Abstract
The putative primase gene and other genes associated with the Sfi21-prototype genome replication module are highly conserved in Streptococcus thermophilus bacteriophages. Expression of antisense RNAs complementary to the putative primase gene (pri3.1) from S. thermophilus phage κ3 provided significant protection from κ3 and two other Sfi21-type phages. Expression of pri3.10-AS, an antisense RNA that covered the entire primase gene, reduced the efficiency of plaquing (EOP) of κ3 to 3 × 10−3 and reduced its burst size by 20%. Mutant phages capable of overcoming antisense inhibition were not recovered. Thirteen primase-specific antisense cassettes of different lengths (478 to 1,512 bp) were systematically designed to target various regions of the gene. Each cassette conferred some effect, reducing the EOP to between 0.8 and 3 × 10−3. The largest antisense RNAs (1.5 kb) were generally found to confer the greatest reductions in EOP, but shorter (0.5 kb) antisense RNAs were also effective, especially when directed to the 5′ region of the gene. The impacts of primase-targeted antisense RNAs on phage development were examined. The expression of pri3.10-AS resulted in reductions in target RNA abundance and the number of phage genomes synthesized. Targeting a key genome replication function with antisense RNA provided effective phage protection in S. thermophilus.
Strains of lactic acid bacteria are used in starter cultures or culture adjuncts during the manufacture of a variety of fermented dairy products. Phage contamination during product manufacture can result in significant loss of starter culture activity and remains the leading cause of failed batch fermentations. These losses are particularly severe when highly specialized strains, which are themselves a valuable product of scientific discovery and product development, become susceptible to phage attack. In this case, costs committed for strain development will not be recovered if the expected lifetime of a new, highly specialized strain is diminished by the appearance of lytic phages capable of attacking it. The crux of the problem is that the dairy environment and fermentation substrate provide a continuous reservoir for the influx of new virulent phages (7, 25), while existing phages adapt by mutation and recombination (4, 13). Together, these events enable the appearance of subpopulations of phages capable of subverting previously resistant cultures and necessitate the development of strains of lactic acid bacteria with enhanced phage resistance properties.
Novel and more efficacious phage defense strategies continue to be developed, including the expression of antisense RNA targeted against phage-encoded transcripts. These antisense RNAs have been constitutively expressed by starter strains (reviewed in references 2, 24, 32, and 34) or triggered in response to phage infection through the use of phage-encoded promoters and/or origins of replication (32, 34). Regardless of the delivery strategy employed, antisense RNAs act to interfere with phage development by promoting the degradation of mRNA transcripts or inhibiting the translation of phage-encoded genes necessary for normal development, albeit at markedly variable and often poor efficiencies (18).
Currently, 6 Streptococcus thermophilus-, 11 Lactococcus-, and 3 Lactobacillus-specific phage genomes have been sequenced completely and subjected to extensive comparative genomic analyses (5, 10), especially between S. thermophilus phages (23). The developmental pathways encoded by these genomes reveal susceptibilities to engineered phage defense systems, including antisense RNA. When used in conjunction with comparative hybridization studies, these analyses enable the elimination of poorly conserved targets in silico, while facilitating the identification of well-conserved targets present in a wide variety of phages (32). This advantage is of great importance for industrial applications, where defense strategies ideally confer resistance against broad groups of related phages.
Among S. thermophilus phages, genome replication functions are catalyzed by two distinct but likely interchangeable clusters of nonorthologous genes, which are exemplified by the phage Sfi21- and 7201-derived prototype modules (23). For several reasons, the genes associated with the Sfi21-type genome replication module were found to be among the best-conserved targets for the expression of phage-inhibitory antisense RNAs (32). First, gross comparisons revealed that six of the seven sequenced phages encoded variants of the Sfi21-type module, while only the remaining phage, 7201, did not. Second, hybridization studies against unsequenced phages have demonstrated that variants of the Sfi21-type module are found in the majority of problematic industrial isolates, suggesting that this module may confer a competitive advantage over phages encoding the 7201-type module (6, 32). Finally, fine-scale comparisons between Sfi21-type module-containing phages revealed that nucleotide sequence similarity dropped off sharply outside the module's boundaries, while the replication modules themselves shared striking sequence conservation, exhibiting greater than 99.9% sequence similarity between variants (5). The Sfi21-type replication module is comprised of a single origin of DNA replication (ori) and several open reading frames that encode a putative primase, a putative helicase, and a number of other proteins of undetermined function (5).
In this study, the putative primase gene, which is a component of the Sfi21-type genome replication module encoded by phage κ3 (pri3.1), was targeted for antisense RNA-mediated gene silencing in S. thermophilus. Regions responsible for antisense efficacy were determined through the characterization of a variety of constructs engineered to constitutively express antisense RNA complementary to various structural or putative regulatory regions of pri3.1. The effects of primase-targeted antisense RNA on phage development were also examined.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The bacterial strains used in this study are listed in Table 1. Unless otherwise indicated, bacteria were propagated as described previously (32).
TABLE 1.
