Abstract
Objective
Several epidemiological studies have evaluated the association between the GNB3 C825T polymorphism and hypertension or stroke. The results of these studies were inconsistent; therefore, we performed a meta-analysis to clarify these discrepancies.
Methods
We systematically searched the PubMed, Embase, Web of Science, CNKI, and CBM databases, and manually searched reference lists of relevant papers, meeting abstracts, and relevant journals. Pooled odds ratios (ORs) and 95% confidence intervals (CIs) were calculated for dominant, recessive, and allelic models. A fixed or random effects model was separately adopted depending on study heterogeneity. Subgroup and sensitivity analyses were performed to detect study heterogeneity and examine result stability, respectively. Publication bias was tested using funnel plots, the Egger's regression test, and Begg's test.
Results
We screened 66 studies regarding hypertension and eight concerning stroke. A combined analysis showed that only the allelic model found a marginal association with hypertension (OR = 1.07, 95% CI = 1.01–1.13) and female gender (OR = 1.11, 95% CI = 0.99–1.24). However, no comparison models found an association with stroke (allelic model: OR = 1.11, 95% CI = 0.94–1.32; dominant model: OR = 1.16, 95% CI = 0.92–1.48; and recessive model: OR = 1.05, 95% CI = 0.97–1.14). Sensitivity analysis suggested that all models did not yield a relationship to hypertension or stroke among Asians. Besides, there was a lack of statistical association with hypertension in Caucasians, which maybe due to a small sample size. When we restricted the included studies to normal populations according to the Hardy–Weinberg equilibrium, no association was found.
Conclusions
There was no evidence indicating that the 825T allele or TT genotype was associated with hypertension or stroke in Asians or hypertension in Caucasians. However, further studies regarding Africans and other ethnicities are needed to identify further correlations.
Introduction
Hypertension is a major risk factor of stroke, cardiovascular disease, and end-stage renal disease and affects about 1 billion adults worldwide, including 3.8 million in Taiwan and 160 million in China [1]. Stroke is a primary contributor to long-term adult disability and the third most common cause of death in developed countries [2], [3]. Blood pressure-lowering therapies are viewed as protective measures against the risk of hypertension and stroke, but both genetic and lifestyle factors are likely involved in the development of these conditions.
Guanine nucleotide-binding proteins (G proteins) are key determinants of specific and temporal characteristics of many signaling processes and are expressed in all cells of the human body to primarily transduce signals from the cell surface into a cellular response. G proteins consist of α, β, and γ subunits and different genes encode for 18 α subunits, 5 β subunits, and 12 γ subunits, which enable the formation of highly variable heterotrimers [4]. Activation of a G protein-coupled receptor results in an exchange of guanosine triphosphate for guanosine diphosphate followed by dissociation of the α subunit from the βγ complex. Different α subunits can then regulate a large variety of intracellular signaling cascades. The α subunit and βγ complex then reassemble as a heterotrimer available for a new activation cycle [5]. Reportedly, the α, β, γ subunit composition of G proteins determine the receptor and effector specificities of particular heterotrimers. Thus, alterations in G protein signaling can cause multiple disorders and it is likely that functionally important genetic polymorphisms in genes that encode human G protein subunits can cause or contribute to various disease phenotypes.
The G protein beta polypeptide 3 (GNB3) gene encodes the Gβ3 subunit of heterotrimeric G proteins and is located on chromosome 12p13 and comprises 11 exons and 10 introns. A polymorphism (C825T, rs5433) was found to be associated with a shortened splice variant of the Gβ3 protein that gives rise to enhanced signal transduction via pertussis toxin-sensitive G proteins [6], [7]. The C825T polymorphism located in exon 10 is in close linkage disequilibrium with the A(-350)G promoter single nucleotide polymorphism (SNP) and the C1429T SNP and can serve as a marker for allele-specific GNB3 expression. However, differential G protein activities associated with the C825T SNP did not result from different transcript amounts associated with specific GNB3 genotypes [8].
Several epidemiological studies have shown an association between the GNB3 825T allele and other features of metabolic syndrome, including obesity, insulin resistance, changes in autonomic nervous function, and dyslipidemia. This polymorphism has also been identified in hypertension, stroke, Alzheimer’s disease, sudden death, tumor progression, and as a genetic marker for drug responses to diuretics, antidepressants, and the antihypertension medications sildenafil, clonidine, and sibutramine [9]–[12].
Recently, many groups have investigated the relationship between the GNB3 C825T polymorphism and hypertension or stroke; however, the results have been inconclusive. Therefore, we designed the present meta-analysis to better clarify the association between the GNB3 C825T polymorphism and hypertension or stroke.
Materials and Methods
Literature Search
This meta-analysis followed the PRISMA (preferred reporting items for systematic reviews and meta-analyses) criteria [13]. We comprehensively searched for related papers in the following electronic databases: PubMed (up to Nov 2012), Embase (1996 to Nov 2012), Web of Science (2003 to Nov 2012), CBM (China Biology Medicine, 1978 to Jul 2012) and CNKI (China National Knowledge Infrastructure, 1999 to Nov 2012) using various keywords, including “hypertension,” “stroke,” “cerebral hemorrhage,” “cerebrovascular disorder,” “cerebrovascular disease,” “mutation,” “variant,” “polymorphism,” “ischemic stroke,” “GNB3,” “G protein beta,” and “G-beta.” Then, we manually searched the relevant journals and co-authors listed in the included studies to find additional studies. Reference lists of all retrieved publications were also checked for missing information. Meeting abstracts, which were previously shown to influence meta-analytical results [14], were also scrutinized. All relevant articles were initially scanned on the basis of title, keywords, and abstract. If this was not possible, the full text was obtained for further evaluation. The literature retrieval was performed independently by three investigators (LG, LLZ, and BZ) and discrepancies were resolved by reaching a consensus among the investigators. If a consensus could not be established, a fourth reviewer (JCL) was consulted to resolve the discrepancy. The last database searches were performed on November 10, 2012.
Inclusion Criteria
Studies were screened that met the following criteria: (1) population-based or hospital-based case-control studies regarding the relationship between the GNB3 C825T polymorphism and essential hypertension or stroke; (2) sufficient data on genotypic and allelic frequencies to determine an odds ratio (OR) with a 95% confidence interval (CI). If multiple publications reported the same or overlapping data, the most recent or complete study or the largest population was included in this meta-analysis as described by Little et al. [15]; (3) to avoid local literature bias, publications in both Chinese and English were considered [16]; (4) studies with related clinical characteristics were limited to those using human subjects; (5) articles regarding cases compounded with other diseases, such as diabetes mellitus and myocardial infarction, were also included; and (6) if patient blood pressure was measured casually or ambulatory (24 h), the latter were used. Hypertension was defined as mean casual blood pressure ≥140/90 mmHg or mean ambulatory blood pressure >134/79 mmHg.
Data Extraction
Data were independently extracted from each study by three investigators (LG, LLZ, and BZ) following the above-mentioned inclusion criteria. Discordance was resolved by discussion or another reviewer (JCL) was consulted. The following data were collected from each of the selected studies: surname of the first author, year of publication, country of origin, population ethnicity, source of control, T allele frequency in controls, genotype variance in the cases and controls, and the Hardy–Weinberg equilibrium (HWE) using the χ2 test. A p-value of <0.05 for the HWE was considered statistically significant.
Quality Score Assessment
The quality of each selected study was assessed independently by the same three investigators according to the Newcastle–Ottawa Scale (NOS) (www.ohri.ca/programs/clinical_epidemiology/oxford.asp). Scores were based on the selection, comparability, and exposure (case-control studies) or outcome (cohort studies) of the studies. To avoid selection bias, studies of poor quality were not rejected in this meta-analysis.
Statistical Analysis
All statistical analyses were conducted using Stata statistical software ver. 11.0 (Stats Corp., College Station, TX, USA) and Review Manager ver. 5.0 (The Cochrane Collaboration, Oxford, UK). All tests were two-sided and a p-value <0.05 was considered statistically significant. The strength of association of the GNB3 C825T polymorphism with hypertension or stroke was measured by calculating summary ORs with corresponding 95% CIs for the dominant model (TT+CT vs. CC), recessive model (TT vs. CT+CC), and allelic model (T allele vs. C allele), respectively.
Heterogeneity between the studies was analyzed using the Cochran’s Q test and the I2 statistic (range, 0–100%) [17], [18]. If the results of the Q test was p<0.1 and the measure of I2 was >50%, indicating significant heterogeneity between studies, the ORs were pooled using a fixed effects Mantel–Haenszel method [19], otherwise the DerSimonian and Laird random effects model was adopted [20], [21]. A Galbraith plot was employed to detect potential sources of heterogeneity [22]. The pooled ORs were recalculated after removing outlier studies identified by the Galbraith plots. To further detect heterogeneity, subgroup analyses were performed using the status of the HWE (yes or no) or the control source.
Sensitivity analysis was conducted by limiting the meta-analysis to high quality studies (NOS score ≥8). We also performed the analyses a second time by limiting the studies according to the HWE and excluding those that included myocardial infarction, obesity, or diabetes mellitus in the cases or controls. Sensitivity analysis was performed to identify alterations in the overall significance of the estimate.
Cumulative meta-analysis was performed to identify the influence of the first published study on the subsequent publications concerning the relationship between the GNB3 C825T polymorphism and hypertension, and to estimate the combined estimate over time [23].
Publication bias was assessed using the Egger's regression test and Begg's test. The Egger’s test detects funnel plot asymmetry by determining whether the intercept deviates significantly from zero in a regression of the standardized effect estimates against their precision [24], [25]. These methods were based on plotting the estimate (logOR) against the corresponding standard error (SE).
