Abstract
Background
Flooding significantly reduces the growth and grain yield of soybean plants. Proteomic and biochemical techniques were used to determine whether the function of cotyledon and root is altered in soybean under flooding stress.
Results
Two-day-old soybean plants were flooded for 2 days, after which the proteins from root and cotyledon were extracted for proteomic analysis. In response to flooding stress, the abundance of 73 and 28 proteins was significantly altered in the root and cotyledon, respectively. The accumulation of only one protein, 70 kDa heat shock protein (HSP70) (Glyma17g08020.1), increased in both organs following flooding. The ratio of protein abundance of HSP70 and biophoton emission in the cotyledon was higher than those detected in the root under flooding stress. Computed tomography and elemental analyses revealed that flooding stress decreases the number of calcium oxalate crystal the cotyledon, indicating calcium ion was elevated in the cotyledon under flooding stress.
Conclusion
These results suggest that calcium might play one role through HSP70 in the cotyledon under flooding stress.
Introduction
Flooding has a significant negative influence on the productivity of arable farmland, as the vast majority of crops cannot grow under the stress conditions induced by flooding [1]. Most studies of flooding stress have focused on relatively flood-tolerant species such as rice, Rumex, and Echinochloa [2]. Soybean, however, is sensitive to flooding stress, and its growth and grain yield are significantly reduced by flooding [3], [4]. Hashiguchi et al. [5] showed that soybean seedling root elongation is suppressed after 1 day of flooding stress and is significantly retarded by 2 days of flooding stress. Suppression of root elongation occurred in the root tip region [6], [7] since it contains the root apical meristem, which is important for root system development and also contains the elongation region [8].
Proteomic studies of the total protein complement of the soybean root have indicated that flooding stress alters the abundance of proteins associated with protein transport, protein storage, ATP synthesis, metabolism, and signal transduction pathways [4]. Recent studies using omics techniques [9] have identified numerous flooding-responsive pathways and systems in plants, including hormonal signaling [10], transcriptional control [10], glucose degradation and sucrose accumulation [11], activation of alcohol fermentation [12], the gamma-aminobutyric acid shunt [4], suppression of reactive oxygen species scavenging system [4], suppression of mitochondria [13], ubiquitin/proteasome-mediated proteolysis [6], [14], and the cell wall [15]. Flooding-induced changes in these systems and pathways have been well-documented with regard to the root and hypocotyl of young soybeans; however, there are no reports on the effect of flooding stress on the function of other soybean organs, such as the cotyledon.
Khatoon et al. [16], [17] used gel-based proteomic techniques to examine organ-specific responses in soybean plants flooded for 12 days. In the root, hypocotyl, and leaf, the abundance of 51, 66, and 51 proteins, respectively, changed significantly in response to flooding stress. The abundance of many metabolism-related proteins increased in the root but decreased in both the hypocotyl and leaf. Isoflavone reductase decreased at the protein level in all 3 organs under flooding stress, but expression of isoflavone reductase mRNA was up-regulated in leaf. In addition, flooding stress led to an increase in biophoton emission in all 3 organs. These results suggested that concurrence of the expression of the isoflavone reductase gene at the mRNA and protein levels, together with imbalances in the levels of disease/defense and metabolism-related proteins, might be responsible for the observed impaired growth of root, hypocotyl, and leaf of flooding-stressed soybean seedlings. Such organ-specific analyses have provided many important insights into the flooding response mechanism in soybean.
Biophotons generated during germination of soybean seeds are composed of two spectral components, a UV component, corresponding to what was originally described as “mitogenetic radiation”, and emission in the red and far-red regions [18]. Biophoton emission has been detected in cut potato tubers infected with Fusarium [19]. In soybean, biophoton emission has been detected in cadmium-stressed leaf [20] and drought-stressed root [21]. Under flooding stress, biophoton emission has also been detected in leaf and root of soybean [16]; however, the molecular mechanisms underlying biophoton emission are unknown, although biophotons are thought to represent spontaneous. In this study, proteomic and biochemical techniques were used to determine whether flooding stress alters root and cotyledon function in soybean. Biochemical techniques such as biophoton emission analysis, computed tomography, and elemental analysis were used to obtain a more detailed understanding of how the function of cotyledon and root during flooding stress.
Results
Flooding-stress-induced Changes in the Abundance of Proteins in the Root and Cotyledon of Soybean Plants
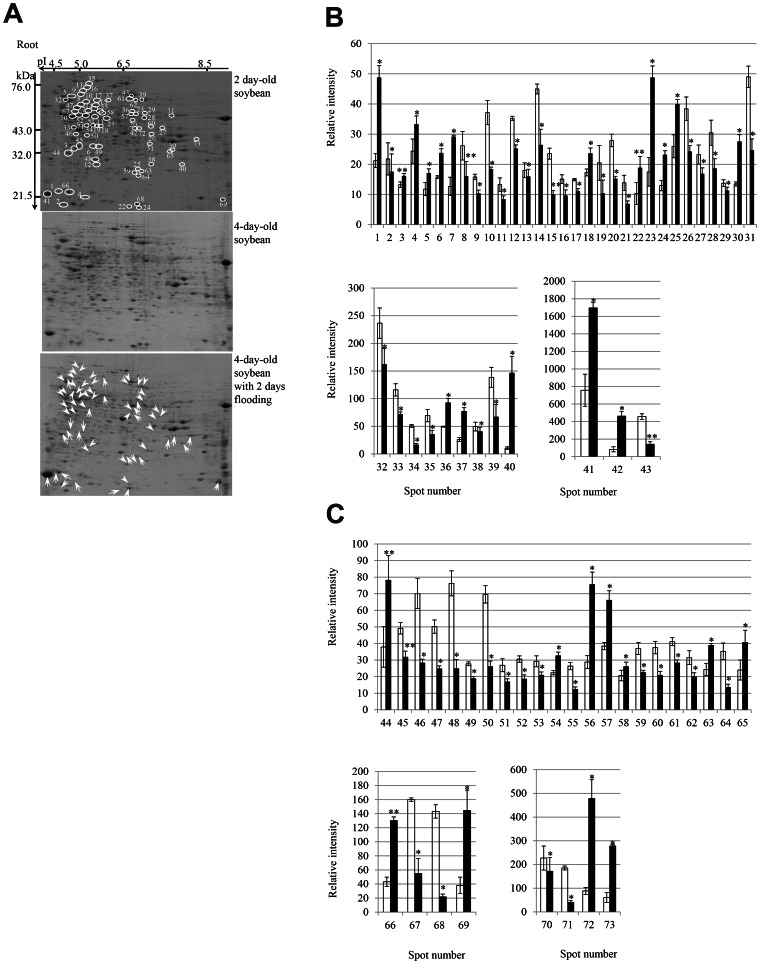
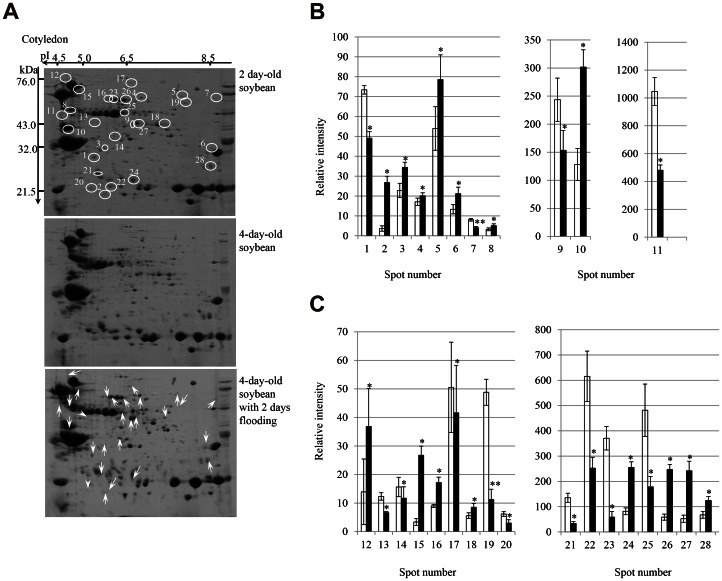
A proteomic approach was used to identify changes in protein abundance in the root and cotyledon during the early stages of growth in flooding-stressed soybean plants. Two-day-old soybean plants were flooded for 2 days. Proteins were extracted from the root and cotyledon, separated using two-dimensional polyacrylamide gel electrophoresis (2-DE), and the gels were stained with Coomassie brilliant blue (CBB) (Figure 1A) (Figure 2A). Three independent experiments were performed as biological replicates (Figure S1) (Figure S2). The relative intensity of protein spots was determined using PDQuest software (Table S1) (Table S2) (Figure 1B, 1C) (Figure 2B, 2C). A total of 615 and 377 protein spots were reproducibly detected on 2-DE gels of the root (Figure 1A and cotyledon (Figure 2A), respectively, of 2-day-old soybeans. Flooding stress resulted in significant changes in the abundance of 73 and 28 proteins in the root (Figure 1C) and cotyledon (Figure 2C), respectively, compared with 2-day-old (Figure 1A) (Figure 2A) and 4-day-old (Figure 1B) (Figure 2B) soybeans. Of the 615 total protein spots comprising the 2-DE pattern of root proteins, the staining intensity of 73 was significantly altered, 27 showing an increase in intensity and 46 showing a decrease in intensity in response to flooding stress (Figure 1). In the cotyledon, the intensity of 28 of 377 protein spots comprising the 2-DE pattern significantly changed in response to flooding stress, and of these 28 spots, 16 had increased in intensity and 12 had decreased (Figure 2).