Bacterial strains, bacteriophages and plasmids
| Strain, bacteriophage, or plasmid | Relevant characteristic(s)a | Source or reference |
|---|---|---|
| Streptococcus thermophilus | ||
| NCK1125 | Industrial isolate; sensitive to phages κ3, κ5, κ6, κ9; Cms | 32 |
| SMQ495 | Industrial isolate; sensitive to phage DT1; Cms | 33 |
| Escherichia coli MC1061 | Transformation host | 17 |
| Lactococcus lactis NCK203 | Sensitive to phage φ31 | 30 |
| Bacteriophage | ||
| κ3 | Encodes Sfi21-type replication module; cos-type encapsidation module | 32 |
| κ5 | Encodes Sfi21-type replication module; cos-type encapsidation module | 32 |
| κ6 | Encodes 7201-type replication module; pac-type encapsidation module | 32 |
| κ9 | Encodes Sfi21-type replication module; cos-type encapsidation module | 32 |
| DT1 | Encodes Sfi21-type replication module; cos-type encapsidation module | 33 |
| φ31 | P335-type lactococcal phage | 1 |
| Plasmid | ||
| pTRK686 | 2.4 kb; deletion derivative of pNZ123; Cmr | 32 |
| pTRK687 | 3.0 kb; pTRK686 containing the P6 promoter in α-orientation | 32 |
| pTRK696 | 3.0 kb; pTRK686 containing the P6 promoter in β-orientation | This study |
| pTRK787::pri3.4-AS | 3.6 kb; pTRK687 containing the 587-bp pri3.4 antisense cassette | This study |
| pTRK788::pri3.7-AS | 4.0 kb; pTRK687 containing the 1,023-bp pri3.7 antisense cassette | This study |
| pTRK789::pri3.8-AS | 3.5 kb; pTRK687 containing the 519-bp pri3.8 antisense cassette | This study |
| pTRK790::pri3.10-AS | 4.5 kb; pTRK687 containing the 1,512-bp pri3.10 antisense cassette | This study |
| pTRK791::pri3.11-AS | 4.0 kb; pTRK687 containing the 1,008-bp pri3.11 antisense cassette | This study |
| pTRK792::pri3.12-AS | 3.5 kb; pTRK687 containing the 504-bp pri3.12 antisense cassette | This study |
| pTRK793::pri3.13-AS | 4.5 kb; pTRK687 containing the 1,506-bp pri3.13 antisense cassette | This study |
| pTRK794::pri3.14-AS | 4.5 kb; pTRK687 containing the 1,486-bp pri3.14 antisense cassette | This study |
| pTRK795::pri3.15-AS | 4.0 kb; pTRK687 containing the 982-bp pri3.15 antisense cassette | This study |
| pTRK796::pri3.16-AS | 3.5 kb; pTRK687 containing the 487-bp pri3.16 antisense cassette | This study |
| pTRK797::pri3.18-AS | 4.0 kb; pTRK687 containing the 1,008-bp pri3.18 antisense cassette | This study |
| pTRK798::pri3.19-AS | 3.5 kb; pTRK687 containing the 504-bp pri3.19 antisense cassette | This study |
| pTRK799::pri3.21-AS | 3.5 kb; pTRK687 containing the 504-bp pri3.21 antisense cassette | This study |
| pTRK800::pri3.12-AS | 3.5 kb; pTRK696 containing the 504-bp pri3.12 antisense cassette | This study |
Cmr, encodes chloramphenicol resistance; Cms, sensitive to chloramphenicol.
Bacteriophages and propagation assays.
The phages used in this study are listed in Table 1. Bacteriophages were propagated as described previously (32). The efficiency of plaquing (EOP) was calculated by dividing the plaque-forming units per milliliter (PFU/ml) on the test strain by the PFU/ml on the control strain, S. thermophilus NCK1125, harboring the base vector used for antisense RNA constructs. The diameters of phage plaques were measured by using a caliper, and values represent the averages of the measurements of 60 random plaques chosen over three independent experiments. Center-of-infection assays were performed as described previously, and the efficiency of center of infection formation (ECOI) was calculated by dividing the number of infective centers on the test strain by the number of infective centers on NCK1125(pTRK687) (31). Adsorption assays were performed as described previously, except that Elliker-BC medium was used (29). Lysis-in-broth assays were performed in Elliker-BC medium in the presence or absence of individual phage isolates at a multiplicity of infection (MOI) of 0.1% ± 20%, as described previously (32). Burst size determinations were performed at 42°C in Elliker-BC medium but otherwise were performed as described previously (21). Lactococcus lactis phages were propagated and enumerated as described previously (13).
PCR and DNA sequencing.
PCR and DNA sequencing and sequence analyses were all performed as described previously (32). When appropriate, restriction endonuclease recognition sites were incorporated into the 5′ ends of oligonucleotide primers to facilitate the cloning of PCR products. The primers used in this study are listed in Table 2.
TABLE 2.
Primers used in this study
| Primera | Nucleic acid sequenceb | Positionc |
|---|---|---|
| S1 | 5′-AAACTGCAGCAGAGAACAATTTGCAAGC-3′ | 28,575 |
| S2 | 5′-AAACTGCAGCAACACCCAAGAGCC-3′ | 28,466 |
| S3 | 5′-AAACTGCAGTAAGGAGGATTGGACTTGAC-3′ | 28,534 |
| S4 | 5′-AAACTGCAGTTGACAACGATTGATTTCG-3′ | 28,549 |
| S5 | 5′-AAACTGCAGATTAATTTTAGTACCATTG-3′ | 29,053 |
| S6 | 5′-AAACTGCAGGGTACATATCGACGTATCG-3′ | 29,557 |
| S7 | 5′-AAACTGCAGTAGCTATATATGATCCAG-3′ | 28,787 |
| S8 | 5′-CCCAAGAGCCTTTGGGCAATAAGG-3′ | 28,471 |
| A1 | 5′-AAACTGCAGGTTGCAATAACCTGCGG-3′ | 30,080 |
| A2 | 5′-AAACTGCAGGTGAGTAACCATAACCAC-3′ | 30,060 |
| A3 | 5′-AAACTGCAGAAACTTATGGTCAAACGATAG-3′ | 29,052 |
| A4 | 5′-AAACTGCAGGTTTGACTTATTCTTAAACAC-3′ | 29,556 |
| A5 | 5′-AAACTGCAGCTTTCCCATTTTCGAGGG-3′ | 28,786 |
| A6 | 5′-CTAAGTAACTAAAGCAACCGAACCC-3′ | 30,135 |
| P6 | 5′-GGAGCGTGATTTTTATGG-3′ | —d |
| T7 | 5′-GCTTCCGGCTCGTATGTTGTGTGG-3′ | — |
S and A primers are derived from the sense and antisense strands, respectively.
PstI (5′-CTGCAG-3′) restriction sites are underlined when appropriate.
The 5′ nucleotide position relative to the DT1 sequence is shown in bold when appropriate.
—, not applicable.
Plasmid construction and bacterial transformation.