Results
Study Selection and Characteristics
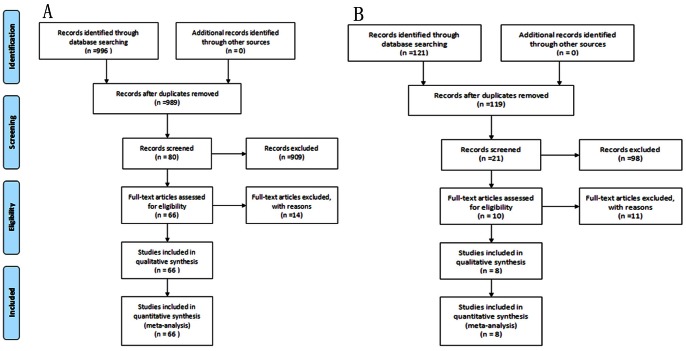
The present study met the PRISMA statement requirements (Appendix S1). Through comprehensive retrieval and evaluation, 66 studies (20,782 cases and 26,141 controls) regarding hypertension and eight studies (3,427 cases and 3,948 controls) regarding stroke met the inclusion criteria and were included in the final meta-analysis. Details of the included studies are presented in Tables 1 and 2 and the selection process is shown in Figure 1.
Table 1. The main characteristics of included studies regarding the association between the GNB3 C825T polymorphism and hypertension.
| Author | Year | Country | Ethnicity | Sample sizeHT/Control, n | HT/Control, n | HT/Control, n | HEWY/N | T frequencyin control | SOCPB/HB | Score | |||
| CC | CT | TT | C | T | |||||||||
| rand [52] | 2003 | Belgian | Caucasian | 352/1160 | 173/542 | 151/511 | 28/107 | 497/1595 | 207/725 | Y | 0.313 | PB | 9 |
| Shioji [53] | 2003 | Japan | Asian | 775/1105 | 177/287 | 385/536 | 213/282 | 739/1110 | 811/1100 | Y | 0.498 | PB | 8 |
| Dong [54] | 1999 | London | African | 185/243 | 3/14 | 61/83 | 121/146 | 67/111 | 303/375 | Y | 0.772 | PB | 8 |
| Yamamoto [55] | 2004 | Japan | Asian | 266/540 | 70/162 | 120/239 | 76/139 | 260/563 | 272/517 | N | 0.479 | PB | 8 |
| Hayakawa [56] | 2007 | Japan | Asian | 156/271 | 42/82 | 76/121 | 38/68 | 160/285 | 152/257 | Y | 0.474 | HB | 9 |
| Khamidullaeva [57] | 2011 | Uzbek | Asian | 174/60 | 64/0 | 93/50 | 17/10 | 221/50 | 127/70 | Y | 0.583 | PB | 9 |
| Hui [58] | 2007 | Japan | Asian | 261/271 | 78/72 | 115/148 | 68/51 | 271/292 | 251/250 | Y | 0.461 | PB | 9 |
| Alioglu [59] | 2008 | Turkey | Asian | 209/82 | 37/27 | 124/40 | 48/15 | 198/94 | 220/70 | Y | 0.427 | PB | 8 |
| Kato [31] | 1998 | Japan | Asian | 718/515 | 187/128 | 359/263 | 172/124 | 733/519 | 703/511 | Y | 0.496 | PB | 9 |
| Tsai [60] | 2000 | China | Asian | 302/199 | 57/43 | 149/96 | 96/60 | 263/182 | 341/216 | Y | 0.543 | PB | 9 |
| Kedzierska [61] | 2006 | Poland | Caucasian | 26/18 | 9/15 | 12/2 | 5/1 | 30/32 | 22/4 | Y | 0.111 | HB | 9 |
| Hager [62] | 2011 | Finland | Caucasian | 74/48 | 32/24 | 27/19 | 15/5 | 91/67 | 57/29 | Y | 0.302 | HB | 8 |
| Marcun Varda [63] | 2006 | Slovenia | Caucasian | 104/200 | 53/104 | 42/80 | 9/16 | 148/288 | 60/112 | Y | 0.280 | HB | 9 |
| Holmen [64] | 2010 | Norway | Caucasian | 1661/1175 | 863/630 | 682/465 | 116/80 | 2408/1725 | 914/625 | Y | 0.266 | PB | 9 |
| Tozawa [65] | 2001 | Japan | Asian | 179/180 | 32/39 | 68/82 | 79/59 | 132/160 | 226/200 | Y | 0.556 | HB | 8 |
| Wang [66] | 2004 | Kazak | Asian | 264/244 | 76/67 | 129/119 | 59/58 | 281/253 | 247/235 | Y | 0.482 | PB | 8 |
| Buchmayer [67] | 2000 | Australia | Caucasian | 174/174 | 85/72 | 70/85 | 19/17 | 240/229 | 108/119 | Y | 0.342 | PB | 8 |
| Yamagishi [68] | 2006 | Japan | Asian | 640/792 | 159/156 | 321/415 | 160/221 | 639/727 | 641/857 | Y | 0.541 | PB | 9 |
| Beige [69] | 1999 | Germany | Caucasian | 479/900 | 204/514 | 224/312 | 51/74 | 632/1340 | 326/460 | N | 0.256 | PB | 9 |
| Zychma [70] | 2000 | Poland | Caucasian | 85/68 | 32/24 | 44/36 | 9/8 | 108/84 | 62/52 | Y | 0.382 | PB | 8 |
| Benjafieid [41] | 1997 | Australia | Caucasian | 110/189 | 27/101 | 71/82 | 12/6 | 125/284 | 95/94 | N | 0.249 | PB | 8 |
| Li(a) [71] | 2005 | China | Asian | 501/503 | 142/137 | 256/259 | 103/107 | 540/533 | 462/473 | Y | 0.470 | PB | 8 |
| Suwazono [51] | 2006 | Japan | Asian | 218/1052 | 47/345 | 121/719 | 50/288 | 215/1409 | 221/1295 | N | 0.479 | PB | 9 |
| Ishikawa(a) [26] | 2000 | Japan | Asian | 304/422 | 43/37 | 90/85 | 48/43 | 184/159 | 186/171 | Y | 0.518 | HB | 9 |
| Ishikawa(b) [26] | 2000 | Japan | Asian | 181/165 | 67/96 | 161/204 | 76/122 | 295/396 | 313/448 | Y | 0.531 | HB | 9 |
| Bae [72] | 2007 | Korea | Asian | 687/924 | 193/217 | 319/469 | 175/238 | 705/903 | 669/945 | Y | 0.511 | PB | 9 |
| Panoulas [73] | 2009 | Britain | Caucasian | 269/114 | 128/50 | 113/54 | 28/10 | 369/154 | 169/74 | Y | 0.325 | HB | 8 |
| Huang [74] | 2003 | China | Asian | 585/580 | 134/126 | 290/303 | 161/151 | 558/555 | 612/605 | Y | 0.522 | PB | 9 |
| Larson [75] | 2000 | America | African | 472/432 | 29/25 | 190/170 | 253/237 | 248/220 | 696/644 | Y | 0.745 | PB | 8 |
| Suwazono [76] | 2004 | Japan | Asian | 332/2289 | 78/574 | 171/1216 | 83/499 | 327/2364 | 337/2214 | N | 0.484 | PB | 8 |
| Brand [77] | 1999 | France/Ireland | Caucasian | 206/467 | 98/226 | 92/197 | 16/44 | 288/649 | 124/285 | Y | 0.305 | PB | 8 |
| Nejatizadeh [78] | 2011 | Iran | Asian | 449/345 | 185/192 | 211/144 | 53/9 | 581/528 | 317/162 | N | 0.235 | PB | 9 |
| Pitsavos [79] | 2006 | Greece | Caucasian | 136/239 | 65/126 | 60/86 | 11/27 | 190/338 | 82/140 | N | 0.293 | PB | 8 |
| Izawa [80] | 2003 | Japan | Asian | 574/533 | 138/159 | 291/261 | 145/113 | 567/579 | 581/487 | Y | 0.457 | PB | 9 |
| Ozkececi [81] | 2008 | Turkey | Asian | 99/45 | 35/26 | 51/15 | 13/4 | 121/67 | 77/23 | Y | 0.256 | PB | 8 |
| Yin [82] | 2009 | China | Asian | 257/865 | 60/224 | 126/424 | 71/217 | 246/872 | 268/858 | Y | 0.496 | PB | 9 |
| Vasudevan [32] | 2009 | Malaysian | Asian | 70/75 | 19/20 | 32/44 | 19/11 | 70/84 | 70/66 | Y | 0.440 | PB | 8 |
| Dong [83] | 2006 | China | Asian | 97/87 | 25/27 | 47/46 | 25/14 | 97/100 | 97/74 | Y | 0.425 | PB | 7 |
| Zhang [84] | 2007 | China | Asian | 143/124 | 68/54 | 59/58 | 16/12 | 195/166 | 91/82 | Y | 0.331 | PB | 8 |
| Li(b) [85] | 2005 | China | Asian | 321/147 | 92/40 | 167/69 | 62/38 | 351/149 | 291/145 | Y | 0.493 | PB | 8 |
| Hu [86] | 2006 | China | Asian | 135/124 | 60/54 | 59/58 | 16/12 | 179/166 | 91/82 | Y | 0.331 | PB | 7 |
| Gai [87] | 2007 | China | Asian | 136/197 | 31/54 | 73/95 | 32/48 | 135/203 | 137/191 | Y | 0.485 | PB | 7 |
| Chen [88] | 2007 | China | Asian | 109/378 | 25/104 | 52/219 | 32/55 | 102/427 | 116/329 | N | 0.435 | PB | 7 |
| Tan(b) [89] | 2003 | China | Asian | 112/112 | 38/66 | 60/40 | 14/6 | 136/172 | 88/52 | Y | 0.232 | PB | 7 |
| Zhang [90] | 2005 | China | Asian | 111/150 | 32/51 | 52/72 | 27/27 | 116/174 | 106/126 | Y | 0.856 | PB | 7 |
| You [91] | 2000 | China | Asian | 98/110 | 25/31 | 47/52 | 26/27 | 97/114 | 99/106 | Y | 0.482 | PB | 7 |
| Jing [92] | 2006 | China | Asian | 354/384 | 96/106 | 152/163 | 106/115 | 344/375 | 364/393 | N | 0.512 | PB | 8 |
| Sun [93] | 2003 | China | Asian | 117/151 | 41/51 | 56/78 | 20/22 | 138/180 | 96/122 | Y | 0.404 | PB | 7 |
| Zhang [94] | 2001 | China | Asian | 146/79 | 36/18 | 101/50 | 9/11 | 173/86 | 119/72 | N | 0.456 | PB | 8 |
| Dou [42] | 2009 | Japan | Asian | 2092/2810 | 480/679 | 1081/1380 | 531/751 | 2041/2738 | 2143/2882 | Y | 0.513 | PB | 9 |
| Song (a) [27] | 2011 | China | Asian | 122/104 | 17/26 | 78/49 | 27/29 | 112/101 | 132/107 | Y | 0.514 | PB | 9 |
| Song (b) [27] | 2011 | China | Asian | 102/92 | 34/18 | 40/43 | 28/31 | 108/79 | 96/105 | Y | 0.571 | PB | 9 |
| Liu [95] | 2009 | China | Asian | 269/229 | 93/67 | 106/100 | 70/62 | 292/234 | 246/224 | Y | 0.489 | PB | 8 |
| Huang (a) [28] | 2005 | China | Asian | 96/87 | 18/20 | 57/47 | 21/20 | 93/87 | 99/87 | Y | 0.500 | PB | 8 |