Figure 1. 2-DE pattern and relative abundance of proteins in the root of flooding-stressed soybean plants.
Two-day-old soybean plants were flooded for 2 days, after which proteins were extracted from the root, separated by 2-DE, and the gels were stained with CBB (A). Open circles denote protein spots showing altered staining intensity. Upward and downward arrows indicate increase or decrease in intensity, respectively. The differential abundance of proteins was determined using PDQuest software and is plotted as the relative intensity (B and C). Results are presented as the mean ± SE of relative protein intensity for gels from 3 biological replicates (Figure S1). Differences were compared using the Student’s t test (*P<0.05, **P<0.01). White and black bars show control and treatment, respectively. Spots numbers are the same as shown in panel A.
Figure 2. 2-DE pattern and relative abundance of proteins in the cotyledon of flooding-stressed soybean plants.
Two-day-old soybean plants were flooded for 2 days, after which proteins were extracted from the cotyledon, separated by 2-DE, and the gels were stained with CBB (A). Open circles denote protein spots showing altered staining intensity. Upward and downward arrows indicate increase or decrease in intensity, respectively. The differential abundance of proteins was determined using PDQuest software and is plotted as the relative intensity (B and C). Results are presented as the mean ± SE of relative protein intensity for gels from 3 biological replicates (Figure S2). Differences were compared using the Student’s t test (*P<0.05, **P<0.01). White and black bars show control and treatment, respectively. Spots numbers are the same as shown in panel A.
Identification of Proteins that Change in Abundance in the Soybean Root and Cotyledon in Response to Flooding Stress
Soybean root and cotyledon proteins that had changed in abundance in response to flooding stress as determined using 2-DE were subsequently identified using nano liquid chromatography (LC)- mass spectrometry (MS)/MS. Peak lists of the identified proteins are provided in Tables (Table S3) (Table S4). A total of 73 and 28 flooding-responsive proteins were identified in the root (Table 1) and cotyledon (Table 2), respectively. A total of 5 root proteins that increased in abundance at least 3-fold in response to flooding stress were identified: decarboxylase isozyme 1 (spot number R37), stem 28 kDa glycoprotein (spot number R40), alcohol dehydrogenase 1F (spot number R42), peptidyl-prolyl cis-trans isomerase 1 (spot number R69), and fructose-bisphosphate aldolase (spot number R73) (Figure 1) (Table 1). A total of 4 root proteins that decreased in abundance at least 3-fold in response to flooding stress were identified: ripening-related protein (spot number R68), S-adenosylmethionine synthetase (spot number R71), and an unknown protein (spot number R72).
Table 1. Flooding-responsive proteins identified in soybean root using LC-MS/MS.
| Theoretical | ||||||||||
| Spot no | Homologous protein | Species | Protein ID | Accession no | Mr (Da) | pI | Score | MP | Cov(%) | BlastScore |
| 1 | Kunitz trypsin protease inhibitor | [Glycine max] | Glyma09g28310.1 | ACA23207 | 22947 | 5.24 | 28 | 2 | 15 | 397 |
| 2 | predicted protein | [Populus trichocarpa] | Glyma03g34820.1 | XP_002327377 | 20260 | 6.13 | 26 | 2 | 15 | 212 |
| 3 | (No significant hits to report) | |||||||||
| 4 | enolase | [Glycine max] | Glyma03g34830.1 | AAS18240 | 47914 | 5.49 | 72 | 2 | 13 | 863 |
| 5 | Heat shock 70 kDa protein | [Glycine max] | Glyma17g08020.1 | P26413 | 71220 | 5.28 | 2152 | 25 | 38 | 1144 |
| 6 | U2 small nuclear ribonucleoprotein A | [Ricinus communis] | Glyma20g18440.1 | XP_002512712 | 32537 | 5.16 | 104 | 3 | 18 | 405 |
| 7 | UDP-glucose 6-dehydrogenase | [Glycine max] | Glyma08g26520.1 | Q96558 | 53478 | 5.74 | 66 | 2 | 14 | 936 |
| 8 | dihydroxyacid dehydratase | [Glycine max] | Glyma13g27810.1 | ACU26534 | 64644 | 5.76 | 314 | 23 | 25 | 1160 |
| 9 | V- ATPase subnit A | [Glycine max] | Glyma08g23990.1 | ABU87506 | 69020 | 5.48 | 281 | 47 | 26 | 1217 |
| 10 | predicted protein | [Populus trichocarpa] | Glyma09g40690.1 | XP_002323696 | 61059 | 5.51 | 1049 | 104 | 30 | 949 |
| 11 | oligopeptidase A | [Medicago truncatula] | Glyma01g02480.1 | ABY48141 | 88745 | 6.03 | 777 | 84 | 43 | 1227 |
| 12 | Proteasome subnit alpha type-6 | [Glycine max] | Glyma06g39710.1 | O48551 | 27481 | 5.58 | 1406 | 107 | 47 | 489 |
| 13 | glutathione reductase | [Vigna unguiculata] | Glyma16g27210.1 | ABB89042 | 54243 | 5.63 | 629 | 79 | 48 | 880 |
| 14 | hypothetical protein | [Vitis vinifera] | Glyma06g48360.1 | XP_002272730 | 67578 | 5.60 | 765 | 89 | 39 | 1075 |
| 15 | isoflavonoid malonyl transferase 2 | [Medicago truncatula] | Glyma18g49240.1 | ABY91222 | 56964 | 5.70 | 601 | 75 | 29 | 430 |
| 16 | predicted protein | [Populus trichocarpa] | Glyma05g37670.1 | XP_002298026 | 112704 | 5.89 | 69 | 9 | 15 | 1470 |
| 17 | hypothetical protein | [Vitis vinifera] | Glyma18g04730.1 | XP_002270157 | 82072 | 6.21 | 225 | 15 | 17 | 1216 |
| 18 | UDP-D-apiose/xylose synthetase | [Gossypium hirsutum] | Glyma11g19550.1 | ACJ11753 | 43680 | 5.83 | 283 | 47 | 27 | 728 |
| 19 | hypothetical protein | [Vitis vinifera] | Glyma02g36530.1 | XP_002276114 | 60039 | 6.69 | 70 | 7 | 16 | 854 |
| 20 | isocitrate dehydrogenase [NADP] | [Glycine max] | Glyma02g40820.1 | Q06197 | 46393 | 5.87 | 1474 | 124 | 40 | 828 |
| 21 | UDP-glucose 6-dehydrogenase | [Glycine max] | Glyma08g26520.1 | Q96558 | 53478 | 5.74 | 141 | 8 | 19 | 936 |
| 22 | seed maturation protein PM31 | [Glycine max] | Glyma02g08400.1 | AAD30865 | 17907 | 6.10 | 57 | 2 | 19 | 323 |
| 23 | elongation factor 1-gamma | [Glycine max] | Glyma16g00360.1 | AAL82617 | 47785 | 5.92 | 117 | 2 | 15 | 619 |
| 24 | nucleoside diphosphate kinase | [Glycine max] | Glyma03g25820.1 | AAN77501 | 16308 | 6.30 | 76 | 2 | 12 | 284 |
