Abstract
Pseudomonas fluorescens Pf-5, a rhizosphere bacterium, produces a suite of secondary metabolites that are toxic to seed- and root-rotting plant pathogens. Among these are the polyketide compounds pyoluteorin and 2,4-diacetylphloroglucinol. We provide evidence that pyoluteorin production is influenced by positive autoregulation. Addition of pyoluteorin to liquid cultures of Pf-5 enhanced pyoluteorin production. In addition, pyoluteorin and 2,4-diacetylphloroglucinol mutually inhibit one another's production in Pf-5. For pyoluteorin, both positive autoregulation and negative influences on production by 2,4-diacetylphloroglucinol were demonstrated at the transcriptional level by measuring activity from transcriptional fusions of an ice nucleation reporter gene (inaZ) to three separate pyoluteorin biosynthetic genes. The occurrence of pyoluteorin autoregulation in the rhizosphere was assessed on cucumber seedlings in pasteurized soil with cross-feeding experiments. In the rhizosphere, expression of a pyoluteorin biosynthesis gene by a pyoluteorin-deficient mutant of Pf-5 was enhanced by pyoluteorin produced by coinoculated cells of Pf-5. These data establish that the polyketide pyoluteorin is an autoregulatory compound and functions as a signal molecule influencing the spectrum of secondary metabolites produced by the bacterial cell.
Microbial biological control agents, such as the rhizosphere bacterium Pseudomonas fluorescens Pf-5, represent alternatives to synthetic chemicals for combating plant disease in agriculture. An important aspect of plant disease suppression by rhizosphere bacteria is the production of low-molecular-weight metabolites with antibiotic properties against certain plant pathogens (reviewed in references 8, 12, 17, 18, and 36). P. fluorescens Pf-5 produces an array of secondary metabolites that inhibit plant pathogens, including pyoluteorin, pyrrolnitrin, 2,4-diacetylphloroglucinol, and hydrogen cyanide (23, 24, 26, 29, 43, 47). Pyoluteorin and 2,4-diacetylphloroglucinol (Fig. 1) are synthesized by polyketide synthase complexes (4, 42, 44) and are secreted from cells of Pf-5. The focus of this research is pyoluteorin, a chlorinated polyketide compound (3) that suppresses the oomycete Pythium ultimum, a seed- and root-rotting pathogen (24, 37). Pyoluteorin production is conferred by a biosynthetic gene cluster encompassing nine structural genes whose predicted functions are sufficient for the biochemical transformations required for pyoluteorin biosynthesis from acetate and proline precursors (11, 42, 44). Also within the pyoluteorin biosynthetic gene cluster is pltR, which encodes a LysR transcriptional regulator and is required for transcription of pyoluteorin biosynthesis genes and resultant pyoluteorin production (42). Pyoluteorin production by Pf-5 is affected by nutrient source (30, 43), temperature (41), and cell density (11). Although the links between these extracellular factors and the intracellular regulatory pathways controlling pyoluteorin production are not yet understood, a complex picture is emerging that links regulation of production of pyoluteorin and other exoproducts to the physiological status of the cell.
FIG. 1.
Structures of pyoluteorin (a) and 2,4-diacetylphloroglucinol (b).
Regulation of pyoluteorin biosynthesis has been dissected both in Pf-5 and in P. fluorescens CHA0, a biocontrol strain that produces a suite of exoproducts similar to that of Pf-5, and utilizes similar regulatory mechanisms to govern their accumulation (18). The production of many antibiotics and exoproducts by Pseudomonas spp. and other gram-negative bacteria requires the histidine protein kinase/response regulator pair GacS/GacA (for example, see reference 21); the signal(s) that activates GacS is not known. In Pf-5, the GacS/GacA pair positively regulates production of several secreted products, including pyoluteorin, 2,4-diacetylphloroglucinol, pyrrolnitrin, hydrogen cyanide, and an extracellular protease (10, 29). At least two common components of stress response systems, the stationary phase and stress response sigma factor RpoS (σS) and Lon protease, are implicated in regulation of antibiotic production in Pf-5. Relative levels of σS and the housekeeping sigma factor RpoD (σD) influence pyoluteorin, 2,4-diacetylphloroglucinol, and pyrrolnitrin production. Abundant σS is required for the production of pyrrolnitrin but is repressive to pyoluteorin and 2,4-diacetylphloroglucinol production (50, 52). Lon, a serine protease induced by heat shock, also is repressive to pyoluteorin production (60). The importance of nutrient status to pyoluteorin production is corroborated by the observation that pyrrolquinoline quinone, a cofactor required by glucose and alcohol dehydrogenases, represses pyoluteorin production (53). Thus, pyoluteorin production in Pf-5 is linked to the physiological status of the cell.
In this report we present evidence that the regulatory circuit governing pyoluteorin production includes positive autoregulation. The most familiar example of positive autoregulation is that of the N-acyl homoserine lactones. In gram-negative bacteria, acyl homoserine lactones are synthesized and perceived, respectively, by LuxI/LuxR homologs, and are postulated to function as signals of cell density and/or confinement. Acyl homoserine lactone production, along with that of other cellular products, is upregulated as a consequence of acyl homoserine lactone accumulation in the extracellular milieu and reentry into producer cells. Acyl homoserine lactones have been demonstrated to function as intercellular signal molecules in the rhizosphere, where their production by rhizosphere inhabitants can induce phenazine antibiotic production by Pseudomonas aureofaciens (48, 61). Non-acyl homoserine lactone extracellular metabolites produced by Pseudomonas spp. also undergo positive autoregulation, including the antibiotics 2,4-diacetylphloroglucinol (54) and oomycin A (16, 25) and the siderophores pyochelin (49) and pyoverdine (31). This report adds pyoluteorin to the growing list of non-acyl homoserine lactone autoinducers known in Pseudomonas spp. Because Pf-5 produces several antibiotics, we investigated the potential for regulation of pyoluteorin production by a heterologous antibiotic. We demonstrate that pyoluteorin production and the transcription of pyoluteorin biosynthesis genes are influenced by the presence of another antibiotic produced by Pf-5, 2,4-diacetylphloroglucinol. Finally, we present evidence that pyoluteorin produced by Pf-5 can induce the expression of pyoluteorin biosynthesis genes by neighboring bacterial cells in the rhizosphere.
MATERIALS AND METHODS
Bacterial strains, plasmids, and culture conditions.
Bacterial strains and plasmids used in this study are listed in Table 1. Pf-5 and derivative strains were cultured routinely on King's Medium B agar (KMB; see reference 28) containing streptomycin (100 μg/ml). KMB was supplemented with other antibiotics (kanamycin, 50 μg/ml; gentamicin, 40 μg/ml) when appropriate. Inoculum of Pf-5 and derivatives was grown overnight with shaking (200 rpm) at 27°C in either KMB broth or KMBGlu, modified from KMB broth by replacing the glycerol with 1% (wt/vol) glucose to repress pyoluteorin production. For experiments evaluating antibiotic production or pyoluteorin biosynthetic gene transcription, cultures were grown at 20°C with shaking (200 rpm) in nutrient broth (pH 6.8; Difco Laboratories, Detroit, Mich.) without selection and were supplemented with either 2% (wt/vol) glycerol for NBGly, a medium conducive to pyoluteorin production, or with 2% (wt/vol) glucose for NBGlu, a medium repressive to pyoluteorin production by Pf-5 (30, 43). For experiments evaluating antibiotic production or pyoluteorin biosynthetic gene transcription in defined medium, bacterial cultures were grown in Ornston-Stanier medium (45) amended with 0.5% (wt/vol) glycerol at 20°C with shaking (200 rpm).
