Abstract
The prevalence and antimicrobial susceptibilities of Campylobacter spp. isolates from bovine feces were compared between organic and conventional dairy herds. Thirty organic dairy herds, where antimicrobials are rarely used for calves and never used for cows, were compared with 30 neighboring conventional dairy farms, where antimicrobials were routinely used for animals for all ages. Fecal specimens from 10 cows and 10 calves on 120 farm visits yielded 332 Campylobacter isolates. The prevalence of Campylobacter spp. in organic and conventional farms was 26.7 and 29.1%, and the prevalence was not statistically different between the two types of farms. Campylobacter prevalence was significantly higher in March than in September, higher in calves than in cows, and higher in smaller farms than in large farms. The rates of retained placenta, pneumonia, mastitis, and abortion were associated with the proportion of Campylobacter isolation from fecal samples. The gradient disk diffusion MIC method (Etest) was used for testing susceptibility to four antimicrobial agents: ciprofloxacin, gentamicin, erythromycin, and tetracycline. Two isolates were resistant to ciprofloxacin, and none of isolates was resistant to gentamicin or erythromycin. Resistance to tetracycline was 45% (148 of 332 isolates). Tetracycline resistance was found more frequently in calves than in cows (P = 0.042), but no difference was observed between organic and conventional farms. When we used Campylobacter spp. as indicator bacteria, we saw no evidence that restriction of antimicrobial use on dairy farms was associated with prevalence of resistance to ciprofloxacin, gentamicin, erythromycin, and tetracycline.
Campylobacter spp. have been recognized as a cause of septic abortion, infectious infertility, and diarrhea in cattle and sheep (25). Abortions in cattle can be caused by Campylobacter fetus subsp. veneralis or C. fetus subsp. fetus; however, C. jejuni and C. coli are also recognized as causal agents of abortions (18, 36). C. hyointestinalis was reported as a cause of ileitis in pigs (12), bovine diarrhea (6), and human gastroenteritis (14). C. jejuni and C. coli can be found in the rumens and small intestines of normal calves and adult cattle, so that the bacteria are considered commensal in cattle (29).
Campylobacter was not recognized as a cause of human enteritis until the mid-1970s, when selective isolation media were developed for human stool culture. At present, campylobacteriosis is the most commonly reported human bacterial gastroenteritis in the United States, and the majority of infections are with C. jejuni (21). The incidence of laboratory-diagnosed campylobacteriosis was 15.7 per 100,000 person-years in FoodNet surveillance sites (5), and an estimated 2 to 2.4 million infections occur in the United States each year (9). Though antimicrobials are not essential for the treatment of most routine human cases of campylobacteriosis, severe or prolonged cases are usually treated with fluoroquinolone or erythromycin. Resistance to ciprofloxacin in human isolates of C. jejuni is reportedly increasing (1, 10, 28).
The majority of sporadic cases of Campylobacter infections are foodborne, and undercooked poultry is the most likely source of infections (9, 24). Contaminated water and unpasteurized milk are common sources of outbreaks; 9 percent of bulk tank milk was found to be culture positive for C. jejuni in a study of 131 dairy herds in South Dakota and Minnesota (16).
Critical control points are largely unknown for reducing preharvest Campylobacter prevalence. Most animal-specific factors (age, gender, breed, etc.) are not amenable to intervention. Herd-level management factors (bedding, sanitation, feeding, stocking rate, etc.) can often be changed, albeit sometimes only with considerable investment in labor and physical facilities. The influence on Campylobacter prevalence of the management factors that constitute “organic dairy production” has not heretofore been investigated.
Organic dairy milk production has been previously described (27). Organic farms in Wisconsin usually graze their cattle during the warm season and do not use hormones, herbicides, insecticides, or anthelmintics, and no antibiotics are permitted for 1 year before milk is marketed. This antibiotic restriction means that dairy calves may receive antibiotics, but antibiotic usage for calves is reportedly very low due to the overall management philosophy of these farmers. It is not known to what extent the management practices embodied in the organic approach may lead to a lower rate of antimicrobial resistance among Campylobacter isolates from cattle on these farms.
We used Campylobacter spp. as indicator bacteria to estimate the spread of antimicrobial resistance determinants in the farm environment and as a means to reveal and reflect the ecological effect of antimicrobial selective pressure on the farm microbial community. The objective of the study was to describe the prevalence and antimicrobial resistance patterns of Campylobacter spp. in healthy calves and cows in organic and conventional dairy farms in Wisconsin.