The plasmids used in this study are listed in Table 1. Unless otherwise indicated, antisense RNA expression vectors were constructed from pTRK687 (Fig. 1). The orientation of cloned PCR products was confirmed by restriction digestion and PCR amplification by using the primer P6 with either of the two primers used to amplify the PCR fragment (Table 2). Electrocompetent Escherichia coli (28), L. lactis (16), and S. thermophilus were prepared and electroporated as described previously (32).
FIG. 1.
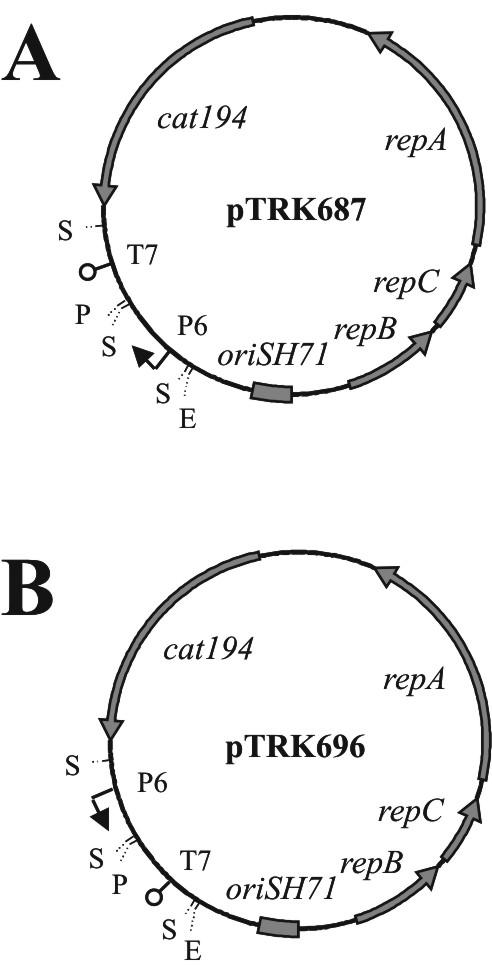
The antisense RNA expression vectors used in this study that contain the P6 promoter cassette cloned in opposite orientations relative to the cat194 gene. (A) pTRK687; (B) pTRK696. Abbreviations: T7, coliphage T7 transcription terminator; P6, L. acidophilus P6 promoter; repBCA, genes encoding plasmid replication factors; cat194, chloramphenicol resistance gene; oriSH71, plasmid origin of DNA replication; pri3.1, phage κ3-derived putative primase. Restriction endonuclease recognition sites: E, EcoRI; S, Sau3AI; P, PstI.
Plasmid, phage, and genomic DNA preparations.
Plasmid DNAs were isolated from E. coli (28) and S. thermophilus (27) as described elsewhere. Phage genomic DNA was isolated from cells infected with phage κ3 at a MOI of 1% ± 20% or from uninfected control cultures as described previously by using the Lambda kit (QIAGEN) (14). DNAs were purified after PCR and extracted from agarose gels as described previously (32).
RNA isolation and RNA-RNA slot blot hybridizations.
Using the Lign'Scribe promoter addition kit (Ambion, Austin, Tex.), DNA adapters containing coliphage T7 promoters were ligated to a 504-bp pri3.12 PCR fragment amplified from the 5′ region of the putative primase gene of phage κ3 by using primers S4 and A3 (Fig. 2; Table 2). Double-stranded DNA templates used during downstream in vitro transcription reactions were generated by PCR by using the primer T7, which was specific to the T7 promoter adapter, and either primer S4 or A3. The T7-S4 and T7-A3 templates were used to generate probes detect-S and detect-AS, which detected the phage-encoded sense mRNA and plasmid-encoded antisense RNA, respectively. In vitro transcription was carried out in the presence of [α-32P]UTP (NEN, Boston, Mass.) by using the high-yield MEGAscript transcription kit (Ambion). Radiolabeled RNA probes were purified by using NucTrap probe purification columns (Stratagene, La Jolla, Calif.). Total RNAs were isolated (i) from cultures 10, 20, 30, and 60 min after infection with phage κ3 at a MOI of 1% ± 20% and (ii) from uninfected control cultures grown in parallel. RNAs were isolated by using the TRIzol reagent (GIBCO-BRL) as described previously (11). RNA-RNA slot hybridizations were performed by using the Bio-Dot-SF apparatus and Zeta-probe membranes (Bio-Rad Laboratories, Richmond, Calif.) according to the manufacturer's instructions.
FIG. 2.
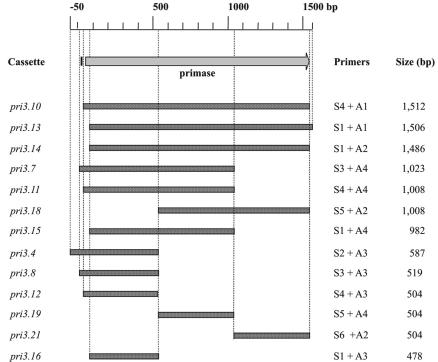
Schematic of the pri3.1 gene (arrow) and upstream putative ribosome binding site (small vertical black bar). The boundaries of the subcloned fragments are shown as bars demarcated with dotted lines. Base pair (bp) coordinates are numbered relative to the 5′ position of the primase translation initiation codon.
Nucleotide sequence accession numbers.
The DNA and deduced protein sequences for phage DT1 (33) and the κ3-derived putative primase have been submitted to the GenBank database under the nucleotide accession numbers AF085222 and AY196178, respectively.
RESULTS
Base RNA expression vectors.
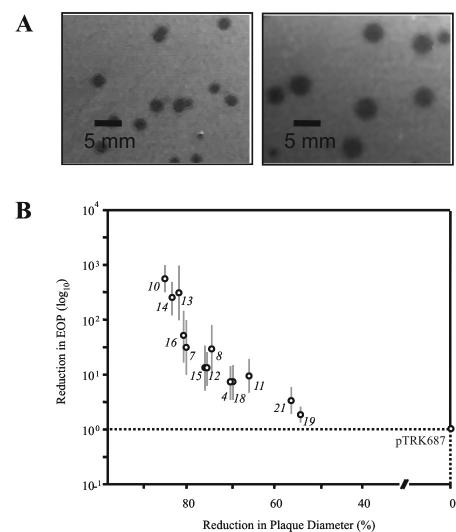
When present in S. thermophilus, the base vector pTRK687 resulted in an increased plaque size but did not impact the EOP of any of the phages tested, as observed previously (32) (Fig. 3A). In contrast, the presence of pTRK696 (Fig. 1) resulted in a 20% reduction in EOP without altering plaque size. Due to the artificial reduction in phage EOP associated with pTRK696 replication, plasmid pTRK687 was used as the basal antisense RNA expression vector in this study unless indicated otherwise. As a result, all EOP and plaque size comparisons were made relative to the strain NCK1125(pTRK687).
