of the study (where time is described as weeks). The fitted difference in change in adjusted CPD between the two conditions (e.g., the interaction between time and interaction between time and treatment condition, with methadone as the reference condition) was small and nonsignificant (β = −0.08/week, SE = 0.05, p = .135). Based on the model, which controlled for study site and maternal age, individuals on methadone experienced no appreciable change in adjusted CPD (β = 0.03/week, SE = 0.04, p = .405), whereas individuals on buprenorphine likewise experienced no appreciable change in adjusted CPD (β = −0.05/week, SE = 0.04, p = .209). Controlling for maternal age in a secondary analysis did not affect the other estimates, and maternal age itself was not a statistically significant predictor of adjusted cigarettes per day.
DISCUSSION
The percentage of opioid-dependent pregnant participants in this study who reported current cigarette smoking was 95%, more than four times higher than in the general pregnant population (Jones et al., 2009; Tong et al., 2009). This high rate of cigarette smoking is alarming because continued smoking during pregnancy is associated with diverse adverse health effects (Cnattingius, 2004). The current findings reveal that the rate of smoking and amount of cigarettes smoked did not differ on the basis of the agonist medication received in this study sample. In Figure 1, women in the methadone condition appeared to smoke a greater number of cigarettes on average per day; however, there was no significant difference when compared with the women in buprenorphine condition throughout the course of the study. Neither medication condition was associated with any statistically significant change (neither an increase nor a reduction) in smoking over the course of pregnancy. These findings further support the need to address cigarette smoking cessation in substance-dependent individuals, including pregnant women (Bada et al., 2005; Bailey & Marien, 2011; Chisolm, Brigham, Lookatch, et al., 2010; Fiore & Jaen, 2008; Haug et al., 2001; Richter & Ahluwalia, 2000; Richter & Arnsten, 2006; Winklbaur et al., 2009).
There are several limitations of this study. First, data were collected as part of a larger randomized double-blind study not designed to compare smoking characteristics. Like previous studies, this study relies on commonly used, broadly defined self-report measures of smoking. A prospective trial examining smoking history and current smoking could include more comprehensive and sensitive measures and yield results supporting stronger conclusions. For example, in this study, the number of CPD was based on self-report and most likely represents an underestimation of the cigarette smoking behavior among the participants (Burstyn et al., 2009). A prospective trial designed to study cigarette smoking would include collection of validated biomarkers, such as urine cotinine levels, which would more accurately reflect smoking prevalence and levels. However, the present data are an important first step in the development of direct comparisons between smoking and agonist treatments for this patient population. Second, the sample sizes of the examined conditions are variable and may limit the power to detect differences in some instances. A larger sample size would have allowed for participants to be further categorized according to smoking severity and perhaps assessed for more subtle differences. Third, all of the participants included in the study are MOTHER completers, which does raise the potential for bias in estimates (because this is not an intention-to-treat analysis) as, unfortunately, many of the key outcomes from the parent study are only measureable near completion of the study (e.g., delivery).
Despite these limitations, this study provides the first data comparing smoking behavior in pregnant women receiving methadone and buprenorphine treatment. Although the health risks of smoking during pregnancy are well known, future research must focus on several important issues: (a) characterizing how these risks are exacerbated or mitigated in drug-dependent pregnant women; (b) how the addictive disorder that is the focus of treatment and smoking together fuel negative biobehavioral outcomes in these women and their offspring; (c) developing and implementing effective behavioral and medication treatments to reduce and eliminate smoking over the course of pregnancy to improve maternal and child health is imperative. In addition, further research comparing methadone and buprenorphine with regard to their interactions with cigarette smoking during pregnancy (including examination of the relationship among opioid agonist, smoking, maternal depression, and/or neonatal abstinence syndrome) is needed.
Overall, this study revealed no significant difference in cigarette smoking between the methadone- and buprenorphine-treated pregnant patients who completed the MOTHER study. In addition, these results add to already existing scientific evidence documenting a high prevalence and lack of significant change (neither increase nor reduction) among cigarette smoking in opioid-dependent, pregnant women. Thus, the current findings underscore the need to develop and implement effective smoking cessation treatments for agonist-treated pregnant patients in an effort to promote improved maternal and infant health outcomes. Future work to target pregnant agonist-treated women is of particular importance. Developing effective smoking cessation treatments has the potential to reduce adverse health outcomes for both the mother and child. Also because pregnancy represents an opportunity to intervene and change problem behaviors, pregnant women engaged in drug treatment may be uniquely motivated for such interventions.