MATERIALS AND METHODS
Data and fecal sample collection.
Cattle fecal samples and management and production data were collected from 30 organic dairy farms and 30 conventional farms in Wisconsin. The organic farms were from an association of about 325 organic dairy farms. All organic farms were certified by an approved certification agency as not using antimicrobials for cows for at least 3 years (mean = 8.0 years) before the start of our study. For each organic farm selected, the nearest conventional dairy farmer (in sequence of geographical proximity) was asked to serve as a control farm. All herds were visited twice: once in March and once in September.
Management and production information was collected at the first visit by using an orally administered questionnaire. Questions and investigator observations regarded milk production, milking practices, housing, grazing, incidence of the major diseases, medical treatments, and other management factors. Also at each visit, environmental and animal sanitation was assessed with a subjective score for cow cleanliness and the amount of moisture and manure in the bedding and exercise areas, as previously described (3).
At each of the two visits, fecal specimens were collected from five lactating cows and five calves (under approximately 6 months of age). Animals were excluded if they had obvious diarrhea or were under treatment for another illness. Adult cows were sampled by walking among the cows and waiting for one to defecate. The fresh fecal sample was taken from the freshly voided fecal pile, taking care to not contact the ground beneath. Fecal samples were obtained from calves when they defecated following anal stimulation. A sterile latex glove was used for each specimen to avoid cross-contamination. Approximately 5 g of fecal sample was collected and placed in a Cary-Blair transport media tube (Medical Chemical Corp., Torrance, Calif.). The specimens were kept on ice and mailed to the Michigan Department of Community Health by overnight courier service for processing within 32 h from the time of sampling.
Bacteria isolation.
The fecal samples from the Cary-Blair tube were streaked directly on Campy blood agar (REMEL, Lenexa, Kans.). The inoculated plates were incubated under a microaerophilic atmosphere (Campy-Pak; BBL Microbiology Systems, Cockeysville, Md.) at 37°C for 48 h. One typical colony was selected and identified by testing by Gram stain, microscopic cell morphology, catalase production, oxidase production, and hippurate hydrolysis in accordance with the standard methods at the Michigan Department of Community Health (21).
Antimicrobial susceptibility testing.
Bacterial isolates were tested for resistance using gradient disk diffusion MIC to ciprofloxacin (0.002 to 32.0 μg/ml), erythromycin (0.016 to 256 μg/ml), gentamicin (0.016 to 256 μg/ml), and tetracycline (0.016 to 256 μg/ml) by Etest (AB Biodisk, Piscataway, N.J.). Sample bacteria were streaked from the frozen stock onto 5% sheep blood agar plates (REMEL) and incubated for 48 h at 37°C under a microaerophilic atmosphere. The colonies were restreaked to new sheep blood agar and incubated for another 24 h to allow recovery after being frozen. The subcultured colonies were examined for purity and emulsified in 4 ml of Mueller-Hinton broth, adjusting the turbidity to that of a 1.0 McFarland standard. The suspension was then inoculated evenly on 150-mm Mueller-Hinton agar plates supplemented with 5% defibrinated sheep blood (REMEL) by swabbing evenly in accordance with the Etest manufacture's instructions. Etest strips containing ciprofloxacin, gentamicin, erythromycin, and tetracycline were placed on the surface of agar plate in a radial pattern with the lowest concentration toward the center. The plates were incubated for 72 h at 37°C under the microaerophilic conditions, and the MICs were read directly from the test strip point where the growth inhibition zone intersected with the test strip, in accordance with the manufacturer's instruction. Quality control was performed daily using C. coli, ATCC 33559.
Since no breakpoints for the MIC for Campylobacter were defined by the NCCLS, our test results were dichotomized based on the breakpoints used by the National Antimicrobial Resistance Monitoring System: for ciprofloxacin, ≥4 μg/ml; for gentamicin, ≥16 μg/ml; for erythromycin, ≥8 μg/ml; and for tetracycline, ≥16 μg/ml (31).
Statistical analysis.
The prevalence of Campylobacter spp. in herds was analyzed using a generalized linear model with logit link function, based on the binomial distribution. The outcome variable was Campylobacter negative (0) or positive (1). Explanatory (independent) variables were farm type (organic or conventional), cow or calf, season, herd size (number of milking cows), purchase of animals during the past year (yes or no), grazing intensity during summer (no grazing, little grazing, or intensive grazing), abortion rate (per 100 cows/year), metritis rate, retained placenta rate (retained over 12 h after calving), calf population, calf mortality rate, and calf diarrhea rate. “Farm” was included as a random effect variable with an independent correlation matrix.