FIG. 3.
(A) Plaque size of phage κ3 when titered on NCK1125 (left) and NCK1125(pTRK687) (right). (B) Comparison of primase-derived antisense RNA constructs on the EOP and plaque size of phage κ3 relative to that of the plasmid control, pTRK687. Horizontal and vertical dotted lines represent an EOP of 1.0 and a plaque size of 4 mm, respectively. The error bars for EOP were calculated from the results of three independent experiments.
Amplification and sequence characteristics of the primase gene.
Using a consensus sequence generated from the alignment of the Sfi21-type genome replication modules from six S. thermophilus phages (32), primers S8 and A6 were used to amplify primase-containing fragments from the S. thermophilus phages listed in Table 1. The expected 1.7-kb fragment, which spanned the entire consensus primase open reading frame, was successfully amplified from the positive control, phage DT1, and other Sfi21-type phages, including κ3, κ4, and κ9 (data not shown). The 1.7-kb fragment was not generated from the negative control, phage κ6, which encodes a variant of the 7201-type genome replication module.
The 1.7-kb PCR fragment amplified from phage κ3 was sequenced and revealed a single open reading frame of 1,515 bp (GenBank accession number AY196178). This open reading frame, designated pri3.1, begins with a 5′-TTG-3′ translation initiation codon, ends with a 5′-TAA-3′ stop codon, and is preceded by a putative ribosome binding site (RBS) (5′-AGGAGG-3′). The deduced Pri3.1 protein is 504 amino acids long and has a predicted molecular mass of 59.0 kDa. A BlastP (3) search for conserved domains detected a conserved Parvo_NS1 domain (pfam01057) within the Pri3.1 primary amino acid sequence. Both DNA helicase and ATPase activities are associated with this domain, which is required for genome replication in double-stranded DNA parvoviruses (26, 35).
Primase-targeted antisense RNA expression.
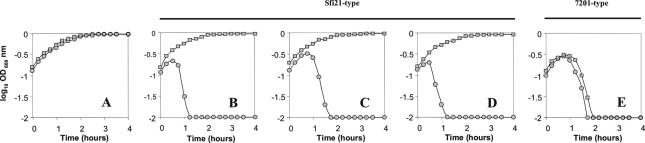
The 1.5-kb fragment designated pri3.10 (Fig. 2) was amplified by PCR from phage κ3 by using primers S4 and A1, digested with PstI, and cloned in the antisense (-AS) orientation into the PstI site of pTRK687, which is downstream from the Lactobacillus acidophilus P6 promoter (12). The resulting vector, pTRK790::pri3.10-AS, was electroporated into NCK1125 and tested for its ability to impede phage replication. The growth of the pri3.10-AS-expressing strain and the parental strain, NCK1125(pTRK687), was evaluated over time in the presence and absence of Sfi21-type phages κ3, κ4, and κ9 and 7201-type phage κ6 at a MOI of 0.1% ± 20% (Fig. 4). The control strain NCK1125(pTRK687) was lysed within 120 min by all phages tested. The pri3.10-AS-expressing strain was similarly lysed by 7201-type phage κ6 but grew nearly unimpeded when challenged with Sfi21-type phages κ3, κ4, and κ9.
FIG. 4.
The effect of various phages on the growth of NCK1125(pTRK687) (•) and NCK1125 (pTRK790::pri3.10-AS) (▪). (A) Growth in the absence of phage. Growth in the presence of phage κ3 (B); κ5 (C); κ9 (D); and κ6 (E). The multiplicities of infection were 0.1% ± 20% for all phages.
Since the antisense RNA cassette was generated from phage κ3, the effects of pri3.10-AS expression on phage κ3 development were further characterized. The expression of pri3.10-AS resulted in a 2.7-log cycle reduction in the EOP and reduced the average plaque size by 85%. Phages recovered from plaques formed on the antisense-expressing hosts remained sensitive to pri3.10-AS inhibition. No mutant phages insensitive to antisense inhibition were recovered. The expression of pri3.10-AS also lowered the ECOI to 0.5 and reduced the burst size per infective center by 20%. No significant difference in phage adsorption was observed between the strain expressing pri3.10-AS and the parent strain, NCK1125(pTRK687) (data not shown).
Identification of regions critical for antisense RNA efficacy.
The importance of antisense RNA length and the region targeted were then examined. Twelve DNA cassettes ranging from 478 to 1,506 bp in length were amplified from the phage κ3-derived putative primase gene (Fig. 2). These cassettes were cloned in an antisense orientation into the PstI site of pTRK687. The antisense constructs were then electroporated into NCK1125, and the resulting transformants were evaluated for their sensitivity to the Sfi21-type phage κ3. The effects of these constructs on both the EOP and average plaque size of phage κ3 are illustrated in Fig. 3B. In most cases, a strong correlation between plaque size and EOP was observed. The largest antisense RNAs (e.g., pri3.10-AS, pri3.13-AS, and pri3.14-AS) were generally found to confer the most significant and consistent reductions in EOP; however, correlations between fragment size and the magnitude of EOP reduction were not observed as the length of the antisense fragments was systemically reduced (e.g., compare pri3.10-AS, pri3.11-AS, and pri3.12-AS). Reductions in EOP mediated by pri3.12-AS, pri3.19-AS, and pri3.21-AS, three 504-bp antisense cassettes designed to target different, nonoverlapping regions of the gene, exhibited significant differences in their ability to reduce the EOP. In general, antisense constructs that contained sequences complementary to the putative RBS, which included pri3.4-AS (587 bp), pri3.7-AS (1,023 bp), and pri3.8-AS (519 bp), reduced the EOP below the level of similarly sized constructs that lacked sequences complementary to the RBS; however, this was not always the case (e.g., compare pri3.7-AS and pri3.15-AS).