| 25 | cysteine proteinase inhibitor | [Glycine max] | Glyma13g25870.1 | BAA19608 | 27708 | 6.57 | 57 | 5 | 10 | 417 |
| 26 | Transaminase mtnE | [Ricinus communis] | Glyma08g06790.1 | XP_002522052 | 50463 | 6.67 | 607 | 54 | 32 | 721 |
| 27 | acetoacetyl-CoA thiolase | [Medicago sativa] | Glyma14g00760.1 | ACX47470 | 41930 | 6.16 | 469 | 46 | 39 | 678 |
| 28 | hypothetical protein isoform 2 | [Vitis vinifera] | Glyma01g06970.1 | XP_002269733 | 53522 | 6.34 | 448 | 44 | 38 | 890 |
| 29 | acetoacetyl-CoA thiolase | [Medicago sativa] | Glyma14g00760.1 | ACX47470 | 41930 | 6.16 | 62 | 2 | 13 | 678 |
| 30 | Gamma-glutamyl hydrolase | [Glycine max] | Glyma13g34290.1 | P93164 | 38282 | 6.72 | 3208 | 212 | 36 | 619 |
| 31 | serine hydroxymethyltransferase 2 | [Glycine max] | Glyma05g28490. | ACM45952 | 52142 | 6.90 | 982 | 67 | 25 | 920 |
| 32 | heat shock protein 70 | [Gossypium hirsutum] | Glyma19g35560.1 | ACJ11741 | 71854 | 5.05 | 3704 | 251 | 37 | 1135 |
| 33 | actin isoform PEAc14-1 | [Pisum sativum] | Glyma02g10170.1 | ADP09679 | 42036 | 5.23 | 682 | 107 | 51 | 754 |
| 34 | glutamate decarboxylase | [Glycine max] | Glyma18g04940.1 | BAF80896 | 57554 | 5.59 | 762 | 71 | 33 | 1011 |
| 35 | predicted protein | [Populus trichocarpa] | Glyma36750.1 | XP_002327728 | 99030 | 5.56 | 999 | 130 | 26 | 1624 |
| 36 | hypothetical protein | [Vitis vinifera] | Glyma16g32960.1 | XP_002267091 | 48231 | 6.06 | 1216 | 56 | 40 | 842 |
| 37 | decarboxylase isozyme 1 | [Glycine max] | Glyma13g30490.1 | P51850 | 64169 | 5.73 | 626 | 97 | 35 | 1060 |
| 38 | Ran3A-1 | [Dimocarpus longan] | Glyma04g07350.1 | AEM97804 | 25505 | 6.38 | 914 | 86 | 53 | 427 |
| 39 | copper amino oxidase | [Glycine max] | Glyma17g02260.1 | CAE47488 | 76061 | 6.21 | 696 | 121 | 32 | 1284 |
| 40 | stem 28 kDa glycoprotein | [Glycine max] | Glyma07g01730.1 | P15490 | 29218 | 8.75 | 1419 | 108 | 51 | 528 |
| 41 | (No significant hits to report) | |||||||||
| 42 | alcohol dehydrogenase-1F | [Phaseolus acutifolius] | Glyma04g41990.1 | Caa80691 | 41638 | 5.97 | 2015 | 183 | 53 | 734 |
| 43 | methionine synthase | [Glycine max] | Glyma16g04240.1 | AAQ08403 | 84401 | 5.93 | 2071 | 243 | 48 | 1419 |
| 44 | hypothetical protein | [Vitis vinifera] | Glyma08g13440.1 | XP_002270155 | 39049 | 5.12 | 2842 | 178 | 42 | 417 |
| 45 | hypothetical protein | [Vitis vinifera] | Glyma13g41960.1 | XP_002268097 | 35546 | 5.29 | 3702 | 207 | 58 | 560 |
| 46 | cytosolic glutamine synthetase GSbeta1 | [Glycine max] | Glyma11g33560.1 | AAG24873 | 39138 | 5.48 | 262 | 20 | 27 | 718 |
| 47 | heat shock 70 kDa protein | [Glycine max] | Glyma08g06950.1 | Q01899 | 74981 | 6.02 | 3835 | 187 | 39 | 1157 |
| 48 | lactoylglutathione lyase | [Gossypium hirsutum] | Glyma09g00660.1 | ACJ11750 | 32504 | 5.76 | 645 | 70 | 31 | 531 |
| 49 | glutamate-1-Semialdehyde2,1-aminomutase | [Glycine max] | Glyma04g00420.1 | P45621 | 50239 | 6.05 | 168 | 7 | 12 | 874 |
| 50 | predicted protein | [Populus trichocarpa] | Glyma02g13330.1 | XP_002305394 | 45064 | 6.10 | 2165 | 185 | 40 | 685 |
| 51 | dihydrolipoamide acetyltransferase | [Cucumis melo] | Glyma07g03930.1 | ADN33731 | 59813 | 8.00 | 109 | 10 | 16 | 697 |
| 52 | predicted protein | [Populus trichocarpa] | Glyma09g40690.1 | XP_002323696 | 61059 | 5.51 | 2019 | 157 | 34 | 949 |
| 53 | 6-phosphogluconate dehydrogenase | [Glycine max] | Glyma18g51260.1 | BAA22812 | 53903 | 5.68 | 699 | 93 | 27 | 927 |
| 54 | glutathione reductase | [Vigna unguiculata] | Glyma16g27210.1 | ABB89042 | 54243 | 5.63 | 1795 | 168 | 35 | 880 |
| 55 | d-3-phosphoglycerate dehydrogenase | [Ricinus communis] | Glyma10g40750.1 | XP_002518687 | 62859 | 6.32 | 41 | 2 | 14 | 878 |
| 56 | (No significant hits to report) | |||||||||
| 57 | argininosuccinate synthase,putative | [Ricinus communis] | Glyma05g03190.1 | XP_002521168 | 52641 | 6.55 | 16 | 2 | 13 | 774 |
| 58 | pyruvate decarboxylase 1 | [Lotus corniculatus] | Glyma18g43460.1 | AAO72533 | 66371 | 5.80 | 273 | 46 | 19 | 1071 |
| 59 | Chalcone-flavonone isomerase 1A | [Glycine max] | Glyma20g38560.1 | Q93XE6 | 23307 | 6.23 | 2359 | 126 | 61 | 394 |
| 60 | 6-phosphogluconate dehydrogenase | [Glycine max] | Glyma08g28230.1 | BAA22812 | 53811 | 6.11 | 1433 | 110 | 39 | 899 |
| 61 | Os05g0553000 | [Oryza sativa] | Glyma10g41330.1 | NP_001056261 | 59913 | 5.80 | 39 | 2 | 15 | 879 |
| 62 | predicted protein | [Populus trichocarpa] | Glyma11g03330.1 | XP_002329902 | 65864 | 6.26 | 585 | 52 | 39 | 854 |
| 63 | (No significant hits to report) | |||||||||
| 64 | quinine oxidoreductase | [Cicer arietinum] | Glyma13g32300.1 | CAD31838 | 21653 | 6.43 | 1017 | 80 | 47 | 384 |
| 65 | ald/keto reductase,putative | [Ricinus communis] | Glyma03g40680.1 | XP_002512220 | 37793 | 6.26 | 201 | 22 | 33 | 510 |
| 66 | Knitz trypsin protease inhibitor | [Glycine max] | Glyma09g28310.1 | ACA23207 | 22947 | 5.24 | 405 | 37 | 33 | 397 |
| 67 | peroxisomal betaine-aldehyde dehydrogenase | [Glycine max] | Glyma06g19820.1 | BAG09377 | 55389 | 5.23 | 965 | 111 | 35 | 1015 |
| 68 | ripening related protein | [Glycine max] | Glyma08g24760.1 | AAD50376 | 17865 | 5.96 | 1338 | 195 | 79 | 263 |
| 69 | peptidyl-prolyl cis-trans isomerase 1 | [Glycine max] | Glyma11g10480.1 | Q8W171 | 18441 | 8.70 | 1440 | 71 | 62 | 321 |
| 70 | Os05g0553000 | [Oryza sativa] | Glyma10g41330.1 | NP_001056261 | 59913 | 5.80 | 3205 | 160 | 50 | 879 |
| 71 | S-adenosylmethionine synthetase | [Cicer arietinum] | Glyma15g21890.1 | ACL14491 | 43425 | 5.50 | 1599 | 98 | 51 | 745 |
| 72 | (No significant hits to report) | |||||||||
| 73 | fructose-bisphosphate aldolase | [Glycine max] | Glyma02g38730 | O65735 | 38469 | 7.12 | 2591 | 148 | 76 | 634 |
Spot no shows Spot number. Protein ID shows Phytozome protein ID. Accession no shows NCBI Accession number. MP shows matched peptides. Cov shows sequence coverage.
Table 2. Flooding-responsive proteins identified in soybean cotyledon using LC-MS/MS.