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Strain collection no. | Relevant characteristicsa | Reference or source |
|---|---|---|---|
| P. fluorescens | |||
| Pf-5 | JL4474 | P. fluorescens Pf-5 rhizosphere isolate; Plt+, Ina− | 23 |
| Pf-5 iceC+ | JL4303 | Pf-5 (pJEL1703); Kmr, Plt+, Ina+ | 34 |
| Pf-5 pltE::inaZ | JL4365 | pltE::Tn3-nice derivative of Pf-5, Plt−, Ina+, Kmr | 30 |
| Pf-5 pltB::inaZ | JL4389 | pltB::Tn3-nice derivative of Pf-5, Plt−, Ina+, Kmr | 30 |
| Pf-5 pltF::inaZ | JL4390 | pltF::Tn3-nice derivative of Pf-5, Plt−, Ina+, Kmr | 30 |
| Pf-5 rpoS::lacZ | JL4489 | rpoS::lacZ derivative of Pf-5, Plt++, Ina− | 59 |
| Pf-5 gacA::aphI | JL4577 | gacA::aphI derivative of Pf-5; Plt−, Ina−, Kmr | 9 |
| Pf-5 pltR::aacCI | JL4563 | pltR::aacCI derivative of Pf-5; Plt−, Ina−; Gmr | 42 |
| Plasmids | |||
| pJEL1703 | iceC from P. syringae cloned into pVSP61 (nucleotide sequences of iceC and inaZ are nearly identical) | 34 | |
| pVSP61 | Stable plasmid vector containing pVS1 replicon and mobilization region and p15A replicon; Mob+, Kmr | W. Tucker, DNA Plant Technology Corp., Oakland, Calif. |
Plt+, produces pyoluteorin; Plt−, does not produce detectable pyoluteorin; Plt++, overproduces pyoluteorin relative to the parental strain; Ina+, expresses ice nucleation activity at −5°C; Ina−, does not express ice nucleation activity at −5°C; Mob+, mobilizable plasmid; Kmr and Gmr, resistant to kanamycin and gentamycin, respectively.
Influence of exogenous antibiotics on antibiotic production and pyoluteorin gene expression by Pf-5.
Cultures of experimental strains were grown overnight in KBGlu, and cells were harvested by centrifugation (2,500 × g, 5 min), washed twice in sterile KMBGlu to eliminate traces of exported metabolites, and resuspended in 5 ml of NBGly to achieve an optical density at 600 nm (OD600) of 0.08. Pure pyoluteorin and 2,4-diacetylphloroglucinol standards dissolved in methanol were added to NBGly immediately prior to inoculation with Pf-5 or derivative strains. An equivalent volume of methanol was added to negative controls. In concentration series, the no-treatment control cultures received the greatest volume of methanol to which experimental cultures were exposed via amendments. Except where noted, antibiotics were added to the medium at 4 μg of pyoluteorin/ml and 12.5 μg of 2,4-diacetylphloroglucinol/ml. These are within the range of concentrations typically detected in supernatants of Pf-5 cultures grown under conditions optimizing antibiotic production (10, 43, 59) and were selected to mimic extracellular antibiotic concentrations that Pf-5 cells would encounter in a stationary-phase culture.
For experiments assessing transcriptional activity of pyoluteorin genes by using the inaZ reporter gene, samples were harvested periodically for 24 h and assessed for ice nucleation activity (INA) as described below. At 18 h after inoculation, both 2,4-diacetylphloroglucinol and pyoluteorin were present at measurable concentrations in the supernatant of Pf-5 cultures grown in NBGly. Therefore, for all experiments that were not harvested at multiple time points, pyoluteorin and 2,4-diacetylphloroglucinol concentrations were determined from cultures harvested 18 h after inoculation. Bacterial population densities were determined by spreading serial dilutions of cultures onto KMB and counting colony-forming units on the plates after 48 h incubation at 27°C. In each experiment, triplicate 5-ml cultures were grown and evaluated; each experiment was performed at least twice; and the results from a representative experiment are presented.
Antibiotic quantification.
Cultures were centrifuged (5,000 × g, 5 min) to pellet cells, and supernatants were extracted for recovery of pyoluteorin and 2,4-diacetylphloroglucinol. Extractions were performed by a modification of the methods of Sarniguet et al. (50). Culture supernatants were acidified to pH ≤2.0 with 1 M HCl and extracted twice with 0.4 vol of ethyl acetate. Ethyl acetate was removed from extracts under vacuum, and the resultant residues were resuspended in 100 μl of methanol, from which 10-μl samples were evaluated by high-performance liquid chromatography (HPLC). Samples were separated over a Nova-Pak C18 reversed-phase column (Waters Corporation, Milford, Mass.) eluted (1 ml/min) with 90% water plus 0.1% acetic acid-10% acetonitrile plus 0.1% (vol/vol) acetic acid for 2 min, followed by a linear gradient to 100% acetonitrile plus 0.1% acetic acid over 18 min. Antibiotics were detected with a photodiode array detector (for pyoluteorin, λ = 310 nm and retention time [tr] = 13.1 min; for 2,4-diacetylphloroglucinol, λ = 270 nm and tr = 15.6 min). Quantification was done by comparison to a standard curve generated from authentic compounds. The detection limit was 0.02 μg/ml for pyoluteorin and 0.01 μg/ml for 2,4-diacetylphloroglucinol. Recovery of compounds by using the extraction procedure described above averaged 70% for pyoluteorin and 45% for 2,4-diacetylphloroglucinol.
Transcriptional activity from pyoluteorin biosynthetic genes.
Transcriptional fusions of a promoterless inaZ gene to three pyoluteorin biosynthetic genes of Pf-5 were constructed previously (30). Transcription of the inaZ reporter gene from an upstream promoter results in expression of the membrane-bound InaZ protein, which forms aggregates that nucleate water molecules to form ice crystals at temperatures slightly below 0°C (32). Expression of INA from inaZ transcriptional fusions was quantified by using a droplet-freezing assay at −5°C as described previously (35) and reported as log10(INA), where INA is ice nuclei per colony-forming unit. The correlation between INA and the concentration of InaZ protein has been defined as nonlinear in Escherichia coli and P. syringae pv. phaseolicola, where the log10INA is related to the square root of InaZ concentrations when INA is below 10−1 nuclei per cell, and the cube root of InaZ concentration at higher INA values (57, 58).
Assessment of pyoluteorin cross-feeding on roots.
Assays were performed in pasteurized Newberg fine sandy loam, wetted 24 h in advance of planting to water holding capacity. Cucumber seeds (Cucumis sativis L. cv. Marketmore 86) were surface disinfested by immersion in 95% ethanol for 1 min, followed by a 20% (vol/vol) sodium hypochlorite solution for 15 min. Seeds were rinsed thoroughly in sterile distilled water and were placed on sterile, wetted filter paper within petri plates to germinate. Petri plates were incubated at 27°C in darkness for two days before seedlings were removed and inoculated with bacterial suspensions. Inoculum of inducing strains Pf-5, Pf-5 pltR::aacC1, and Pf-5 rpoS::lacZ was produced in KMB broth to encourage pyoluteorin production. Inoculum of reporter strain Pf-5 pltB::inaZ was produced in KMBGlu to discourage pltB induction. Cultures were grown with shaking (200 rpm) overnight at 27°C. Cells were then harvested by centrifugation (2,500 × g, 5 min) and resuspended in 20 mM potassium phosphate buffer (pH 7.0). Suspensions of three inducing strains, Pf-5, Pf-5 pltR::aacC1, and Pf-5 rpoS::lacZ, were adjusted to an OD600 of 1.0 while a suspension of Pf-5 pltB::inaZ was adjusted to an OD600 of 0.1. The suspension of reporter strain Pf-5 pltB::inaZ was mixed 1:1 with suspensions of each of the other three strains, providing three inoculum mixtures in which the reporter and inducing strains were present at final cell density ratios of 1:10. Cucumber seedlings were inoculated by soaking them in the bacterial suspensions for 10 to 20 min. Inoculated seedlings were planted individually into cells of plastic punch trays (35 mm diameter; 25 cm3 of soil per cell). Treatments within trays were arranged by a complete randomized design. Punch trays were placed in closed plastic containers and were incubated in darkness at 20°C and 100% humidity. At 0, 10, 24, and 48 h, seedlings were retrieved from 10 replicate cells, placed in culture tubes containing 20 mM potassium phosphate buffer (pH 7.0), and sonicated for 5 min in a bath-style sonicator to remove bacterial cells from roots of the seedlings. Serial 10-fold dilutions were made in 20 mM potassium phosphate buffer (pH 7.0) for determination of INA and CFU. Total CFU per sample were determined by spreading diluted samples onto KMB agar containing streptomycin, while CFU of the reporter strain Pf-5 pltB::inaZ were enumerated separately on KMB agar amended with streptomycin and kanamycin.