None of the antisense RNA cassettes were found to negatively impact the replication of S. thermophilus phage κ6, which encodes a variant of the heterologous 7201-type genome replication module. The lactococcal P335-type phage φ31 encodes a primase orthologue (AF208055) that exhibits short patches of local nucleotide sequence similarity to the phage κ3-encoded pri3.1 (13). Given this finding, the pri3.10-AS construct was also transformed into L. lactis NCK203 and tested for its ability to impede the replication of phage φ31. This antisense construct, however, had no effect on φ31.
Monitoring primase sense mRNA expression during phage infection.
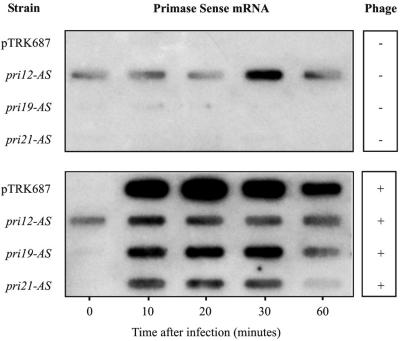
The impact of antisense RNA expression on transcript abundance over the course of a phage κ3 lytic infection was monitored by RNA-RNA slot blot hybridization (Fig. 5). The single-stranded RNA probes detect-S and detect-AS were used to detect the phage κ3-encoded primase sense (-S) mRNA and the plasmid-encoded antisense RNA (-AS), respectively. Both probes were specific for the region comprised by the pri3.12 PCR fragment (Fig. 2). In the absence of phage κ3 infection, no primase mRNA was detected from NCK1125(pTRK687) or from strains expressing pri3.19-AS or pri3.21-AS. A weak primase mRNA-specific hybridization signal was observed only in the pri3.12-AS-expressing strain, indicating that a basal level of transcription occurred through the coliphage T7 transcriptional terminator (Fig. 1).
FIG. 5.
RNA-RNA slot blot hybridization of three antisense RNA-expressing constructs and the vector control strain NCK1125(pTRK687) with detect-S, a pri3.12-derived single-stranded RNA probe that was complementary to the sense strand of the phage κ3-encoded primase mRNA (Fig. 2). RNA was isolated from cells in the absence (top panel) or presence (bottom panel) of phage κ3.
It was postulated that expression of the pri3.12-S transcript might have led to decreased levels of pri3.12-AS by means of the antisense effect. In order to address this, the pri3.12 cassette was cloned in the antisense orientation into pTRK696, a pTRK686 derivative containing the P6 promoter cassette cloned in the opposite orientation relative to the cat194 gene found in pTRK687 (Fig. 1). The resultant construct, designated pTRK800::pri3.12-AS, was electroporated into NCK1125 and tested for its ability to inhibit phage replication. The strain harboring pTRK800::pri3.12-AS failed to reduce the EOP of phage κ3 beyond the level conferred by pTRK792::pri3.12-AS. These results indicated that the basal level of pri3.12-S expressed from pTRK792::pri3.12-AS did not result in a significant reduction in the efficacy of the expressed pri3.12-AS antisense RNA.
After infection with phage κ3 and throughout the lytic cycle, a robust primase mRNA-specific signal was observed from NCK1125(pTRK687) (Fig. 5). The signal intensity weakened 60 min postinfection, correlating with culture lysis. When strains expressing pri3.12-AS, pri3.19-AS, and pri3.21-AS were infected with phage κ3, the observed signal intensities from each strain were significantly weaker at every time point tested, relative to that of the NCK1125(pTRK687) control. Of these, the phage-infected strain expressing pri3.19-AS yielded the strongest sense mRNA signal, while those of infected strains expressing pri3.12-AS and pri3.21-AS were weaker. Notably, pri3.12-AS, which was most effective in reducing the EOP of phage κ3 (Fig. 3B), appeared to have the greatest impact on lowering sense mRNA, especially if the background level of vector-derived pri3.12-S sense mRNA is considered.
Monitoring primase antisense RNA.
Total RNAs from NCK1125(pTRK687) and the strain expressing pri3.12-AS were probed with detect-AS. As expected, primase-specific antisense RNAs were detected in the strain expressing pri3.12-AS. When NCK1125(pTRK792::pri3.12-AS) was infected with phage κ3, the intensity of the primase antisense RNA-specific signal was significantly weaker at every time point tested relative to that of the uninfected control culture, which suggests its probable interaction with the sense mRNA expressed by the infecting phage. Primase-specific antisense RNAs were not detected in the presence or absence of phage κ3 in NCK1125(pTRK687).
Interference with intracellular bacteriophage DNA replication.
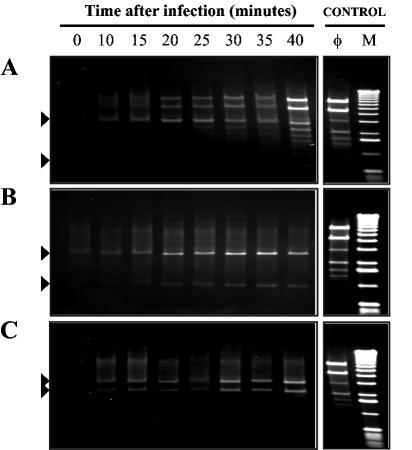
NCK1125(pTRK687) and strains expressing pri3.8-AS and pri3.10-AS were infected with phage κ3 at a MOI of 1% ± 20%. Total genomic DNAs were isolated from infected cells over the course of the lytic cycle and digested with HindIII. The HindIII-digested DNA fragments were then subjected to agarose gel electrophoresis in order to determine if antisense RNA expression retarded the accumulation of phage-specific DNA bands over time (Fig. 6). Relative to that for NCK1125(pTRK687), the accumulation of phage-specific DNA fragments over time was greatly diminished in both antisense RNA-expressing strains.
FIG. 6.