| Theoretical | ||||||||||
| Spot no | Homologous protein | Species | Protein ID | Accession no | Mr (Da) | pI | Score | MP | Cov (%) | Blast Score |
| 1 | sucrose-binding protein | [Glycine max] | Glyma10g03390.1 | Q04672 | 58353 | 6.08 | 22 | 3 | 17 | 723 |
| 2 | (No significant hits to report) | |||||||||
| 3 | lactoylglutathione lyase | [Gossypium hirsutum] | Glyma09g00660.1 | ACJ11750 | 32504 | 5.76 | 393 | 15 | 21 | 531 |
| 4 | (No significant hits to report) | |||||||||
| 5 | glycinin G2 | [Glycine max] | Glyma03g32020.1 | P04405 | 54927 | 5.46 | 202 | 26 | 20 | 799 |
| 6 | NADPH-protochlorophyllide oxidoreductase | [Vigna radiata] | Glyma06g38160.1 | AAF89208 | 43247 | 9.12 | 132 | 15 | 19 | 714 |
| 7 | (No significant hits to report) | (No significant hits to report) | ||||||||
| 8 | beta-conglycinin alpha prime subunit | [Glycine max] | Glyma10g39150.1 | BAE02726 | 72469 | 5.50 | 105 | 19 | 15 | 893 |
| 9 | beta-conglycinin alpha subunit | [Glycine max] | Glyma20g28650.1 | BAE44299 | 70549 | 5.12 | 82 | 22 | 17 | 783 |
| 10 | (No significant hits to report) | |||||||||
| 11 | beta-conglycinin alpha subunit | [Glycine max] | Glyma20g28650.1 | BAE44299 | 70549 | 5.12 | 121 | 33 | 17 | 783 |
| 12 | hypothetical protein | [Vitis vinifera] | Glyma04g35950.1 | XP_002281671 | 91567 | 5.11 | 63 | 6 | 11 | 1446 |
| 13 | predicted protein | [Populus trichocarpa] | Glyma01g05060.1 | XP_002323767 | 48123 | 5.61 | 81 | 2 | 13 | 539 |
| 14 | cytosolic malate dehydrogenase | [Glycine max] | Glyma02g00810.1 | AAS18241 | 35787 | 5.91 | 140 | 7 | 13 | 631 |
| 15 | heat shock 70 kDa protein | [Glycine max] | Glyma17g08020.1 | P26413 | 71220 | 5.28 | 130 | 16 | 24 | 1144 |
| 16 | sucrose-binding protein | [Glycine max] | Glyma10g03390.1 | Q04672 | 58353 | 6.08 | 475 | 38 | 14 | 723 |
| 17 | (No significant hits to report) | |||||||||
| 18 | (No significant hits to report) | |||||||||
| 19 | glyoxysomal isocitrate lyase isoform 1 | [Glycine max] | Glyma06g45950.1 | ABD28288 | 65144 | 7.26 | 30 | 2 | 12 | 1075 |
| 20 | (No significant hits to report) | |||||||||
| 21 | ferrous iron transport protein b | [Ralstonia solanacearum] | Glyma02g08980.1 | AEG70893 | 217645 | 5.32 | 21 | 2 | 10 | 22.7 |
| 22 | glycinin G2[Glycine max] | [Glycine max] | Glyma03g32030.1 | P04405 | 54927 | 5.46 | 382 | 23 | 19 | 799 |
| 23 | sucrose-binding protein | [Glycine max] | Glyma10g03390.1 | Q04672 | 58353 | 6.08 | 1359 | 102 | 36 | 723 |
| 24 | glycinin G3 | [Glycine max] | Glyma19g34780.1 | P11828 | 54835 | 5.73 | 53 | 9 | 16 | 796 |
| 25 | (No significant hits to report) | |||||||||
| 26 | sucrose-binding protein | [Glycine max] | Glyma10g03390.1 | Q04672 | 58353 | 6.08 | 2101 | 142 | 54 | 723 |
| 27 | beta-conglycinin alpha subunit | [Glycine max] | Glyma20g28650.1 | BAE44299 | 70549 | 5.12 | 584 | 96 | 33 | 783 |
| 28 | ferrous iron transport protein b | [Ralstonia solanacearum] | Glyma03g42290.1 | AEG70893 | 217201 | 6.16 | 15 | 2 | 10 | 22.7 |
Spot no shows Spot number. Protein ID shows Phytozome protein ID. Accession no shows NCBI Accession number. MP shows matched peptides. Cov shows sequence coverage.
In the cotyledon, 7 proteins that increased in abundance at least 2-fold in response to flooding stress were identified: heat shock 70 kDa protein (HSP70) (spot number C15), glycinin G3 (spot number C24), sucrose-binding protein (spot number C26), beta-conglycinin alpha subunit (spot number C27), hypothetical protein (spot number C12), and 2 unknown proteins (spot number C2 and spot number C10). A total of 4 cotyledon proteins that decreased in abundance at least 2-fold in response to flooding stress were identified: glycinin G2 (spot number C22), sucrose-binding protein (spot number C23), beta-conglycinin alpha subunit (spot number C11), and ferrous iron transport protein b (spot number C21) (Figure 2) (Table 2).
Flooding-stress-induced Changes in the Abundance of Proteins Common to the Root and Cotyledon of Soybean Plants
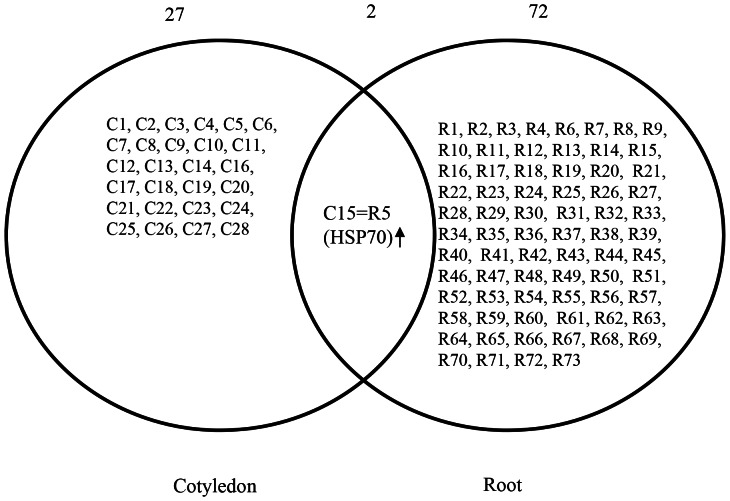
Analysis of the 2-DE pattern of root and cotyledon proteins of flooding-stressed soybean plants showed that the abundance of only one protein common to both organs, HSP70 (Glyma17g08020.1) (spot numbers R5 and C15), increased in both the root and cotyledon following flooding stress (Figure 3) (Table S5). The ratio of protein abundance of HSP70 in the cotyledon was higher than that detected in the root under flooding stress. However, HSP70 with accession number Glyma19g35560.1 (spot numbers R32) and Glyma08g06950.1 (spot numbers R47) decreased in root under flooding stress. The remaining proteins showed organ-specific changes in response to flooding, indicating that each organ responds differently to flooding stress.
Figure 3. Venn diagram analysis of root and cotyledon proteins of flooding-stressed soybean plants.
Diagram shows root and cotyledon proteins that changed in abundance in response to flooding stress. The overlapping area shows the common protein to both root and cotyledon that decreased in abundance in both organs. Numbers at the top of the diagram indicate the number of proteins. Downward arrow indicates a decrease in staining intensity. The numbers within the circles correspond to the proteins listed in Table 1 and Table 2.
Biophoton Emission in the Root and Cotyledon of Soybean Plants under Flooding Stress
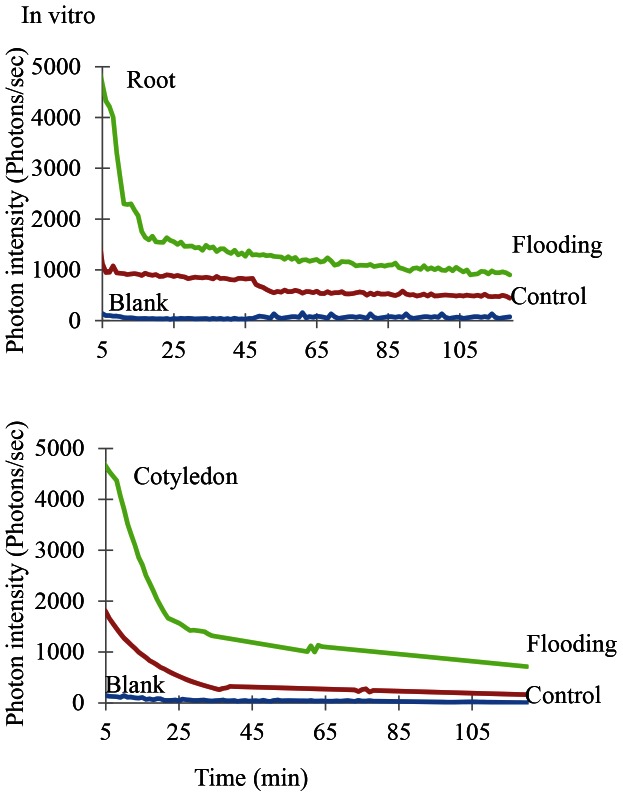
To determine the effect of flooding stress on the physiological state of soybean plants, biophoton emission in the root and cotyledon was measured both in vitro and in vivo using a photon counter. Two-day-old soybean was flooded for 2 days and the results were compared to measurements of control soybean plants that had not been flooded. In vitro measurements showed that the rate of biophoton emission in both the root and cotyledon was higher in flooding-stressed plants than in control untreated plants (Figure 4) (Figure S3). Furthermore, the ratio of biophoton emission of both in vivo and in vitro in the cotyledon was higher than that detected in the root under flooding stress (Figure 4) (Figure 5).
Figure 4. In vitro biophoton emission patterns of flooding-stressed soybean plants.
Two-day-old soybean plants were flooded for 2 days, after which proteins were extracted from the root and cotyledon and H2O2 was added. Emission of photons from these extracts was then measured. Three independent biological replicate analyses of the root and cotyledon were performed (Figure S3). Ground photon emission by the empty Petri dish served as a blank (blue). Green lines represent biophoton emission from flooding-stressed soybean plants, while red lines represent biophoton emission from untreated soybean plants.
Figure 5. In vivo biophoton emission patterns of flooding-stressed soybean plants.