Data analysis.
Treatment means were compared using a Student's t test following an analysis of variance. Two-tailed P values are reported. Statistical analyses were performed by using JMP, version 3 (SAS Institute, Cary, N.C.).
RESULTS
Pyoluteorin production is autoregulated.
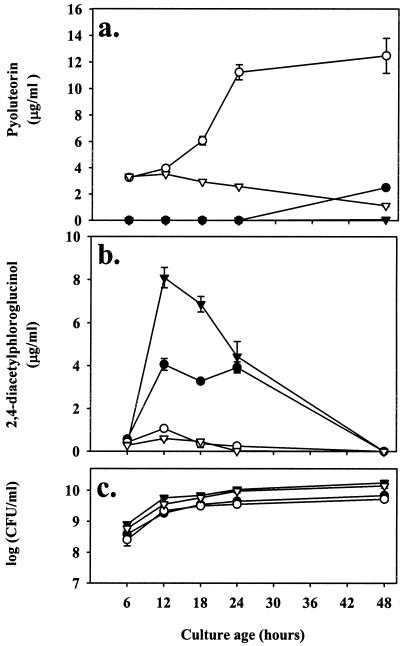
To evaluate the possibility that pyoluteorin induces its own production in Pf-5, pyoluteorin was added to NBGly prior to inoculation with either Pf-5 pltB::inaZ or Pf-5 iceC+. In preliminary experiments, Pf-5 and Pf-5 iceC+ were similar in production of pyoluteorin and 2,4-diacetylphloroglucinol (data not shown), so Pf-5 iceC+ filled a dual role as an experimental pyoluteorin-producing strain for evaluation of antibiotic production and a positive control for INA. Similarly, Pf-5 pltB::inaZ provided a negative control for pyoluteorin production while serving as a reporter of pyoluteorin biosynthetic gene transcription. As expected, no pyoluteorin was detected in cultures of Pf-5 pltB::inaZ (a pyoluteorin-deficient strain) grown in nonamended medium. The concentrations of pyoluteorin in pyoluteorin-amended cultures of Pf-5 pltB::inaZ initially reflected the amount detectable in supernatant extracts as a result of pyoluteorin amendment. Over time, pyoluteorin concentrations declined in these cultures, probably because of degradation (Fig. 2a). Although some variation occurred in the timing and levels of pyoluteorin production across multiple time course experiments that we performed, amendment of the culture medium with 4 μg of pyoluteorin/ml consistently hastened pyoluteorin production by Pf-5 or Pf-5 iceC+ and enhanced it between 4.5- and 20-fold (after accounting for the pyoluteorin amendment) by late stationary phase (Fig. 2a). Addition of 4 μg of pyoluteorin/ml to the culture medium did not significantly alter the population sizes of Pf-5 iceC+ or Pf-5 pltB::inaZ (all P > 0.05; Fig. 2c), indicating that the influence of exogenous pyoluteorin on pyoluteorin production was not achieved through indirect effects on bacterial growth.
FIG. 2.
Influence of exogenous pyoluteorin on pyoluteorin and 2,4-diacetylphloroglucinol production by derivatives of Pf-5. Cultures of Pf-5 iceC+ (•,○) and Pf-5 pltB::inaZ (▿,▾) were grown in nonamended NBGly (filled symbols) or in NBGly amended with 4 μg of pyoluteorin/ml (open symbols). At the indicated sampling times following inoculation, pyoluteorin concentrations (a), 2,4-diacetylphloroglucinol concentrations (b), and population densities of cultures (c) were assessed. Population sizes did not differ significantly between pyoluteorin-amended and nonamended treatments at any time point. All three panels represent data from the same experiment. Error bars denote one standard error.
Pyoluteorin and 2,4-diacetylphloroglucinol exhibit mutual inhibition.
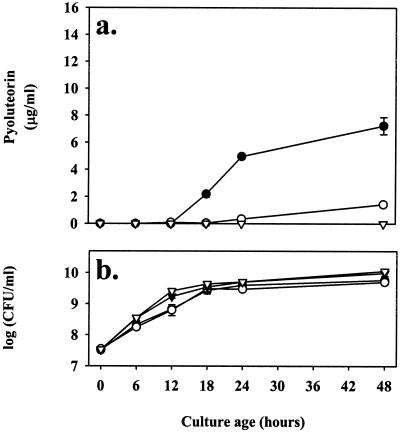
Pyoluteorin-deficient mutants produced more 2,4-diacetylphloroglucinol than did pyoluteorin-producing strains, as exemplified by comparing 2,4-diacetylphloroglucinol production in cultures of Pf-5 iceC+ and Pf-5 pltB::inaZ (Fig. 2b). This observation is consistent with the hypothesis that pyoluteorin represses 2,4-diacetylphloroglucinol production in Pf-5. To test this hypothesis, the influence of pyoluteorin on 2,4-diacetylphloroglucinol production was determined for Pf-5 iceC+ and Pf-5 pltB::inaZ. Exogenous pyoluteorin (4 μg/ml) added to NBGly repressed 2,4-diacetylphloroglucinol production by both strains. At 12 h, when 2,4-diacetylphloroglucinol was at peak levels, pyoluteorin repressed 2,4-diacetylphloroglucinol production in Pf-5 iceC+ and Pf-5 pltB::inaZ by a factor of 3.8 and 13.6, respectively (Fig. 2b). The reverse scenario was also tested: we examined whether 2,4-diacetylphloroglucinol repressed pyoluteorin production by Pf-5. Prior to inoculation with Pf-5 iceC+ and Pf-5 pltB::inaZ, NBGly was amended with 12.5 μg of 2,4-diacetylphloroglucinol/ml. Amendment of the medium with 2,4-diacetylphloroglucinol reduced pyoluteorin production by Pf-5 iceC+ at 18 h after inoculation, when pyoluteorin production was first detected, and at each time point thereafter (Fig. 3a). As expected, Pf-5 pltB::inaZ did not produce detectable pyoluteorin. Again, despite some variation in timing and levels of pyoluteorin production across multiple time course experiments, the addition of 12.5 μg of 2,4-diacetylphloroglucinol/ml to the initial culture medium always slowed pyoluteorin production in Pf-5 or Pf-5 iceC+ and repressed it between two- and fivefold by late stationary phase. Addition of 12.5 μg of 2,4-diacetylphloroglucinol/ml to the culture medium did not significantly alter the population sizes of Pf-5 iceC+ or Pf-5 pltB::inaZ (P ≥ 0.09; Fig. 3b), indicating that differences in pyoluteorin production could not be attributed to an impact of 2,4-diacetylphloroglucinol amendments on growth.
FIG. 3.