A regression model (generalized linear model with logit link function) was used to estimate the effect of farm type, animal age, and season on the prevalence of antimicrobial agent-resistant bacteria. The data were also analyzed using a proportional odds model with a generalized estimating equation. The proportional odds model with a generalized estimating equation provides a method for analyzing an ordinal-level repeated dependent variable and several categorical and continuous-level explanatory variables with fixed or random effects (30). Farm type, season, and animal age were included as fixed effects, and the farm was included as a random effect. All statistical analysis was performed using SAS statistical software (version 8.02; SAS Institute, Cary, N.C.).
RESULTS
The organic dairies had converted to organic farming methods at least 3 years before the initiation of our study (mean = 8.0 years). Organic farmers indicated that no antimicrobials were used for cows on their dairy farms, but four organic farmers reported using antimicrobials for calves if they had serious diarrhea or pneumonia. In 26 of the 30 conventional dairy herds, cows routinely received antimicrobial infusions into the udder at the cessation of each lactation cycle (“dry-cow treatment”). Cephapirin or penicillin was used most for this purpose. Eighteen conventional dairy producers reported using infusion of antimicrobials into the udder for the treatment of clinical mastitis. For severe cases of clinical mastitis, eight conventional dairy producers used systemic antimicrobials.
A total of 332 Campylobacter spp. isolates were obtained from 1,191 fecal specimens (27.9%). A total of 234 (70.5%) were identified as C. jejuni on the basis of the hippurate test (72.9% of the organic isolates and 68.2% of the conventional isolates). The rest of the isolates were not identified to the species level but were presumed to be primarily C. coli. No Campylobacter isolates were obtained from one conventional farm or from three organic farms; thus, 6.7% of farms were culture negative. On the 56 Campylobacter-positive farms, 5 to 70% of the collected specimens were culture positive. The prevalence was significantly higher in calves (32.7%) than in cows (23.2%) and significantly higher in March (36.8%) than in September (18.9%) (Table 1).
TABLE 1.
Number of Campylobacter isolates in each group
| Month | No. (%) of Campylobacter isolates
|
|||
|---|---|---|---|---|
| Conventional dairy farms (n = 30)
|
Organic dairy farms (n = 30)
|
|||
| Calf | Cow | Calf | Cow | |
| March | 56 (37.8) | 54 (36.0) | 65 (43.3) | 45 (30.0) |
| September | 42 (28.6) | 21 (14.0) | 30 (20.5) | 19 (12.7) |
There was no significant difference in Campylobacter prevalence between organic and conventional farms in the multivariate analysis (P = 0.5253) (Tables 2 and 3). Rates of retained placenta, pneumonia incidence rate, and abortion were positively associated with Campylobacter prevalence, whereas herd size (number of lactating cows and dry cows) and mastitis rate were negatively associated with Campylobacter prevalence (P < 0.05). The calf mortality was nearly significantly associated with the prevalence (P = 0.0511).
TABLE 2.
Odds ratios for Campylobacter spp. isolation (generalized linear model analysis)
| Risk factor | No. of specimens campylobacter positive/no. negative (%) | Odds | Odds ratio | 95% Confidence intervala | Type 3 GEE chi-square result (P value)b |
|---|---|---|---|---|---|
| Animal maturity | |||||
| Calf | 193/398 (48.5) | 0.485 | 1.635 | 1.180 < OR < 2.656 | 0.0031 |
| Cow | 139/461 (30.2) | 0.302 | 1 | ||
| Season | |||||
| March | 220/378 (58.2) | 0.582 | 2.524 | 1.748 < OR < 3.646 | <0.0001 |
| September | 112/481 (23.3) | 0.233 | 1 | ||
| Farm type | |||||
| Conventional | 173/422 (41.0) | 0.410 | 1.138 | 0.742 < OR < 1.743 | 0.5541 |
| Organic | 159/437 (36.4) | 0.368 | 1 |
OR, odds ratio.
GEE, generalized estimating equation.
TABLE 3.