FUNDING
MOTHER grants are from the National Institute on Drug Abuse (NIDA) unless noted otherwise: Brown University, R01 DA015778; Johns Hopkins University, R01 DA015764; Medical University of Vienna, R01 DA018417; Thomas Jefferson University, R01 DA015738; University of Toronto, R01 DA015741; University of Vermont, R01 DA018410 and M01 RR109; Vanderbilt University, R01 DA017513 and M01 RR00095, and Wayne State University, R01 DA15832.
DECLARATION OF INTERESTS
HJ discloses that she has received reimbursement for time and travel from Reckitt Benckiser. All other authors declare no competing financial interests. No contractual constraints on publishing have been imposed by any agency from which an author has received funding. The clinical trial was registered with ClinicalTrials.gov (Identifier: NCT00271219; Title: RCT Comparing Methadone and Buprenorphine in Pregnant Women).
REFERENCES
- Baca C. T., Yahne C. E. (2009). Smoking cessation during substance abuse treatment: What you need to know. Journal of Substance Abuse Treatment, 36, 205–219.10.1016/j.jsat.2008.06.003 [DOI] [PubMed] [Google Scholar]
- Bada H. S., Das A., Bauer C. R., Shankaran S., Lester B. M., Gard C. C. … Higgins R. (2005). Low birth weight and preterm births: Etiologic fraction attributable to prenatal drug exposure. Journal of Perinatology, 25, 631–637.10.1038/sj.jp.7211378 [DOI] [PubMed] [Google Scholar]
- Bailey G. P., Marien D. (2011). The value of juvenile animal studies “what have we learned from preclinical juvenile toxicity studies? II”. Birth Defects Research.Part B, Developmental and Reproductive Toxicology, 92, 273–291.10.1002/bdrb.20328; 10.1002/bdrb.20328 [DOI] [PubMed] [Google Scholar]
- Ball S. A., Rounsaville B. J., Tennen H., Kranzler H. R. (2001). Reliability of personality disorder symptoms and personality traits in substance-dependent inpatients. Journal of Abnormal Psychology, 110, 341–352 [DOI] [PubMed] [Google Scholar]
- Burstyn I., Kapur N., Shalapay C., Bamforth F., Wild T. C., Liu J., LeGatt D. (2009). Evaluation of the accuracy of self-reported smoking in pregnancy when the biomarker level in an active smoker is uncertain. Nicotine & Tobacco Research, 11, 670–678.10.1093/ntr/ntp048 [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) (2008a). Smoking prevalence among women of reproductive age--united states, 2006. MMWR.Morbidity and Mortality Weekly Report, 57, 849–852 [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) (2008b). Smoking-attributable mortality, years of potential life lost, and productivity losses--united states, 2000–2004. MMWR.Morbidity and Mortality Weekly Report, 57, 1226–1228 [PubMed] [Google Scholar]
- Chait L. D., Griffiths R. R. (1984). Effects of methadone on human cigarette smoking and subjective ratings. Journal of Pharmacology and Experimental Therapeutics, 229, 636–640 [PubMed] [Google Scholar]
- Chisolm M. S., Brigham E. P., Lookatch S. J., Tuten M., Strain E. C., Jones H. E. (2010). Cigarette smoking knowledge, attitudes, and practices of patients and staff at a perinatal substance abuse treatment center. Journal of Substance Abuse Treatment, 39, 298–305.10.1016/j.jsat.2010.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisolm M. S., Brigham E. P., Tuten M., Strain E. C., Jones H. E. (2010). The relationship between antidepressant use and smoking cessation in pregnant women in treatment for substance abuse. American Journal of Drug and Alcohol Abuse, 36, 46–51.10.3109/00952990903544844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisolm M. S., Tuten M., Brigham E. C., Strain E. C., Jones H. E. (2009). Relationship between cigarette use and mood/anxiety disorders among pregnant methadone-maintained patients. American Journal on Addictions/American Academy of Psychiatrists in Alcoholism and Addictions, 18, 422–429.10.3109/10550490903077721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choo R. E., Huestis M. A., Schroeder J. R., Shin A. S., Jones H. E. (2004). Neonatal abstinence syndrome in methadone-exposed infants is altered by level of prenatal tobacco exposure. Drug and Alcohol Dependence, 75, 253–260 [DOI] [PubMed] [Google Scholar]