Influence of exogenous 2,4-diacetylphloroglucinol on pyoluteorin production by derivatives of Pf-5. Cultures of Pf-5 iceC+ (•,○) and Pf-5 pltB::inaZ (▿,▾) were grown in nonamended NBGly (filled symbols) or in NBGly amended with 12.5 μg of 2,4-diacetylphloroglucinol/ml (open symbols). At the indicated sampling times following inoculation, pyoluteorin concentrations (a) and population densities of cultures (b) were assessed. Population sizes did not differ significantly between 2,4-diacetylphloroglucinol-amended and nonamended treatments at any time point. Error bars denote one standard error. Symbols denoting pyoluteorin concentrations for unamended Pf-5 pltB::inaZ cultures are occluded by those denoting 2,4-diacetylphloroglucinol-amended Pf-5 pltB::inaZ cultures.
Low concentrations of exogenous pyoluteorin and 2,4-diacetylphloroglucinol affect antibiotic production in Pf-5.
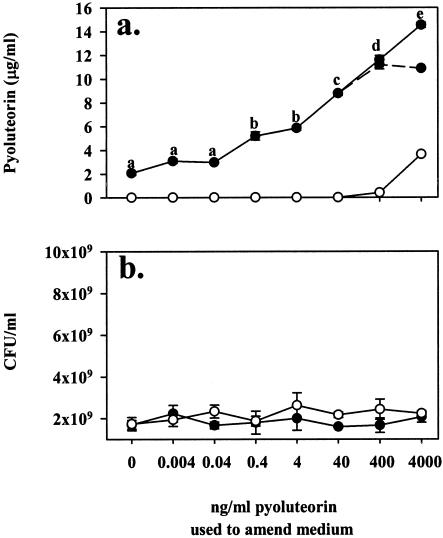
To estimate the minimum concentration of pyoluteorin needed to induce pyoluteorin autoregulation, Pf-5 was grown in NBGly amended with pyoluteorin at concentrations ranging from 0.004 to 4,000 ng/ml (Fig. 4a). Amendment of NBGly with pyoluteorin at concentrations greater than or equal to 0.4 ng/ml (1.47 nM) enhanced pyoluteorin production by Pf-5 (P = 0.002), with pyoluteorin production increasing with increasing concentrations of pyoluteorin up to 4,000 ng/ml in the initial growth medium. Addition to the growth medium of pyoluteorin at 4 μg/ml or lower concentrations did not significantly alter the population sizes of Pf-5 iceC+ or Pf-5 pltB::inaZ (P ≥ 0.53; Fig. 4b).
FIG. 4.
Minimum effective concentration of pyoluteorin for autoinduction in Pf-5. Pyoluteorin concentrations (a) were measured in Pf-5 (•) cultured for 18 h in NBGly that was amended with the concentrations of pyoluteorin indicated on the abscissa. To determine how much of the recovered pyoluteorin could be attributed to the original medium amendments, pyoluteorin was also measured in cultures of the pyoluteorin-deficient strain Pf-5 pltB::inaZ (○) that had been amended with identical amounts of pyoluteorin. Filled symbols connected by a solid line (—•—) represent actual concentrations of pyoluteorin recovered from Pf-5, and filled symbols connected by a dashed line (- - -•- - -) represent pyoluteorin that resulted from biosynthesis by Pf-5, approximated as (pyoluteorin recovered from Pf-5 cultures) − (pyoluteorin recovered from Pf-5 pltB::inaZ cultures at the same amendment rate). CFU (b) were also enumerated in each sample. Pyoluteorin amendments did not significantly alter population sizes. Differing letters above symbols denote treatments significantly different from one another (P ≤ 0.05; two-sample t test). Error bars denote one standard error.
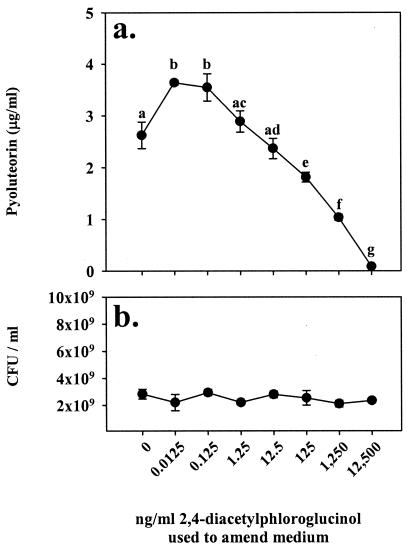
To estimate the minimum concentration of 2,4-diacetylphloroglucinol needed to inhibit pyoluteorin production, Pf-5 was grown in NBGly amended with 2,4-diacetylphloroglucinol concentrations ranging from 0.0125 to 12,500 ng/ml (Fig. 5a). Amendment of NBGly with 2,4-diacetylphloroglucinol at concentrations greater than or equal to 125 ng/ml (0.595 μM) inhibited pyoluteorin production by Pf-5, with pyoluteorin production decreasing as the initial concentrations of 2,4-diacetylphloroglucinol in the growth medium exceeded that level (P ≤ 0.04). In contrast, when cultures were amended with very low concentrations (between 0.0125 and 0.125 ng/ml, or 59.5 pM to 0.595 nM) of 2,4-diacetylphloroglucinol, pyoluteorin production was reproducibly enhanced over that in nonamended cultures (P = 0.04). Addition to the growth medium of 2,4-diacetylphloroglucinol at concentrations of 12,500 ng/ml or lower did not significantly alter the population sizes of Pf-5 iceC+ or Pf-5 pltB::inaZ (P ≥ 0.55; Fig. 5b).
FIG. 5.
Minimum effective concentration of 2,4-diacetylphloroglucinol for repression of pyoluteorin production in Pf-5. Pyoluteorin concentrations (a) and CFU (b) were measured in Pf-5 after culturing for 18 h in NBGly that was amended with the concentrations of 2,4-diacetylphloroglucinol indicated on the abscissa. 2,4-Diacetylphloroglucinol amendments did not significantly alter population sizes. Differing letters above symbols denote treatments significantly different from one another (P ≤ 0.05; two-sample t test). Error bars denote one standard error.
Effects of pyoluteorin and 2,4-diacetylphloroglucinol on pyoluteorin production occur at the transcriptional level.
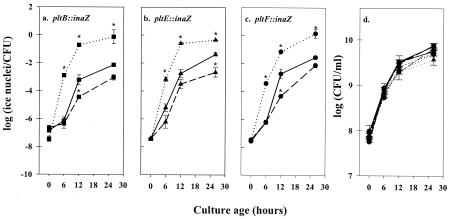
The effects of exogenous pyoluteorin and 2,4-diacetylphloroglucinol on pyoluteorin production were accompanied by corresponding effects on transcription from pyoluteorin biosynthetic gene promoters, as measured by activity of the ice nucleation transcriptional reporter gene (inaZ) fused to three separate chromosomal pyoluteorin biosynthesis genes, pltB, pltE, and pltF. Because preliminary experiments indicated that inaZ expression from plt promoters reached a maximum by 24 h after inoculation, INA was measured periodically from 0 to 24 h after inoculation (Fig. 6). INA was enhanced in all three reporter strains when grown in NBGly amended with 4 μg of pyoluteorin/ml. At 24 h after inoculation, INA expressed by Pf-5 derivatives containing pltB::inaZ, pltE::inaZ, and pltF::inaZ was enhanced by 10- to 100-fold by the addition of pyoluteorin. Conversely, transcription of pyoluteorin biosynthetic genes was reduced when NBGly was amended with 2,4-diacetylphloroglucinol (12.5 μg/ml) prior to inoculation. At 24 h after inoculation, 2,4-diacetylphloroglucinol amendment of the culture medium reduced INA expressed by Pf-5 derivatives containing pltB::inaZ, pltE::inaZ, and pltF::inaZ by 3- to 12-fold, respectively. These data provide evidence that pyoluteorin autoinduction, as well as repression of pyoluteorin production by 2,4-diacetylphloroglucinol, occur at least in part at the level of transcription from pyoluteorin biosynthetic gene promoters. Addition of 4 μg of pyoluteorin/ml or 12.5 μg of 2,4-diacetylphloroglucinol/ml to the growth medium did not significantly alter the population sizes of any strain at any time points sampled (all P > 0.05; Fig. 6d).
