Abstract
In contrast to mammals that have limited proliferation and neurogenesis capacities, the Xenopus frog exhibit a great potential regarding proliferation and production of new cells in the adult brain. This ability makes Xenopus a useful model for understanding the molecular programs required for adult neurogenesis. Transcriptional factors that control adult neurogenesis in vertebrate species undergoing widespread neurogenesis are unknown. NeuroD1 is a member of the family of proneural genes, which function during embryonic neurogenesis as a potent neuronal differentiation factor. Here, we study in detail the expression of NeuroD1 gene in the juvenile and adult Xenopus brains by in situ hybridization combined with immunodetections for proliferation markers (PCNA, BrdU) or in situ hybridizations for cell type markers (Vimentin, Sox2). We found NeuroD1 gene activity in many brain regions, including olfactory bulbs, pallial regions of cerebral hemispheres, preoptic area, habenula, hypothalamus, cerebellum and medulla oblongata. We also demonstrated by double staining NeuroD1/BrdU experiments, after long post-BrdU administration survival times, that NeuroD1 gene activity was turned on in new born neurons during post-metamorphic neurogenesis. Importantly, we provided evidence that NeuroD1-expressing cells at this brain developmental stage were post-mitotic (PCNA-) cells and not radial glial (Vimentin+) or progenitors (Sox2+) cells.
Introduction
Adult neurogenesis is a fascinating biological trait, which has captivated researchers since many years. In mammals and under normal conditions, adult neurogenesis has been identified in two anatomical regions: the subventricular zone (SVZ) lining the lateral ventricles and the subgranular zone (SGZ) of the hippocampal dentate gyrus (reviewed by [1]). Interestingly, adult neurogenesis seems to be more abundant in birds, reptiles, amphibians and fish than in mammals (reviewed by [2]–[5]. Recently, the detailed neuroanatomical mappings of proliferative activity in the adult brain were provided in two non-mammalian vertebrate models, the fish Danio rerio (reviewed in [6]–[8] and the amphibian Xenopus laevis [9]. In these two vertebrates, a widespread proliferation and neurogenic activities were detected. In the adult fish Danio rerio brain, more than ten distinct adult proliferative zones have been identified along the whole brain axis in areas such as olfactory bulb, telencephalon, thalamus, epithalamus, preoptic region, hypothalamus, tectum, cerebellum, rhombencephalon, and spinal cord [10], [11]. In the zebrafish, several studies have demonstrated that radial progenitors exhibit proliferative activity and give birth to at least part of the newborn neurons [12]. In post-metamorphic Xenopus laevis brain, at both juvenile and adult stages, cell proliferation activity was also found in various brain regions, namely, olfactory bulbs, cerebral hemispheres, preoptic region, ventral hypothalamus and cerebellum [9]. Most importantly, new differientiated neurons and oligodendrocytes were clearly detected at these stages [9]. These studies fully confirmed that amphibian and fish adult brains are characterized by a greater number of proliferation and neurogenic compartments than previously described in other vertebrates. However, the molecular mechanisms, and among them the transcriptional program, underlying the widespread neurogenesis found in adult frog and fish brains remains unknown.
Studies in mammals have indicated that several transcription factors of the basic helix-loop-helix (bHLH) family play critical roles not only during during embryonic neurogenesis but also during post-natal neurogenesis [13], [14]. In an effort to identify transcription factors involved in Xenopus post-metamorphic neurogenesis, we have focused our analysis on the NeuroD1 factor, also known as NeuroD or Beta2. NeuroD1 is a proneural basic helix-loop-helix (bHLH) transcription factor that belongs to the Ath (Drosophila Atonal) group, which also includes Math and Neurogenin subfamilies (reviews in [15], [16]. In Xenopus embryo, all primary neurons, neurogenic placodes, and retina strongly express the neuronal diffentiation gene NeuroD1 [17], [18]. When ectopically expressed in Xenopus embryos, NeuroD1 can convert non-neuronal ectodermal cells into fully differentiated neurons, indicating that it can be a potent neuronal differentiation factor [17]. In mammals, NeuroD1 is widely expressed during brain development and is essential for the development of the central nervous sytem, particularly for the generation of granule cells in the hippocampus and cerebellum [19], [20].
To gain further insight into the transcriptional program that controls adult neurogenesis in non-mammalian vertebrates, this study examined in details the NeuroD1 gene expression, together with various cellular markers (Vimentin, Sox2, PCNA and BrdU), in post-metamorphic (juvenile and adult) Xenopus brains. The data show that a high number of NeuroD1-expressing cells can be detected in various brain areas and that NeuroD1 gene is up-regulated during post-metamorphic neurogenesis. Moreover, we provide evidence that NeuroD1 is expressed in post-mitotic (PCNA-) neuronal cells and not in radial glial (Vimentin+) or neural progenitors (Sox2+) cells.
Results
Strong and spatially restricted expression of the NeuroD1 gene in juvenile and adult Xenopus brains
In order to identify the brain sub-divisions that express the NeuroD1 gene, in situ hybridization experiments, using high stringent conditions, were performed on coronal sections throughout the whole juvenile and adult brains. The detailed NeuroD1 expression pattern is illustrated in figures 1 and 2 and described below. There were no significant differences between males and females in any brain regions.
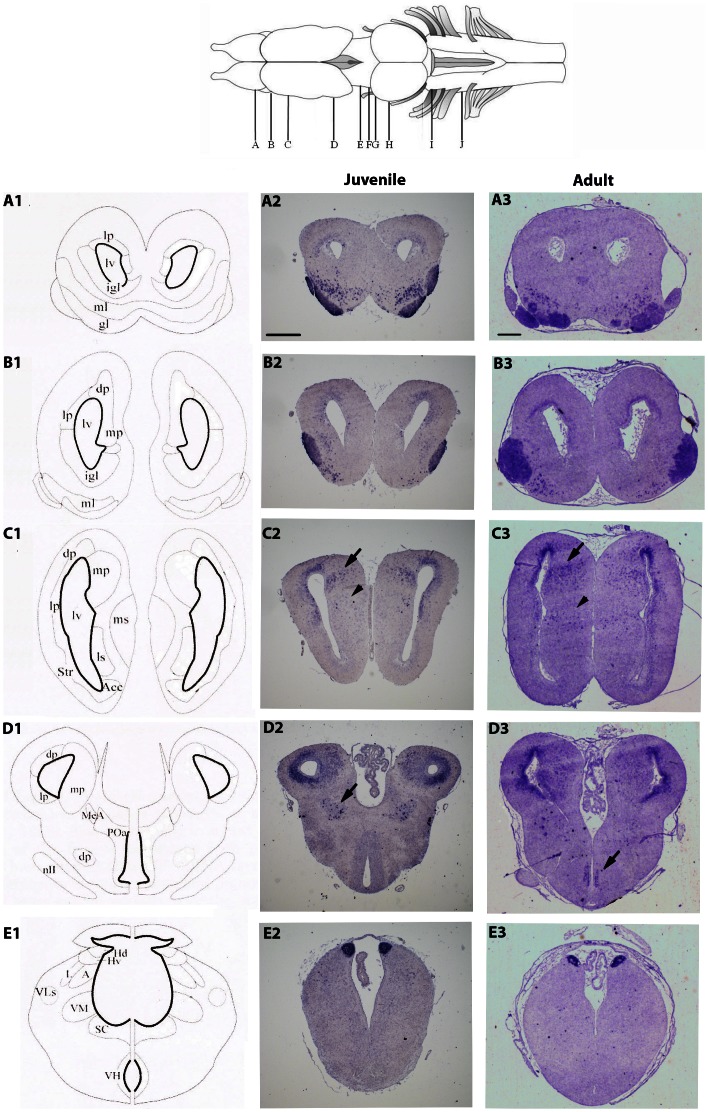
Figure 1. Expression pattern of NeuroD1 in the juvenile (A2–E2) and adult (A3–E3) X. laevis brains.