| Huang (b) [28] | 2005 | China | Asian | 34/151 | 9/37 | 21/97 | 4/17 | 39/171 | 29/131 | N | 0.434 | PB | 8 |
| Lu [96] | 2009 | China | Asian | 162/180 | 48/52 | 94/101 | 20/27 | 190/205 | 134/155 | Y | 0.431 | PB | 7 |
| Li(c) [97] | 2005 | China | Asian | 310/151 | 89/42 | 161/70 | 60/39 | 339/154 | 281/148 | Y | 0.490 | PB | 8 |
| Zhao [98] | 2009 | China | Asian | 331/293 | 117/52 | 179/137 | 35/104 | 413/241 | 249/345 | Y | 0.589 | PB | 7 |
| Wang [99] | 2011 | China | Asian | 92/110 | 30/34 | 50/70 | 12/6 | 110/138 | 74/82 | N | 0.373 | PB | 7 |
| Wang [100] | 2003 | China | Asian | 408/140 | 131/39 | 182/66 | 95/35 | 444/144 | 372/136 | Y | 0.486 | PB | 7 |
| Li (a) [101] | 2006 | China | Asian | 334/267 | 59/54 | 149/113 | 126/100 | 267/221 | 401/313 | N | 0.586 | PB | 8 |
| Huang [102] | 2007 | China | Asian | 502/489 | 142/135 | 257/252 | 103/102 | 541/522 | 463/456 | Y | 0.466 | PB | 8 |
| Li (b) [103] | 2006 | China | Asian | 268/218 | 47/48 | 132/85 | 89/85 | 226/181 | 310/255 | N | 0.585 | PB | 7 |
| Dai [104] | 2002 | China | Asian | 133/257 | 28/70 | 73/127 | 32/60 | 129/267 | 137/247 | Y | 0.481 | PB | 7 |
| Zhang [105] | 2006 | China | Asian | 100/100 | 19/32 | 46/53 | 35/15 | 84/117 | 116/83 | Y | 0.415 | PB | 7 |
| Yang [106] | 2007 | China | Asian | 170/196 | 53/60 | 98/118 | 19/18 | 204/238 | 136/154 | N | 0.393 | PB | 8 |
| Li [39] | 2003 | China | Asian | 641/370 | 119/85 | 313/157 | 209/128 | 551/327 | 731/413 | N | 0.558 | PB | 8 |
| Liu [107] | 2003 | China | Asian | 163/339 | 50/125 | 79/157 | 34/57 | 179/407 | 147/271 | Y | 0.400 | PB | 8 |
| Tan(a) [108] | 2003 | China | Asian | 40/31 | 11/14 | 25/15 | 4/2 | 47/43 | 33/19 | Y | 0.306 | HB | 7 |
HT, hypertension; SOC, source of control; PB, population-based, controls were blood donors, healthy controls matched for age, gender and domicile and participants in an health service programme from the same geographical region without clinically detectable hypertension; HB, hospital-based, controls were patients admitted to hospital without hypertension matched for age, gender and domicile; HWE, Hardy–Weinberg equilibrium; and MAF, minor allele frequency; Three publications [26]–[28] contained more than one independent population, therefore, we considered them as different studies. Two studies [57], [80] were limited to the relationship in males. The samples [54], [75] were from individuals of African descent.
Table 2. The main characteristics of the included studies regarding association between the GNB3 C825T polymorphism and stroke.
| Author | Year | Country | Ethnicity | Sample sizeStroke/Control, n | Stroke/Control, n | Stroke/Control, n | HEWY/N | T frequency in control | SOCPB/HB | Score | |||
| CC | CT | TT | C | T | |||||||||
| Zhang [12] | 2005 | China | Asian | 922/1124 | 212/244 | 512/569 | 198/311 | 936/1057 | 908/1191 | Y | 0.530 | PB | 8 |
| Morrison [11] | 2001 | America | Caucasian | 990/1124 | 266/311 | 512/569 | 212/244 | 1044/1191 | 936/1057 | Y | 0.470 | PB | 9 |
| Zhao [34] | 2001 | China | Asian | 294/280 | 89/93 | 144/133 | 61/54 | 322/319 | 266/241 | Y | 0.430 | PB | 7 |
| Tan [35] | 2003 | China | Asian | 100/100 | 32/65 | 58/32 | 10/3 | 122/162 | 78/38 | Y | 0.190 | PB | 7 |
| Wang [36] | 2011 | China | Asian | 80/110 | 26/34 | 46/70 | 8/6 | 98/138 | 62/82 | N | 0.373 | PB | 7 |
| Zhao [38] | 2000 | China | Asian | 715/668 | 196/195 | 348/338 | 171/135 | 740/728 | 690/608 | Y | 0.455 | PB | 8 |
| Li [39] | 2003 | China | Asian | 144/352 | 36/64 | 70/175 | 38/113 | 142/303 | 146/401 | Y | 0.570 | PB | 7 |
| Zhao [37] | 2004 | China | Asian | 182/190 | 35/55 | 87/92 | 60/43 | 157/202 | 207/178 | Y | 0.468 | PB | 7 |
HT, hypertension; SOC, source of control; PB, population-based, controls were blood donors, healthy controls matched for age, gender and domicile and participants in an health service programme from the same geographical region without clinically detectable hypertension; HB, hospital-based, controls were patients admitted to hospital without hypertension matched for age, gender and domicile; HWE, Hardy–Weinberg equilibrium; and MAF, minor allele frequency. Five studies [11], [12], [34]–[36] regarding the association of the GNB3 C825T polymorphism and ischemic stroke were identified while one [37] was regarding cerebral hemorrhage and the other two [38], [39] included ischemic stroke or cerebral hemorrhage cases.
Figure 1. A flow diagram of the literature search for associations between the GNB3 C825T polymorphism and hypertension (A) or stroke (B).
Of the 66 studies, eight compared males and females to assess an association between the GNB3 C825T polymorphism and hypertension. Among these articles, three publications [26]–[28] contained more than one independent population, and thus, we considered them as different studies that should be counted twice. Two studies [29], [30], which did not supply all of the required information regarding case or control genotypes were excluded from this meta-analysis. We only retrieved information on hypertensive patients and controls without diabetes mellitus from three studies [31]–[33]. Five studies [11], [12], [34]–[36] regarding the association of the GNB3 C825T polymorphism and ischemic stroke were identified while one [37] was regarding cerebral hemorrhage and the other two [38], [39] included ischemic stroke or cerebral hemorrhage cases.
All of the included studies were case-controlled in design. The main characteristics of the included studies are summarized in Tables 1–3. In all of the included studies, genotyping was analyzed via polymerase chain reaction and restriction fragment length polymorphisms. Stroke cases were evaluated by strict neurological examination: computed tomography, nuclear magnetic resonance imaging or both.
Table 3. The association between the GNB3 C825T polymorphism and hypertension among males and females.
| Male(HT/Control),n | Female(HT/Control),n | ||||||||||||
| Author | Year | Country | Ethnicity | CC | CT | TT | C | T | CC | CT | TT | C | T |
| Khamidullaeva [57] | 2011 | Uzbek | Asian | 64/0 | 93/50 | 17/10 | 221/50 | 127/70 | Not available | ||||
| Hui [58] | 2007 | Japan | Asian | 57/50 | 69/100 | 44/32 | 183/200 | 157/164 | 21/22 | 46/48 | 24/19 | 88/101 | 94/117 |
| Tsai [60] | 2000 | China | Asian | 28/21 | 70/39 | 30/30 | 126/81 | 130/99 | 29/22 | 79/57 | 58/30 | 137/101 | 195/117 |
| Holmen [64] | 2010 | Norway | Caucasian | 404/245 | 340/194 | 58/41 | 1148/684 | 456/276 | 459/385 | 340/271 | 58/39 | 1258/1041 | 456/349 |
| Buchmayer [67] | 2000 | Australia | Caucasian | 40/33 | 36/43 | 11/11 | 116/109 | 58/65 | 45/39 | 34/42 | 8/6 | 124/120 | 50/54 |
| Suwazono [51] | 2006 | Japan | Asian | 35/180 | 90/372 | 30/171 | 160/732 | 150/714 | 12/165 | 31/347 | 20/117 | 55/677 | 71/581 |
| Suwazono [76] | 2004 | Japan | Asian | 58/300 | 135/614 | 63/282 | 251/1214 | 261/1178 | 20/274 | 36/602 | 20/217 | 76/1150 | 76/1036 |
| Izawa [80] | 2003 | Japan | Asian | 138/159 | 291/261 | 145/113 | 567/579 | 581/487 | Not available | ||||
HT, hypertension.
