Abstract
Background
Hemolytic reactions (HTRs) can occur from ABO-incompatible platelet transfusions. After a series of cases at our institution, a procedure to screen all plateletpheresis donors for high-titer ABO antibodies was implemented.
Study design and methods
Plasma samples from plateletpheresis donors were screened using pooled 0.8% A1 and 0.8% B RBC in buffered gel. Dilutions of 1 in 150, 1 in 200, and 1 in 250 were sequentially evaluated. A component testing positive for high-titer ABO antibodies is restricted to ABO-identical or group O recipients, or washed.
Results
At the initial dilution of 1 in 150, half of group O components were labeled as high-titer. At the current dilution of 1 in 250, 25% of group O components are labeled as high-titer. No platelet-associated HTR has been reported since screening began.
Conclusion
Universal screening for high-titer ABO antibodies in plateletpheresis donors can be implemented efficiently to reduce the risk of HTRs. The cutoff for classifying a unit as high-titer depends on the serologic method used, and may be customized by the individual facility. Our screening method uses one gel test per donation regardless of blood group, and a plasma dilution of 1 in 250 with pooled A1/B RBCs in buffered gel.
Introduction
Platelets express ABO antigens on their surface, and platelet components are usually suspended in the original donor plasma. Under ideal circumstances, patients should receive ABO-identical platelets. However, in the face of limited supply, adult patients are often transfused without regard to ABO compatibility. Transfusion of platelets containing ABO-incompatible plasma carries the risk of hemolytic transfusion reactions (HTR). Contributing risk factors include the small blood volume of the patient, exposure to a large cumulative volume of incompatible plasma over time, and high-titer anti-A, anti-B or both in donor plasma.1,2
Our institution utilizes apheresis platelets exclusively. AABB Standards for Blood Banks and Transfusion Services require transfusion services to have a policy concerning transfusion of components containing significant amounts of incompatible ABO antibodies. Our existing transfusion service policy mandates volume reduction of ABO-incompatible platelets for patients under 40 kg. Within a three-year period (during which 12,299 plateletpheresis components were transfused), five HTRs occurred in association with ABO-incompatible platelets; one of these cases was not recognized as a reaction and occurred despite volume reduction in accordance with our policy.3 These observations prompted us to screen all plateletpheresis donors for high-titer ABO antibodies. In our index series of platelet-associated HTRs, the lowest implicated donor titer was 128 in tube at the saline phase. We therefore set our initial dilution threshold at 1 in 150.
Materials and methods
Evaluation of pooled A1 and B RBC in tube and gel techniques
A 1 in 150 dilution was prepared by adding 5 uL of plasma to 745 uL of normal saline and vortexing to mix. Pooled A1and B RBC were prepared by mixing equal volumes of commercial 0.8% A1 and 0.8% B reagent RBC. Each sample was tested by adding 50 uL diluted plasma to 50 uL pooled A1and B RBCs in buffered gel cards (Ortho Clinical Diagnostics, Raritan, NJ), and also was tested in buffered gel cards with separate 0.8% A1 and 0.8% B cells. The samples were tested in parallel in tube with pooled 3% A1 and 3% B RBCs, and with 3% A1 and 3% B cells separately, using 2 drops of diluted plasma and one drop of cell suspension. Following a 15-minute room temperature incubation and centrifugation, all tests were read as positive or negative.
Universal screening for donors with high titer ABO antibodies
Universal screening was implemented using the EDTA tubes collected on donors at the time of apheresis, regardless of prior donation/testing history. Our validated process allows us to test all samples uniformly regardless of ABO group. We initiated screening at a dilution of 1 in 150. After a ten-month period the dilution was increased to 1 in 200, and subsequently to 1 in 250, which is our current cutoff. All positive results are entered in the blood bank information system as a donor comment. High-titer status is appropriately designated on the component bag. A component that screens positive for high-titer ABO antibodies is restricted to ABO-identical recipients, group O recipients or washed prior to transfusion.
Results
We first validated our proposed serologic test method, which is manual buffered gel cards using pooled A1 and B cells, and compared these results to gel and tube techniques using separate A1 and B cells. Upon successful validation of the serologic method, we initiated universal screening of all apheresis platelet components at a dilution of 1 in 150.