FIG. 6.
Positive and negative influences by pyoluteorin and 2,4-diacetylphloroglucinol, respectively, on transcriptional activity within the pyoluteorin biosynthetic gene cluster, assessed from transcriptional fusions of the ice nucleation reporter gene (inaZ) to three pyoluteorin biosynthetic genes (pltB, pltE, and pltF). Panels a to c depict the response of individual reporter strains to exogenous pyoluteorin and 2,4-diacetylphloroglucinol. Pf-5 pltB::inaZ (▪) (a); Pf-5 pltE::inaZ (▴) (b); and Pf-5 pltF::inaZ (•) (c); were grown in unamended NBGly (solid lines) or in NBGly amended with 4 μg of pyoluteorin/ml (dotted lines) or 12.5 μg of 2,4-diacetylphloroglucinol/ml (dashed lines). Population densities (d) are shown for all strains and did not differ significantly among treatments at any time point. Asterisks above symbols denote treatment means which differ significantly from the mean of the unamended treatment at the identical time point (P ≥ 0.05; Student's t test). Error bars denote one standard error.
Pyoluteorin autoinduction is superseded by genetically and environmentally repressive backgrounds.
In NBGlu, a medium that is not conducive to pyoluteorin production by Pf-5 (30, 43), the addition of 4 μg of pyoluteorin/ml did not result in detectable pyoluteorin production by the bacterium (data not shown). Thus, pyoluteorin amendments in NBGly and NBGlu had different effects on pyoluteorin production by Pf-5, suggesting that the pyoluteorin autoinduction phenomenon was overridden by glucose repression. Similarly, Pf-5 pltR::aacC1 and Pf-5 gacA::aphI derivatives grown in NBGly failed to produce detectable concentrations of pyoluteorin, even in the presence of exogenous pyoluteorin (data not shown), suggesting that autoinduction does not supersede control of pyoluteorin production by the LysR transcriptional regulator PltR or the by the GacS/GacA histidine kinase/response regulator pair. Other workers observed that autoinduction of 2,4-diacetylphloroglucinol is not entirely subordinate to gacA in CHA0 (54), indicating that the mechanisms of autoinduction may differ between the two systems.
Pyoluteorin autoinduction occurs in the rhizosphere.
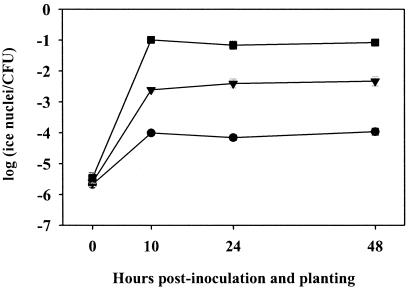
To determine whether pyoluteorin could influence pyoluteorin biosynthetic gene expression by bacterial populations in the rhizosphere as well as in culture, we performed a pyoluteorin cross-feeding assay (Fig. 7). The pyoluteorin-deficient strain Pf-5 pltB::inaZ was used as a reporter strain to detect the presence of pyoluteorin produced by other bacteria in the rhizosphere. Cucumber seedlings were coinoculated, at a 1:10 ratio, with Pf-5 pltB::inaZ and one of three other Pf-5 derivatives that differ in pyoluteorin production: Pf-5, which produces pyoluteorin; Pf-5 rpoS::lacZ, which overproduces pyoluteorin; or pltR::aacC1, which does not produce detectable pyoluteorin. When coinoculated with pltR::aacC1 in the rhizosphere of cucumber, Pf-5 pltB::inaZ produced a mean of −4.0 log10(INA) from 10 to 48 h after inoculation, which represented a basal level of pltB::inaZ expression in the absence of pyoluteorin cross-feeding. When coinoculated with the pyoluteorin-producing strain Pf-5, rhizosphere populations of Pf-5 pltB::inaZ expressed significantly greater amounts of INA [-2.5 log10(INA)]. The greatest level of INA expressed by Pf-5 pltB::inaZ in the rhizosphere, averaging −1.1 log10(INA) from 10 to 48 h after inoculation, occurred when Pf-5 pltB::inaZ was coinoculated with the pyoluteorin-overproducer Pf-5 rpoS::lacZ. Thus, pyoluteorin produced by Pf-5 and the overproducing derivative Pf-5 rpoS::lacZ was perceived by coinoculated cells of Pf-5 pltB::inaZ in the rhizosphere, as evidenced by enhanced transcription of pltB in those coinoculated cells. Population sizes (mean log CFU/seedling) of the inducing strains averaged 6.5 at the time of inoculation, and increased to 6.8 over 48 h. Log CFU/seedling for the reporter strain coinoculated with each inducer averaged 5.3 at the time of inoculation and increased to 5.9 over 48 h. While slight differences in the rhizosphere population sizes of Pf-5 pltB::inaZ (data not shown) were observed among treatments at two time points, these differences were not found to significantly affect INA determinations.
FIG. 7.
Autoinduction of pyoluteorin as a result of cross-feeding in the rhizosphere. A pyoluteorin-deficient reporter strain (Pf-5 pltB::inaZ) was coinoculated onto cucumber seedlings at a cell density ratio of 1:10 with each of the following inducing strains: Pf-5 (parental strain [▾]), Pf-5 pltR::aacC1 (pyoluteorin deficient [•]) and Pf-5 rpoS::lacZ (pyoluteorin overproducer [▪]). After seedlings were grown in pasteurized soil for the durations indicated, bacteria were harvested and evaluated for INA conferred by Pf-5 pltB::inaZ in the presence of each coinoculant. Values represent the mean of 10 replicate seedlings, and error bars indicate one standard error, which are obscured by the symbols for some time points.
DISCUSSION
The data presented in this paper provide strong evidence that pyoluteorin serves as an autoregulator, positively influencing its own production in P. fluorescens Pf-5. Three lines of evidence from experiments with Pf-5 and derivative strains support this conclusion: (i) exogenous pyoluteorin enhanced pyoluteorin production, (ii) exogenous pyoluteorin enhanced transcriptional activity of three pyoluteorin biosynthetic genes, and (iii) coinoculation with pyoluteorin-producing cells induced expression of pyoluteorin biosynthetic gene transcription by a pyoluteorin-deficient reporter strain in the rhizosphere. Furthermore, pyoluteorin functioned as an inducer of its own production when present in a culture medium at an extremely low concentration (1.47 nM). In this respect, pyoluteorin is similar to established signaling molecules, such as the A factor of Streptomyces griseus (19, 22, 27) or acyl homoserine lactones of Vibrio fischeri and Serratia liquefaciens (13, 14), which influence cell physiology and gene expression when extracellular concentrations are in the nanomolar range. In addition to its autoregulatory role, pyoluteorin influenced the production of at least one other secondary metabolite, 2,4-diacetylphloroglucinol. Exogenous pyoluteorin repressed 2,4-diacetylphloroglucinol production by Pf-5, as was previously reported for the related strain, P. fluorescens CHA0 (54). Thus, in addition to its established extracellular role as an antibiotic, pyoluteorin contributes to regulation of at least two metabolic pathways within the bacterial cell.
The roles of antibiotics in the ecology and physiology of microorganisms have been debated for decades, and evidence is accruing to support intracellular roles for bacterial antibiotics. For example, P. aeruginosa produces 2-heptyl-3-hydroxy-4-quinolone which, when present in micromolar concentrations, plays an integral role in signal transduction of producing bacterial cells (38, 46). Although toxicity has not been demonstrated for 2-heptyl-3-hydroxy-4-quinolone itself (46), related molecules in the 4-quinolone class have antibacterial activity and are used medicinally (55). More recently, it has been shown that exposure to subinhibitory concentrations of several commonly used antibiotics results in altered transcription of as many as 5% of genes in Salmonella enterica serovar Typhimurium (15); by extension, one might predict that these antibiotics also have physiological relevance in the cells in which they are produced. The finding that nanomolar concentrations of pyoluteorin can enhance pyoluteorin production and biosynthetic gene expression by Pf-5 provides another example of a regulatory role for antibiotics.