Quantitative Synthesis
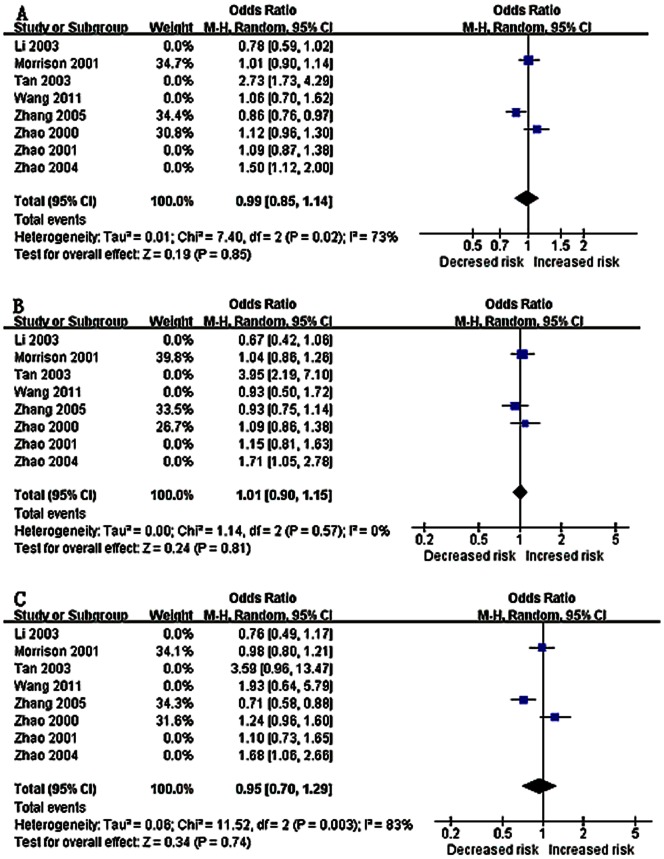
All models concerning the association of the GNB3 C825T polymorphism and hypertension or stroke were identified using the random effects model for I2>50%, which suggested significant heterogeneity. However, in most of the models, I2 was ≥70%, which indicated high heterogeneity [40], thus we pooled the ORs because of the significant results. The main results of this meta-analysis are presented in Tables 4 and 5. A significant overall association between the GNB3 C825T polymorphism and the risk of hypertension was only detected in the allelic model (OR = 1.07, 95% CI = 1.01–1.13). No evidence of significance was identified in the dominant model (OR = 1.08, 95% CI = 0.98–1.81) or the recessive model (OR = 1.05, 95% CI = 0.97–1.14). However, none of the comparison models found an association between the GNB3 C825T polymorphism and stroke (allelic model: OR = 1.11, 95% CI = 0.94–1.32; dominant model: OR = 1.16, 95% CI = 0.92–1.48; and recessive model: OR = 1.05, 95% CI = 0.97–1.14, respectively) (Figure 2). After excluding the outlier studies identified by the Galbraith plots, heterogeneity was effectively nonexistent or decreased and the pooled ORs were similar to those when the outlier studies regarding stroke cases were included; however, the association to hypertension was significant using the dominant model (OR = 1.05, 95% CI = 1.00–1.11). These results suggested that carriers of the T allele or TT genotype may have a higher risk of hypertension than non-carriers; however, the GNB3 C825T polymorphism was not a risk factor for stroke.
Table 4. The main results of meta-analysis of the association between the GNB3 C825T polymorphism and hypertension.
| T allele vs. C allele (allelic model) | TT+CT vs. CC (dominant model) | TT vs. CT+CC (recessive model) | |||||||
| Study group | OR (95%CI) | p | I 2 | OR (95%CI) | p | I2 | OR (95%CI) | p | I2 |
| Overall | 1.07 (1.01,1.13) | 0.02 | 71% | 1.08 (0.98,1.81) | 0.11 | 74% | 1.05 (0.97,1.14) | 0.23 | 58% |
| Excluding outlier studies | 1.03 (1.00,1.06) | 0.06 | 0% | 1.05 (1.00,1.11) | 0.03 | 0% | 1.00 (0.95,1.05) | 0.92 | 0% |
| Male | 0.93 (0.79,1.11) | 0.43 | 71% | 1.01 (0.80,1.28) | 0.92 | 59% | 1.02 (0.87,1.18) | 0.82 | 45% |
| Female | 1.11 (0.99,1.24) | 0.08 | 0% | 1.05 (0.90,1.24) | 0.53 | 0% | 1.35 (1.07,1.70) | 0.01 | 0% |
| Caucasian | 1.18 (1.00,1.39) | 0.05 | 76% | 1.22 (0.97,1.54) | 0.09 | 79% | 1.10 (0.90,1.34) | 0.36 | 20% |
| Asian | 1.05 (0.99,1.11) | 0.12 | 68% | 1.05 (0.94,1.16) | 0.39 | 72% | 1.04 (0.95,1.15) | 0.37 | 63% |
| HWE | |||||||||
| Y | 1.03 (0.97,1.10) | 0.32 | 68% | 1.04 (0.96,1.14) | 0.34 | 58% | 1.02 (0.93,1.11) | 0.71 | 52% |
| N | 1.18 (1.06,1.33) | 0.004 | 70% | 1.13 (0.86,1.48) | 0.39 | 88% | 1.19 (0.96,1.47) | 0.11 | 71% |
| Source of control | |||||||||
| HB | 1.07 (0.99,1.16) | 0.07 | 0% | 1.15 (0.92,1.44) | 0.23 | 35% | 1.11 (0.89,1.39) | 0.34 | 17% |
| PB | 1.07 (1.00,1.13) | 0.05 | 74% | 1.07 (0.97,1.18) | 0.21 | 76% | 1.04 (0.96,1.14) | 0.34 | 62% |
| Normal population* | 1.04 (0.97,1.12) | 0.25 | 69% | 1.05 (0.96,1.16) | 0.30 | 61% | 1.03 (0.93,1.14) | 0.57 | 59% |
| Score≥8 | 1.08 (1.01,1.16) | 0.03 | 76% | 1.07 (0.97,1.18) | 0.20 | 75% | 1.03 (0.97,1.11) | 0.34 | 32% |
p, a p-value of combined effect; CI, confidence interval;
, We conducted the analyses by limiting the studies according to the HWE and excluding those that included myocardial infarction, obesity, or diabetes mellitus in the cases or controls.
Table 5. The main results of meta-analysis of association between the GNB3 C825T polymorphism and stroke.
| T allele vs. C allele (allelic model) | TT+CT vs. CC (dominant model) | TT vs. CT+CC (recessive model) | |||||||
| Study group | OR (95%CI) | p | I 2 | OR (95%CI) | P | I2 | OR (95%CI) | p | I2 |
| Overall | 1.11 (0.94,1.32) | 0.22 | 81% | 1.16 (0.92,1.48) | 0.21 | 76% | 1.08 (0.84,1.38) | 0.54 | 71% |
| Excluding outlier studies | 1.06 (0.97,1.15) | 0.20 | 0% | 1.05 (0.94,1.17) | 0.36 | 13% | 1.11 (0.96,1.29) | 0.16 | 34% |
| Asian | 1.15 (0.92,1.43) | 0.22 | 84% | 1.21 (0.89,1.63) | 0.23 | 79% | 1.13 (0.82,1.56) | 0.45 | 76% |
| Ischemic stroke | 1.50 (1.12,2.00) | 0.28 | 84% | 1.24 (0.89,1.73) | 0.21 | 81% | 1.01 (0.74,1.38) | 0.95 | 68% |
| HWE (Y) | 1.12 (0.93,1.34) | 0.23 | 84% | 1.19 (0.92,1.54) | 0.18 | 79% | 1.05 (0.82,1.35) | 0.68 | 74% |
| Score≥8 | 0.99 (0.85,1.14) | 0.85 | 73% | 1.01 (0.90,1.15) | 0.81 | 0% | 0.95 (0.70,1.29) | 0.74 | 83% |
p, a p-value of combined effect; CI: confidence interval.
Figure 2. A forest plot for (A) the allelic model (T allele vs. C allele), (B) the dominant model (GG+GA vs. AA), and (C) the recessive model (TT vs. CT+CC).
Random effects models were used with I2 values of 81, 76, and 71%. No evidence of association between the GNB3 C825T polymorphism and stroke were detected in the allelic model (OR = 1.11, 95% CI = 0.94–1.32), dominant model (OR = 1.16, 95% CI = 0.92–1.48), or recessive model (OR = 1.08, 95% CI = 0.84–1.38).
We also performed a meta-analysis to detect any association between males and females; however, only the recessive model (OR = 1.35, 95% CI = 1.07) identified a risk of hypertension among females.
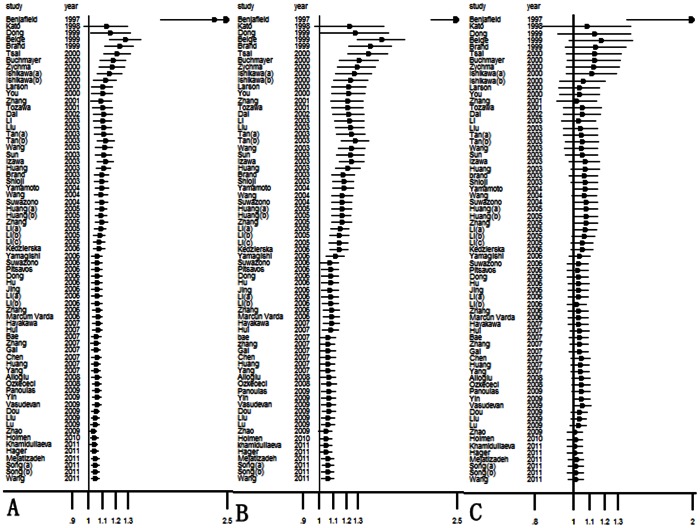
In the cumulative meta-analysis by year of publication, the ORs and 95% CIs became more stable (Figure 3). Study by Benjafield et al. [41] was the first publication to report a significant association between the GNB3 C825T polymorphism and hypertension and triggered the identification of subsequent related studies that tried to replicate the initial results. In the allelic, dominant, and recessive models, the study by Benjafield et al. [41] was the most influential and made the overall estimation more significant in the present cumulative meta-analysis. After the study by Dou et al. [42] was included, the overall estimation became more accurate for the larger sample size.