The impact of antisense RNA expression on the in vivo accumulation of HindIII-digested phage κ3-specific DNA fragments over time. (A) Vector control strain NCK1125(pTRK687); (B) NCK1125(pTRK789::pri3.8-AS); (C) NCK1125(pTRK790::pri3.10-AS). Each plasmid encoded two HindIII sites; plasmid-specific DNA bands are demarked with black triangles. Abbreviations: φ, purified phage κ3 genomic DNA; M, 1-kb molecular size marker (GIBCO-BRL).
DISCUSSION
Comparative genomics was successfully used to identify conserved and early-expressed genes in S. thermophilus phages that could be targeted by antisense RNA-based phage defense strategies. Antisense RNAs directed against the conserved putative primase gene, which is a component of the Sfi21-type genome replication module, retarded phage genome replication, significantly reduced the EOP, and severely limited the number of progeny phages released from an infected cell. The expression of 13 different antisense RNA constructs targeting specific regions of the S. thermophilus phage κ3-encoded putative primase gene was also evaluated in an attempt to approximate key regions that are more or less sensitive to antisense targeting. The expression of antisense RNA that covered the entire gene (pri3.10-AS) was the most effective of the constructs, resulting in an 85% reduction in plaque size, a 2.7-log cycle reduction in EOP, and a 50% reduction in the ECOI formation. Thus, only one of every two phage-infected cells released progeny phage, while those that released progeny phages exhibited a 20% reduction in the burst size. Overall, this is among the strongest levels reported for inhibition of phage via antisense RNA.
Primase-targeted expression of pri3.10-AS antisense RNA also provided significant protection in broth lysis experiments from all Sfi21-type phages tested, including κ3, κ4, and κ9—three phages that were each isolated from different dairy facilities across the continental United States. In contrast, phage κ6, which encodes a heterologous 7201-type genome replication module, was not inhibited. Together, these results illustrate that the antisense targeting of the highly conserved, early-expressed putative primase is broadly effective against S. thermophilus phages that encode the conserved Sfi21-type replication module. It was further noted that mutant phages insensitive to primase antisense RNA were not recovered after numerous attempts to select or enrich for phage derivatives. This result was not expected in light of the prior work with RNA coliphage SP that demonstrated the appearance of antisense-insensitive phages by point mutation (8).
Given the effectiveness of pri3.10-AS expression on phage κ3 development, efforts were made to identify regions that were more or less important for optimal efficacy in vivo. To address this, the pri3.10-AS region was systematically reduced through the construction of 12 additional subclones that spanned various structural or putative regulatory regions of the primase gene. The expression of all 13 antisense constructs resulted in statistically significant reductions in EOP that ranged from 0.2 to 2.7 log cycles. In general, the largest antisense RNAs (1.5 kb) were found to confer the largest reductions in EOP; however, shorter (478 bp) antisense RNAs designed to the 5′ region of the gene retained much of the inhibitory function. The superior efficacy of larger antisense RNAs may stem from the fact that they have more opportunities over their length to maximize intermolecular base pairing and thus exert their inhibitory effects, perhaps through multiple associations within the target RNA (15, 22). Alternatively, larger antisense RNAs may simply exhibit decreased stability when bound to the target RNA. Considering both the length of antisense RNA and the potential for multiple associations, we suspect that these factors could limit the ease with which phages might overcome antisense inhibition via point mutation(s).
As the length of the antisense fragments was reduced, however, a correlation between fragment size and the magnitude of EOP reduction was not observed. For instance, reductions in EOP mediated by pri3.12-AS, pri3.19-AS, and pri3.21-AS, three antisense cassettes of equal length (504 bp) designed to target different, nonoverlapping regions of the entire gene, exhibited significant differences in their ability to reduce the EOP of phage κ3. The observed variation in the effectiveness of these antisense RNAs may result from differences in the primary nucleotide sequence of the expressed antisense RNAs, which dictates the formation of higher-order intramolecular structures, and/or intrinsic, regional differences in the phage-encoded transcript that were strategically targeted (e.g., 5′ or 3′ regions). RNA-RNA slot blot analysis indicated that the expression of pri3.12-AS, pri3.19-AS, and pri3.21-AS resulted in marked decreases in the abundance of the sense, phage-encoded primase transcript (Fig. 5). These three antisense RNAs generally reduced the abundance of the target transcript in a manner consistent with the observed reductions in EOP (Fig. 3). Further, expression of antisense RNAs that strongly inhibited plaque formation, as measured by EOP, also resulted in the synthesis of fewer phage genomes over time, indicating a correlation between the lowered abundance of primase transcripts, lowered levels of genome replication, and interference with progeny phage development.
In general, antisense constructs that contained sequences complementary to the putative ribosome-binding site reduced the EOP below the level of similarly sized constructs that lacked sequences complementary to the RBS; however, this was not always the case (e.g., compare pri3.4-AS and pri3.16-AS). This phenomenon is believed to be due to the formation of a double-stranded RNA over the length of the RBS, thus preventing efficient ribosome loading and reducing translation of the targeted gene (18), which may not only affect the translation of the targeted gene but may also result in the polar expression of translationally coupled genes located downstream from the target.
A previous study revealed that a 1.5-kb antisense RNA complementary to the complete S. thermophilus phage κ3-derived putative helicase gene (hel3.1-AS) inhibited the proliferation of Sfi21-type phages, mediating a phage-specific 40 to 70% reduction in the EOP with a concomitant reduction in plaque size (32). In L. lactis, Kim et al. (20) found that antisense expression of two polycistronic open reading frames, designated gp18C and gp24C, inhibited the P335-type phage φ7-9, as measured by a 55% reduction in EOP. The reduction in EOP dropped to 30% if the RBS and coding region for the first 15 amino-terminal residues of gp18C were omitted from the antisense construct. In both cases, the plaque size was also reduced by approximately 10-fold. Chung et al. (9) obtained variable reductions in EOP, which ranged between 0.5 and 0.8, as they expressed different lengths of the φF4-1 major coat protein (mcp) gene. Kim and Batt (19) found that antisense expression of the full-length, phage φ7-9-derived gp15C mediated a 100-fold reduction in the EOP of φ7-9 and other gp15C-containing phages.