Generalized linear model analysis of management factors for Campylobacter spp. prevalence
| Parameter | Estimate | P value |
|---|---|---|
| Season (March/September) | 1.0225 | <0.0001 |
| Retained placenta incidence rate | 0.0460 | <0.0001 |
| Herd size | −0.0234 | 0.0031 |
| Cow or calf (calf/cow) | 0.5304 | 0.0032 |
| Pneumonia incidence rate | 0.0266 | 0.0187 |
| Mastitis rate | −0.0131 | 0.0486 |
| Abortion rate | 0.0531 | 0.0437 |
| Calf mortality | 0.5909 | 0.0511 |
| Metritis rate | 0.0133 | 0.1532 |
| Open herd | 0.1691 | 0.4359 |
| Milk production per cow | 0.0001 | 0.5165 |
| Organic or conventional | 0.1166 | 0.5253 |
| Grazing with housing | −0.1697 | 0.5204 |
| No grazing (tie stall, free stall) | 0.1178 | 0.7051 |
| SCC | 0.0003 | 0.7693 |
| Cow mortality | −0.0062 | 0.8391 |
Only two isolates of Campylobacter spp. from geographically distant conventional dairy herds were resistant to ciprofloxacin (MICs of >32 and 24 μg/ml). For the other 330 isolates, MICs were between 0.012 and 0.25 μg/ml. None of the 332 isolates was resistant to gentamicin or erythromycin. The ranges of MICs were 0.047 to 2 μg/ml for gentamicin and 0.047 to 4 μg/ml for erythromycin (Table 4). A total of 148 isolates resistant to tetracycline were obtained (Table 4).
TABLE 4.
Proportion (%) of isolates which were inhibited by antimicrobials at each concentrationa
| Antimicrobial concn (μg/ml) | % of isolates inhibited by:
|
|||
|---|---|---|---|---|
| Ciprofloxacin (n = 332) | Gentamicin (n = 332) | Erythromycin (n = 332) | Tetracycline (n = 332) | |
| 0.012 | 0.3 | 3.6 | ||
| 0.016 | 1.8 | 2.7 | ||
| 0.023 | 6.9 | 7.8 | ||
| 0.032 | 28.6 | 19.6 | ||
| 0.047 | 29.2 | 0.6 | 0.3 | 11.4 |
| 0.064 | 22.9 | 0.9 | 0.9 | 4.5 |
| 0.094 | 6.0 | 7.5 | 1.8 | 1.2 |
| 0.125 | 2.4 | 17.2 | 7.2 | 1.5 |
| 0.19 | 0.9 | 18.1 | 24.7 | 0.6 |
| 0.25 | 0.3 | 28.0 | 23.2 | 0.6 |
| 0.38 | 13.3 | 14.5 | 0.9 | |
| 0.5 | 6.9 | 12.7 | 0.3 | |
| 0.75 | 3.9 | 6.6 | ||
| 1 | 0.9 | 3.3 | ||
| 1.5 | 1.5 | 1.8 | ||
| 2 | 1.2 | 1.8 | ||
| 3 | - - - - - - - - - | 0.9 | ||
| 4 | 0.3 | |||
| 6 | - - - - - - - - - | 0.3 | ||
| 8 | ||||
| 12 | - - - - - - - - | 0.3 | ||
| - - - - - - - - - | ||||
| 16 | 1.8 | |||
| 24 | 0.3 | 1.5 | ||
| 32 | 3.9 | |||
| 48 | 2.1 | |||
| 64 | 0.3 | 3.3 | ||
| 96 | 3.3 | |||
| 128 | 2.4 | |||
| 192 | 0.6 | |||
| 256 | 0.6 | |||
| >256 | 25.0 | |||
| Total | 100 | 100 | 100 | 100 |
The dashed lines indicate the NARMS breakpoints (Ciprofloxacin, ≥4 μg/ml; Gentamicin, ≥16 μg/ml; Erythromycin, ≥8 μg/ml; and Tetracycline, ≥16 μg/ml).
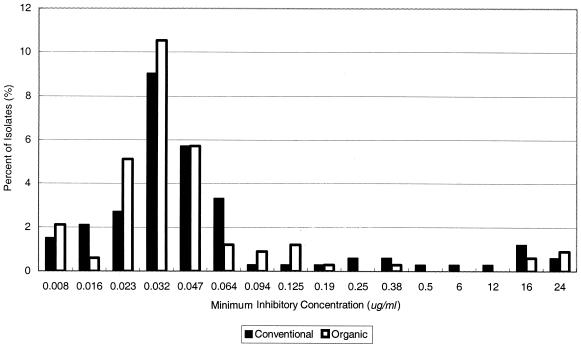
The analysis of the dichotomized tetracycline resistance data indicated a higher prevalence of resistant Campylobacter spp. among calf isolates than among cow isolates (P = 0.0419), with the estimated odds ratio (OR) of 1.81 (1.0221 < OR < 3.2059). Farm type (organic or conventional) and season of specimen collection were not significant predictors of tetracycline resistance (P = 0.4971 and 0.1729, respectively). The proportional odds model analysis using all antimicrobial dilution levels did not find a significant difference of MIC distributions for tetracycline between the two types of farm (Fig. 1). For ciprofloxacin, gentamicin, and erythromycin resistance, the proportional odds model found no significant effect on MIC distribution by farm type (organic or conventional), animal age, or season of specimen collection.