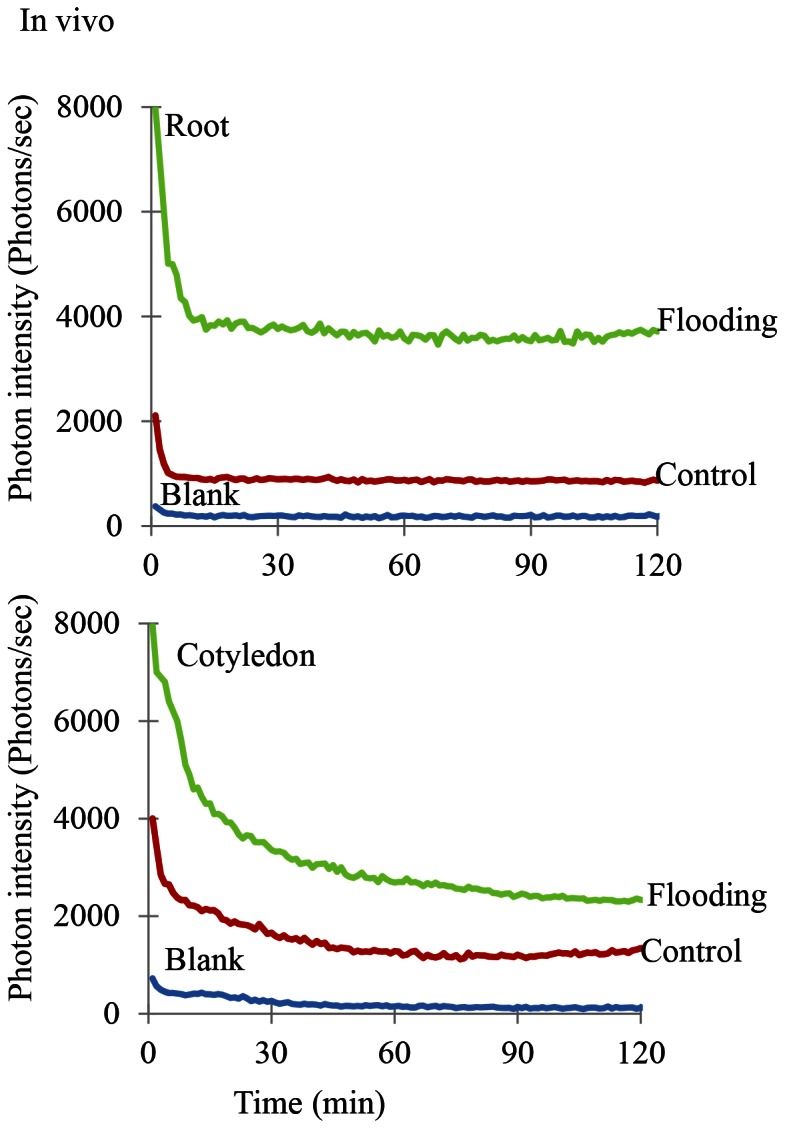
Two-day-old soybean plants were flooded for 2 days, after which the root and cotyledon were treated with luminal solution and photon emission was measured. Three independent biological replicate analyses of the root and cotyledon were performed (Figure S4). Ground photon emission by the empty Petri dish served as a blank (blue). Green lines represent biophoton emission from flooding-stressed soybean plants, while red lines represent biophoton emission from untreated soybean plants.
For in vivo measurement of biophoton emission, luminal solution was applied to fresh samples which were then transferred to Petri plates and placed on the rotating disk of the photon counter. These analyses confirmed that the intensity of biophoton emission was also higher in both the root and cotyledon of flooding-stressed soybeans (Figure 5) (Figure S4).
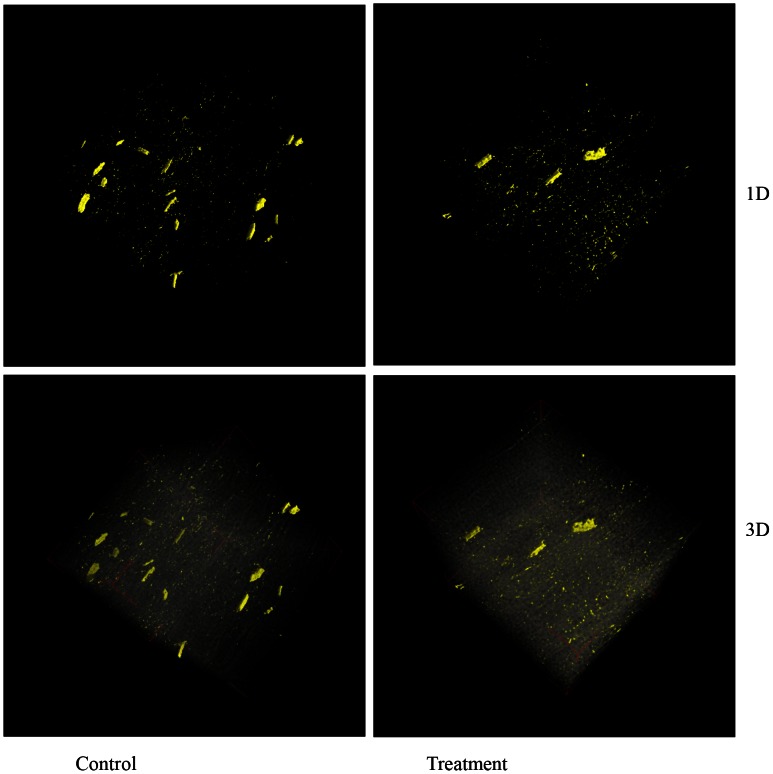
X-Ray Computed Tomography and Scanning Electron Microscopy/Energy Dispersive X-Ray Analysis
Two-day-old soybean plants were flooded for 2 days, and the cotyledon was collected and fixed. Changes in components in the cotyledon of flooding-stressed plants were analyzed using X-ray computed tomography (Figure 6) (Video S1) (Video S2). Flooding stress resulted in a decrease in the number of crystals in the cotyledon (Figure 6).
Figure 6. X-ray computed tomography analysis.
Two-day-old soybean plants were flooded for 2 days, after which the cotyledon was collected and fixed. Untreated plants were used as control. Digital geometry processing was used to generate a three-dimensional image of the inside of the cotyledon from a large series of two-dimensional X-ray images taken around a single axis of rotation. The “1D” and “3D” show the first-dimension and three-dimension.
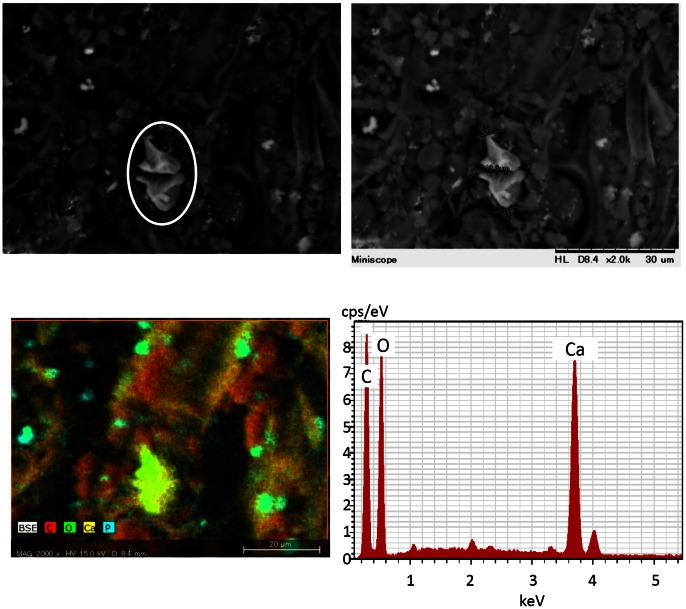
Analysis of the cotyledon crystals using scanning electron microscopy and energy dispersive X-rays showed that the element of crystal contained the carbon (C), oxygen (O), and calcium (Ca); which indicated that it was composed of calcium oxalate, CaC2O4 (Figure 7) (Figure S5) (Figure S6). These results indicated that flooding stress leads to a decrease in the number of calcium oxalate crystals in the cotyledon.
Figure 7. Scanning electron microscopy and energy dispersive X-ray analysis.
Two-day-old soybean plants were flooded for 2 days, after which the cotyledon was collected and fixed. Scanning electron micrographs and energy dispersive X-ray spectra are shown.
Discussion
Soybean plants are sensitive to flooding stress, and their growth is significantly reduced by flooding [4]. Root elongation of soybean clearly retarded by 2 days of flooding stress [5], [22]. Flooding-associated suppression of root elongation may occur in the root tip because this region contains the root apical meristem and is the site of elongation [8]. The root apical meristem is also important for root system development. Prolonged flooding of soybean seedlings has also been shown to induce the cell death of root tip [6], [7], [14], which is one of the symptoms of flooding injury in soybean seedlings. While a number of reports have described the flood response mechanisms of soybean root [4], there are no reports describing the changes that occur in the cotyledon in response to flooding. In this study, flooding-stress-induced changes in the soybean cotyledon were examined using biological and proteomic technique to obtain a further current understanding of the flooding-stress response in this important agricultural crop.
Changes in components in the cotyledon of soybeans under flooding stress were analyzed using X-ray computed tomography, and flooding stress resulted in a decrease in the number of crystals in the cotyledon (Figure 6). Furthermore, scanning electron microscopy and energy dispersive X-rays showed that it was composed of calcium oxalate (Figure 7). These results indicated that flooding stress leads to a decrease in the number of calcium oxalate crystals in the cotyledon. Calcium oxalate crystals modulate physiological calcium levels within plant tissues [23], [24]. Depending on the calcium requirement of the developing soybean seed, the number and distribution of calcium oxalate crystals change as the seed develops and matures [25]. Chiera and Grabau [26] provided evidence that D-myo-inositol-3-phosphate synthase is associated with oxalate crystal idioblasts. Furthermore, the cell death of cotyledon in flooding-stressed soybean plants has been documented [9].