Pyoluteorin biosynthesis genes were transcribed in early- to mid-log phase cultures of Pf-5 but pyoluteorin did not accumulate to detectable levels until cells were entering or in stationary phase. The lag between induction of gene expression and accumulation of detectable levels of pyoluteorin might be explained by slow rates of pyoluteorin biosynthesis or by posttranscriptional control mechanisms, like those affecting the antibiotics 2,4-diacetylphloroglucinol and hydrogen cyanide in the related P. fluorescens strains CHA0 and F113 (1, 5, 6, 20). Transcripts of genes for biosynthesis of hydrogen cyanide and 2,4-diacetylphloroglucinol are sequestered by a small RNA-binding protein (RsmA), but a noncoding RNA, PrrB (RsmZ), whose expression is regulated by GacA/GacS, can in turn bind RsmA, and thus relieve RsmA suppression in these strains. An alternative hypothesis is that low levels of pyoluteorin, below our detection limits, may gradually accumulate in cultures until a threshold rate of pyoluteorin reentry into producer cells is achieved, whereupon pyoluteorin begins autoinduction. Such a mechanism is reminiscent of the prototypic LuxI/LuxR autoinduction systems, which require accumulation of an autoinducer in the extracellular milieu for induction of gene expression (reviewed in 39).
Established positive autoregulation systems, such as LuxI/LuxR, rely upon diffusion or secretion and reuptake of the signal molecule across the membrane and include an intracellular signal receptor(s) that mediates signal transduction within the cell. Neither a pyoluteorin receptor nor a mechanism for pyoluteorin transport across the bacterial membrane is known in Pf-5, but candidates for both are found within the pyoluteorin biosynthetic gene cluster. The nucleotide sequence of the pltHIJ cluster, which is directly adjacent to the biosynthesis genes pltLABCDEFG, includes genes encoding members of the ABC transporter family (7). Transcription of pltI and pltJ is enhanced by exogenous pyoluteorin (7), indicating that regulation of the genes is coordinated with pyoluteorin production, as would be expected if the region encodes proteins that function in transport of pyoluteorin into or out of the cell. A candidate pyoluteorin receptor(s) is PltR, a LysR transcriptional regulator required for transcriptional activity of pltB, pltE, and pltF and for the accumulation of detectable levels of pyoluteorin in culture supernatants (42). LysR family members generally require a cofactor for activity (reviewed in reference 51).
The findings of this study demonstrate that regulation of 2,4-diacetylphloroglucinol and pyoluteorin production is interrelated in Pf-5; both compounds were repressive of the other's production. In P. fluorescens strain CHA0, 2,4-diacetylphloroglucinol autoinduction and repression by pyoluteorin, salicylate, and a fungal metabolite, fusaric acid, are mediated by PhlF (54), whose repressive binding to the phlACBD promoter is directly stabilized by salicylate but is alleviated by 2,4-diacetylphloroglucinol (2). Whether the mechanism of 2,4-diacetylphloroglucinol autoregulation and repression in Pf-5 is similar in to that in CHA0 remains to be determined; we have not ruled out the possibility that precursor competition (e.g., for malonyl-coenzyme A needed to extend the growing polyketide chains) contributes to pyoluteorin repression of 2,4-diacetylphloroglucinol in Pf-5.
The concentration of 2,4-diacetylphloroglucinol (595 nM) required for repression of pyoluteorin production in Pf-5 was much higher than the concentration of pyoluteorin (1.47 nM) required for autoinduction. Furthermore, the bimodal response of pyoluteorin production to increasing concentrations of 2,4-diacetylphloroglucinol suggests a concentration-dependent role reversal of 2,4-diacetylphloroglucinol from activator to inhibitor. The reason for this shift is unknown, but precedents exist. For example, gene expression in response to acyl homoserine lactone in V. fischeri (13) or to the small peptide glu-arg-gly-met-thr in Bacillus subtilis (56) is enhanced at low concentrations but is inhibited at high concentrations.
This study demonstrated that pyoluteorin has the ability to serve as an intercellular signal between distinct populations of bacterial cells coinhabiting the rhizosphere. Our observation that pyoluteorin biosynthetic gene expression is upregulated by exogenous pyoluteorin in the rhizosphere implies that pyoluteorin autoinduction contributes significantly to overall regulation of pyoluteorin production by Pf-5 in vivo. To our knowledge, this is the first report of an antibiotic functioning as a signal molecule in a natural environment.
Pyoluteorin is an antibiotic toxic to Pythium ultimum and other plant pathogenic oomycetes and has been shown to be an important factor in biological control of such pathogens (24, 37). The finding that pyoluteorin production by Pf-5 cells in the rhizosphere can influence pyoluteorin gene expression by neighboring bacterial cells has important implications for biological control of plant disease. Pyoluteorin can now be added to the growing list of microbial metabolites shown to function as signal molecules influencing the expression of traits that contribute to biological control by Pseudomonas spp. in the rhizosphere. Other examples include acyl homoserine lactones (48, 61), which influence the expression of phenazine biosynthesis genes by P. aureofaciens; fusaric acid (40), which represses the expression of 2,4-diacetylphloroglucinol biosynthesis genes by P. fluorescens CHA0; and siderophores (33), which influence the expression of pyoverdine biosynthesis genes by P. putida. When taken together, these examples provide convincing evidence that the expression of biocontrol traits by Pseudomonas spp. is influenced profoundly by signal molecules produced by their coinhabitants in the rhizosphere. It seems likely that secreted metabolites with various functions in the extracellular environment, retrieved or intercepted by producing or neighboring cells, supply information about the ecological and physiological status of the surrounding community. Therefore, the composition and physiological status of the microbial community is likely to be an important factor influencing the expression of biocontrol traits by bacterial antagonists in the rhizosphere, and consequently, their success as biological control agents.
Acknowledgments
We thank Brian Nowak-Thompson and Regina Notz for providing purified pyoluteorin and synthetic 2,4-diacetylphloroglucinol, respectively. We are grateful to Meadow Anderson, Walter Mahaffee, Dmitri Mavrodi, Caroline Press, Brenda Shaffer, and Virginia Stockwell for critical reviews of the manuscript.
This research was supported in part by a fellowship to M.B. from the U.S. Environmental Protection Agency Science to Achieve Results program (U-91582801-0).
REFERENCES
- 1.Aarons, S., A. Abbas, C. Adams, A. Fenton, and F. O'Gara. 2000. A regulatory RNA (PrrB RNA) modulates expression of secondary metabolite genes in Pseudomonas fluorescens F113. J. Bacteriol. 182:3913-3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abbas, A., J. P. Morrissey, P. Carnicero Marquez, M. M. Sheehan, I. R. Delany, and F. O'Gara. 2002. Characterization of interactions between the transcriptional repressor PhlF and its binding site at the phlA promoter in Pseudomonas fluorescens F113. J. Bacteriol. 184:3008-3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bailey, D., R. E. Johnson, and U. J. Salvador. 1973. Pyrrole antibacterial agents. 1. Compounds related to pyoluteorin. J. Med. Chem. 16:1298-1300. [DOI] [PubMed] [Google Scholar]
- 4.Bangera, M. G., and L. S. Thomashow. 1999. Identification and characterization of a gene cluster for synthesis of the polyketide antibiotic 2, 4-diacetylphloroglucinol from Pseudomonas fluorescens Q2-87. J. Bacteriol. 181:3155-3163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blumer, C., and D. Haas. 2000. Multicopy suppression of a gacA mutation by the infC operon in Pseudomonas fluorescens CHA0: competition with the global translational regulator RsmA. FEMS Microbiol. Lett. 187:53-58. [DOI] [PubMed] [Google Scholar]
- 6.Blumer, C., S. Heeb, G. Pessi, and D. Haas. 1999. Global GacA-steered control of cyanide and exoprotease production in Pseudomonas fluorescens involves specific ribosome binding sites. Proc. Natl. Acad. Sci. USA 96:14073-14078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brodhagen, M. 2003. Ph.D. thesis. Oregon State University, Corvallis.