A1–E1) Schematic coronal illustrations of the corresponding transverse sections of a juvenile X. laevis brain (NF stage 66). The drawing at the top of the figure shows a dorsal view of the X. laevis brain. The letters correspond to the rostro-caudal location of sections as depicted in the whole brain drawing. Arrows and arrowheads in C2, C3, D2, and D3 highlight less conspicuous areas of labeling. Abbreviations are defined in Table 1. The anatomical drawings are from [55], with modifications of basal ganglia subdivisions according to [56]. For all images, dorsal is to the top. Scale bar = 400 µm in A2–E2, and 100 µm in A3–E3.
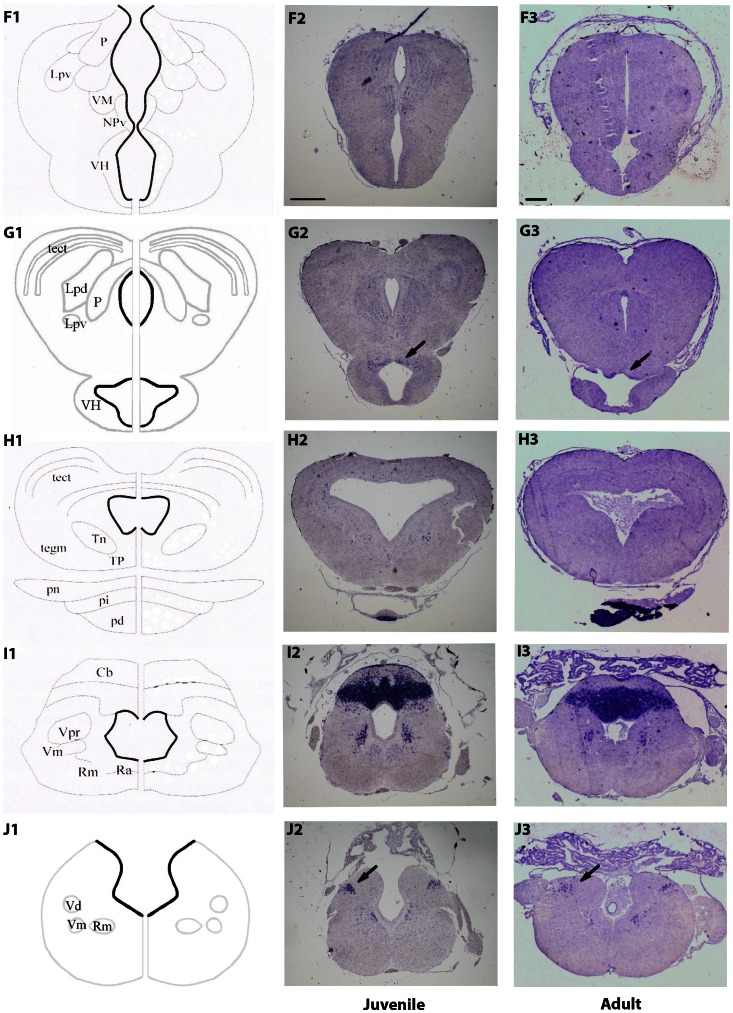
Figure 2. Expression pattern of NeuroD1 in the juvenile (F2–J2) and adult (F3–J3) X. laevis brains.
(F1–J1) Schematic coronal illustrations of the corresponding transverse sections of a juvenile X. laevis brain (NF stage 66). The letters correspond to the rostro-caudal location of sections as depicted in the whole brain drawing (see Figure 1). Arrows and arrowheads in G2, G3, J2 and J3 highlight less conspicuous areas of labeling. Abbreviations are defined in Table 1. The anatomical drawings, with the exceptions of G1 and J1, are from [55], with modifications of basal ganglia subdivisions according to [56]. For all images, dorsal is to the top. Scale bar = 400 µm in F2–J2, and 100 µm in F3–J3.
Table 1. Abbreviations of major neuro-anatomical landmarks.
| A | anterior thalamic nucleus | P | posterior thalamic nucleus |
| Acc | nucleus accumbens | pd | pars distalis |
| Cb | cerebellum | pi | pars intermedia |
| dp | dorsal pallium | pn | pars nervosa |
| gl | glomerular layer | POA | preoptic area |
| Hd | dorsal habenular nucleus | Ra | raphe nucleus |
| Hv | ventral habenular nucleus | Rm | nucleus reticularis medius |
| igl | internal granule cell layer | SC | suprachiasmatic nucleus |
| L | lateral thalamic nucleus | Str | striatum |
| lp | lateral pallium | tect | optic tectum |
| Lpv | lateral thalamic nucleus | tegm | mesencephalic tectum |
| ls | lateral septum | Tn | tegmental nuclei |
| lv | lateral ventricle | TP | posterior tuberculum |
| MeA | medial amygdala | VH | ventral hypothalamic nucleus |
| ml | mitral layer | VLs | superficial ventral nucleus |
| mp | medial pallium | VM | ventromedial thalamic nucleus |
| ms | medial septum | Vm | nucleus motorius nervi trigemini |
| nII | cranial nerve II | Vpr | nucleus sensorius principalis nervi trigemini |
In the more rostral portions of the telencephalon, NeuroD1 expression was strongly detected in the glomerular and mitral cell layers of the olfactory bulbs (Figs. 1, A1–A3 and B1–B3). No expression of NeuroD1 was detected in the inner granule cell layer. Moving caudally towards mid-telencephalic levels, NeuroD1-positive cells were predominantly found in the dorsal, lateral and medial (arrows) territories of the pallia (Figs. 1, B1–B3 and C1–C3). Scattered cells could be also observed in the medial septum (arrowheads in Fig. 1, C2 and C3). No NeuroD1 labeling was observed in ventricular cells directly at the ventricle but rather in the migrated cells in the mantle zone. In more caudal portions of the telencephalon, NeuroD1-expressing cells were still detected in the dorsal, lateral and medial pallium (Figs. 1, D1–D3). In addition, NeuroD1 was expressed in part of the medial amygdala and bed nucleus striae medullaris (arrow in Figs. 1, D2). Ventrally, NeuroD1-expressing cells were also present in the medial portion of the anterior preoptic telencephalic area, such labeling being manifest in the adult brain section (arrow in Figs. 1, D3). In both pallial and preoptic areas, numerous NeuroD1-expressing cells were localized close to the ventricles, mainly in the subventricular layers from where the newborn post-mitotic neuronal cells migrate to their more peripheral final destination.