Figure 3. A cumulative plot by publication year for (A) the allelic model, (B) the dominant model, and (C) the recessive model.
The ORs and associated 95% CIs became more stable over time. The study by Benjafield et al. [41] was the first report to show a significant association between the GNB3 C825T polymorphism and the risk of hypertension, and this study likely influenced the overall estimation.
Subgroup Analysis
To further clarify heterogeneity among the studies, we performed subgroup analysis. Regarding the hypertension study population, the status of the HWE and the source of control had a critical role in heterogeneity (detailed data is presented in Table 4). Interestingly, only the allelic model, which was not consistent with the HWE, yielded a marginally significant risk of hypertension (OR = 1.18, 95% CI = 1.06–1.33), but no evidence of an association was found in the source of the control studies (controls were population-based or hospital-based).
Only one publication regarding Caucasians was screened in an analysis of the association between the GNB3 C825T polymorphism and stroke, and all of the control sources were population-based, thus we did not perform subgroup analysis by ethnicity. Similarly, there were only two studies regarding an African population and hypertension, further indicating that subgroup analysis by ethnicity was to be avoided.
Sensitivity Analysis
To further strengthen the confidence of the results of this meta-analysis, sensitivity analysis was conducted by limiting the included studies with NOS scores ≥8 or restricted analysis on hypertension populations according to the HWE and without other diseases or only included Asian and/or Caucasian populations. All comparative models found no association with hypertension, which suggested that the T allele or TT genotype may not be a risk factor for hypertension (detailed data is presented in Table 4). Importantly, the sensitivity analysis results were slightly out of agreement with those of the initial analysis; therefore, the results should be interpreted cautiously.
As to the association of stroke, when we restricted the analyses by limiting the included studies according to the HWE, the recalculated pooled OR values did not alter the initial results, suggesting that the TT genotype or T allele was not a risk factor of stroke (detailed data are presented in Table 5). Similarly, when we evaluated the ischemic stroke population or Asian population, no evidence of statistical association was obtained.
Publication Bias
Funnel plots were constructed and the Egger's test was performed to assess publication bias of the studies. Funnel plots should be symmetrical when no publication bias exists (Figures 4 and 5). Regarding the hypertension population, only the recessive model displayed an asymmetric funnel plot, while the Egger's regression test confirmed the presence of moderate publication bias (p = 0.043). No statistical evidence of publication bias was identified regarding the GNB3 C825T polymorphism and its association with stroke.
Figure 4. Funnel plots for the GNB3 C825T polymorphism and its association with hypertension.
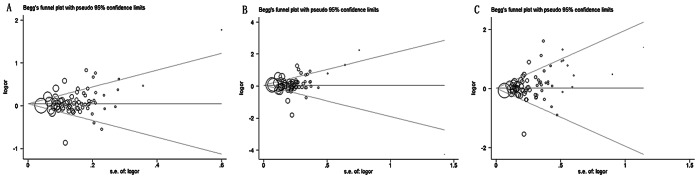
(A) the allelic model (T allele vs. C allele, p = 0.150), (B) the dominant model (TT+CT vs. CC, p = 0.565), and (C) the recessive model (TT vs. CT+CC, p = 0.043). The funnel plots should be symmetrical when no publication bias occurs; however, the funnel plot of the recessive model was asymmetrical (p = 0.043), suggesting publication bias. The other two were symmetrical (p = 0.150 and 0.565, respectively). SE, standard error; OR, odds ratio.
Figure 5. Funnel plots for the GNB3 C825T polymorphism and its association with stroke.
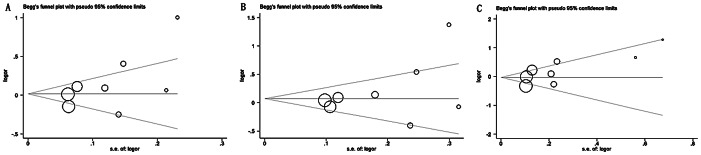
(A) the allelic model (T allele vs. C allele, p = 0.145), (B) the dominant model (TT+CT vs. CC, p = 0.281), and (C) the recessive model (TT vs. CT+CC, p = 0.116). The funnel plots should be symmetrical when no publication bias occurs. No evidence of publication bias was detected in the three models. SE, standard error; OR, odds ratio.
Discussion
Stroke is a significant event that leads to increased mortality and morbidity and hypertensive individuals reportedly have a greater incidence of stroke than normotensive individuals. Genetic factors as well as obesity, high sodium intake, physical inactivity, low potassium diets, and alcohol consumption contribute to the occurrence of hypertension, and essential hypertension status may play a role in the etiology of stroke either through effects on blood pressure levels or through separate pathways [11], [43]. The established relationship between hypertension and stroke suggested that these disorders may have at least some genes in common. Recently, several studies reported that the GNB3 825T polymorphism was associated with an increased risk of hypertension, obesity, metabolic syndrome, atherosclerosis, and diabetes mellitus. Besides, the GNB3 825T allele was found to significantly increase the risk of clinical ischemic stroke in Caucasians, but not subclinical cerebral infarct [12], [44]. However, the present meta-analysis was designed to confirm the association between the GNB3 C825T polymorphism and essential hypertension or stroke.
Overall, our meta-analytical results showed that the GNB3 825T allele had a weak association with essential hypertension. However, after we restricted the studies according to the HWE and included only those without other diseases, such as diabetes and myocardial infarction, all of the compared models failed to identify an association between the GNB3 825T allele and hypertension. Similarly, when we performed sensitivity analysis with the inclusion criteria of “Asian” or “Caucasian,” no evidence of an association was obtained, which might be due to heterogeneity between the studies. Besides, the funnel plot was asymmetric in the recessive model for p = 0.043, so publication bias must also be considered. In addition, our results were consistent with those reported in previous studies [45], [46], but were slightly less discrepant with others [47], which might have resulted from the greater number of studies included in our meta-analysis. However, there were only two studies concerning an African population, thus a larger sample size is needed to further address the relationship between the GNB3 C825T polymorphism and essential hypertension in Africans.
Interestingly, the GNB3 C825T polymorphism was not associated with stroke. When we retrieved studies on ischemic stroke cases or limited the studies according to the HWE or an NOS score of ≥8, similar results were obtained, suggesting that our initial results were reliable and in line with most of the included studies. But, considering that most of the included stroke patients were Asian, our results cannot be directly used to extrapolate a correlation between the GNB3 c825T polymorphism and stroke in Caucasians, Africans, or other ethnicities.
In addition, we tested the T allele frequency in controls (hypertensive population) (Table 1), and found that there was statistical significance between Asian, Caucasian, and African groups (p = 0.0001). This result was in agreement with a previous study [48] that reported varied frequencies of the T allele among different ethnic groups, in which the highest rate occurred in Africans (T = 79%), followed by Asians (T = 46%), and then Caucasians (T = 33%). However, no statistical significance was found between males and females (p = 0.337). Therefore, it is likely that a higher T allele frequency is not necessarily indicative of an increased occurrence of hypertension.
Generally, the GNB3 825T allele was only slightly associated with an increased risk of essential hypertension compared to non-carriers. But, the GNB3 C825T polymorphism failed to contribute to the risk of stroke, thus it was clear that the polymorphism contributed to hypertension and stroke differently. Therefore, gene-gene interactions should be taken into consideration. Until now, >500 candidate genes for hypertension have been suggested from a variety of genetic studies, and this number continues to increase [1], but not all of these genes were associated with an increased risk of stroke. Distribution of the C825T genotypes varies greatly in different ethnicities and the frequency of the T allele is highest in Africans, lowest in Caucasians, and intermediate in Asians. However, the CC genotype is rare in Africans and the distribution of East Asian genotypes is roughly 25% TT, 50% TC, and 25% CC [4]. Thus, individuals from different ethnicities may develop cardiovascular disorders, such as hypertension or stroke, which more or less differ in pathogenesis/pathophysiology of a given disorder due to different genetic backgrounds. In our meta-analysis, individual studies on Africans, Asians, and other ethnicities were deficient; therefore, additional evidence regarding the correlation of the GNB3 C825T polymorphism with hypertension or stroke is required. The interactions between environmental and genetic factors constitute a key issue in the pathogenesis of hypertension and stroke. Most of the susceptibility genes for common diseases such as hypertension do not have a strong primary etiological role in disease predisposition, but rather code response elements to exogenous environmental factors. Therefore, a genetic marker may have only a modest affect on calculating risk in individuals who minimize exposure to environmental factors, but a major effect in individuals exposed to high-risk environment factors [49]. Young et al. [50] reported that latitude was an ecological factor that affected blood pressure via temperature and humidity. Likewise, GNB3 presents a number of functional alleles that influence hypertension susceptibility. Therefore, those populations that have a high prevalence of the GNB3 825T allele also have a higher prevalence of heat-adapted alleles at other SNPs. Physical inactivity, increased body mass, obesity, and smoking may also influence the risk of hypertension and stroke differently.