More recently, six genes putatively involved in lactococcal P335-type phage genome replication were targeted with antisense RNA (24). The targeted genes were orf14 (encoding a putative topoisomerase), orf15 (encoding a putative single-stranded DNA binding protein), orf16 (encoding a putative replisome organizer), orf18 (encoding a putative methylase), and two orfs encoding proteins of undetermined function (i.e., orf17 and orf19). For each gene, the expressed antisense RNAs were complementary to the complete open reading frame, including its upstream putative RBS. When challenged with four different P335-type phages, the authors found that the expression of antisense RNAs specific for orf14, orf15, and orf18 each reduced the EOP of phage Tuc2009 10-fold but did not have any effect on phage Q30, Q33, or ul36. In contrast, the expression of orf16 and orf17 conferred significant but highly variable resistance to all four phages, as measured by 0.5- to 10−6-log reductions in EOP. Antisense RNA specific for orf19 failed to inhibit any of the four phages.
In general, antisense RNAs targeting early-expressed genes involved in genome replication (24, 32) have been more effective targets than genes expressed later in the lytic cycle (24, 34). Note, however, that some genes involved in genome replication are not effective targets. Polzin et al. (K. M. Polzin, L. J. Collins, M. W. Lubbers, and A. W. Jarvis, Abstr. 5th Symp. Lactic Acid Bacteria, abstr. F2, 1996) found that the antisense expression of four early open reading frames, including e5 (encoding a putative subunit of DNA polymerase), e12 (putative transcription regulator), and e15 (putative recombinase), was ineffective in inhibiting the replication of the lactococcal prolate-headed phage c2, regardless of the gene dosage tested.
In these and other studies, the effectiveness of antisense RNA-based phage defense strategies has been highly variable, exhibiting both target- and phage-specific differences. The collective observations thus far do suggest some key characteristics of an ideal antisense RNA target. Genes that are transiently expressed, expressed at a very low level, and/or coded for by unstable, inefficiently translated mRNAs should make excellent candidates for antisense RNA targeting. From a practical standpoint, the target RNA must be essential for phage development or at least be critical to the synthesis or maturation of virulent progeny phages. In this study, selection of a vital, early gene target was very effective at restricting phage development and further limiting the appearance of mutant phages insensitive to antisense inhibition. In addition, expression of a sufficient dose of antisense RNA at the appropriate time during the lytic cycle is important to the effectiveness of antisense RNA-based phage resistance strategies. Walker and Klaenhammer (34) found that two middle-expressed open reading frames, including orf1 and orf2, and four late-expressed open reading frames, orf3 through orf6, were ineffective at inhibiting the L. lactis P335-type phage φ31 when expressed from the high-copy-number vector pTRKH2. In order to increase the ratio of antisense RNA to sense RNA during the later stages of the lytic infection, the authors cloned the aforementioned antisense expression cassettes into a low-copy-number vector containing the φ31 putative origin of DNA replication (ori31) and a phage-inducible promoter. Following φ31 infection, the expression of phage-derived DNA replication factors triggered both the expression of antisense RNA and explosive replication of the plasmid replicon, thereby elevating levels of antisense RNA later in the lytic cycle (34).
When phage-encoded resistance systems are engineered, there are potential benefits in implementing comparative genomics as an initial screen for choosing potential targets, as described here. These analyses expedited the identification of well-conserved genes and cis regulatory elements shared between the various genomes, while conversely enabling the elimination of poorly conserved targets in silico. These advantages are of critical importance when engineering defense strategies intended for use in large-scale industrial settings, where protection is required against both the residing and potentially emerging phage populations.
Acknowledgments
This study was supported in part by the USDA-NRICGP, project number 97-35503-4468, and by Rhodia, Inc., of Madison, Wis. Joseph Sturino was supported by a National Institutes of Health Biotechnology Training Program fellowship.
We thank Eric Altermann for critical reading of the manuscript and extend our gratitude to Sylvain Moineau for kindly providing phage DT1 and its propagating host, S. thermophilus SMQ495.
REFERENCES
- 1.Alatossava, T., and T. R. Klaenhammer. 1991. Molecular characterization of three small isometric-headed bacteriophages which vary in their sensitivity to the lactococcal phage resistance plasmid pTR2030. Appl. Environ. Microbiol. 57:1346-1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allison, G. E., and T. R. Klaenhammer. 1998. Phage resistance mechanisms in lactic acid bacteria. Int. Dairy J. 8:207-226. [Google Scholar]
- 3.Altschul, S. F., T. L. Madden, A. A. Schäffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST, a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bouchard, J. D., and S. Moineau. 2000. Homologous recombination between a lactococcal bacteriophage and the chromosome of its host strain. Virology 270:65-75. [DOI] [PubMed] [Google Scholar]
- 5.Brüssow, H., and F. Desiere. 2001. Comparative phage genomics and the evolution of Siphoviridae: insights from dairy phages. Mol. Microbiol. 39:213-222. [DOI] [PubMed] [Google Scholar]
- 6.Brüssow, H., A. Probst, M. Fremont, and J. Sidoti. 1994. Distinct Streptococcus thermophilus bacteriophages share an extremely conserved DNA fragment. Virology 200:854-857. [DOI] [PubMed] [Google Scholar]
- 7.Bruttin, A., F. Desiere, N. d'Amico, J. P. Guerin, J. Sidoti, B. Huni, et al. 1997. Molecular ecology of Streptococcus thermophilus bacteriophage infections in a cheese factory. Appl. Environ. Microbiol. 63:3144-3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bull, J. J., A. Jacobson, M. R. Badgett, and J. Molineux. 1998. Viral escape from antisense RNA. Mol. Microbiol. 28:835-846. [DOI] [PubMed] [Google Scholar]