Serologic validation results
On the initial validation set of 11 samples (Table 1), all specimens that were positive when tested with A1 and B RBCs separately were also positive when tested with the pooled RBCs, in gel and in tube. Mixed field reactivity was observed with some group O samples as expected. Five samples were negative by both methods (including the two control AB samples), 5 samples were positive by both methods, and one group O sample was positive by gel for anti-A but negative by tube, consistent with gel being a more sensitive technique.
Table 1.
Results of ABO antibody testing in gel and in tube at a dilution of 1in150
| Buffer gel card (0.8% RBC) |
Tube (3% RBC) |
||||||
|---|---|---|---|---|---|---|---|
| ABO* | Pooled A1 & B |
A1 | B | Pooled A1 & B |
A1 | B | Samples (n) |
| O | + | − | + | + | − | + | 1 |
| O | + | + | + | + | + | + | 1 |
| O | + | + | − | − | − | − | 1 |
| A | − | nd | − | − | nd | − | 2 |
| A | + | nd | + | + | nd | + | 2 |
| B | + | + | nd | + | + | nd | 1 |
| B | − | − | nd | − | − | nd | 1 |
| AB | − | − | − | − | − | − | 2 |
All specimens exhibited the expected reactivity in reverse typing.
+, positive; −, negative; nd, not done.
Universal screening results
During the first 6 months of implementation, a total of 1277 components were screened, of which 1261 units were collected in-house from 504 donors. We excluded 71 group AB units from analysis. Half of the group O units had high-titer anti-A and/or anti-B at the initial dilution of 1:150 and 14% of group A units had high-titer anti-B. The percentage of platelets washed during this 6-month period was 5.7%, compared to 3.3% the prior year. We did not specifically track indications for washing platelets, so the difference may be partially attributable to platelet transfusions given to patients requiring washed components for clinical indications.
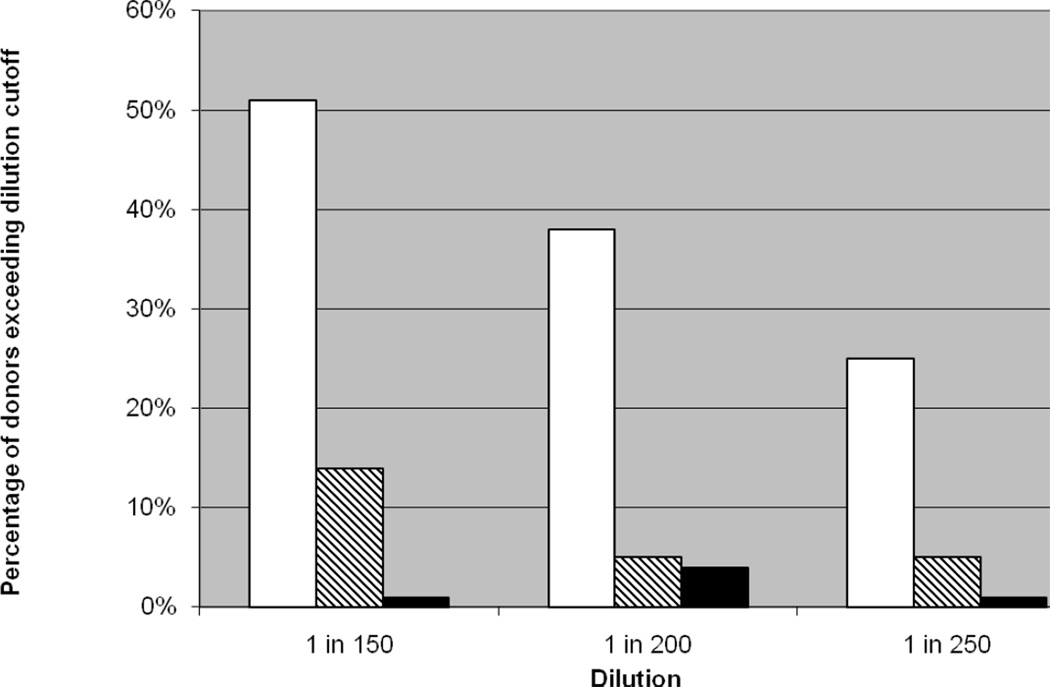
After 10 months of screening, we increased the dilution from 1 in 150 to 1 in 200 for two months. At this threshold 36% of group O platelets were still classified as high-titer. Most recently, for the past 8 months, we have adopted a dilution of 1 in 250. Using this cutoff, 25% of group O platelets and 5% of group A platelets are classified as high-titer (Figure 1). In the two years since screening began, our platelet utilization has increased to 4900 units per year, and no platelet-associated HTRs have been reported.