FIG. 1.
Comparison of tetracycline resistance: distribution of MICs of tetracycline for Campylobacter spp. from conventional and organic dairy farms. The proportional odds model analysis using all antimicrobial dilution levels did not find significant difference of MIC distributions for tetracycline between the two types of farm.
DISCUSSION
The estimation of Campylobacter spp. prevalence may be affected by factors such as location, season, use of transport medium, time before processing, use of enrichment media, and the use of various isolation methods (media, temperature, atmosphere, and time). The selection of farms in the present study was not random but rather constituted a cluster of organic herds and neighboring conventional herds in a particular region of Wisconsin. The fecal samples were collected from presumably healthy cows and calves, after having excluded animals with obvious diarrhea or that were under treatment for some other disease. This selection strategy may have resulted in lower measures of Campylobacter spp. prevalence in our study than in other studies, if cows with diarrhea are more likely to have been infected with Campylobacter spp. It has been reported that the Campylobacter spp. isolation rate was decreased approximately 16% by storing feces at 4°C for 24 h (17), and our samples took 24 to 36 h to be transported to the laboratory. However, the Cary-Blair transport medium with ice packs should have enabled Campylobacter spp. to maintain sufficient viability (19, 34, 35).
The enrichment techniques are beneficial for the detection of Campylobacter spp. when present at low concentrations. Perhaps our measured prevalence estimate would have been higher had we used an enrichment technique (4, 20). Nielsen (23) found 9 out of 77 positive samples were positive only after growth in enrichment broth. We used a Campy blood agar plate, which contains cephalothin, polymyxin B, vancomycin, trimethoprim, and amphotericin B. The culture medium is optimized for C. jejuni and C. coli but not for other Campylobacter spp. in cattle. C. jejuni subsp. doylei, C. fetus subsp. fetus, C. upsaliensis, and C. hyointestinalis are known to be inhibited by cephalothin (21). Though we used an incubation temperature of 37°C, other studies of Campylobacter spp. used an incubation temperature of 42°C to optimize the growth of thermophilic Campylobacter species, such as C. jejuni, C. coli, or C. lari, with the decreased ability to isolate nonthermophilic species (C. fetus and C. jejuni subsp. doylei). The incubation temperature of 37°C may have resulted in a lower prevalence of Campylobacter in our study. Atabay and Corry (2) used three kinds of media, an enrichment technique, a membrane filtration technique, and three different incubation temperatures. They found 62% overall prevalence in 136 cattle in three farms in the United Kingdom. The major species in their study were C. hyointestinalis (32%), C. sputorum bv. paraureolyticus (21%), C. fetus subsp. fetus (11%), and C. jejuni subsp. jejuni (7%). Giacoboni et al. (13) also found C. fetus subsp. fetus in 17% of cattle and C. hyointestinalis in 19% of cattle, whereas the dominant species was C. jejuni, found in 29% of 94 cattle in Japan. Our study design emphasized the isolation of C. jejuni and C. coli, which are species of public health importance.
A higher prevalence of Campylobacter spp. was found on dairy farms in March than was found in September, and the prevalence was higher for calves than for cows. These observations generally agreed with those of previous population-based studies (23, 37). The housing and grazing styles in our study were very different between organic and conventional dairy herds. Free stalls were used for nine conventional herds, in contrast to being used for only four organic herds. Half of the organic herds (15 farms) applied intensive grazing during summer, whereas only two conventional herds used intensive grazing. We saw no evidence that use of antimicrobials on dairy farms had any effect on Campylobacter spp. prevalence, since farm type was not significantly associated with prevalence after controlling for housing and grazing in the regression analysis.
Resistance to antimicrobials in Campylobacter spp.
It is known that C. jejuni and C. coli have different susceptibility profiles (11). However, resistance traits are known to be readily transferred among species of Campylobacter (32), so a separate analysis for each species of Campylobacter was inadvisable given the ecological nature of our objectives regarding the use of Campylobacter spp. as an indicator of antimicrobial selective pressure on the entire bacterial community.