In the diencephalon, the level of NeuroD1 expression was moderate compared to telencephalon. The most densely labeled cell group was located in the epithalamus, namely, in the dorsal and ventral nuclei of the habenula (Figs. 1, E1 and E2). This expression pattern was maintained at adult stage (Figs. 1, E3). More caudally, a low number of moderately labeled cells was also found in posterior and ventromedial thalamic areas (Fig. 2, F2), which was no longer detected at adult stage (Fig. 2, F3). In addition, a discrete cell population located dorsally in the hypothalamic region, and very close to the infundibular recess, obviously expressed NeuroD1 in both juvenile and adult brains (arrows in Figs. 2, G1–G3). At mesencephalic level, only very few and dispersed NeuroD1-expressing cells were present in the tectum and tegmentum at juvenile stage (Figs. 2, H2). This weak labeling was not found in corresponding adult mesencephalic sections (Fig. 2, H3).
Within the metencephalon, very high levels of NeuroD1 labeling were found in the region of the cerebellum, such expression being restricted to the granular layer (Figs. 2, I1–I3). Interestingly, this strong NeuroD1 expression was maintained in adult cerebellum (Figs. 2, I3). Few NeuroD1-expressing cells were also found in areas corresponding to the nuclei of the trigeminal nerves (Figs. 2, I1 and I3). Posterior to the cerebellum, in the medulla oblongata (Figs. 2, J1–J3), NeuroD1 expression was still detected in nuclei of trigeminal nerves and, more dorsally, close to the lateral vestibular area (arows in Figs. 2, J2 and J3). Importantly, throughout the juvenile and adult brains, we never observed NeuroD1-expressing cells in the ependymal layer.
NeuroD1-expressing cells are not radial glial or progenitors cells
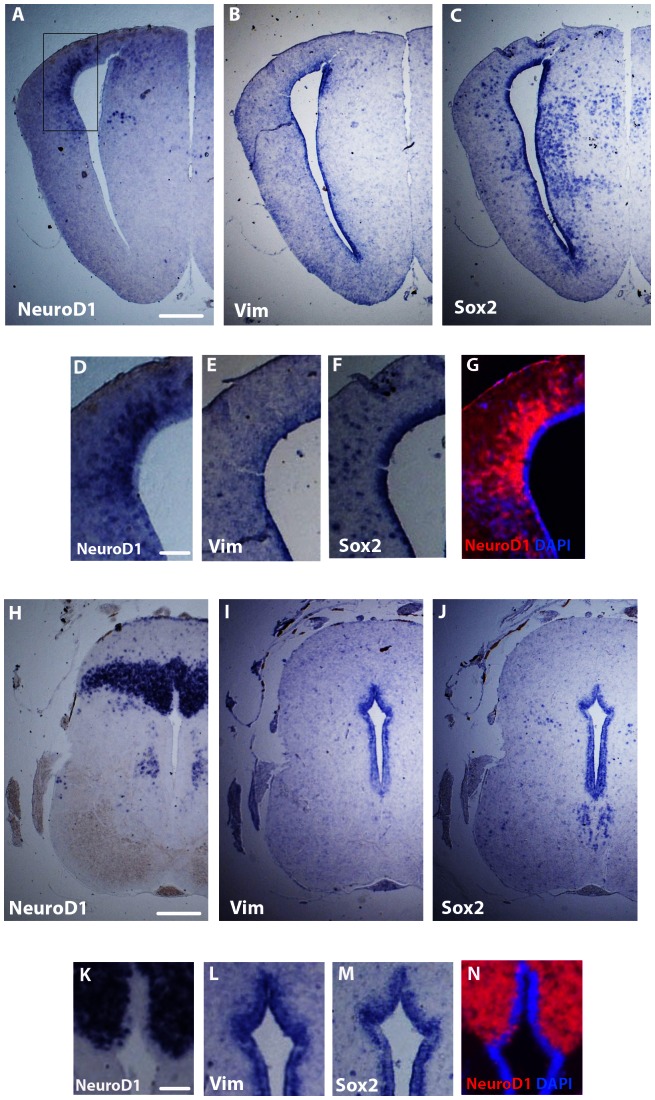
As previously described, NeuroD1-positive cells could be observed both in the parenchyma and in the subventricular layers, but never in the ventricular zone adjacent to the ventricle. To more precisely define the cellular expression of NeuroD1 within the brain, serial in situ hybridizations were performed on thin adjacent sections using the NeuroD1 probe, but also Vimentin and Sox2 probes, as markers of radial glial cells and neural progenitor cells, respectively [21], [22]. Radial glial cells, known to behave as neural stem cell (reviewed in Kriegstein and Alvarez-Buylla, 2009), were previously identified in the ventricular layers of post-metamorphic Xenopus brain [9]. We focused our analyses on the pallial and cerebellar regions of juvenile brains because these two regions displayed heavy NeuroD1 expressions (see Figs. 1 and 2). As confirmed in figure 3, strong NeuroD1 expression domains were detected in the dorso-lateral pallium (Fig. 3, A) and in the cerebellum (Fig. 3, H). Using adjacent sections, in situ hybridizations with Vimentin (Fig. 3, B and I) and Sox2 (Fig. 3, C and J) probes showed strong expressions of both genes in ventricular cells of the pallium and sub-pallium. Sox2 labeling was also detected in few cells localized in the parenchyma (Fig. 3, C and J). Most importantly, higher magnifications of the dorso-lateral pallium (Figs. 3, D–F) and the granular layer of the cerebellum (Figs. 3, K–M) provided evidence that NeuroD1-positive cells were detected in large amount outside the ventricular layer cells that expressed Vimentin and Sox2 markers (compared Figs. 3 D to E and F; Figs. 3 K to L and M). These observations were reinforced by performing double labelings NeuroD1/DAPI on the same sections (Figs. 3, G and N). Taken together, these in situ hybridization experiments demonstrated that expression domains of NeuroD1 were excluded from the ventricular layers as these domains did not overlap with radial glia or neural progenitor markers.