Figure 1.
Donors identified with high-titer ABO antibodies as a function of plasma dilution. Dilutions were evaluated at 1in150 (n = 1,206), 1 in 200 (n = 507) and 1in 250 (n = 923). The percentage of identified donors is shown for blood group O (open bar □), A (hatched bar  ), and B (closed bar ■).
), and B (closed bar ■).
Look back studies in recipients of platelets screened at the 1 in 250 threshold
Some donors who were classified as below the cut-off on one donation were classified as high-titer on subsequent donations. We examined clinical and laboratory data on 5 recipients of 6 prior plateletpheresis components from donors who were identified as high-titer at the time of a subsequent donation (Table 2). The components transfused had met our release criteria for the earlier donation and no transfusion reactions had been reported. Three group O platelets were transfused to group A recipients, two components were O to B, and one A to B. We observed no significant change in hemoglobin, LDH or bilirubin levels before and after the transfusions. Direct antiglobulin tests had not been performed on any of these recipients.
Table 2.
Look back for signs of hemolysis in 6 transfusion episodes from 5 plateletpheresis donors with marginal high-titer ABO antibodies
| Platelet transfusion (mean ± SD) |
P† (n = 6) |
||
|---|---|---|---|
| Parameter* | Before | After | |
| Hemoglobin (g/dL) | 9.3 ± 0.5†† | 9.5 ± 0.5†† | nd |
| Platelets (103/µL) | 15.5 ± 10.3 | 28.2 ± 15.2 | nd |
| LDH (U/L) | 253 ± 119 | 267 ± 143 | 0.31 |
| Bilirubin, total (mg/dL) | 1.10 ± 0.47 | 1.25 ± 0.58 | 0.18 |
| Bilirubin, direct (mg/dL) | 0.33 ± 0.05 | 0.45 ± 0.18 | 0.15 |
Reference ranges are 13.7 g/dL – 17.5 g/dL for hemoglobin, 161,000/µL – 347,000/ µL for platelets, 113 U/L – 226 U/L for LDH, 0.1 mg/dL – 1.0 mg/dL for total bilirubin and 0.0 mg/dL – 0.2 mg/dL for direct bilirubin.
Wilcoxon matched-pairs signed-ranks W test (two tailed). P < 0.05 would be considered statistically significant.
n = 5, because 1 transfusion episode was excluded due to a oncomitant RBC unit transfusion.
nd – not done
Discussion
We implemented a practical strategy to reduce the risk of passive hemolysis caused by plateletpheresis products. Our initial choice of 1 in150 in buffered gel (IgM) as a threshold was based on data from our index series of patients and literature review. We felt universal screening that included group A platelets would be more effective than limiting screening to group O platelets. Our screening method using pooled A1 and B RBCs in one gel test can be applied uniformly to all donors regardless of ABO group, which may be unknown at the time of screening. As with all quality improvement initiatives, a screening program requires ongoing evaluation to assess program effectiveness and operational efficiency. In our case, the initial threshold classified 50% of group O platelets as high-titer. We progressively increased the threshold such that we are now using the dilution of 1 in 250, which classifies 25% of group O platelets as high-titer. This is a pragmatic decision to avoid earmarking half of our group O platelets as requiring special handling, and reflects the observations that most cases of platelet-mediated HTRs are associated with very high titers of ABO antibodies.1,2 Furthermore, the lookback review on patients who received components from donors who subsequently tested positive for high-titer ABO antibodies was reassuring.