In addition to race, gender also seems to be an important risk factor for adverse cardiovascular events, such as hypertension and stroke. Suwazono et al. [51] reported that the 825T allele was an independent risk factor for hypertension in Japanese females, whereas Beige et al. [52] found that the T allele in males was associated with higher blood pressure. In our analysis, the GNB3 TT genotype was marginally associated with hypertension among females, and no evidence of an association with hypertension was found in males. In the cumulative meta-analysis, three models showed evidence of a non-association between GNB3 alleles and hypertension or stroke. Two primary causes may account for this discrepancy. First, females have dominant parasympathetic and subordinate sympathetic activities compared to males, and, secondly, estrogen plays an important role in gender-related differences in the autonomic nervous system [51]. Thus, it seems that different automatic functions between genders altered the association of the GNB3 825T allele with hypertension.
Some limitations of the present meta-analysis should be considered. Firstly, all of the included studies mostly involved Caucasians and Asians, thus studies on other ethnic populations are needed. Secondly, all of the included studies were case-controlled and all of the cases involved survivors of hypertension and stroke. Finally, the number of stroke cases were limited and had relatively weak statistical power to detect potential risks of the GNB3 C825T polymorphism. Thus, more population-based studies with large sample sizes are required. Despite these limitations, this meta-analysis was designed to overcome the limitations of individual studies, thus the results should be more reliable. Since the GNB3 C825T polymorphism appears to be a useful marker to predict the relative risk of diseases, such as hypertension and stroke, this meta-analysis is better suited in a preventive aspect to identify certain genotypes that will be most likely to benefit from pharmacological interventions.
In summary, the overall analysis of available evidence suggested that the GNB3 825T allele may be a good indicator of hypertension; however, it had no association with hypertension in Asians and Caucasians and there was lack of evidence to support an association with stroke in Asians. Therefore, multiethnic studies with much larger sample-sizes are required to better evaluate the association between the GNB3 C825T polymorphism and hypertension or stroke.
Supporting Information
PRISMA Checklist.
(DOC)
Funding Statement
This work was financially supported by the National Natural Science Foundation of China (81271282, 30970998) and by the Chongqing Natural Science Foundation (CSTC2011BB5031). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Kuo TY, Kang MJ, Chen JW, Ho HY, Ting CT, et al. (2012) A two-stage matched case-control study on multiple hypertensive candidate genes in Han Chinese. Am J Hypertens 25: 804–811. [DOI] [PubMed] [Google Scholar]
- 2. Dichgans M (2007) Genetics of ischaemic stroke. The Lancet Neurology 6: 149–161. [DOI] [PubMed] [Google Scholar]
- 3. Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, et al. (2011) Heart Disease and Stroke Statistics–2012 Update: A Report From the American Heart Association. Circulation 125: e2–e220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Siffert W (2005) G protein polymorphisms in hypertension, atherosclerosis, and diabetes. Annu Rev Med 56: 17–28. [DOI] [PubMed] [Google Scholar]
- 5. Klenke S, Kussmann M, Siffert W (2011) The GNB3 C825T polymorphism as a pharmacogenetic marker in the treatment of hypertension, obesity, and depression. Pharmacogenet Genomics 21: 594–606. [DOI] [PubMed] [Google Scholar]
- 6. Turner ST, Schwartz GL, Chapman AB, Boerwinkle E (2001) C825T Polymorphism of the G Protein 3-Subunit and Antihypertensive Response to a Thiazide Diuretic. Hypertension 37: 739–743. [DOI] [PubMed] [Google Scholar]
- 7. Siffert W, Rosskopf D, Siffert G, Busch S, Moritz A, et al. (1998) Association of a human G-protein beta3 subunit variant with hypertension. Nat Genet 18: 45–48. [DOI] [PubMed] [Google Scholar]
- 8. Sun A, Ge J, Siffert W, Frey UH (2005) Quantification of allele-specific G-protein beta3 subunit mRNA transcripts in different human cells and tissues by Pyrosequencing. Eur J Hum Genet 13: 361–369. [DOI] [PubMed] [Google Scholar]
- 9. Lee JY, Kim SU, Cho JK, Woo SK, Kang HS (2009) GNB3 C825T polymorphism and elevated blood pressure. Int J Sports Med 30: 892–897. [DOI] [PubMed] [Google Scholar]
- 10. Weinstein LS, Chen M, Xie T, Liu J (2006) Genetic diseases associated with heterotrimeric G proteins. Trends Pharmacol Sci 27: 260–266. [DOI] [PubMed] [Google Scholar]
- 11. Morrison AC, Doris PA, Folsom AR, Nieto FJ, Boerwinkle E, et al. (2001) G-Protein 3 Subunit and -Adducin Polymorphisms and Risk of Subclinical and Clinical Stroke Editorial Comment : Candidate Genes for Stroke: If Elected, Will They Serve? Stroke 32: 822–829. [DOI] [PubMed] [Google Scholar]
- 12. Zhang L, Zhang H, Sun K, Song Y, Hui R, et al. (2005) The 825C/T polymorphism of G-protein beta3 subunit gene and risk of ischaemic stroke. J Hum Hypertens 19: 709–714. [DOI] [PubMed] [Google Scholar]
- 13. Moher D, Liberati A, Tetzlaff J, Altman DG (2010) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg 8: 336–341. [DOI] [PubMed] [Google Scholar]
- 14. McAuley L, Pham B, Tugwell P, Moher D (2000) Does the inclusion of grey literature influence estimates of intervention effectiveness reported in meta-analyses? Lancet 356: 1228–1231. [DOI] [PubMed] [Google Scholar]
- 15. Little J, Bradley L, Bray MS, Clyne M, Dorman J, et al. (2002) Reporting, appraising, and integrating data on genotype prevalence and gene-disease associations. Am J Epidemiol 156: 300–310. [DOI] [PubMed] [Google Scholar]
- 16. Pan Z, Trikalinos TA, Kavvoura FK, Lau J, Ioannidis JP (2005) Local literature bias in genetic epidemiology: an empirical evaluation of the Chinese literature. PLoS Med 2: e334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Petiti DB (1999) Meta-analysis, decision analysis, and cost-effectiveness analysis: methods for quantitative synthesis in medicine. Oxford University Press. [Google Scholar]
- 19. Mantel N, Haenszel W (1959) Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst 22: 719–748. [PubMed] [Google Scholar]
- 20. DerSimonian R, Kacker R (2007) Random-effects model for meta-analysis of clinical trials: an update. Contemp Clin Trials 28: 105–114. [DOI] [PubMed] [Google Scholar]
- 21. DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7: 177–188. [DOI] [PubMed] [Google Scholar]
- 22.Galbraith RF (1988) Graphical Display of Estimates Having Differing Standard Erro. Technometrics. 271–281. [Google Scholar]
- 23. Ioannidis JP, Trikalinos TA (2005) Early extreme contradictory estimates may appear in published research: the Proteus phenomenon in molecular genetics research and randomized trials. J Clin Epidemiol 58: 543–549. [DOI] [PubMed] [Google Scholar]
- 24. Begg CB, Mazumdar M (1994) Operating characteristics of a rank correlation test for publication bias. Biometrics 50: 1088–1101. [PubMed] [Google Scholar]
- 25. Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315: 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ishikawa K, Imai Y, Katsuya T, Ohkubo T, Tsuji I, et al. (2000) Human G-protein beta3 subunit variant is associated with serum potassium and total cholesterol levels but not with blood pressure. Am J Hypertens 13: 140–145. [DOI] [PubMed] [Google Scholar]
- 27. Song J, Lu S, Gao XH, Zhang SJ (2011) Polymorphism of G protein β3 subunit 825C/T in Korean population and Han population with essential hypertension in Mudanjiang region. Journal of Clinical Rehabilitative Tissue Engineering Research 15: 9491–9495. [Google Scholar]
- 28. Huang XM, Jiang XD, Duan YF, Zhang S, Yao LP, et al. (2005) Polymorphism: analysis of 825C/T of the G-protein B3 subunit gene in high risk population of hypertension of Dong nationality group in Huaihua district of Huna. Chinese Journal of a birth Health and Heredity 13: 21–23. [Google Scholar]
- 29. Zeltner R, Delles C, Schneider M, Siffert W, Schmieder RE (2001) G-Protein 3 Subunit Gene (GNB3) 825T Allele Is Associated With Enhanced Renal Perfusion in Early Hypertension. Hypertension 37: 882–886. [DOI] [PubMed] [Google Scholar]
- 30.Chen YY, Li GW, Li CM, Huang XH, Ju ZY, et al.. (2003) Association between G-protein B3 subunit (GNB3 ) gene C825T polymorphism, hypertension, insulin resistance and obesity. Nat Med J China. [PubMed] [Google Scholar]
- 31. Kato N, Sugiyama T, Morita H, Kurihara H, Yamori Y, et al. (1998) G Protein 3 Subunit Variant and Essential Hypertension in Japanese. Hypertension 32: 935–938. [DOI] [PubMed] [Google Scholar]