- Cnattingius S. (2004). The epidemiology of smoking during pregnancy: Smoking prevalence, maternal characteristics, and pregnancy outcomes. Nicotine & Tobacco Research, 6(Suppl. 2), S125–S140.10.1080/14622200410001669187 [DOI] [PubMed] [Google Scholar]
- Conter V., Cortinovis I., Rogari P., Riva L. (1995). Weight growth in infants born to mothers who smoked during pregnancy. British Medical Journal (Clinical research ed.), 310, 768–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz P. M., England L. J., Shapiro-Mendoza C. K., Tong V. T., Farr S. L., Callaghan W. M. (2010). Infant morbidity and mortality attributable to prenatal smoking in the U.S. American Journal of Preventive Medicine, 39, 45–52.10.1016/j.amepre.2010.03.009 [DOI] [PubMed] [Google Scholar]
- Dolan-Mullen P., Ramírez G., Groff J. Y. (1994). A meta-analysis of randomized trials of prenatal smoking cessation interventions. American Journal of Obstetrics and Gynecology, 171, 1328–1334 [DOI] [PubMed] [Google Scholar]
- D’Onofrio B. M., Turkheimer E. N., Eaves L. J., Corey L. A., Berg K., Solaas M. H., Emery R. E. (2003). The role of the children of twins design in elucidating causal relations between parent characteristics and child outcomes. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 44, 1130–1144 [DOI] [PubMed] [Google Scholar]
- Dupont W. D., Plummer W. D., Jr (1990). Power and sample size calculations. A review and computer program. Controlled clinical trials, 11, 116–128 [DOI] [PubMed] [Google Scholar]
- Elkader A. K., Brands B., Selby P., Sproule B. A. (2009). Methadone-nicotine interactions in methadone maintenance treatment patients. Journal of Clinical Psychopharmacology, 29, 231–238.10.1097/JCP.0b013e3181a39113 [DOI] [PubMed] [Google Scholar]
- Fiore M. C., Jaen C. R. (2008). A clinical blueprint to accelerate the elimination of tobacco use. JAMA: The Journal of the American Medical Association, 299, 2083–2085.10.1001/jama.299.17.2083 [DOI] [PubMed] [Google Scholar]
- Floyd R. L., Rimer B. K., Giovino G. A., Mullen P. D., Sullivan S. E. (1993). A review of smoking in pregnancy: Effects on pregnancy outcomes and cessation efforts. Annual Review of Public Health, 14, 379–411.10.1146/annurev.pu.14.050193.002115 [DOI] [PubMed] [Google Scholar]
- Friedmann P. D., Jiang L., Richter K. P. (2008). Cigarette smoking cessation services in outpatient substance abuse treatment programs in the united states. Journal of Substance Abuse Treatment, 34, 165–172.10.1016/j.jsat.2007.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller B. E., Guydish J., Tsoh J., Reid M. S., Resnick M., Zammarelli L, … McCarty D. (2007). Attitudes toward the integration of smoking cessation treatment into drug abuse clinics. Journal of Substance Abuse Treatment, 32, 53–60.10.1016/j.jsat.2006.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haug N. A., Stitzer M. L., Svikis D. S. (2001). Smoking during pregnancy and intention to quit: A profile of methadone-maintained women. Nicotine & Tobacco Research, 3, 333–339.10.1080/14622200110050493 [DOI] [PubMed] [Google Scholar]
- Heil S. H., Higgins S. T., Bernstein I. M., Solomon L. J., Rogers R. E., Thomas C. S, … Lynch M. E. (2008). Effects of voucher-based incentives on abstinence from cigarette smoking and fetal growth among pregnant women. Addiction (Abingdon, England), 103, 1009–1018.10.1111/j.1360-0443.2008.02237.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson R., Diggle P., Dobson A. (2000). Joint modelling of longitudinal measurements and event time data. Biostatistics (Oxford, England), 1, 465–480.10.1093/biostatistics/1.4.465 [DOI] [PubMed] [Google Scholar]
- Hurt R. D., Offord K. P., Croghan I. T., Gomez-Dahl L., Kottke T. E., Morse R. M., Melton L. J., 3rd (1996). Mortality following inpatient addictions treatment. Role of tobacco use in a community-based cohort. JAMA: The Journal of the American Medical Association, 275, 1097–1103 [DOI] [PubMed] [Google Scholar]