Issues to consider for a universal platelet screening program include the serologic method (manual tube versus gel versus an automated platform), the choice of IgM or IgG titers, determining the threshold for a critical titer, performance of serial titrations versus testing a single pre-determined dilution, and interlaboratory variation in antibody titrations4,5. Reported donor anti-A titers implicated in HTRs range from 32–16,384 in saline (median 512), to 32–32,000 (median 4096) in the anti-globulin phase2. Many countries outside North America have adopted universal screening, although plateletpheresis components account for a minority of total platelet use in much of Europe: a critical IgM titer is 64–100 in tube or gel, and a critical IgG titer is 200–512.6,7 Screening for high-titer anti-A and/or anti-B in the United Kingdom using a single dilution cut-off of 1 in 100 by automated direct agglutination has not eliminated platelet-mediated HTR, and Scotland now uses a dilution cutoff of 1 in 50.8
Screening experience in the United States is currently limited to approximately 2% of institutions.2 In one report9, the median IgM titer was 32 in gel out of 100 group O apheresis donors, and 28% of samples were labeled as high-titer using 64 or greater as the cutoff. However, the plasma: RBC ratio used for buffered gel testing in this report appears to be lower (25 uL plasma to 50 uL RBCs) than recommended by the manufacturer. Cooling and colleagues10 tested 124 pools of group O platelets (each pool containing 5 platelet concentrates) using buffered gel technique and found the median anti-A titer to be 64 and the median anti-B titer to be 32. Our screening in buffered gel with the initial dilution of 1:150 classified 50% of our group O units as high-titer. This does not necessarily imply that the median titer is 150, since a single dilution does not always yield equivalent results to those obtained by serial two-fold titration testing.8
Strategies to reduce the risk of platelet-associated HTRs include volume-reduction of platelets, screening donor plasma for high-titer antibodies if the component is needed for an out-of-group patient, setting a maximum volume of incompatible plasma per defined time period, or screening donor plasma for high-titer antibodies at the donor center, prior to distribution. None of these methods is guaranteed to eliminate platelet-associated HTRs, nor is the use of platelet storage media.11 With our own hospital-based donor center, it was operationally easier and more efficient to screen all units prior to component release to general inventory. Screening a unit at assignment to an out-of-group recipient would delay component release for transfusion. The decision to screen all platelet donors regardless of historical information was based on the concern that some donors may develop higher titers over time, with pregnancies, immunizations, or ingestion of live bacteria such as those in certain yogurt products and probiotic formulations.3
The true incidence of HTR associated with platelet transfusion is unknown. Estimates range from 1:2500 to 1:9000.12,13 We observed an incidence of 1:2460 before we implemented screening. Our series includes three recipients from one donor; two of these cases were not recognized as HTRs at the time of transfusion. Data from the hemovigilance system in the United Kingdom suggest that platelets account for 20% (9/44) of acute HTRs overall,14 and 33% (3/9) of acute HTRs in children.15 Although most cases recover with appropriate supportive care, reactions can be fatal. Our own experience suggests that platelet-mediated HTRs may be under-recognized,3 because patients receiving platelet transfusions are frequently anemic at baseline, and symptoms and signs associated with hemolysis may be mild or atypical and not appreciated as such, particularly in outpatients.
Acknowledgments
The authors thank Minh T. Tran for her technical assistance in performing the validation assays. The current affiliations of K. Quillen and J. Daniel-Johnson are Boston University Medical Center (Boston, MA) and Columbia University Medical Center (New York, NY), respectively.
Footnotes
There are no conflicts of interest to disclose. The views expressed in this paper are those of the authors and are not to be construed as the official position of the United States Department of Health and Human Services.
Authors’ contribution. KQ, SLS and WAF collated and interpreted the data and KQ initiated the study. SLS and WAF completed the study. JADJ compiled and analyzed data. AHLS provided technical assistance with initial study design and performed serologic titration studies.