The proteomic technique was used to examine whether calcium related proteins are involved in soybean under flooding stress. The proteomic analysis of the root and cotyledon showed that only one protein, HSP70, increased in abundance in both organs in response to flooding stress (Figure 3). HSP70 had a weak ATPase activity that was stimulated by interaction with DnaJ proteins [27]. Enhanced ATPase activity was required for stable binding and proper folding of HSP70’s client proteins [28]. HSP70 was involved in many cellular processes, including protein folding, protein translocation across membranes, and regulation of protein degradation. Cha et al. [29] reported that HSP70 bound to calmodulin-2 in the presence of calcium ion via a conserved calmodulin-binding domain. Suri and Dhindsa [30] reported that heat-induced accumulation of HSP70 required a heat-activated MAP kinase. Heat activation of MAP kinase was rapid and transient, and required an influx of calcium ion from the apoplast and the activity of an upstream MEK-related MAPKK. Interestingly, the ratio of protein abundance of HSP70 in the cotyledon was significantly higher than that detected in the root under flooding stress (Figure 1) (Figure 2). These observations and our result indicate that calcium might be one candidate of the factor that operates in soybean cotyledon when responding to the flooding stress.
In addition, biophoton emission was measured because calcium is related to photon emission. The intensity of photon emission was higher in the root and cotyledon of flood-stressed plants than in untreated soybean plants. Furthermore, the ratio of biophoton emission in the cotyledon was higher than that in the root under flooding stress (Figure 4) (Figure 5). Bennett et al. [31] demonstrated that ultra-weak photon emission or biophoton generation was associated with hypersensitive cell death. Biophoton emission required an intact R-signaling network and increased as levels of cytosolic calcium and nitric oxide rise, but elevated levels of reactive oxygen species were not necessary [31]. Subbaiah and Sachs [7] indicated that early rise in cytosolic calcium ion, as well as a quick establishment of ionic homeostasis, may be essential for the induction of adaptive changes at the cellular as well as organism-level. These reports and our result suggest that an increase in calcium level in the cotyledon might stimulate biophoton emission.
A role for calcium oxalate production is as part of a high-capacity mechanism for regulating bulk calcium levels in plant tissues and organs [24]. A decrease in calcium oxalate crystal suggests that calcium ions increase in soybean cotyledon under flooding stress. Decomposition of calcium oxalate crystals produces calcium ions that may be available for HSP70 signal transduction. Since flooding stress leads to a decrease in the amount of calcium oxalate crystals, which means an increase of calcium ion, HSP70 signal transduction might be stimulated. In conclusion, these results suggest that calcium ion supplied from calcium oxalate crystals might play one role for signal transduction through HSP70 in the cotyledon, and that a part of the passing way of flooding stress may induce HSP70-mediated signal transduction.
Materials and Methods
Plant Growth and Treatments
Seeds of soybean (Glycine max [L.] Merrill cultivar Enrei) were sterilized in a sodium hypochlorite solution and germinated on silica sand. Two-day-old soybean plants were flooded with water for 2 days in a growth chamber illuminated with white fluorescent light (200 µmol m−2s−1; 12-h light period/day) at 25°C and 70% relative humidity. To examine organ-specific responses, cotyledon and root were collected from the 4-day-old plants after 2 days of flooding. For all experiments, untreated equivalent plants were sampled as controls, and 3 independent experiments were performed as biological replicates.
Protein Extraction for Two-Dimensional Polyacrylamide Gel Electrophoresis
A portion (500 mg) of fresh sample was ground to powder in liquid nitrogen with a mortar and pestle. The powder was transferred to 10% trichloroacetic acid and 0.07% 2-mercaptoethanol in acetone and the mixture was vortexed. The suspension was sonicated for 5 min and then incubated for 1 h at −20°C. After incubation, the suspension was centrifuged at 9,000 × g for 20 min at 4°C. The supernatant was discarded and resulting pellet was washed twice with 0.07% 2-mercaptoethanol in acetone. The resulting pellet was dried using a Speed-Vac concentrator (Savant Instruments, Hicksville, NY, USA) and resuspended by vortexing for 1 h at 25°C in a solution of 8 M urea, 2 M thiourea, 5% CHAPS, and 2 mM tributylphosphine. The resulting suspension was centrifuged at 20,000 × g for 20 min at 25°C and the supernatant was collected for two-dimensional polyacrylamide gel electrophoresis (2-DE). Protein concentrations were determined using the Bradford method [32] with bovine serum albumin as the standard.
Two-Dimensional Polyacrylamide Gel Electrophoresis
Protein samples (500 µg) in a final volume of 180 µL of lysis buffer containing 0.4% ampholytes pH 3–10 (Bio-Lyte, Bio-Rad, Hercules, CA, USA) were loaded into a focusing tray. Immobilized pH gradient strips (3–10 NL, 11 cm, Bio-Rad) were passively rehydrated for 2.5 h and then actively rehydrated for 14 h at 50 V. Isoelectric focusing (IEF) was carried out using a Protean IEF Cell system (Bio-Rad) under the following conditions: 250 V for 15 min with a linear ramp, 8,000 V for 1 h with a linear ramp, and finally 8,000 V for 35,000 V-h with a rapid ramp. After IEF, the strips were incubated for 30 min in equilibration buffer I containing 6 M urea, 2% SDS, 0.375 M Tris-HCl (pH 8.8), 20% glycerol, and 130 mM dithiothreitol. The strips were then incubated for 30 min in equilibration buffer II containing 6 M urea, 2% SDS, 0.375 M Tris-HCl (pH 8.8), 20% glycerol, and 135 mM iodoacetamide. The equilibrated strips were then placed onto 15% SDS-PAGE gels and sealed with 1% low-melting-temperature agarose. Second dimension electrophoresis was performed at a constant current of 30 mA. After electrophoresis, the gels were stained for 1 h with Coomassie brilliant blue (CBB) (Phast Gel™ Blue R, GE Healthcare, Piscataway, NJ, USA) in 35% methanol and 10% acetic acid, and then destained for 12 h in 35% methanol and 10% acetic acid. The images were analyzed as described below.
Gel Image Analysis
CBB-stained gels were scanned using a high-resolution scanner (GS-800 Calibrated Imaging Densitometer, Bio-Rad). Protein spots were detected and quantified on the basis of relative intensity using PDQuest software (version 8.0.1, Bio-Rad). The intensity of a given protein spot was expressed in terms of its volume, which was defined as the sum of the intensities of all pixels constituting the spot in the image. To compensate for subtle differences in sample loading, gel staining, and gel destaining, the volume of each spot was normalized as a percentage of the total volume of all the spots present in the gel. Manual editing was carried out after automated detection and matching.
Student’s t test was used to assess the statistical significance of differences in protein abundance between control and treatment samples. Proteins exhibiting a fold change of more than 2.0 and a P-value <0.05 relative to the control were considered to be significantly altered in abundance.
Peptide Preparation for Mass Spectrometry Analysis
Proteins were identified using mass spectrometry (MS). Protein spots were excised from 2-DE gels and washed with water. Proteins in the excised gel pieces were then reduced by incubating them for 1 h at 60°C in 100 mM NH4HCO3 buffer containing 10 mM dithiothreitol followed by incubation for 30 min in 100 mM NH4HCO3 buffer containing 40 mM iodoacetamide. The gel pieces were digested at 37°C overnight in 100 mM NH4HCO3 containing 1 pM trypsin (Wako, Osaka, Japan). The tryptic peptides were extracted from the gel grains 3 times using 0.1% trifluoroacetic acid in 50% acetonitrile. The procedure described above was performed with DigestPro (Intavis Bioanalytical Instruments AG, Cologne, Germany). The resulting peptide solutions were desalted with C-Tip pipet tips (Nikkyo Technos, Tokyo, Japan) and analyzed by nano-liquid chromatography (LC)-tandem mass spectrometry (LC)-MS/MS.
Protein Identification by Mass Spectrometry
Peptides in 0.1% formic acid were loaded onto a C18 PepMap trap column (300-µm ID × 5 mm) and eluted using an Ultimate 3000 nanoLC system (Dionex, Germering, Germany). The peptides were separated on a nano-capillary column (NTTC-360/75-3, Nikkyo Technos) with 0.1% formic acid in acetonitrile at a flow rate of 200 nL/min and introduced with a spray voltage of 1.8 kV into a nanospray LTQ XL Orbitrap MS (Thermo Fisher Science, San Jose, CA, USA) operated in data-dependent acquisition mode with the installed XCalibur software. Full-scan mass spectra were acquired over the m/z range 150–2,000 with a resolution of 15,000. The 3 most intense ions above the 1,000 threshold were selected for collision-induced fragmentation in the linear ion trap at a normalized collision energy of 35% after accumulation to a target value of 1,000. Dynamic exclusion was employed within 30 s to prevent repetitive selection of peptides.