Agar disk diffusion, broth dilution, agar dilution, and gradient disk diffusion (Etest) have commonly been used to determine Campylobacter susceptibilities in vitro. The agar dilution test was recently set by NCCLS as a reference standard susceptibility testing method for veterinary isolates of Campylobacter spp. (22); however, the agar dilution test is costly in both time and supplies and therefore is not ideal for most surveillance purposes. Ge et al. (11) reported that MICs measured with the Etest were generally lower than the results obtained with the agar dilution method. The agreement (±1 dilution range) of MICs between two test methods depended on the antimicrobials used: ciprofloxacin (85%), gentamicin (92.6%), erythromycin (65.6%), and tetracycline (57.7%). The Etest MIC results for the quality control strain (C. jejuni ATCC 33560) were consistently one to several dilutions lower than the corresponding agar dilution results. Huang et al. (15) also compared the Etest method with the agar dilution method and reported slightly lower MICs with the Etest than with the agar dilution. The percent agreement (±1 dilution range) were 90.4% for ciprofloxacin, 83.0% for gentamicin, 94.1% for erythromycin, and 77.5% for tetracycline. In our study, any bias due to the testing procedure should not have affected our comparison between organic and conventional farms. Any such systematic error or bias would have been a nondifferential misclassification bias that would have equally affected the organic and conventional farms (26). However, direct comparisons of MICs obtained from different methods should be interpreted with caution.
We isolated two ciprofloxacin-resistant Campylobacter spp. from conventional dairy farms. Fluoroquinolone is not commonly used in the dairy industry. Sarafloxacin was approved in the United States for poultry in 1995, but the approval was withdrawn in 2001 (8). The Center for Veterinary Medicine of the Food and Drug Administration proposed to withdraw approval of enrofloxacin for poultry use because of the possible transfer of fluoroquinolone-resistant Campylobacter spp. from poultry to humans (7). Though the use of enrofloxacin in beef cattle is approved for treatment of bovine respiratory disease, the extralabel use of any fluoroquinolones on dairy cattle has been clearly prohibited by the Food and Drug Administration. The resistance to fluoroquinolone is rendered by (i) decreased permeability of the bacterial cell wall; (ii) increased efflux pump activity; and (iii) mutation of the DNA gyrase. Thus, the decreased permeability and/or the increased efflux pump can also confer resistance to other antimicrobial agents, such as tetracycline (33). Since our ciprofloxacin-resistant Campylobacter spp. were not resistant to the other three antimicrobials, it is speculated that the resistant isolates arose by point mutation.
Conclusions.
The prevalence of Campylobacter spp. was not significantly different between organic and conventional dairy farms in Wisconsin. Campylobacter prevalence was significantly higher in March than in September, higher in calves than in cows, and higher on smaller farms than on larger farms. Rates of retained placenta, pneumonia, and abortion were positively associated with the Campylobacter spp. prevalence. The proportion of tetracycline-resistant Campylobacter spp. was higher in isolates derived from calves. The prevalence of resistance to ciprofloxacin, gentamicin and erythromycin was very low. We saw no evidence that restricted antimicrobial use on dairy farm had any association with antimicrobial resistance to ciprofloxacin, gentamicin, erythromycin, and tetracycline in Campylobacter spp.
Acknowledgments
We gratefully acknowledge the participation of dairy producers in the study. We greatly appreciate that the Michigan Department of Community Health provided laboratory space, supplies, and technical advice.
This study was made possible by grants from FDA/CDC (grant no. US1/ccu516219 03 2 CCUS1).