- Jones H. E., Heil S. H., O’Grady K. E., Martin P. R., Kaltenbach K., Coyle M. G. … Fischer G. (2009). Smoking in pregnant women screened for an opioid agonist medication study compared to related pregnant and non-pregnant patient samples. American Journal of Drug and Alcohol Abuse, 35, 375–380.10.1080/00952990903125235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones H. E., Kaltenbach K., Heil S. H., Stine S. M., Coyle M. G., Arria A. M. … Fischer G. (2010). Neonatal abstinence syndrome after methadone or buprenorphine exposure. New England Journal of Medicine, 363, 2320–2331.10.1056/NEJMoa1005359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami N., Takatsuka N., Inaba S., Shimizu H. (1999). Development of a screening questionnaire for tobacco/nicotine dependence according to ICD-10, DSM-III-R, and DSM-IV. Addictive Behaviors, 24, 155–166 [DOI] [PubMed] [Google Scholar]
- Knopik V. S., Sparrow E. P., Madden P. A., Bucholz K. K., Hudziak J. J., Reich W., Heath A. C. (2005). Contributions of parental alcoholism, prenatal substance exposure, and genetic transmission to child ADHD risk: A female twin study. Psychological Medicine, 35, 625–635 [DOI] [PubMed] [Google Scholar]
- Lacroix I., Berrebi A., Garipuy D., Schmitt L., Hammou Y., Chaumerliac C. … Damase-Michel C. (2011). Buprenorphine versus methadone in pregnant opioid-dependent women: A prospective multicenter study. European Journal of Clinical Pharmacology, 67, 1053–1059.10.1007/s00228-011-1049-9 [DOI] [PubMed] [Google Scholar]
- Lasser K., Boyd J. W., Woolhandler S., Himmelstein D. U., McCormick D., Bor D. H. (2000). Smoking and mental illness: A population-based prevalence study. JAMA: The Journal of the American Medical Association, 284, 2606–2610 [DOI] [PubMed] [Google Scholar]
- Lumley J., Chamberlain C., Dowswell T., Oliver S., Oakley L., Watson L. (2009). Interventions for promoting smoking cessation during pregnancy. Cochrane Database of Systematic Reviews (Online), 3, CD001055.10.1002/14651858.CD001055.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin B. K., Meinert C. L., Breitner J. C. ADAPT Research Group (2002). Double placebo design in a prevention trial for Alzheimer’s disease. Controlled Clinical Trials, 23, 93–99 [DOI] [PubMed] [Google Scholar]
- McCowan L., Horgan R. P. (2009). Risk factors for small for gestational age infants. Best Practice & Research.Clinical Obstetrics & Gynaecology, 23, 779–793.10.1016/j.bpobgyn.2009.06.003 [DOI] [PubMed] [Google Scholar]
- McLellan A. T., Cacciola J., Alterman A. I., Rikoon S. H., Carise D. (2006). The addiction severity index at 25: Origins, contributions and transitions. American Journal of Addiction, 15, 113–124 [DOI] [PubMed] [Google Scholar]
- McLellan A. T., Kushner H., Metzger D., Peters R., Smith I., Grissom G. … Argeriou M. (1992). Fifth edition of the addiction severity index. Journal of Substance Abuse Treatment, 9, 199–213 [DOI] [PubMed] [Google Scholar]
- Mello N. K., Lukas S. E., Mendelson J. H. (1985). Buprenorphine effects on cigarette smoking. Psychopharmacology, 86, 417–425 [DOI] [PubMed] [Google Scholar]
- Mello N. K., Mendelson J. H., Sellers M. L., Kuehnle J. C. (1980). Effects of heroin self-administration on cigarette smoking. Psychopharmacology, 67, 45–52 [DOI] [PubMed] [Google Scholar]
- Minnes S., Lang A., Singer L. (2011). Prenatal tobacco, marijuana, stimulant, and opiate exposure: Outcomes and practice implications. Addiction Science and Clinical Practice, 6, 57–70 [PMC free article] [PubMed] [Google Scholar]
- Mokdad A. H., Marks J. S., Stroup D. F., Gerberding J. L. (2004). Actual causes of death in the united states, 2000. JAMA: The Journal of the American Medical Association, 291, 1238–1245.10.1001/jama.291.10.1238 [DOI] [PubMed] [Google Scholar]
- Mutschler N. H., Stephen B. J., Teoh S. K., Mendelson J. H., Mello N. K. (2002). An inpatient study of the effects of buprenorphine on cigarette smoking in men concurrently dependent on cocaine and opioids. Nicotine & Tobacco Research, 4, 223–228.10.1080/14622200210124012 [DOI] [PubMed] [Google Scholar]