Figure 3. Expression patterns of NeuroD1, Sox2 and Vimentin in the juvenile X. laevis brain.
In situ hybridizations on coronal sections of cerebral hemispheres (A–G) and cerebellum (H–N). In cerebral illustrations, D, E and F are high magnifications of A, B and C, respectively. In cerebellum illustrations, K, L and M are high magnifications of H, I and J, respectively. To allow merge with the DAPI staining, colors of high magnification illustrations D and K were negatively inverted in photos G and N, respectively. For all images, dorsal is to the top. Scale bar = 220 µm in A–C and H–J; 95 µm in D–G and K–N.
NeuroD1-expressing cells are post-mitotic
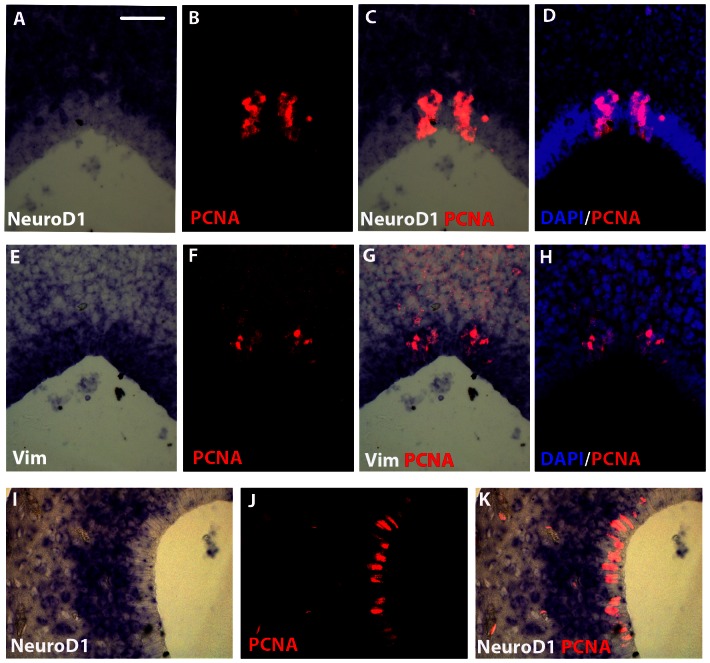
Our detailed analysis of NeuroD1 gene activity have revealed that, throughout juvenile and adult brains, NeuroD1 transcripts were never detected in the ventricular layers, where both neural stem cells and mitotic neuronal precursor cells were located (Figure 3). This consistent NeuroD1 expression pattern, together with our previous published data that identified proliferating cells specifically in the ventricular layers of juvenile and adult brains [9], strongly suggested that NeuroD1-expressing cells were post-mitotic cells. To further investigate this hypothesis, we designed double labeling experiments combining NeuroD1 RNA in situ hybridization and immunocytochemistry for the PCNA proliferation marker on coronal sections of pallial and cerebellar regions (Figure 4). As shown at high magnifications of the cerebellum (Fig. 4, A–H), the PCNA positive cells were clearly found restricted to the ventricular layer (Fig. 4, B–D and F–H) while NeuroD1-expressing cells were observed both in sub-ventricular layers and parenchyma (Fig. 4, A and C). No significant co-localization of NeuroD1 transcripts and PCNA in the same cell could be detected (Fig. 4C). As expected, using adjacent coronal section, PCNA-positive cells perfectly co-localized with Vimentin-expressing cells in the ventricular layer (Figs. 4, E–H). Using tissue sections of the pallial region, we also revealed NeuroD1 expression in post-mitotic cells, i.e outside the ventricular zone containing PCNA-positive cells (Fig. 4, I–K). Identical results were obtained using the BrdU proliferation marker after short (2 days) post-BrdU administration survival time (data not shown). Overall, our data demonstrated that NeuroD1 expression in juvenile Xenopus brain was only detected in post-mitotic cells undergoing neuronal differentiation, supporting the previous observation that NeuroD1 expression was not detected in progenitor and radial glial cells.
Figure 4. NeuroD1/PCNA and Vimentin/PCNA double stainings in the cerebellar and pallial regions of juvenile X. laevis brain.
Coronal sections at the level of cerebellum (A–H) or pallium (I–K). In situ hybridization using a NeuroD1 (A–D and I–K) or a Vimentin (E–H) probe combined with PCNA immunohistochemistry. For all images, dorsal is to the top. Scale bar = 30 µm.
NeuroD1 gene is up-regulated in new born cells during post-metamorphic neurogenesis
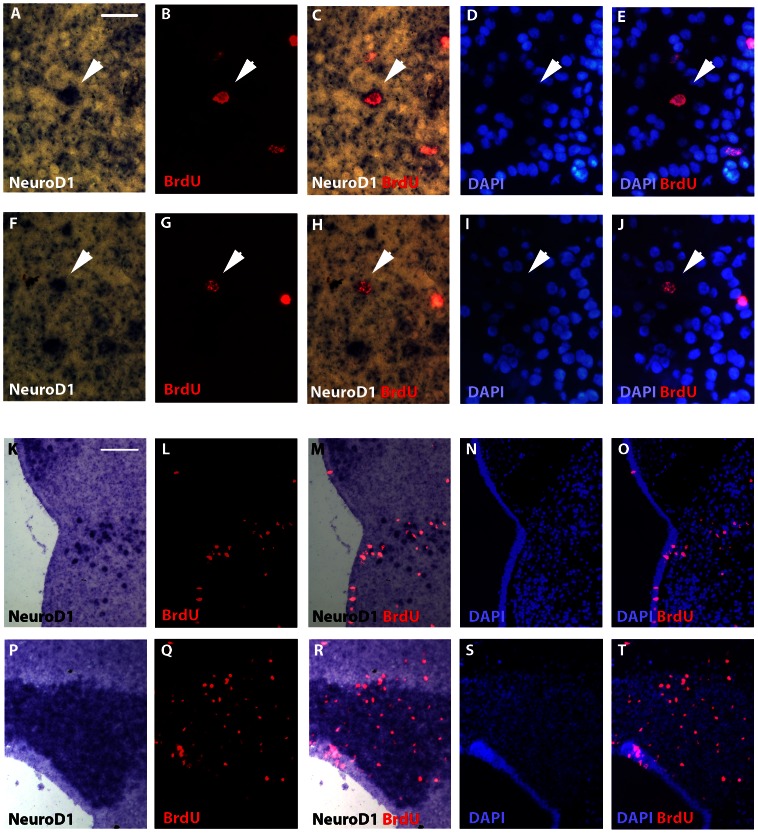
We additionally asked wether NeuroD1 transcription was turned on in post-metamorphic neurogenesis, i.e during the differentiation process of juvenile new born cells. In order to adress this question, we followed the fate of proliferative cells 14 days after injections of BrdU at juvenile stage. In situ hybridizations experiments for NeuroD1 transcripts combined with immunodectection for BrdU were then performed on thin telencephalic and cerebellar coronal sections. In the telencephalon, 14 days post-injections, BrdU-positive cells were found in large amount outside the ventricular layers in the medial and lateral septum, striatum and nucleus accumbens, and to a lesser extent in the pallium (Fig. 5, K–O and D'Amico et al., 2011). In the cerebellum, BrdU-positive cells were mainly detected in the granular layer (about 90%) and very few were found in the molecular layer (Fig. 5, P–T; D'Amico et al., 2011). In addition, both in telencephalon and cerebellum, the majority of BrdU-positive cells were also observed in regions with high cell density (see Fig. 5, O and T). In order to make undoubtedly NeuroD1/BrdU cellular co-localization analysis, we focused our double staining illustrations on the medial pallium area because NeuroD1 expressing cells were found more scattered than in the dorso-lateral pallium and cell density was also weak. As shown by the high magnification images of the figure 5 (A–J), we found individual BrdU- positive cells (Fig. 5, B and G) that perfectly matched with nuclei stained with DAPI (Fig. 5, D–E and I–J). Most importantly, a strong NeuroD1 expression was detected in some of these BrdU-positive cells (arrowheads in Fig. 5, A–C and F–H). Cell counting analysis in these low cell density areas revealed that approximately 1.2% of the cells were labeled for both NeuroD1 and BrdU. Unfortunately, the experimental procedure did not allow to quantify double-positive cells in high cell density areas (Fig. 5, N and S), in particular in the granular layer of cerebellum where strong NeuroD1 gene activity and BrdU labelling were detected (Figure 5, P–T). Nevertheless, these data demonstrated that new born cells in the medial pallium of a juvenile brain can turn on NeuroD1 gene expression during the neuronal differentiation process.