- 9.Chung, D. K., S. K. Chung, and C. A. Batt. 1992. Antisense RNA directed against the major capsid protein of Lactococcus lactis subsp. cremoris bacteriophage F4-1 confers partial resistance to the host. Appl. Microbiol. Biotechnol. 37:79-83. [DOI] [PubMed] [Google Scholar]
- 10.Desiere, F., S. Lucchini, C. Canchaya, M. Ventura, and H. Brussow. 2002. Comparative genomics of phages and prophages in lactic acid bacteria. Antonie Leeuwenhoek 82:73-91. [PubMed] [Google Scholar]
- 11.Dinsmore, P. K., and T. R. Klaenhammer. 1997. Molecular characterization of a genomic region in a Lactococcus bacteriophage that is involved in its sensitivity to the phage defense system AbiA. J. Bacteriol. 179:2949-2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Djordjevic, G., B. Bojovic, N. Miladinov, and L. Topisirovic. 1997. Cloning and molecular analysis of promoter-like sequences isolated from the chromosomal DNA of Lactobacillus acidophilus ATCC 4356. Can. J. Microbiol. 43:61-69. [DOI] [PubMed] [Google Scholar]
- 13.Durmaz, E., and T. R. Klaenhammer. 2000. Genetic analysis of chromosomal regions of Lactococcus lactis acquired by recombinant lytic phages. Appl. Environ. Microbiol. 66:895-903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hill, C., I. J. Massey, and T. R. Klaenhammer. 1991. Rapid method to characterize lactococcal bacteriophage genomes. Appl. Environ. Microbiol. 57:283-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hjalt, T. A., and E. G. Wagner. 1995. Bulged-out nucleotides in an antisense RNA are required for rapid target RNA binding in vitro and inhibition in vivo. Nucleic Acids Res. 23:580-587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holo, H., and I. F. Nes. 1989. High-frequency transformation, by electroporation, of Lactococcus lactis subsp. cremoris grown with glycine in osmotically stabilized media. Appl. Environ. Microbiol. 55:3119-3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huynh, T. V., R. A. Young, and R. W. Davis. 1985. Construction and screening cDNA libraries in λgt10 and λgt11, p. 49-78. In D. M. Glover (ed.), DNA cloning, vol. 1. IRL Press Ltd., Oxford, United Kingdom.
- 18.Inouye, M. 1988. Antisense RNA: its functions and applications in gene regulation—a review. Gene 72:25-34. [DOI] [PubMed] [Google Scholar]
- 19.Kim, S. G., and C. A. Batt. 1991. Antisense mRNA-mediated bacteriophage resistance in Lactococcus lactis subsp. lactis. Appl. Environ. Microbiol. 57:1109-1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim, S. G., Y. C. Bor, and C. A. Batt. 1992. Bacteriophage resistance in Lactococcus lactis subsp. lactis using antisense ribonucleic acid. J. Dairy Sci. 75:1761-1767. [DOI] [PubMed] [Google Scholar]
- 21.Klaenhammer, T. R., and R. B. Sanozky. 1985. Conjugal transfer from Streptococcus lactis ME2 of plasmid encoding phage resistance, nisin resistance and lactose-fermenting ability: evidence for a high-frequency conjugative plasmid responsible for abortive infection of virulent bacteriophage. J. Gen. Microbiol. 131:1531-1541. [DOI] [PubMed] [Google Scholar]
- 22.Kolb, F. A., E. Westhof, C. Ehresmann, B. Ehresmann, E. Gerhart, H. Wagner, and P. Romby. 2001. Bulged residues promote the progression of a loop-loop interaction to a stable and inhibitory antisense-target RNA complex. Nucleic Acids Res. 29:3145-3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lucchini, S., F. Desiere, and H. Brüssow. 1999. Comparative genomics of Streptococcus thermophilus phage species supports a modular evolution theory. J. Virol. 73:8647-8656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McGrath, S., G. F. Fitzgerald, and D. van Sinderen. 2001. Improvement and optimization of two engineered phage resistance mechanisms in Lactococcus lactis. Appl. Environ. Microbiol. 67:608-616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moineau, S., M. Borkaev, B. J. Holler, S. A. Walker, J. K. Kondo, E. R. Vedamuthu, and P. A. Vandenbergh. 1996. Isolation and characterization of lactococcal bacteriophages from cultured buttermilk plants in the United States. J. Dairy Sci. 79:2104-2111. [Google Scholar]
- 26.Nuesch, J. P., and P. Tattersall. 1993. Nuclear targeting of the parvoviral replicator molecule NS1: evidence for self-association prior to nuclear transport. Virology 196:637-651. [DOI] [PubMed] [Google Scholar]
- 27.O'Sullivan, D. J., and T. R. Klaenhammer. 1993. Rapid mini-prep isolation of high-quality plasmid DNA from Lactococcus and Lactobacillus spp. Appl. Environ. Microbiol. 59:2730-2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sambrook, J., E. Fritsch, and T. Maniatis. 1982. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 29.Sanders, M. E., and T. R. Klaenhammer. 1980. Restriction and modification in group N streptococci: effect of heat on development of modified lytic bacteriophage. Appl. Environ. Microbiol. 40:500-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sanders, M. E., P. J. Leonhard, W. D. Sing, and T. R. Klaenhammer. 1986. Conjugal strategy for construction of fast acid-producing, bacteriophage-resistant lactic streptococci for use in dairy fermentations. Appl. Environ. Microbiol. 52:1001-1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sing, W. D., and T. R. Klaenhammer. 1990. Characteristics of phage abortion conferred in lactococci by the conjugal plasmid pTR2030. J. Gen. Microbiol. 136:1807-1815. [Google Scholar]
- 32.Sturino, J. M., and T. R. Klaenhammer. 2002. Expression of antisense RNA targeted against Streptococcus thermophilus bacteriophages. Appl. Environ. Microbiol. 68:588-596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tremblay, D. M., and S. Moineau. 1999. Complete genomic sequence of the lytic bacteriophage DT1 of Streptococcus thermophilus. Virology 255:63-76. [DOI] [PubMed] [Google Scholar]
- 34.Walker, S. A., and T. R. Klaenhammer. 2000. An explosive antisense RNA strategy for inhibition of a lactococcal bacteriophage. Appl. Environ. Microbiol. 66:310-319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang, D., W. Yuan, I. Davis, and C. R. Parrish. 1998. Nonstructural protein-2 and the replication of canine parvovirus. Virology 240:273-281. [DOI] [PubMed] [Google Scholar]