- 8.Cook, R. J., L. S. Thomashow, D. M. Weller, D. Fujimoto, M. Mazzola, G. Bangera, and D.-S. Kim. 1995. Molecular mechanisms of defense by rhizobacteria against root disease. Proc. Natl. Acad. Sci. USA 92:4197-4201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Corbell, N. 1999. Ph.D. thesis. Oregon State University, Corvallis.
- 10.Corbell, N., and J. E. Loper. 1995. A global regulator of secondary metabolite production in Pseudomonas fluorescens Pf-5. J. Bacteriol. 177:6230-6236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cuppels, D. A., C. R. Howell, R. D. Stipanovic, A. Stoessl, and J. B. Stothers. 1986. Biosynthesis of pyoluteorin: a mixed polyketide-tricarboxylic acid cycle origin demonstrated by [1, 2-13C2]acetate incorporation. Z. Naturforsch. 41c:532-536. [Google Scholar]
- 12.Dowling, D. N., and F. O'Gara. 1994. Metabolites of Pseudomonas involved in the biocontrol of plant disease. Trends Biotechnol. 12:133-141. [Google Scholar]
- 13.Eberhard, A., A. L. Burlingame, C. Eberhard, G. L. Kenyon, K. H. Nealson, and J. J. Oppenheimer. 1981. Structural identification of autoinducer of Photobacterium fischeri luciferase. Biochemistry 20:2444-2449. [DOI] [PubMed] [Google Scholar]
- 14.Eberl, L., M. K. Winson, C. Sternberg, G. S. A. B. Stewart, G. Christiansen, S. R. Chhabra, B. Bycroft, P. Williams, S. Molin, and M. Givskov. 1996. Involvement of N-acyl-L-homoserine lactone autoinducers in controlling the multicellular behaviour of Serratia liquefaciens. Mol. Microbiol. 20:127-136. [DOI] [PubMed] [Google Scholar]
- 15.Goh, E.-B., G. Yim, W. Tsui, J. McClure, M. G. Surette, and J. Davies. 2002. Transcriptional modulation of bacterial gene expression by subinhibitory concentrations of antibiotics. Proc. Natl. Acad. Sci. USA 99:17025-17030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gutterson, N., J. S. Ziegle, G. J. Warren, and T. J. Layton. 1988. Genetic determinants for catabolite induction of antibiotic biosynthesis in Pseudomonas fluorescens HV37a. J. Bacteriol. 170:380-385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haas, D., C. Blumer, and C. Keel. 2000. Biocontrol ability of fluorescent pseudomonads genetically dissected: importance of positive feedback regulation. Curr. Opin. Biotechnol. 11:290-297. [DOI] [PubMed] [Google Scholar]
- 18.Haas, D., and C. Keel. 2003. Regulation of antibiotic production in root-colonizing Pseudomonas spp. and relevance for biological control of plant disease. Annu. Rev. Phytopathol. 41:117-153. [DOI] [PubMed] [Google Scholar]
- 19.Hara, O., and T. Beppu. 1982. Mutants blocked in streptomycin production in Streptomyces griseus - the role of A-factor. J. Antibiot. 35:349-358. [DOI] [PubMed] [Google Scholar]
- 20.Heeb, S., C. Blumer, and D. Haas. 2002. Regulatory RNA as mediator in GacA/RsmA-dependent global control of exoproduct formation in Pseudomonas fluorescens CHA0. J. Bacteriol. 184:1046-1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heeb, S., and D. Haas. 2001. Regulatory roles of the GacS/GacA two-component system in plant-associated and other gram-negative bacteria. Mol. Plant-Microbe Interact. 14:1351-1363. [DOI] [PubMed] [Google Scholar]
- 22.Horinouchi, S. 1999. γ-Butyrolactones that control secondary metabolism and cell differentiation in Streptomyces, p. 193-207. In G. M. Dunny and S. C. Winans (ed.), Cell-cell signaling in bacteria. ASM Press, Washington, D.C.
- 23.Howell, C. R., and R. D. Stipanovic. 1979. Control of Rhizoctonia solani on cotton seedlings with Pseudomonas fluorescens and with an antibiotic produced by the bacterium. Phytopathology 69:480-482. [Google Scholar]
- 24.Howell, C. R., and R. D. Stipanovic. 1980. Suppression of Pythium ultimum-induced damping-off of cotton seedlings by Pseudomonas fluorescens and its antibiotic, pyoluteorin. Phytopathology 70:712-715. [Google Scholar]
- 25.Howie, W. J., and T. V. Suslow. 1991. Role of antibiotic biosynthesis in the inhibition of Pythium ultimum in the cotton spermosphere and rhizosphere by Pseudomonas fluorescens. Mol. Plant-Microbe Interact. 4:393-399. [Google Scholar]
- 26.Keel, C., U. Schnider, M. Maurhofer, C. Voisard, J. Laville, U. Burger, P. Wirthner, D. Haas, and G. Defago. 1992. Suppression of root diseases by Pseudomonas fluorescens CHA0: importance of the bacterial secondary metabolite 2,4-diacetylphloroglucinol. Mol. Plant-Microbe Interact. 5:4-13. [Google Scholar]
- 27.Khokhlov, A. S. 1988. Results and perspectives of actinomycete autoregulators studies, p. 338-345. In Y. Okami, T. Beppu, and H. Ogawara (ed.), Biology of actinomycetes '88. Japan Scientific Societies Press, Tokyo, Japan.
- 28.King, E. O., M. K. Ward, and D. E. Raney. 1954. Two simple media for the demonstration of pyocyanin and fluorescein. J. Lab. Clin. Med. 44:301-307. [PubMed] [Google Scholar]
- 29.Kraus, J., and J. E. Loper. 1992. Lack of evidence for a role of antifungal metabolite production by Pseudomonas fluorescens Pf-5 in the biological control of Pythium damping-off of cucumber. Phytopathology 82:264-271. [Google Scholar]
- 30.Kraus, J., and J. E. Loper. 1995. Characterization of a genomic region required for the production of the antibiotic pyoluteorin by the biological control agent Pseudomonas fluorescens Pf-5. Appl. Environ. Microbiol. 61:849-854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lamont, I. L., P. A. Beare, U. Ochsner, A. I. Vasil, and M. L. Vasil. 2002. Siderophore-mediated signaling regulates virulence factor production in Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 99:7072-7077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lindgren, P. B., R. Frederick, A. G. Govindarajan, N. J. Panopoulos, B. J. Staskawicz, and S. E. Lindow. 1989. An ice nucleation reporter gene system: identification of inducible pathogenicity genes in Pseudomonas syringae pv. phaseolicola. EMBO J. 8:1291-1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Loper, J. E., and M. D. Henkels. 1999. Utilization of heterologous siderophores enhances levels of iron available to Pseudomonas putida in the rhizosphere. Appl. Environ. Microbiol. 65:5357-5363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Loper, J. E., and S. E. Lindow. 1994. A biological sensor for iron available to bacteria in their habitats on plant surfaces. Appl. Environ. Microbiol. 60:1934-1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Loper, J. E., and S. E. Lindow. 2002. Reporter gene systems useful in evaluating in situ gene expression by soil and plant-associated bacteria, p. 627-637. In C. J. Hurst, R. L. Crawford, G. R. Knudsen, J. J. McInerney, and L. D. Stetzenbach (ed.), Manual of environmental microbiology, 2nd ed. ASM Press, Washington, D.C.