Figure 5. NeuroD1/BrdU double stainings on telencephalic and cerebellar sections of a juvenile X. laevis brain.
(A–J) Telencephalon high magnifications of NeuroD1 in situ hybridizations (A, C, F and H) combined with BrdU immunodetections (B, C, F and H) after 14-days BrdU post-administration time. Arrows indicate double stained cells. DAPI stainings are indicated to certify the presence of the nucleus. (K–T) Low magnifications of the above NeuroD1/BrdU/DAPI triple labelling experiments showing larger view of telencephalon (K–O) and cerebellum (P–T). For all images, dorsal is to the top. Scale bar = 15 µm in A–J; 45 µm in K–T.
Discussion
To gain further insight into the activation of NeuroD1 gene in non mammalian vertebrates, we examined the precise expression pattern of NeuroD1 by in situ hybridization on coronal sections throughout the whole juvenile and adult Xenopus laevis brains. Expression data analysis revealed NeuroD1 gene activity in various brain regions, including olfactory bulbs, pallial regions of cerebral hemispheres, preoptic area, habenula, hypothalamus, cerebellum and medulla oblongata. In previous reports, NeuroD1 gene expression has only been studied in embryonic and larval Xenopus stages (up to stage 48) in the context of primary neurogenesis and secondary neurogenesis, respectively [17], [23]–[26]. At larva stage, NeuroD1 gene activity was identified in pallia, dorsal thalamus (habenula), pretectum, posterior tuberculum, nucleus of the medial longitudinal fascicle, mesencephalic optic tectum, torus semicircularis, tegmentum and medulla oblongata [23]. Thus, our study demonstrates that some of the previously identified larva brain regions maintained NeuroD1 gene activity untill juvenile and adult stages. This is obvious for the pallial, habenula and cerebellar regions in which we detected the heaviest NeuroD1 expressions. Importantly, our study also revealed that two additional post-metamorphic brain regions, compared to larva stage, expressed NeuroD1, namely the preoptic area and the hypothalamus. Previously, our laboratory performed studies to identify proliferation in the brain of both juvenile and adult Xenopus [9]. Interestingly, the patterns of NeuroD1 expression were consistent with the majority of the proliferation zones that we mapped. In mammals, where most of NeuroD1 studies were performed, it was shown that structures such as the olfactory bulbs, cerebellum and hippocampus, maintained significant levels of NeuroD1 mRNA expression at post-natal stages and also throughout adulthood in humans and mice [14], [27]–[30]. Recent studies in adult zebrafish telencephalon have also identified NeuroD1 gene activity in the pallial region [31]. Wether or not the Xenopus NeuroD1 protein is regionally expressed at a similar level than the NeuroD1 transcripts remains to be demonstrated. Unfortunately, due to the lack of available valid NeuroD1 antibodies, this could not be investigated.
In juvenile and adult Xenopus brains, we identified a large amount of NeuroD1 expressing cells in the cerebellum, in particular in the granular layer. Interestingly, this strong NeuroD1 gene activity is both conserved during Xenopus cerebellar development and across species. Indeed, in Xenopus and zebrafish larvae, a very strong NeuroD1 expression in the developing cerebellum was previously detected [23], [32]. In mouse, at post-natal stages, NeuroD1 was also clearly detected in both external and internal granular layers of the cerebellum, and the internal granular layers expression was shown to stably persist until adulthood [19], [27], [28]. In addition, systemic or conditional NeuroD1 null mice experiments have shown that the absence of NeuroD1 leads to a lack of foliation and the complete loss of granular cells in the posterior half of the cerebellum, whereas a substantial number of granular cells survive and differentiate in the anterior lobules [19], [33], [34]. It will be interesting to investigate, in nonmammalian vertebrates, if there is a similar anterior-posterior differential requirement of NeuroD1 for granular cell maintenance.
The present study also shows that the medial pallium was another domain of abundant gene activity in juvenile and adult brains. In perfect agreement, we previously identified migrating cells and new born mature neurons in the medial pallium (D'Amico et al., 2011). Interestingly, the amphibian medial pallium is regarded as the homologue of the mammalian hippocampus [26], [35]. In other vertebrates, the hippocampus is also known to be one of the very few brain regions in which adult neurogenesis continues into adult stages of development, as demonstrated in reptiles, birds, fish and mammals [10], [36]–[43]. In mammals, after birth, NeuroD1 is prominently expressed in the hippocampus, particularly in the granule cells of the dentate gyrus and pyramidal cells in CA1 and CA3 [27], [44]–[46]. As expected from the high expression level in the granule cell of the dentate gyrus, mice lacking the NeuroD1 gene revealed striking abnormalities in the hippocampal formation [19], [20], [47]. Although the pyramidal layers appear normal, the mutant brains lack the dentate granule cell layer and have no organized dentate hilus [20]. More recently, it was found that overexpression of NeuroD1 was sufficient to promote neuronal differentiation in adult hippocampal neural progenitors [48], whereas inducible NeuroD1 gene ablation resulted in decreased survival and maturation of newborn neurons [29]. In our study, the presence of a high NeuroD1 gene activity in the hippocampus of Xenopus laevis strongly suggest that NeuroD1 might also promote neuronal differentiation in this brain area in juvenile and adult animals.