- 32. Vasudevan R, Ismail P, Stanslas J, Shamsudin N (2009) Analysis of three genetic polymorphisms in Malaysian essential hypertensive and type 2 diabetic subjects. African Journal of Biotechnology 8: 2069–2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sun LL, Wang HY, Ji LN, Han XY, Zhu YG (2003) Association of G protein subunit 825T allele with diabetes mellitus and diabetic nephropathy in Chinese. Chin J Diabete 11: 110–113. [Google Scholar]
- 34. Zhao Y, Ma LY, Liu YC, Wang XY, Liu LS, et al. (2001) The relationship between GNB3 gene C825T polymorphism and ischemic stroke. Stroke And nervous Diseases 8: 14–16. [Google Scholar]
- 35. Tan JC, Zhu SJ, Zhu ZM, Yu CQ, Wang L, et al. (2003) The G-protein B3 subunit gene C825T polymorphism and related risk factors in patients with ischemic stroke. Chin J Geriatr Cardiovasc Cerebr vasc Dis 5: 177–180. [Google Scholar]
- 36. Wang HL, Li ZZ, Zhang JF, Wang ZZ, SHi FP (2011) C825T polymorphism of G protein B3 subunit in cerebral infarction patients with a history of hypertension. Int J cerebrovasc Dis 19: 580–584. [Google Scholar]
- 37.Zhao Y, Wang JC, Sun H, Ma LY, Sun JF, et al.. (2004) Lack of association of G-protein subunit gene C825T polymorphism with hemorrhagic stroke in Chinese population. J Apoplexy and Nervous Diseases 21–23. [Google Scholar]
- 38.Zhao Y (2000) Relationship between stroke and its related gene polymorphism. Chinese Doctoral Dissertation Full-text Database. [Google Scholar]
- 39.Li QX (2003) Association of Candidate Gene With Hypertension, target-organ Complication Chinese Doctoral Dissertation Full-text Database. [Google Scholar]
- 40. Higgins JPT, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. British Medical Journal 327: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Benjafield AV, Jeyasingam CL, Nyholt DR, Griffiths LR, Morris BJ (1998) G-protein beta3 subunit gene (GNB3) variant in causation of essential hypertension. Hypertension 32: 1094–1097. [DOI] [PubMed] [Google Scholar]
- 42. Dou GW, Qi Y, Wu ZH (2009) Relationship between GNb3 (G-protein B3 subunit) gene C825T polymorphism and essential hypertension. Journal of Biology 26: 18–20. [Google Scholar]
- 43. Montasser ME, Gu D, Chen J, Shimmin LC, Gu C, et al. (2011) Interactions of genetic variants with physical activity are associated with blood pressure in Chinese: the GenSalt study. Am J Hypertens 24: 1035–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Chistiakov DA, Spitsina EV, Nikitin AG, Strokov IA, Nosikov VV (2009) A splice variant of GNB3 and peripheral polyneuropathy in type 1 diabetes. Dis Markers 26: 111–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Niu WQ, Qi Y (2011) Association of alpha-Adducin and G-Protein beta 3 Genetic Polymorphisms with Hypertension: A Meta-Analysis of Chinese Populations. PLoS One 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lu JP, Guo QQ, Zhang L, Wang W (2012) Association between the G-protein beta 3 subunit C825T polymorphism with essential hypertension: a meta-analysis in Han Chinese population. Molecular Biology Reports 39: 8937–8944. [DOI] [PubMed] [Google Scholar]
- 47. Bagos PG, Elefsinioti AL, Nikolopoulos GK, Hamodrakas SJ (2007) The GNB3 C825T polymorphism and essential hypertension: a meta-analysis of 34 studies including 14,094 cases and 17,760 controls. Journal of Hypertension 25: 487–500. [DOI] [PubMed] [Google Scholar]
- 48. Siffert W, Forster P, Jockel KH, Mvere DA, Brinkmann B, et al. (1999) Worldwide ethnic distribution of the G protein beta3 subunit 825T allele and its association with obesity in Caucasian, Chinese, and Black African individuals. J Am Soc Nephrol 10: 1921–1930. [DOI] [PubMed] [Google Scholar]
- 49. Sarzynski MA, Rankinen T, Sternfeld B, Fornage M, Sidney S, et al. (2011) SNP-by-fitness and SNP-by-BMI interactions from seven candidate genes and incident hypertension after 20 years of follow-up: the CARDIA Fitness Study. J Hum Hypertens 25: 509–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Young JH, Chang YP, Kim JD, Chretien JP, Klag MJ, et al. (2005) Differential susceptibility to hypertension is due to selection during the out-of-Africa expansion. PLoS Genet 1: e82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Suwazono Y, Kobayashi E, Uetani M, Miura K, Morikawa Y, et al. (2006) G-protein beta3 subunit variant C825T is a risk factor for hypertension in Japanese females–a prospective cohort study over 5 years. Ann Hum Genet 70: 767–777. [DOI] [PubMed] [Google Scholar]
- 52. Brand E, Wang JG, Herrmann SM, Staessen JA (2003) An epidemiological study of blood pressure and metabolic phenotypes in relation to the Gbeta3 C825T polymorphism. Journal of Hypertension 21: 729–737. [DOI] [PubMed] [Google Scholar]
- 53. Shioji K, Kokubo Y, Mannami T, Inamoto N, Morisaki H, et al. (2004) Association between hypertension and the alpha-adducin, beta1-adrenoreceptor, and G-protein beta3 subunit genes in the Japanese population; the Suita study. Hypertens Res 27: 31–37. [DOI] [PubMed] [Google Scholar]
- 54. Dong Y, Zhu H, Sagnella GA, Carter ND, Cook DG, et al. (1999) Association between the C825T polymorphism of the G protein beta3-subunit gene and hypertension in blacks. Hypertension 34: 1193–1196. [DOI] [PubMed] [Google Scholar]
- 55. Yamamoto M, Abe M, Jin JJ, Wu Z, Tabara Y, et al. (2004) Association of GNB3 gene with pulse pressure and clustering of risk factors for cardiovascular disease in Japanese. Biochem Biophys Res Commun 316: 744–748. [DOI] [PubMed] [Google Scholar]
- 56. Hayakawa T, Takamura T, Abe T, Kaneko S (2007) Association of the C825T polymorphism of the G-protein beta3 subunit gene with hypertension, obesity, hyperlipidemia, insulin resistance, diabetes, diabetic complications, and diabetic therapies among Japanese. Metabolism 56: 44–48. [DOI] [PubMed] [Google Scholar]
- 57. Khamidullaeva GA, Eliseyeva MR, Nagay AV, Abdullaeva GJ (2011) C825T polymorphism of the G-protein beta3 subunit and its association with essential hypertension in Uzbek males. Turk Kardiyol Dern Ars 39: 198–204. [DOI] [PubMed] [Google Scholar]
- 58. Hui P, Nakayama T, Morita A, Sato N, Hishiki M, et al. (2007) Common single nucleotide polymorphisms in Japanese patients with essential hypertension: aldehyde dehydrogenase 2 gene as a risk factor independent of alcohol consumption. Hypertens Res 30: 585–592. [DOI] [PubMed] [Google Scholar]
- 59. Alioglu E, Ercan E, Tengiz I, Yildiz A, Onsel Turk U, et al. (2008) G protein beta3 subunit gene polymorphism in Turkish hypertensives. Anadolu Kardiyol Derg 8: 331–335. [PubMed] [Google Scholar]
- 60.Tsai CH, Yeh HI, Chou Y, Liu HF, Yang TY, et al.. (2000) G protein beta3 subunit variant and essential hypertension in Taiwan - a case-control study. Int J Cardiol 73: 191–195; discussion 197–198. [DOI] [PubMed] [Google Scholar]
- 61. Kedzierska K, Ciechanowski K, Safranow K, Bober J, Golembiewska E, et al. (2006) GNB3 C825T and ACE I/D polymorphisms on the sodium-proton exchanger and the prevalence of essential hypertension in males. Arch Med Res 37: 150–157. [DOI] [PubMed] [Google Scholar]
- 62. Hager A, Bildau J, Kreuder J, Kaemmerer H, Hess J (2011) Impact of genomic polymorphism on arterial hypertension after aortic coarctation repair. Int J Cardiol 151: 63–68. [DOI] [PubMed] [Google Scholar]
- 63. Marcun Varda N, Zagradisnik B, Herodez SS, Kokalj Vokac N, Gregoric A (2006) Polymorphisms in four candidate genes in young patients with essential hypertension. Acta Paediatr 95: 353–358. [DOI] [PubMed] [Google Scholar]
- 64. Holmen OL, Romundstad S, Melien O (2010) Association between the G protein beta3 subunit C825T polymorphism and the occurrence of cardiovascular disease in hypertensives: The Nord-Trondelag Health Study (HUNT). Am J Hypertens 23: 1121–1127. [DOI] [PubMed] [Google Scholar]
- 65. Tozawa Y (2001) G protein beta3 subunit variant: tendency of increasing susceptibility to hypertension in Japanese. Blood Press 10: 131–134. [DOI] [PubMed] [Google Scholar]
- 66. Wang X, Wang S, Lin R, Jiang X, Cheng Z, et al. (2004) GNB3 gene C825T and ACE gene I/D polymorphisms in essential hypertension in a Kazakh genetic isolate. J Hum Hypertens 18: 663–668. [DOI] [PubMed] [Google Scholar]
- 67. Buchmayer H, Sunder-Plassmann G, Hirschl MM, Kletzmayr J, Woisetschlager C, et al. (2000) G-protein beta3 subunit gene (GNB3) polymorphism 825C–>T in patients with hypertensive crisis. Crit Care Med 28: 3203–3206. [DOI] [PubMed] [Google Scholar]
- 68. Yamagishi K, Tanigawa T, Cui R, Tabata M, Ikeda A, et al. (2006) G-protein beta-3 subunit C825T polymorphism, sodium and arterial blood pressure: a community-based study of Japanese men and women. Ann Hum Genet 70: 759–766. [DOI] [PubMed] [Google Scholar]
- 69. Beige J, Hohenbleicher H, Distler A, Sharma AM (1999) G-Protein beta3 subunit C825T variant and ambulatory blood pressure in essential hypertension. Hypertension 33: 1049–1051. [DOI] [PubMed] [Google Scholar]