Acquired MS/MS spectra were converted to individual DTA files using BioWorks software (version 3.3.1) (Thermo Fisher Science). The following parameters were used to create peak lists: parent ions in the mass range with no limitation, 1 grouping of MS/MS scans, and threshold of 100. The resulting peptide mass data were used to search the database using the MASCOT search engine (Matrix Science, London, UK). Soybean genome sequences were downloaded from the soybean genome database [33] (Phytozome, version 6.0, http://www.phytozome.net/soybean) and converted to FASTA format. Carbamidomethylation of cysteines was set as a fixed modification and oxidation of methionine was set as a variable modification. Trypsin was specified as the proteolytic enzyme and 1 missed cleavage was allowed. The search parameters were peptide mass tolerance = 10 ppm, fragment mass tolerance = 0.2 Da, maximum missed cleavages = 1, and peptide charges = +1, +2, and +3. The minimal requirements for accepting protein identifications were as follows: (i) the score, indicating the probability of a true positive identification, must be at least 100, (ii) there must be at least 2 peptide sequence matches above the identity threshold, and (iii) the coverage of the protein sequence by the matching peptides must be at least 7%. Positive matches were BLASTP searched against the NCBI protein database for updated annotation and identification of homologous proteins.
Measurement of Biophoton Emission
For in vitro measurement of biophoton emission, a portion (250 mg) of fresh sample was homogenized with a mortar and pestle in 2.5 mL of 25 mM potassium phosphate buffer (pH 7.8) containing 2% polyvinylpolypyrrolidone, 0.4 mM EDTA, and 1 mM ascorbic acid. After centrifugation at 15,000 × g for 20 min at 4°C, the supernatant was collected as protein extract. The solution used for the measurement of biophoton emission consisted of 880 µL of reaction mixture, 100 µL of protein extract, and 20 µL of 0.056% H2O2. The reaction mixture was composed of 50 mM phosphate buffer (pH 7.8), 0.5 mM sodium ascorbate, and 0.1 mM EDTA. For in vivo measurement of biophoton emission, the fresh samples were treated directly with 0.5 mM luminal solution in phosphate buffer.
In order to observe the time-dependent variation in photon emission intensity, a C1230 photon counter (Hamamatsu Photonics, Hamamatsu, Japan) with a built-in high-voltage stabilized direct-current power source was used. The device counts the number of photons detected by a R208 photomultiplier tube (Hamamatsu Photonics) with a bi-alkali photocathode, providing a spectral response from 185–650 nm [19] Biophoton emission was measured in the root, hypocotyl, and leaf at 1-min intervals for 2 h at 25°C. Ground photon emission by the empty Petri dish served as a blank. Each experiment involved 3 independent biological replicates.
X-Ray Computed Tomography
The cotyledons were excised from soybean plants and fixed in 4% paraformaldehyde (TAAB, Berks, UK) in 0.1 M phosphate buffer (pH 7.4) for overnight at 4°C. The cotyledons thus fixed were immersed in a solution of 100 mM Tris-HCl (pH 8.0) for 60 min at room temperature, followed by washing and dehydration with 100% ethanol. The dehydrated cotyledons were subjected to X-ray computed tomography analysis using a TOHKEN-SkyScan2011 scanner (MARS TOHKEN X-RAY INSPECTION Co. Ltd., Tokyo, Japan). The computed tomography was performed under the following conditions: X-ray emission with 200 µA at 20 kV, resolution of 360 nm/pixel, and slice thickness of 360 nm. Photographing of each of the 900 slices was done using a CCD camera with an exposure time of 8 s. Digital geometry processing is used to generate a three-dimensional image of the inside of an object from a large series of two-dimensional X-ray images taken around a single axis of rotation (Tsukuba GeneTech Lab,Tsukuba, Japan).
Scanning Electron Microscopy and Energy Dispersive X-Ray Analysis
The cotyledons were excised from soybean plants and fixed in 4% paraformaldehyde (TAAB, Berks, UK) in 0.1 M phosphate buffer (pH 7.4) for overnight at 4°C. The cotyledons thus fixed were immersed in a solution of 100 mM Tris-HCl (pH 8.0) for 60 min at room temperature, followed by washing and dehydration with 100% ethanol. Composite membrane morphology was analyzed using a scanning electron microscopy, Hitachi TM3000 equipped with Quantax70 (Hitachi, Tokyo, Japan), operated with an accelerating voltage of 15 kV. Energy dispersive X-ray spectra of composite membranes were obtained with the SEM at 15 kV, and used for elemental analysis of the surface of samples.
Supporting Information
2-DE patterns in the root proteins of flooding-stressed soybean plants. Two-day-old soybean plants were flooded for 2 days, after which proteins were extracted from the root, separated by 2-DE, and the gels were stained with CBB. Results are presented as the mean ± SE of relative protein intensity for gels from 3 biological replicates. 2-DE patterns were shown 3 biological replicates.
(TIF)
2-DE patterns in the cotyledon proteins of flooding-stressed soybean plants. Two-day-old soybean plants were flooded for 2 days, after which proteins were extracted from the cotyledon, separated by 2-DE, and the gels were stained with CBB. Results are presented as the mean ± SE of relative protein intensity for gels from 3 biological replicates. 2-DE patterns were shown 3 biological replicates.
(TIF)
In vitro biophoton emission patterns of flooding-stressed soybean plants. Two-day-old soybean plants were flooded for 2 days, after which proteins were extracted from the root and cotyledon and H2O2 was added. Emission of photons from these extracts was then measured. R1, R2, and R3 represent 3 independent biological replicate analyses of the root and cotyledon. Ground photon emission by the empty Petri dish served as a blank (blue). Sky blue and purple lines represent biophoton emission from flooded soybean plants, while yellow and pink lines represent biophoton emission from untreated soybean plants.
(TIF)
In vivo biophoton emission patterns of flooding-stressed soybean plants. Two-day-old soybean plants were flooded for 2 days, after which the root and cotyledon were treated with luminal solution and photon emission was measured. R1, R2, and R3 represent biophoton emission from 3 independent biological replicate analyses of the root and cotyledon. Ground photon emission by the empty Petri dish served as a blank (blue). Sky blue and purple lines represent biophoton emission from flooded soybean plants, while yellow and pink lines represent biophoton emission from untreated soybean plants.
(TIF)
Scanning electron microscopy and energy dispersive X-ray analysis of Ca, O, and C. Two-day-old soybean plants were flooded for 2 days, after which the cotyledon was collected and fixed. A scanning electron micrograph and energy dispersive X-ray spectra of Ca, O, and C are shown.
(TIF)
Scanning electron microscopy and energy dispersive X-ray analysis of F, K, Na, P, and S. Two-day-old soybean plants were flooded for 2 days, after which the cotyledon was collected and fixed. A scanning electron micrograph and energy dispersive X-ray spectra of F, K, Na, P, and S are shown.
(TIF)
The results of Student’s t test for each spot in 2-DE pattern of root proteins.
(XLSX)
The results of Student’s t test for each spot in 2-DE pattern of cotyledon proteins.
(XLSX)
Peak lists for proteins identified in the root of flooding-stressed soybean plants.
(DOCX)
Peak lists for proteins identified in the cotyledon of flooding-stressed soybean plants.
(DOCX)
Common proteins to both cotyledon and root of flooding-stressed soybean plants.
(DOCX)
Video detailing X-ray computed tomography analysis of the cotyledon of a 4-day-old soybean plant (control).
(ZIP)
Video detailing X-ray computed tomography analysis of the cotyledon of a 4-day-old soybean plant after 2 days of flooding.
(ZIP)
Acknowledgments
The authors thank Dr. Yohei Nanjo, Dr. Keito Nishizawa, and Mr. Hideki Saito in National Institute of Crop Science for their kind support and discussion to the research. The authors also thank Ms. Mutsumi Ishimoto in MARS TOHKEN X-RAY INSPECTION Co. Ltd., Tokyo, Japan, and Ms. Amana Khatoon, Ms. MyeongWon Oh in National Institute of Crop Science for their technical support to the research.