REFERENCES
- 1.Allos, B. 2001. Campylobacter jejuni infections: update on emerging issues and trends. Clin. Infect. Dis. 32:1201-1206. [DOI] [PubMed] [Google Scholar]
- 2.Atabay, H. I., and J. E. L. Corry. 1998. The isolation and prevalence of campylobacters from dairy cattle using a variety of methods. J. Appl. Microbiol. 84:733-740. [DOI] [PubMed] [Google Scholar]
- 3.Bartlett, P. C., G. Y. Miller, S. E. Lance, and L. E. Heider. 1992. Managerial risk factors of intramammary infection with Streptococcus agalactiae in dairy herds in Ohio. Am. J. Vet. Res. 53:1715-1721. [PubMed] [Google Scholar]
- 4.Bolton, F. J., and L. Robertson. 1982. A selective medium for isolating Campylobacter jejuni/coli. J. Clin. Pathol. 35:462-467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. 2001. Preliminary FoodNet data on the incidence of foodborne illnesses—selected sites, United States. Morb. Mortal. Wkly. Rep. 50:241-246. [PubMed] [Google Scholar]
- 6.Diker, K. S., S. Diker, and M. B. Ozlem. 1990. Bovine diarrhea associated with Campylobacter hyointestinalis. J. Vet. Med. B 37:158-160. [DOI] [PubMed] [Google Scholar]
- 7.Federal Register. 2000. Enrofloxacin for poultry: opportunity for hearing. Fed. Regist. 65:64954-64965. [Google Scholar]
- 8.Federal Register. 2001. Animal drugs, feeds, and related products; sarafloxacin for poultry; withdrawal of approval of NADAs. Fed. Regist. 66:21282. [Google Scholar]
- 9.Friedman, C. R., J. Neimann, H. C. Wegener, and R. V. Tauxe. 2000. Epidemiology of Campylobacter jejuni infections in the United States and other industrialized nations, p. 121-138. In I. Nachamkin and M. J. Blaser (ed.), Campylobacter, 2nd ed. ASM Press, Washington, D.C.
- 10.Gaudreau, C., and H. Gilbert. 1998. Antimicrobial resistance of clinical strains of Campylobacter jejuni subsp. jejuni isolated from 1985 to 1997 in Quebec, Canada. Antimicrob. Agents Chemother. 42:2106-2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ge, B., S. Bodeis, R. D. Walker, D. G. White, S. Zhao, and P. F. McDermott. 2002. Comparison of the Etest and agar dilution for in vitro antimicrobial susceptibility testing of Campylobacter. J. Antimicrob. Chemother. 50:487-494. [DOI] [PubMed] [Google Scholar]
- 12.Gebhart, C. J., G. E. Ward, K. Chang, and H. J. Kurtz. 1983. Campylobacter hyointestinalis (new species) isolated from swine with lesions of proliferative ileitis. Am. J. Vet. Res. 44:361-367. [PubMed] [Google Scholar]
- 13.Giacoboni, G. I., K. Itoh, K. Hirayama, E. Takahashi, and T. Mitsuoka. 1993. Comparison of fecal Campylobacter in calves and cattle of different ages and areas in Japan. J. Vet. Med. Sci. 55:555-559. [DOI] [PubMed] [Google Scholar]
- 14.Gorkiewicz, G., G. Feierl, R. Zechner, and E. L. Zechner. 2002. Transmission of Campylobacter hyointestinalis from a pig to a human. J. Clin. Microbiol. 40:2601-2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang, M. B., C. N. Baker, S. Banerjee, and F. C. Tenover. 1992. Accuracy of the E test for determining antimicrobial susceptibilities of Staphylococci, Enterococci, Campylobacter jejuni, and gram-negative bacteria resistant to antimicrobial agents. J. Clin. Microbiol. 30:3243-3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jayarao, B. M., and D. R. Henning. 2001., Prevalence of foodborne pathogens in bulk tank milk. J. Dairy Sci. 84:2157-2162. [DOI] [PubMed] [Google Scholar]
- 17.Ladron de Guevara, C., M. T. Perez-Pomata, A. Aguila, F. J. Merino, P. A. Villasante, and A. C. Velasco. 1989. Recovery of campylobacter from human faeces stored at 4°C. Epidemiol. Infect. 102:281-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Larson, D. J., I. V. Wesley, and L. J. Hoffman. 1992. Use of oligodeoxynucleotide probes to verify Campylobacter jejuni as a cause of bovine abortion. J. Vet. Diagn. Investig. 4:348-351. [DOI] [PubMed] [Google Scholar]
- 19.Luechtefeld, N. W., W. L. L. Wang, M. J. Blaser, and L. B. Reller. 1981. Evaluation of transport and storage techniques for isolation of Campylobacter fetus subsp. jejuni from turkey cecal specimens. J. Clin. Microbiol. 13:438-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martin, W. T., C. M. Patton, G. K. Morris, M. E. Potter, and N. D. Puhr. 1983. Selective enrichment broth for isolation of Campylobacter jejuni. J. Clin. Microbiol. 17:853-855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nachamkin, I. 1999. Campylobacter and Arcobacter, p. 716-726. In P. R. Murray, E. J. Baron, M. A. Pfaller, F. C. Tenover, and R. H. Yolken (ed.), Manual of clinical microbiology, 7th ed. ASM Press, Washington, D.C.