- 70. Zychma MJ, Zukowska-Szczechowska E, Ossowska-Szymkowicz I, Trautsolt W, Grzeszczak W (2000) G-Protein beta(3) subunit C825T variant, nephropathy and hypertension in patients with type 2 (Non-insulin-dependent) diabetes mellitus. Am J Nephrol 20: 305–310. [DOI] [PubMed] [Google Scholar]
- 71. Li B, Ge D, Wang Y, Zhao W, Zhou X, et al. (2005) G protein beta 3 subunit gene variants and essential hypertension in the northern Chinese Han population. Ann Hum Genet 69: 468–473. [DOI] [PubMed] [Google Scholar]
- 72. Bae Y, Park C, Han J, Hong YJ, Song HH, et al. (2007) Interaction between GNB3 C825T and ACE I/D polymorphisms in essential hypertension in Koreans. J Hum Hypertens 21: 159–166. [DOI] [PubMed] [Google Scholar]
- 73. Panoulas VF, Smith JP, Stavropoulos-Kalinoglou A, Douglas KM, Nightingale P, et al. (2009) Lack of an association of GNB3 C825T polymorphism and blood pressure in patients with rheumatoid arthritis. Clin Exp Hypertens 31: 428–439. [DOI] [PubMed] [Google Scholar]
- 74. Huang X, Ju Z, Song Y, Zhang H, Sun K, et al. (2003) Lack of association between the G protein beta3 subunit gene and essential hypertension in Chinese: a case-control and a family-based study. J Mol Med (Berl) 81: 729–735. [DOI] [PubMed] [Google Scholar]
- 75. Larson N, Hutchinson R, Boerwinkle E (2000) Lack of Association of 3 Functional Gene Variants With Hypertension in African Americans. Hypertension 35: 1297–1300. [DOI] [PubMed] [Google Scholar]
- 76. Suwazono Y, Okubo Y, Kobayashi E, Miura K, Morikawa Y, et al. (2004) Lack of association of human G-protein beta3 subunit variant with hypertension in Japanese workers. Journal of Hypertension 22: 493–500. [DOI] [PubMed] [Google Scholar]
- 77. Brand E, Herrmann SM, Nicaud V, Ruidavets JB, Evans A, et al. (1999) The 825C/T polymorphism of the G-protein subunit beta3 is not related to hypertension. Hypertension 33: 1175–1178. [DOI] [PubMed] [Google Scholar]
- 78. Nejatizadeh A, Kumar R, Stobdan T, Pasha MQ (2011) Association of GNB3 C825T polymorphism with plasma electrolyte balance and susceptibility to hypertension. Genet Mol Biol 34: 553–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Pitsavos C, Papadimitriou L, Panagiotakos D, Chrysohoou C, Skoumas J, et al. (2006) Lack of association between the C825T polymorphism in G-protein beta-3 subunit and arterial blood pressure levels in cardiovascular disease free individuals: the ATTICA epidemiological study. J Hum Hypertens 20: 303–305. [DOI] [PubMed] [Google Scholar]
- 80. Izawa H, Yamada Y, Okada T, Tanaka M, Hirayama H, et al. (2003) Prediction of genetic risk for hypertension. Hypertension 41: 1035–1040. [DOI] [PubMed] [Google Scholar]
- 81. Ozkececi E, Gulyasar T, Sener S (2008) The association of hypertension and C825T polymorphism of the gene encoding the G-protein beta-3 subunit (GNB3) in a group of Turkish hypertensive patients. Trakya Universitesi Tip Fakultesi Dergisi 25: 100–104. [Google Scholar]
- 82. Ruixing Y, Jinzhen W, Weixiong L, Yuming C, Dezhai Y, et al. (2009) The environmental and genetic evidence for the association of hyperlipidemia and hypertension. Journal of Hypertension 27: 251–258. [DOI] [PubMed] [Google Scholar]
- 83. Dong HY, Li QR, Wang Q, Luo ZG (2006) Association analysis between genetic polymorphism of ADD-1 gene and GNB3 gene and essential hypertension. South China Journal of Cardiovascular Diseases 12: 258–261. [Google Scholar]
- 84. Zhang CY, zhao SG, Niu GM, Hu RL, Wang ZG, et al. (2007) Genetic predisposition to essential hypertension in a Mongolian population. Neural Regeneration Research 2: 146–150. [Google Scholar]
- 85. Li DB, Hua Q, Pi L, Xu J, Liu RK (2005) The Effects of C825T Polymorphism in G Protein B3 Subunit Gene on Efficacy of Antihypertensive Treatment with Amlodipine. Journal of Capital University of Medical Sciences 26: 725–728. [Google Scholar]
- 86. Hu RL, Zhao SG, Niu GM, H RY, Zhang CY, et al. (2006) The association research between C825T polymorphism of G proteinums B3 subunit gene and Mongolian patient with essential hypertension Chinese Journal of Birth Health and Heredity. 14: 15–17. [Google Scholar]
- 87. Gai XQ, Shi JP, Zhao YY, Dai SP, Fu LY, et al. (2007) Multivariate analysis on the relationship between G protein B3 subunit gene 825C/T polymorphism and essential hypertension Chin J Epidemiol. 28: 413–414. [PubMed] [Google Scholar]
- 88. Chen XJ, Wang DW, Wu JB, Xiong SD, Wang CX (2007) C825T polymorphism of the G protein B3 subunit and its relationship with essential hypertension J Clin Intern Med. 24: 333–334. [Google Scholar]
- 89. Tan JC, Zhu ZM, Zhu SJ, Yu CQ, Wang L, et al. (2003) The relationship between G - protein B3 subunit gene C825T polymorphism and the pathogenesis of essential hypertension. Acta Academiae Medicinae Militaris Tertiae 25: 1381–1384. [Google Scholar]
- 90. Zhang J, Li L, Zhang ZX, Cui TX (2005) Association of G protein B3 subunit polymorphism with essential hypertension in Chinese Medical Information. 18: 358–360. [Google Scholar]
- 91. You T, Huang JQ, L JY (2000) GNB3 subunit gene variant in the causation of essential hypertension Medical Journal of Liaoning. 14: 179–180. [Google Scholar]
- 92. Jing JY, Wang D, Jiao Y, wang XF, Wen H, et al. (2006) Association study on GNB3 gene polymorphism with essential hypertension in Xinjiang Uiygur group Fudan Univ J Med Sci. 33: 433–437. [DOI] [PubMed] [Google Scholar]
- 93. Sun NL, Wang HY, Ji LN, Han XY, Zhu YG (2003) Association of G protein B3 subunit 825T allele with diabetes mellitus and diabetic nephrology in Chinese Chin J Diabetes. 11: 110–113. [Google Scholar]
- 94. Zhang MH, Liu GS, Shi Y, Shi J, Chen Y, et al. (2001) G-protein B3 subunit gene polymorphism and essential hypertension Chinese Journal of Hypertension. 9: 288–291. [Google Scholar]
- 95.liu WJ, Yang WQ, Wang L, Wang XF, Gan ZM (2009) Association Between ADRB2 Gene, ENaC Gene and GNB3 Gene Polymorphism and Hypertension in Uygur Population chinese Circulation Journal 24. [Google Scholar]
- 96. Lu FH, Yang JM, Zhou XH, Wei F, Liu ZD, et al. (2009) Synergistic effects of GNB3 gene C825T and ATG gene Mellitus 35T polymorphism on familial hypertension Chin J Public Health. 25: 20–22. [Google Scholar]
- 97. Li DB, Hua Q, Pi L (2005) Synergistic Effects between eNOS Gene G894T and GNB3 Gene C825T Polymorphisms in Essential Hypertension. Journal of Capi tal Univ er sity o f Medical Sciences 27: 480–484. [Google Scholar]
- 98. Zhao HY, Cao J, Zhou L, Wang B, Qiu CC (2009) Genetic analysis of a-ADDUCIN and GNB3 in essential hypertension patient Basic and Clinical Medicine. 29: 393–396. [Google Scholar]
- 99. Wang HL, Li ZZ, Chen N, Zhou Y, Zhang RF, et al. (2011) Association between gene polymorphism of GNB3 and essential hypertension Clinical Focus. 26: 766–769. [Google Scholar]
- 100. Wang HY, Sun NL, Gao Y, Gou SQ (2003) G protein B3 subunit C825T polymorphism and essential hypertension in Chinese. Journal of PekingUniversity (Health Sciences ) 35: 423–425. [PubMed] [Google Scholar]
- 101. Li QX, Ci WP, Zhang YQ, Guo LF, Zhu XL, et al. (2006) Relationship between essential hypertension and two related gene polymorphism. Chin J Publ ic Health 22: 1334–1135. [Google Scholar]
- 102. Huang WT, Yu HJ, Lu XF, Zhao WY, Wang YL, et al. (2007) Combined Action of ACE Gene I/D and GNB3 Gene C825T polymorphism on essential Hypertension in Northern Han Chinese Progress in Biochemistry and Biophysics. 34: 471–478. [Google Scholar]
- 103. Li QX, Li FY, Du RY, Zhang Y, Q., Yan HB, et al. (2006) C825T Polymorphism of the G-protein B3 Subunit Gene and Hypertension in Changchun Region in China. Journal of Chinese Microcirculation 10: 205–207. [Google Scholar]
- 104. Dai SP, Shi JP, Ding Q, Wang HL, Dong LY, et al. (2002) Polymorphism Analysis of 825C/T of the G -protein B3 Subunit in High Risk Population of Hypertension in the Northeast China. Acta Genetica Sinica 29: 294–298. [PubMed] [Google Scholar]
- 105.Zhang LH (2006) Correlation of 825C/T polymorphism of G-protein beta3 subunit with essential hypertension and concentration of serum vWF. Chinese Master Thesis Full-text Database. [Google Scholar]
- 106.Yang JM (2007) The Study on Risk factors for Cardiovascular Diseases and Candidate Genes for Hypertension in Shandong Population. Chinese Master Thesis Full-text Database. [Google Scholar]
- 107.Liu LF (2003) Hypertension and artery stiffness in relationship to four candidate genes in the Han nationality population Chinese Doctoral Dissertation Full-text Database. [Google Scholar]
- 108. Tan JC, Yu CQ, Wang L, Liu XL, Wang HY (2003) Study on the polymorphism of G -proteinB3 subunit gene in patient s with strok in Chongqing. Chongqing Medicine 32: 13–15. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PRISMA Checklist.
(DOC)