In the course of this study, we identified in frog post-metamorphic brains new NeuroD1 expression domains in the habenular, preoptic area and hypothalamic areas. To our knowledge, these NeuroD1 expression domains have not been described in any adult vertebrate brain examined thus far. Wether or not the NeuroD1 factor has a critical function in these brain areas remains to be investigated. In particular, in preoptic and hypothalamic areas, it would be interesting to examine if the proliferative and neurogenic activities that we and others previously observed [9] are correlated, at cellular level, with up-regulation of a NeuroD1 gene activity.
In post-metamorphic Xenopus brains, several arguments strongly suggest that NeuroD1 expressing cells were not in a proliferation state. 1) In our detailed in situ hybridization analysis in juvenile and adult brains, we could never find NeuroD1 expressing cells in the ventricular wall, in particular no co-localization with the radial glia or neural progenitor cells markers could be identified; 2) NeuroD1 in situ hybridization combined with PCNA immunodetection did not allow identifying any co-localization of both factors; 3) Positive NeuroD1/BrdU double stainings were only found after long (14 days) post-BrdU administration survival time but never with short survival time (2 days). Therefore, we conclude that NeuroD1 expressing cells in frog post-metamorphic brains are postmitotic. Interestingly, this feature seems to be conserved during Xenopus central nervous development. In Xenopus embryo, during primary neurogenesis, it was clear established that NeuroD1 is expressed transiently in a subset of neurons in the central and peripheral nervous systems at the time of their terminal differentiation into mature neurons [17]. During Xenopus secondary neurogenesis, i.e. at early larval stages, NeuroD1 gene expression was also excluded to the most ventricularly located cells in proliferation zones, in particular mitotic cells expressing Ngnr-1 and Delta1 genes [23]. Interestingly, in post-embryonic and adult zebrafish brains, NeuroD1-expressing cells were also identified from one to several cell rows away from the ventricular surface [31], [32], [49]. Taken together, NeuroD1 expression studies in non-mammalian vertebrates have indicated that NeuroD1-expressing cells were post-mitotic at embryonic, larval and adult brain stages. Surprisingly, in murine NeuroD1 expression was detected not only in post-mitotic but also in mitotic cells, as was evident in its expression in external granular layer of cerebellum and granule cells of the dentate gyrus during post-natal development. This observation suggested that NeuroD1 protein may have a unique role in proliferation and/or differentiation of granule cells of the cerebellum and dentate gyrus [19], [20], [27], [29], [30]. As NeuroD1 expression was restricted to post-mitotic cells in the sub-ventricular zone and parenchyma, the present study suggests that NeuroD1 may play an important role in neuronal cell differentiation in the late stages of neurogenesis rather than proliferation stages in post-metamorphic Xenopus brain.
In an effort to understand the molecular cascade involved in frog adult neurogenesis, further investigations with others proneural and/or neurogenic factors have to be conducted in the future. During primary neurogenesis, and retinal neurogenesis, expression of NeuroD1 is known to follow the expression of the bHLH gene neurogenin-related-1 (X-ngnr-1), a vertebrate neuronal determination gene, also known as Neurogenin 2 (Ngn2) in mammalians [17], [50], [51]. During primary neurogenesis, overexpression of X-ngnr-1 induces formation of ectopic neurons in nonneuronal ectoderm and induces ectopic expression of NeuroD1 ([50]. These data demonstrate that X-ngnr-1 and NeuroD 1 function to regulate successive stages of neuronal differentiation in the developing neural plate. Preliminary studies in our laboratory suggest that X-Ngnr-1 might not be a key factor during adult neurogenesis as we were not able to detect X-Ngnr-1 expression, using stringent hybridization conditions, in any juvenile or adult brain areas, including regions with high proliferative and neurogenic capacities (data not shown). Wether or not other members of the Neurogenin family, such as Xenopus Neurogenin 1 or Neurogenin 3 [52], are able to compensate X-Ngnr-1/Ngn2 expression in the adult neurogenetic network remains an open question.
Materials and Methods
Animals
For the present study, juvenile and adult Xenopus laevis of both sexes were used. Juveniles (NF stage 66) stage were classified according to Nieuwkoop and Faber (Nieuwkoop and Faber, 1967). All procedures involving animals were conducted in accordance with the guidelines of Ethical Committee at our institutions (University of Rennes 1, CNRS and INSERM) and in accordance with European Union regulations concerning the protection of experimental animals (Directive 86/609/EEC). The protocols were approved by the Ethical Committee CREEA (Comité Rennais d'Ethique en matière d'Expérimentation Animale) and performed under the supervision of authorized investigators (Permit number: 75-390). All steps have been taken to reduce suffering of animals. Animals were deeply anesthetized with 0.05% tricaine methane sulphonate (MS-222; Sigma) and killed by decapitation. The whole heads of juvenile frogs were fixed 2 hours in freshly prepared 0.5M, pH 7.4 phosphate–buffered saline (PBS) containing 4% paraformaldehyde (three specimens per stage). After two washes in PBS, brains were then carefully removed from the skull, post-fixed in fresh fixative overnight at 4°C and then stored, for no more than one week, in PBS untill sectioning.
BrdU incorporation
To follow the fate of proliferating cells, juvenile X. laevis were anesthetized (as decribed above) and injected intraperitoneally with approximately 50 µL/g body weight of labeling reagent (Amersham Cell Proliferation kit; RPN20). After survival periods ranging between 2 days and 14 days post-injections, frogs were deeply anesthetized and killed as described above.
In situ hybridization and immunohistochemistry
Brains were embedded in paraffin and sectionned coronally. Consecutive/adjacent thin sections of 8 µM thickness were placed on different slide sets allowing each individual brain to undergo in situ hybridization with different probes and/or antibodies (as described in the following). Sections were subjected to stringent in situ hybridization (ISH) as described [53]. The following ISH probes were used: NeuroD1 [17], Vimentin [21] and Sox2 [54]. For double ISH/immunodetections, the brains were first processed for ISH, then for immunocytochemistry. PCNA and BrdU immunodetections were performed as previously described [9]. All sections were photographed and analyzed under a Olympus PROVIS AX70 microscope with a digital camera (Olympus SP71), and a Nikon multizoom AZ100 macroscope with a DS-Ri1 color camera. Cell counting was doned manually under the microscope by two of the authors and by an observer unfamiliar with experiments. Red blood cells were clearly identified using 20× and 40× microscope objectives and were not counted.