- 22.NCCLS. 2002. Performance standards for antimicrobial disk and dilution susceptibility test for bacteria isolated from animals; approved standard, 2nd ed. NCCLS document M31-A2. NCCLS, Wayne, Pa.
- 23.Nielsen, E. M. 2002. Occurrence and strain diversity of thermophilic campylobacters in cattle of different age groups in dairy herds. Lett. Appl. Microbiol. 35:85-89. [DOI] [PubMed] [Google Scholar]
- 24.Pearson, A. D., M. H. Greenwood, J. Donaldson, T. D. Healing, D. M. Jones, M. Shahamat, R. K. A. Feltham, and R. R. Golwell. 2000. Continuous source outbreak of campylobacteriosis traced to chicken. J. Food Prot. 63:309-314. [DOI] [PubMed] [Google Scholar]
- 25.Radostits, O. M., C. C. Gay, D. C. Blood, K. W. Hinchcliff, and J. H. Arundel. 2000. Diseases caused by bacteria, p. 967. In Veterinary medicine, 9th ed. The W. B. Saunders Co., Philadelphia, Pa.
- 26.Rothman K. J., and S. Greenland. 1998. Nondifferential misclassification, p. 127-132. In Modern epidemiology. Lippincott-Raven Publishers, Philadelphia, Pa.
- 27.Sato, K., P. C. Bartlett, P. Ruegg, J. B. Kaneene, B. Robinson-Dunn, F. P. Downes, and R. J. Erskine. 2002. Milk production and mastitis on Midwestern organic dairy farms. Dairy Food Environ. Sanit. 22:178-183. [Google Scholar]
- 28.Smith, K. E., J. M. Besser, G. W. Hedberg, F. T. Leano, J. B. Bender, J. H. Wicklund, B. P. Johnson, K. A. Moore, M. T. Osterholm, and the Investigation Team. 1999. Quinolone-resistant Campylobacter jejuni infections in Minnesota, 1992-1998. N. Engl. J. Med. 340:1525-1532. [DOI] [PubMed] [Google Scholar]
- 29.Stanley, K. N., J. S. Wallace, J. E. Currie, P. J. Diggle, and K. Jones. 1998. The seasonal variation of thermophilic campylobacters in beef cattle, dairy cattle and calves. J. Appl. Microbiol. 85:472-480. [DOI] [PubMed] [Google Scholar]
- 30.Stokes, M. E., C. S. Davis, and G. G. Koch. 2000. Logistic regression II: polytomous response, p. 243-270. In Categorical data analysis using the SAS system. SAS Institute, Inc., Cary, N.C.
- 31.U.S. Department of Agriculture. Accessed on 3 March 2003. National Antimicrobial Resistance Monitoring System (NARMS), Annual veterinary isolates data, EB 2000, Campylobacter jejuni-2000. [Online.] http://www.arru.saa.ars.usda.gov/narms.htm.
- 32.Velazquez, J., A. Jimenez, B. Chomon, and T. Villa. 1995. Incidence and transmission of antibiotic resistance in Campylobacter jejuni and Campylobacter coli. J. Antimicrob Chemother. 35:173-178. [DOI] [PubMed] [Google Scholar]
- 33.Walker, R. D. 2000. Fluoroquinolones, p. 315-338. In J. F. Prescott, J. D. Baggot, and R. D. Walker (ed.), Antimicrobial therapy in veterinary medicine, 3rd ed. Iowa State University Press, Ames, Iowa.
- 34.Wang, W.-L. L., L. B. Reller, B. Smallwood, N. W. Luechtefeld, and M. J. Blaser. 1983. Evaluation of transport media for Campylobacter jejuni in human fecal specimens. J. Clin. Microbiol. 18:803-807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wasfy, M., B. Oyofo, A. Elgindy, and A. Churilla. 1995. Comparison of preservation media for storage of stool samples. J. Clin. Microbiol. 33:2176-2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Welsh, R. D. 1984. Campylobacter jejuni abortion in a heifer. J. Am. Vet. Med. Assoc. 185:549-551. [PubMed] [Google Scholar]
- 37.Wesley, I. V., S. J. Wells, K. M. Harmon, A. Green, L. Schroeder-Tucker, M. Glover, and I. Siddique. 2000. Fecal shedding of Campylobacter and Arcobacter spp. in dairy cattle. Appl. Environ. Microbiol. 66:1994-2000. [DOI] [PMC free article] [PubMed] [Google Scholar]