- 36.Loper, J. E., B. Nowak-Thompson, C. A. Whistler, M. J. Hagen, N. A. Corbell, M. D. Henkels, and V. O. Stockwell. 1997. Biological control mediated by antifungal metabolite production and resource competition: an overview, p. 73-79. In A. Ogoshi, K. Kobayashi, Y. Homma, F. Kodama, N. Kondo, and S. Akino (ed.), Proceedings of the Fourth International Workshop on Plant Growth Promoting Rhizobacteria, Sapporo, Japan.
- 37.Maurhofer, M., C. Keel, U. Schnider, C. Voisard, D. Haas, and G. Defago. 1992. Influence of enhanced antibiotic production in Pseudomonas fluorescens strain CHA0 on its disease suppressive capacity. Phytopathology 82:190-195. [Google Scholar]
- 38.McKnight, S. L., B. H. Iglewski, and E. C. Pesci. 2000. The Pseudomonas quinolone signal regulates rhl quorum sensing in Pseudomonas aeruginosa. J. Bacteriol. 182:2702-2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nealson, K. H. 1999. Early observations defining quorum-dependent gene expression. In G. M. Dunny and S. C. Winans (ed.), Cell-cell signaling in bacteria. ASM Press, Washington, D.C.
- 40.Notz, R., M. Maurhofer, H. Dubach, D. Haas, and G. Defago. 2002. Fusaric acid-producing strains of Fusarium oxysporum alter 2,4-diacetylphloroglucinol biosynthetic gene expression in Pseudomonas fluorescens CHA0 in vitro and in the rhizosphere of wheat. Appl. Environ. Microbiol. 68:2229-2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nowak-Thompson, B. 1997. Ph.D. thesis. Oregon State University, Corvallis.
- 42.Nowak-Thompson, B., N. Chaney, S. J. Gould, and J. E. Loper. 1999. Characterization of the pyoluteorin biosynthetic gene cluster of Pseudomonas fluorescens Pf-5. J. Bacteriol. 181:2166-2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nowak-Thompson, B., S. J. Gould, J. Kraus, and J. E. Loper. 1994. Production of 2,4-diacetylphloroglucinol by the biocontrol agent Pseudomonas fluorescens Pf-5. Can. J. Microbiol. 40:1064-1066. [Google Scholar]
- 44.Nowak-Thompson, B., S. J. Gould, and J. E. Loper. 1997. Identification and sequence analysis of the genes encoding a polyketide synthase required for pyoluteorin biosynthesis in Pseudomonas fluorescens Pf-5. Gene 204:17-24. [DOI] [PubMed] [Google Scholar]
- 45.Ornston, L. N., and R. Y. Stanier. 1966. The conversion of catechol and protocatechuate to β-ketoadipate by Pseudomonas putida. J. Biol. Chem. 241:3776-3786. [PubMed] [Google Scholar]
- 46.Pesci, E. C., J. B. Milbank, J. P. Pearson, S. McKnight, A. S. Kende, E. P. Greenberg, and B. H. Iglewski. 1999. Quinolone signaling in the cell-to-cell communication system of Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 96:11229-11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pfender, W. F., J. Kraus, and J. E. Loper. 1993. A genomic region from Pseudomonas fluorescens Pf-5 required for pyrrolnitrin production and inhibition of Pyrenophora tritici-repentis in wheat straw. Phytopathology 83:1223-1228. [Google Scholar]
- 48.Pierson, E. A., D. W. Wood, J. A. Cannon, F. M. Blachere, and L. S. Pierson III. 1998. Interpopulation signaling via N-acyl-homoserine lactones among bacteria in the wheat rhizosphere. Mol. Plant-Microbe Interact. 11:1078-1084. [Google Scholar]
- 49.Reimmann, C., L. Serino, M. Beyeler, and D. Haas. 1998. Dihydroaeruginoic acid synthetase and pyochelin synthetase, products of the pchEF genes, are induced by extracellular pyochelin in Pseudomonas aeruginosa. Microbiology 144:3135-3148. [DOI] [PubMed] [Google Scholar]
- 50.Sarniguet, A., J. Kraus, M. D. Henkels, A. M. Muehlchen, and J. E. Loper. 1995. The sigma factor σs affects antibiotic production and biological control activity of Pseudomonas fluorescens Pf-5. Proc. Natl. Acad. Sci. USA 92:12255-12259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schell, M. A. 1993. Molecular biology of the LysR family of transcriptional regulators. Annu. Rev. Microbiol. 47:597-626. [DOI] [PubMed] [Google Scholar]
- 52.Schnider, U., C. Keel, C. Blumer, J. Troxler, G. Defago, and D. Haas. 1995. Amplification of the housekeeping sigma factor in Pseudomonas fluorescens CHA0 enhances antibiotic production and improves biocontrol abilities. J. Bacteriol. 177:5387-5392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schnider, U., C. Keel, C. Voisard, G. Defago, and D. Haas. 1995. Tn5-directed cloning of pqq genes from Pseudomonas fluorescens CHA0: mutational inactivation of the genes results in overproduction of the antibiotic pyoluteorin. Appl. Environ. Microbiol. 61:3856-3864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schnider-Keel, U., A. Seematter, M. Maurhofer, C. Blumer, B. Duffy, C. Gigot-Gonnefoy, C. Reimmann, R. Notz, G. Défago, D. Haas, and C. Keel. 2000. Autoinduction of 2,4-diacetylphloroglucinol biosynthesis in the biocontrol agent Pseudomonas fluorescens CHA0 and repression by the bacterial metabolites salicylate and pyoluteorin. J. Bacteriol. 182:1215-1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Smith, J. T., and H.-J. Zeiler. 1998. History and introduction, p. 1-11. In J. Kuhlmann, A. Dalhoff, and H.-J. Zeiler (ed.), Quinolone antibacterials. Springer, Berlin, Germany.
- 56.Solomon, J. M., B. A. Lazazzera, and A. D. Grossman. 1996. Purification and characterization of an extracellular peptide factor that affects two different developmental pathways in Bacillus subtilis. Genes Dev. 10:2014-2024. [DOI] [PubMed] [Google Scholar]
- 57.Warren, G. J. 1995. Identification and analysis of ina genes and proteins, p. 85-99. In R. E. Lee, Jr., G. J. Warren, and L. V. Gusta (ed.), Biological ice nucleation and its applications. APS Press, St. Paul, Minn.
- 58.Watanabe, N. M., M. W. Southworth, G. J. Warren, and P. K Wolber. 1990. Rates of assembly and degradation of bacterial ice nuclei. Mol. Microbiol. 4:1871-1879. [DOI] [PubMed] [Google Scholar]
- 59.Whistler, C. A., N. A. Corbell, A. Sarniguet, W. Ream, and J. E. Loper. 1998. The two-component regulators GacS and GacA influence accumulation of the stationary-phase sigma factor σs and the stress response in Pseudomonas fluorescens Pf-5. J. Bacteriol. 180:6635-6641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Whistler, C. A., V. O. Stockwell, and J. E. Loper. 2000. Lon protease influences antibiotic production and UV tolerance of Pseudomonas fluorescens Pf-5. Appl. Environ. Microbiol. 66:2718-2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wood, D. W., F. Gong, M. M. Daykin, P. Williams, and L. S. Pierson III. 1997. N-acyl-homoserine lactone-mediated regulation of phenazine gene expression by Pseudomonas aureofaciens 30-84 in the wheat rhizosphere. J. Bacteriol. 179:7663-7670. [DOI] [PMC free article] [PubMed] [Google Scholar]