Acknowledgments
We sincerely thank Dr Lee, Dr Kato and Dr Casey for the gift of NeuroD1, Vimentin and Sox2, respectively. The authors are grateful to Dr Elisabeth Pellegrini, Colette Vaillant and Olivier Kah for helpfull comments and critical reading of the manuscript.
Funding Statement
This research was supported by funds from the Bretagne region, the Ministry of Nationale Education and by an undergraduate fellowship from Bretagne region. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Zhao C, Deng W, Gage FH (2008) Mechanisms and functional implications of adult neurogenesis. Cell 132: 645–660. [DOI] [PubMed] [Google Scholar]
- 2. Kaslin J, Ganz J, Brand M (2008) Proliferation, neurogenesis and regeneration in the non-mammalian vertebrate brain. Philos Trans R Soc Lond B Biol Sci 363: 101–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chapouton P, Jagasia R, Bally-Cuif L (2007) Adult neurogenesis in non-mammalian vertebrates. Bioessays 29: 745–757. [DOI] [PubMed] [Google Scholar]
- 4. Lindsey BW, Tropepe V (2006) A comparative framework for understanding the biological principles of adult neurogenesis. Prog Neurobiol 80: 281–307. [DOI] [PubMed] [Google Scholar]
- 5. Nottebohm F (2004) The road we travelled: discovery, choreography, and significance of brain replaceable neurons. Ann N Y Acad Sci 1016: 628–658. [DOI] [PubMed] [Google Scholar]
- 6. Kizil C, Kaslin J, Kroehne V, Brand M (2012) Adult neurogenesis and brain regeneration in zebrafish. Dev Neurobiol 72: 429–461. [DOI] [PubMed] [Google Scholar]
- 7. Zupanc GK (2006) Neurogenesis and neuronal regeneration in the adult fish brain. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 192: 649–670. [DOI] [PubMed] [Google Scholar]
- 8. Zupanc GK, Sirbulescu RF (2011) Adult neurogenesis and neuronal regeneration in the central nervous system of teleost fish. Eur J Neurosci 34: 917–929. [DOI] [PubMed] [Google Scholar]
- 9. D'Amico LA, Boujard D, Coumailleau P (2011) Proliferation, migration and differentiation in juvenile and adult Xenopus laevis brains. Brain Res 1405: 31–48. [DOI] [PubMed] [Google Scholar]
- 10. Zupanc GK, Hinsch K, Gage FH (2005) Proliferation, migration, neuronal differentiation, and long-term survival of new cells in the adult zebrafish brain. J Comp Neurol 488: 290–319. [DOI] [PubMed] [Google Scholar]
- 11. Grandel H, Kaslin J, Ganz J, Wenzel I, Brand M (2006) Neural stem cells and neurogenesis in the adult zebrafish brain: origin, proliferation dynamics, migration and cell fate. Dev Biol 295: 263–277. [DOI] [PubMed] [Google Scholar]
- 12. Pellegrini E, Mouriec K, Anglade I, Menuet A, Le Page Y, et al. (2007) Identification of aromatase-positive radial glial cells as progenitor cells in the ventricular layer of the forebrain in zebrafish. J Comp Neurol 501: 150–167. [DOI] [PubMed] [Google Scholar]
- 13. Guillemot F (2007) Spatial and temporal specification of neural fates by transcription factor codes. Development 134: 3771–3780. [DOI] [PubMed] [Google Scholar]
- 14. Roybon L, Deierborg T, Brundin P, Li JY (2009) Involvement of Ngn2, Tbr and NeuroD proteins during postnatal olfactory bulb neurogenesis. Eur J Neurosci 29: 232–243. [DOI] [PubMed] [Google Scholar]
- 15. Chae JH, Stein GH, Lee JE (2004) NeuroD: the predicted and the surprising. Mol Cells 18: 271–288. [PubMed] [Google Scholar]
- 16. Cho JH, Tsai MJ (2004) The role of BETA2/NeuroD1 in the development of the nervous system. Mol Neurobiol 30: 35–47. [DOI] [PubMed] [Google Scholar]
- 17. Lee JE, Hollenberg SM, Snider L, Turner DL, Lipnick N, et al. (1995) Conversion of Xenopus ectoderm into neurons by NeuroD, a basic helix-loop-helix protein. Science 268: 836–844. [DOI] [PubMed] [Google Scholar]
- 18. Schlosser G, Northcutt RG (2000) Development of neurogenic placodes in Xenopus laevis. J Comp Neurol 418: 121–146. [PubMed] [Google Scholar]
- 19. Miyata T, Maeda T, Lee JE (1999) NeuroD is required for differentiation of the granule cells in the cerebellum and hippocampus. Genes Dev 13: 1647–1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Liu M, Pleasure SJ, Collins AE, Noebels JL, Naya FJ, et al. (2000) Loss of BETA2/NeuroD leads to malformation of the dentate gyrus and epilepsy. Proc Natl Acad Sci U S A 97: 865–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kiyota T, Kato A, Altmann CR, Kato Y (2008) The POU homeobox protein Oct-1 regulates radial glia formation downstream of Notch signaling. Dev Biol 315: 579–592. [DOI] [PubMed] [Google Scholar]
- 22. Yoshida M (2001) Intermediate filament proteins define different glial subpopulations. J Neurosci Res 63: 284–289. [DOI] [PubMed] [Google Scholar]
- 23. Wullimann MF, Rink E, Vernier P, Schlosser G (2005) Secondary neurogenesis in the brain of the African clawed frog, Xenopus laevis, as revealed by PCNA, Delta-1, Neurogenin-related-1, and NeuroD expression. J Comp Neurol 489: 387–402. [DOI] [PubMed] [Google Scholar]
- 24. Schlosser G, Koyano-Nakagawa N, Kintner C (2002) Thyroid hormone promotes neurogenesis in the Xenopus spinal cord. Dev Dyn 225: 485–498. [DOI] [PubMed] [Google Scholar]
- 25. Schlosser G (2003) Hypobranchial placodes in Xenopus laevis give rise to hypobranchial ganglia, a novel type of cranial ganglia. Cell Tissue Res 312: 21–29. [DOI] [PubMed] [Google Scholar]
- 26. Roth G, Laberge F, Muhlenbrock-Lenter S, Grunwald W (2007) Organization of the pallium in the fire-bellied toad Bombina orientalis. I: Morphology and axonal projection pattern of neurons revealed by intracellular biocytin labeling. J Comp Neurol 501: 443–464. [DOI] [PubMed] [Google Scholar]
- 27. Lee JK, Cho JH, Hwang WS, Lee YD, Reu DS, et al. (2000) Expression of neuroD/BETA2 in mitotic and postmitotic neuronal cells during the development of nervous system. Dev Dyn 217: 361–367. [DOI] [PubMed] [Google Scholar]
- 28. Yokoyama M, Nishi Y, Miyamoto Y, Nakamura M, Akiyama K, et al. (1996) Molecular cloning of a human neuroD from a neuroblastoma cell line specifically expressed in the fetal brain and adult cerebellum. Brain Res Mol Brain Res 42: 135–139. [DOI] [PubMed] [Google Scholar]