References
- 1.Cooling L. ABO and platelet transfusion therapy. Immunohematol. 2007;23:20–33. [PubMed] [Google Scholar]
- 2.Fung MK, Downes KA, Shulman I. Transfusion of platelets containing ABO-incompatible plasma. Arch Pathol Lab Med. 2007;131:909–916. doi: 10.5858/2007-131-909-TOPCAP. [DOI] [PubMed] [Google Scholar]
- 3.Daniel-Johnson J, Leitman S, Klein H, Alter H, Lee-Stroka A, Scheinberg P, Pantin J, Quillen K. Probiotic-associated high-titer anti-B in a group A platelet donor as a cause of severe hemolytic transfusion reactions. Transfusion. 2009;49:1845–1849. doi: 10.1111/j.1537-2995.2009.02208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Josephson CD, Castillejo M, Grima K, Hillyer CD. ABO-mismatched platelet transfusions: strategies to mitigate patient exposure to naturally occurring hemolytic antibodies. Transfus Apher Sci. 2010;42:83–88. doi: 10.1016/j.transci.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 5.AuBuchon JP, de Wildt-Eggen J, Dumont LJ for the BEST collaborative and the Transfusion Medicine Resource Committee of the CAP. Reducing the variation in performance of antibody titrations. Vox Sang. 2008;95:57–65. doi: 10.1111/j.1423-0410.2008.01043.x. [DOI] [PubMed] [Google Scholar]
- 6.Pietersz R, Engelfriet C, Reesink H. Transfusion of apheresis platelets and ABO groups. Vox Sang. 2005;88:207–221. doi: 10.1111/j.1423-0410.2005.00615.x. [DOI] [PubMed] [Google Scholar]
- 7.Novaretti MC, Gallucci AO, Dorlhiac-Llacer PE, Chamone DA. Rapid detection of high titer anti-A/anti-B in blood donors using an Olympus PK7200 equipment. Transfusion. 2009;49S:116A. (abstract) [Google Scholar]
- 8.Sadani DT, Urbaniak SJ, Bruce M, Tighe JE. Repeat ABO-incompatible platelet transfusions leading to hemolytic transfusion reaction. Transf Med. 2006;16:375–379. doi: 10.1111/j.1365-3148.2006.00684.x. [DOI] [PubMed] [Google Scholar]
- 9.Josephson CD, Mullis NC, Van Demark C, Hillyer CD. Significant numbers of apheresis-derived group O platelet units have “high-titer” anti-A/A,B: implications for transfusion policy. Transfusion. 2004;44:805–808. doi: 10.1111/j.1537-2995.2004.03290.x. [DOI] [PubMed] [Google Scholar]
- 10.Cooling L, Downs T, Butch S, Davenport R. Anti-A and anti-B titers in pooled group O platelets are comparable to apheresis platelets. Transfusion. 2008;48:2106–2113. doi: 10.1111/j.1537-2995.2008.01814.x. [DOI] [PubMed] [Google Scholar]
- 11.Valbonesi M, De Luigi MC, Lercari G, Florio G, Bruni R, Van Lint MT, Occhini D. Acute intravascular hemolysis in two patients transfused with dry-platelet units obtained from the same ABO incompatible donor. Int J Artif Organs. 2000;23:642–646. [PubMed] [Google Scholar]
- 12.Mair B, Benson K. Evaluation of changes in hemoglobin levels associated with ABO-incompatible plasma in apheresis platelets. Transfusion. 1998;38:51–55. doi: 10.1046/j.1537-2995.1998.38198141498.x. [DOI] [PubMed] [Google Scholar]
- 13.Larsson LG, Welsh VJ, Ladd DJ. Acute intravascular hemolysis secondary to out-of-group platelet transfusion. Transfusion. 2000;40:902–906. doi: 10.1046/j.1537-2995.2000.40080902.x. [DOI] [PubMed] [Google Scholar]
- 14.Stainsby D, Jones H, Asher D, Atterbury C, Boncinelli A, Brant L, Chapman CE, Davison K, Gerrard R, Gray A, Knowles S, Love EM, Milkins C, McClelland DBL, Norfolk DR, Soldan K, Taylor C, Revill J, Williamson LM, Cohen H for the SHOT Steering Group. Serious hazards of transfusion: a decade of hemovigilance in the UK. Transf Med Rev. 2006;20:273–282. doi: 10.1016/j.tmrv.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 15.Stainsby D, Jones H, Wells AW, Gibson B, Cohen H for the SHOT Steering Group. Adverse outcomes of blood transfusion in children: analysis of UK reports to the serious hazards of transfusion scheme 1996–2005. Brit J Haematol. 2008;141:73–79. doi: 10.1111/j.1365-2141.2008.07022.x. [DOI] [PubMed] [Google Scholar]