- Nahvi S., Richter K., Li X., Modali L., Arnsten J. (2006). Cigarette smoking and interest in quitting in methadone maintenance patients. Addictive Behaviors, 31, 2127–2134.10.1016/j.addbeh.2006.01.006 [DOI] [PubMed] [Google Scholar]
- Office of the Surgeon General (US) & Office on Smoking and Health (US) (2004). 2004 Surgeon General’s Report–The Health Consequences of Smoking. Retrieved from http://www.cdc.gov/tobacco/data_statistics/sgr/2004/index.htm [PubMed]
- Pajusco B., Chiamulera C., Quaglio G., Moro L., Casari R., Amen G. … Lugoboni L. (2012). Tobacco addiction and smoking status in heroin addicts under methadone vs. buprenorphine therapy. International Journal of Environmental Research and Public Health, 9, 932–942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter K. P., Ahluwalia J. S. (2000). A case for addressing cigarette use in methadone and other opioid treatment programs. Journal of Addictive Diseases, 19, 35–52 [DOI] [PubMed] [Google Scholar]
- Richter K. P., Arnsten J. H. (2006). A rationale and model for addressing tobacco dependence in substance abuse treatment. Substance Abuse Treatment, Prevention, and Policy, 1, 23.10.1186/1747-597X-1–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter K. P., Gibson C. A., Ahluwalia J. S., Schmelzle K. H. (2001). Tobacco use and quit attempts among methadone maintenance clients. American Journal of Public Health, 91, 296–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salihu H. M., Sharma P. P., Getahun D., Hedayatzadeh M., Peters S., Kirby R. S. … Gaafer-Ahmed H. (2008). Prenatal tobacco use and risk of stillbirth: A case-control and bidirectional case-crossover study. Nicotine & Tobacco Research, 10, 159–166.10.1080/14622200701705431 [DOI] [PubMed] [Google Scholar]
- Stroud L. R., Paster R. L., Papandonatos G. D., Niaura R., Salisbury A. L., Battle C., Lester B. (2009). Maternal smoking during pregnancy and newborn neurobehavior: Effects at 10 to 27 days. Journal of Pediatrics, 154, 10–16.10.1016/j.jpeds.2008.07.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiriez G., Bouhaddi M., Mourot L., Nobili F., Fortrat J. O., Menget A. … Regnard J. (2009). Heart rate variability in preterm infants and maternal smoking during pregnancy. Clinical Autonomic Research, 19, 149–156.10.1007/s10286-009-0003-8 [DOI] [PubMed] [Google Scholar]
- Tong V. T., Jones J. R., Dietz P. M., D’Angelo D., Bombard J. M. Centers for Disease Control and Prevention (CDC) (2009). Trends in smoking before, during, and after pregnancy - pregnancy risk assessment monitoring system (PRAMS), united states, 31 sites, 2000–2005. MMWR.Surveillance Summaries: Morbidity and Mortality Weekly Report.Surveillance Summaries/ CDC, 58, 1–29 [PubMed] [Google Scholar]
- Tuten M., Fitzsimons H., Chisolm M. S., Nuzzo P. A., Jones H. E. (2012). Contingent incentives reduce cigarette smoking among pregnant, methadone-maintained women: Results of an initial feasibility and efficacy randomized clinical trial. Addiction, 107, 1868–1877. 10.1111/j.1360-0443.2012.03923.x; 10.1111/j.1360-0443.2012.03923.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Preventive Services Task Force (2009). Counseling and interventions to prevent tobacco use and tobacco-caused disease in adults and pregnant women: U.S. preventive services task force reaffirmation recommendation statement. Annals of Internal Medicine, 150, 551–555 [DOI] [PubMed] [Google Scholar]
- Winklbaur B., Baewert A., Jagsch R., Rohrmeister K., Metz V., Aeschbach Jachmann C. … Fischer G. (2009). Association between prenatal tobacco exposure and outcome of neonates born to opioid-maintained mothers. Implications for treatment. European Addiction Research, 15, 150–156.10.1159/000216466 [DOI] [PubMed] [Google Scholar]
- World Health Organization (2010). World No Tobacco Day–May 31, 2010: Women are huge potential market for the tobacco industry. Retrieved from http://www.euro.who.int/en/what-we-publish/information-for-the-media/sections/latest-press-releases/world-no-tobacco-day-may-31,-2010-women-are-huge-potential-market-for-the-tobacco-industry