Funding Statement
This work was supported by a Grant-In-Aid for Challenging Exploratory Research (23658063) from Japan Society for the Promotion of Science. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Setter TL, Waters I (2003) Review of prospect for germplasm improvement for water-logging tolerance in wheat, barley and oats. Plant Soil 253: 1–34. [Google Scholar]
- 2. Bailey-Serres J, Voesenek LACJ (2008) Flooding stress: acclimations and genetic diversity. Annu Rev Plant Biol 59: 313–339. [DOI] [PubMed] [Google Scholar]
- 3. Githiri SM, Watanabe S, Harada K, Takahashi R (2006) QTL analysis of flooding tolerance in soybean at an early vegetative growth stage. Plant Breed 125: 613–618. [Google Scholar]
- 4. Komatsu S, Hiraga S, Yanagawa Y (2012) Proteomics techniques for the development of flood tolerant crops. J Proteome Res 11: 68–78. [DOI] [PubMed] [Google Scholar]
- 5. Hashiguchi A, Sakata K, Komatsu S (2009) Proteome analysis of early-stage soybean seedlings under flooding stress. J Proteome Res 8: 2058–2069. [DOI] [PubMed] [Google Scholar]
- 6. Komatsu S, Nanjo Y, Nishimura M (2013a) Proteomic analysis of the flooding tolerance mechanism in mutant soybean. J Proteomics 79: 231–250. [DOI] [PubMed] [Google Scholar]
- 7. Subbaiah CC, Sachs MM (2003) Molocular and cellular adaptations of maize to flooding stress. Ann Bot 90: 119–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mathesius U, Djordjvic MA, Oakes M, Goffard N, Haerizadeh F, et al. (2011) Comparative proteomic profiles of the soybean (Glycine max) root apex and differentiated root zone. Proteomics 11: 1707–1719. [DOI] [PubMed] [Google Scholar]
- 9.Komatsu S, Shirasaka N, Sakata K (2013b) ‘Omics’ techniques for identifying flooding-response mechanisms in soybean. J Proteomics (on line). [DOI] [PubMed]
- 10. Nanjo Y, Maruyama K, Yasue H, Yamaguchi-Shinozaki K, Shinozaki K, et al. (2011) Transcriptional responses to flooding stress in roots including hypocotyl of soybean seedlings. Plant Mol Biol 77: 129–144. [DOI] [PubMed] [Google Scholar]
- 11. Nanjo Y, Skultety L, Ashraf Y, Komatsu S (2010) Comparative proteomic analysis of early-stage soybean seedlings responses to flooding by using gel and gel-free techniques. J Proteome Res 9: 3989–4002. [DOI] [PubMed] [Google Scholar]
- 12. Komatsu S, Deschamps T, Hiraga S, Kato M, Chiba M, et al. (2011a) Characterization of a novel flooding stress-responsive alcohol dehydrogenase expressed in soybean roots. Plant Mol Biol 77: 309–322. [DOI] [PubMed] [Google Scholar]
- 13. Komatsu S, Yamamoto A, Nakamura T, Nouri MZ, Nanjo Y, et al. (2011b) Comprehensive analysis of mitochondria in roots and hypocotyls of soybean under flooding stress using proteomics and metabolomics techniques. J Proteome Res 10: 3993–4004. [DOI] [PubMed] [Google Scholar]
- 14. Yanagawa Y, Komatsu S (2012) Ubiquitin/proteasome-mediated proteolysis is involved in the response to flooding stress in soybean roots, independent of oxygen limitation. Plant Sci 185–186: 250–258. [DOI] [PubMed] [Google Scholar]
- 15. Komatsu S, Sugimoto T, Hoshino T, Nanjo Y, Furukawa K (2010) Identification of flooding stress responsible cascades in root and hypocotyl of soybean using proteome analysis. Amino Acids 38: 729–738. [DOI] [PubMed] [Google Scholar]
- 16. Khatoon A, Rehman S, Hiraga S, Makino T, Komatsu S (2012a) Organ-specific proteomics analysis for identification of response mechanism in soybean seedlings under flooding stress. J Proteomics 75: 5706–5723. [DOI] [PubMed] [Google Scholar]
- 17. Khatoon A, Rehman S, Salavati A, Komatsu S (2012b) A comparative proteomics analysis in roots of soybean to compatible symbiotic bacteria under flooding stress. Amino Acids 43: 2512–2525. [DOI] [PubMed] [Google Scholar]
- 18. Kobayashi M, Devaraj B, Usa M, Tanno Y, Takeda M, et al. (1997) Two-dimensional imaging of ultraweak photon emission from germinating soybean seedlings with a highly sensitive CCD camera. Photochem Photobiol 65: 535–537. [Google Scholar]
- 19. Makino T, Kato K, Iyozumi H, Honzawa H, Tachiiri Y, et al. (1996) Ultraweak luminescence generated by sweet potato and Fusarium oxysporum interactions associated with a defense response. Photochem Photobiol 64: 953–956. [DOI] [PubMed] [Google Scholar]
- 20. Hossain Z, Makino T, Komatsu S (2012) Proteomic study of β-aminobutyricacid-mediated cadmium stress alleviation in soybean. J Proteomics 75: 4151–4164. [DOI] [PubMed] [Google Scholar]
- 21. Kausar R, Hossain Z, Makino T, Komatsu S (2012) Characterization of ascorbate peroxidase in soybean under flooding and drought stresses. Mol Biol Rep 39: 10573–10579. [DOI] [PubMed] [Google Scholar]
- 22. Komatsu S, Yamamoto R, Nanjo Y, Mikami Y, Yunokawa H, et al. (2009) A comprehensive analysis of the soybean genes and proteins expressed under flooding stress using transcriptome and proteome techniques. J Proteome Res 8: 4766–4778. [DOI] [PubMed] [Google Scholar]
- 23. Webb MA (1999) Cell-mediated crystallization of calcium oxalate in plants. Plant Cell 11: 751–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Franceschi VR, Nakata PA (2005) Calcium oxalate in plants: formation and function. Annu Rev Plant Biol 56: 41–71. [DOI] [PubMed] [Google Scholar]
- 25. Ilarslan H, Palmer RG, Horner HT (2001) Calcium oxalate crystals in developing seeds of soybean. Ann Bot 88: 243–257. [PubMed] [Google Scholar]
- 26. Chiera JM, Grabau EA (2007) Localization of myo-inositol phosphate synthase (GmMIPS-1) during the early stages of soybean seed development. J Exp Bot 58: 2261–2268. [DOI] [PubMed] [Google Scholar]
- 27. Fan CY, Lee S, Cyr DM (2003) Mechanisms for regulation of Hsp70 function by Hsp40. Cell Stress Chaperon 8: 309–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Caplan AJ, Cyr DM, Douglas MG (1993) Eukaryotic homologs of Escherichia coli Dnaj - a diverse protein family that functions with Hsp70 stress proteins. Mol Biol Cell 4: 555–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cha JY, Su’udi M, Kim WY, Kim DR, Kwak YS, et al. (2012) Functional characterization of orchardgrass cytosolic Hsp70 (DgHsp70) and the negative regulation by Ca2+/AtCaM2 binding. Plant Physiol Biochem 58: 29–36. [DOI] [PubMed] [Google Scholar]
- 30. Suri SS, Dhindsa RS (2008) A heat-activated MAP kinase (HAMK) as a mediator of heat shock response in tobacco cells. Plant Cell Environ 31: 218–226. [DOI] [PubMed] [Google Scholar]
- 31. Bennett M, Mehta M, Grant M (2005) Biophoton imaging: A nondestructive method for assaying R gene responses. Mol. Plant–Microbe Interact 18: 95–102. [DOI] [PubMed] [Google Scholar]
- 32. Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254. [DOI] [PubMed] [Google Scholar]
- 33. Schmutz J, Cannon SB, Schlueter J, Ma J, Mitros T, et al. (2010) Genome sequence of the palaeopolyploid soybean Nature. 463: 178–183. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
2-DE patterns in the root proteins of flooding-stressed soybean plants. Two-day-old soybean plants were flooded for 2 days, after which proteins were extracted from the root, separated by 2-DE, and the gels were stained with CBB. Results are presented as the mean ± SE of relative protein intensity for gels from 3 biological replicates. 2-DE patterns were shown 3 biological replicates.
(TIF)
2-DE patterns in the cotyledon proteins of flooding-stressed soybean plants. Two-day-old soybean plants were flooded for 2 days, after which proteins were extracted from the cotyledon, separated by 2-DE, and the gels were stained with CBB. Results are presented as the mean ± SE of relative protein intensity for gels from 3 biological replicates. 2-DE patterns were shown 3 biological replicates.
(TIF)
In vitro biophoton emission patterns of flooding-stressed soybean plants. Two-day-old soybean plants were flooded for 2 days, after which proteins were extracted from the root and cotyledon and H2O2 was added. Emission of photons from these extracts was then measured. R1, R2, and R3 represent 3 independent biological replicate analyses of the root and cotyledon. Ground photon emission by the empty Petri dish served as a blank (blue). Sky blue and purple lines represent biophoton emission from flooded soybean plants, while yellow and pink lines represent biophoton emission from untreated soybean plants.
(TIF)
In vivo biophoton emission patterns of flooding-stressed soybean plants. Two-day-old soybean plants were flooded for 2 days, after which the root and cotyledon were treated with luminal solution and photon emission was measured. R1, R2, and R3 represent biophoton emission from 3 independent biological replicate analyses of the root and cotyledon. Ground photon emission by the empty Petri dish served as a blank (blue). Sky blue and purple lines represent biophoton emission from flooded soybean plants, while yellow and pink lines represent biophoton emission from untreated soybean plants.
(TIF)
Scanning electron microscopy and energy dispersive X-ray analysis of Ca, O, and C. Two-day-old soybean plants were flooded for 2 days, after which the cotyledon was collected and fixed. A scanning electron micrograph and energy dispersive X-ray spectra of Ca, O, and C are shown.
(TIF)
Scanning electron microscopy and energy dispersive X-ray analysis of F, K, Na, P, and S. Two-day-old soybean plants were flooded for 2 days, after which the cotyledon was collected and fixed. A scanning electron micrograph and energy dispersive X-ray spectra of F, K, Na, P, and S are shown.
(TIF)
The results of Student’s t test for each spot in 2-DE pattern of root proteins.
(XLSX)
The results of Student’s t test for each spot in 2-DE pattern of cotyledon proteins.
(XLSX)
Peak lists for proteins identified in the root of flooding-stressed soybean plants.
(DOCX)
Peak lists for proteins identified in the cotyledon of flooding-stressed soybean plants.
(DOCX)
Common proteins to both cotyledon and root of flooding-stressed soybean plants.
(DOCX)
Video detailing X-ray computed tomography analysis of the cotyledon of a 4-day-old soybean plant (control).
(ZIP)
Video detailing X-ray computed tomography analysis of the cotyledon of a 4-day-old soybean plant after 2 days of flooding.
(ZIP)