- 29. Gao Z, Ure K, Ables JL, Lagace DC, Nave KA, et al. (2009) Neurod1 is essential for the survival and maturation of adult-born neurons. Nat Neurosci 12: 1090–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Roybon L, Hjalt T, Stott S, Guillemot F, Li JY, et al. (2009) Neurogenin2 directs granule neuroblast production and amplification while NeuroD1 specifies neuronal fate during hippocampal neurogenesis. PLoS One 4: e4779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ganz J, Kaslin J, Freudenreich D, Machate A, Geffarth M, et al. (2012) Subdivisions of the adult zebrafish subpallium by molecular marker analysis. J Comp Neurol 520: 633–655. [DOI] [PubMed] [Google Scholar]
- 32. Mueller T, Wullimann MF (2003) Anatomy of neurogenesis in the early zebrafish brain. Brain Res Dev Brain Res 140: 137–155. [DOI] [PubMed] [Google Scholar]
- 33. Cho JH, Tsai MJ (2006) Preferential posterior cerebellum defect in BETA2/NeuroD1 knockout mice is the result of differential expression of BETA2/NeuroD1 along anterior-posterior axis. Dev Biol 290: 125–138. [DOI] [PubMed] [Google Scholar]
- 34. Schwab MH, Bartholomae A, Heimrich B, Feldmeyer D, Druffel-Augustin S, et al. (2000) Neuronal basic helix-loop-helix proteins (NEX and BETA2/Neuro D) regulate terminal granule cell differentiation in the hippocampus. J Neurosci 20: 3714–3724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Westhoff G, Roth G (2002) Morphology and projection pattern of medial and dorsal pallial neurons in the frog Discoglossus pictus and the salamander Plethodon jordani. J Comp Neurol 445: 97–121. [DOI] [PubMed] [Google Scholar]
- 36. Lopez-Garcia C, Molowny A, Garcia-Verdugo JM, Ferrer I (1988) Delayed postnatal neurogenesis in the cerebral cortex of lizards. Brain Res 471: 167–174. [DOI] [PubMed] [Google Scholar]
- 37. Kaplan MS, Bell DH (1984) Mitotic neuroblasts in the 9-day-old and 11-month-old rodent hippocampus. J Neurosci 4: 1429–1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gould E, Gross CG (2002) Neurogenesis in adult mammals: some progress and problems. J Neurosci 22: 619–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Barnea A, Nottebohm F (1994) Seasonal recruitment of hippocampal neurons in adult free-ranging black-capped chickadees. Proc Natl Acad Sci U S A 91: 11217–11221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Altman J, Das GD (1965) Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol 124: 319–335. [DOI] [PubMed] [Google Scholar]
- 41. Gould E, Reeves AJ, Graziano MS, Gross CG (1999) Neurogenesis in the neocortex of adult primates. Science 286: 548–552. [DOI] [PubMed] [Google Scholar]
- 42. Kornack DR, Rakic P (1999) Continuation of neurogenesis in the hippocampus of the adult macaque monkey. Proc Natl Acad Sci U S A 96: 5768–5773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Seri B, Garcia-Verdugo JM, McEwen BS, Alvarez-Buylla A (2001) Astrocytes give rise to new neurons in the adult mammalian hippocampus. J Neurosci 21: 7153–7160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Pleasure SJ, Collins AE, Lowenstein DH (2000) Unique expression patterns of cell fate molecules delineate sequential stages of dentate gyrus development. J Neurosci 20: 6095–6105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Uittenbogaard M, Chiaramello A (2000) Differential expression patterns of the basic helix-loop-helix transcription factors during aging of the murine brain. Neurosci Lett 280: 95–98. [DOI] [PubMed] [Google Scholar]
- 46. Seki T (2002) Expression patterns of immature neuronal markers PSA-NCAM, CRMP-4 and NeuroD in the hippocampus of young adult and aged rodents. J Neurosci Res 70: 327–334. [DOI] [PubMed] [Google Scholar]
- 47. Liu M, Pereira FA, Price SD, Chu MJ, Shope C, et al. (2000) Essential role of BETA2/NeuroD1 in development of the vestibular and auditory systems. Genes Dev 14: 2839–2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hsieh J, Nakashima K, Kuwabara T, Mejia E, Gage FH (2004) Histone deacetylase inhibition-mediated neuronal differentiation of multipotent adult neural progenitor cells. Proc Natl Acad Sci U S A 101: 16659–16664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Mueller T, Wullimann MF (2002) Expression domains of neuroD (nrd) in the early postembryonic zebrafish brain. Brain Res Bull 57: 377–379. [DOI] [PubMed] [Google Scholar]
- 50. Ma Q, Kintner C, Anderson DJ (1996) Identification of neurogenin, a vertebrate neuronal determination gene. Cell 87: 43–52. [DOI] [PubMed] [Google Scholar]
- 51. Perron M, Opdecamp K, Butler K, Harris WA, Bellefroid EJ (1999) X-ngnr-1 and Xath3 promote ectopic expression of sensory neuron markers in the neurula ectoderm and have distinct inducing properties in the retina. Proc Natl Acad Sci U S A 96: 14996–15001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Nieber F, Pieler T, Henningfeld KA (2009) Comparative expression analysis of the neurogenins in Xenopus tropicalis and Xenopus laevis. Dev Dyn 238: 451–458. [DOI] [PubMed] [Google Scholar]
- 53. Coumailleau P, Duprez D (2009) Sim1 and Sim2 expression during chick and mouse limb development. Int J Dev Biol 53: 149–157. [DOI] [PubMed] [Google Scholar]
- 54. Rogers CD, Archer TC, Cunningham DD, Grammer TC, Casey EM (2008) Sox3 expression is maintained by FGF signaling and restricted to the neural plate by Vent proteins in the Xenopus embryo. Dev Biol 313: 307–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Tuinhof R, Ubink R, Tanaka S, Atzori C, van Strien FJ, et al. (1998) Distribution of pro-opiomelanocortin and its peptide end products in the brain and hypophysis of the aquatic toad, Xenopus laevis. Cell Tissue Res 292: 251–265. [DOI] [PubMed] [Google Scholar]
- 56. Marin O, Smeets WJ, Gonzalez A (1998) Basal ganglia organization in amphibians: chemoarchitecture. J Comp Neurol 392: 285–312. [PubMed] [Google Scholar]