Abstract
Background and Objectives
The cytolethal distending toxin (Cdt) is a highly conserved exotoxin that are produced by a number of Gram negative bacteria, including Aggregatibacter actinomycetemcomitans, and affects mammalian cells by inhibiting cell division and causing apoptosis. A complete cdt-operon is present in the majority of A. actinomycetemcomitans, but the proportion of isolates that lack cdt-encoding genes (A, B and C) varies according to the population studied. The objectives of this study were to examine serotype, Cdt-genotype, and Cdt-activity in isolates of A. actinomycetemcomitans collected from an adolescent West African population and to examine the association between the carrier status of A. actinomycetemcomitans and the progression of attachment loss (AL).
Materials and Methods
A total of 249 A. actinomycetemcomitans isolates from 200 Ghanaian adolescents were examined for serotype and cdt-genotype by PCR. The activity of the Cdt-toxin was examined by DNA-staining of exposed cultured cells and documented with flow cytometry. The periodontal status of the participants was examined at baseline and at a two-year follow-up.
Results
Presence of all three cdt-encoding genes was detected in 79% of the examined A. actinomycetemcomitans isolates. All these isolates showed a substantial Cdt-activity. The two different cdt-genotypes (with and without presence of all three cdt-encoding genes) showed a serotype-dependent distribution pattern. Presence of A. actinomycetemcomitans was significantly associated with progression of AL (OR = 5.126; 95% CI = [2.994–8.779], p<0.001).
Conclusion
A. actinomycetemcomitans isolated from the Ghanaian adolescents showed a distribution of serotype and cdt-genotype in line with results based on other previously studied populations. Presence of A. actinomycetemcomitans was significantly associated with disease progression, in particular the b serotype, whereas the association with disease progression was not particularly related to cdt-genotype, and Cdt-activity.
Introduction
Colonization of bacteria that adhere to and develop biofilm on teeth and the surrounding tissues are involved in the pathogenesis of periodontitis [1], [2]. Pathogens located in the subgingival biofilm release components that induce processes in the host response that can result in loss of the tooth supporting tissues [3].
More than 700 different bacterial species can be detected in samples from the subgingival plaque biofilm and other sites of the oral cavity [4]. The majority of these species can be detected in samples from both healthy and periodontally diseased subjects. Some of these species are detected in increased numbers or proportions in plaque samples from diseased subjects and have the capacity to express unique virulence factors associated with pathogenic mechanisms [3].
By use of molecular genetic tools, a biodiversity has been demonstrated in the oral microbiota. Among the periodontal pathogens, Aggregatibacter actinomycetemcomitans is often found in high numbers and proportions in plaque samples from subjects with periodontitis, specifically of its localized aggressive form (LAP) [5]. This Gram-negative capnophilic coccobacillus is genetically heterogeneous and comprises distinct clonal lineages that may have different virulence potentials [6]–[8].
A. actinomycetemcomitans possesses a number of important virulence factors [9]. One of them is a leukotoxin, which is a large pore-forming protein of the RTX (repeats in toxin) family that specifically activates and lyses human leukocytes and induces a substantial release of IL-1β from macrophages [10]. A specific clone (JP2) of A. actinomycetemcomitans has a significantly enhanced expression of the leukotoxin and is strongly associated with LAP in adolescents of African descent [11], [12]. The cytolethal distending toxin (Cdt), also an exotoxin, blocks cell cycle progression in all types of host cells [13], [14].
Seven serotypes (a–g) have been identified among A. actinomycetemcomitans isolates, representing distinct clonal lineages [15]–[18]. There is convincing evidence of differences in serotype distribution related to geography and/or ethnic groups [6], [11], [12]. A. actinomycetemcomitans isolates from individuals in European countries are usually represented by almost equal proportions of a, b, and c serotypes [19]–[21]. In contrast, several studies showed a clear predominance of serotype c in populations living in Asia and America [22]–[24]. The serotype distribution in the West African population is unknown. The high genetic diversity in isolates of the same serotype of A. actinomycetemcomitans [7], [8] indicates that the serotype might be a weak marker for the pathogenic potential of an isolate. It has been suggested that serotype b of A. actinomycetemcomitans has a higher pathogenic potential than the other serotypes [25], [26].
Cdt is a highly conserved exotoxin produced by a number of Gram negative bacteria. It affects mammalian cells by inhibiting cell division and causing apoptosis [27]. The active holo-toxin is a heterotrimeric complex of CdtA, CdtB, and CdtC. CdtA and CdtC are necessary for the secretion of the toxin, while CdtB is responsible for the biologic activity [13]. CdtB has a sequence homology with mammalian DNase I, indicating a critical role for nuclease activity in host parasite interactions [28]. The Cdt was firstly discovered in A. actinomycetemcomitans by Sugai and co-workers in 1998 [29]. Apart from blocking cell cycle progression, Cdt also induces expression of the receptor activator of NF-κappaB ligand (RANKL) in human periodontal fibroblasts and lymphocytes [30], [31]. RANKL is a key cytokine for bone resorption and could therefore be associated with the pathogenic mechanisms of periodontitis [32]. In addition, Cdt affects the oral epithelium ex vivo and therefore might contribute to impair the barrier function of this cell layer against invading microbes [33].
A functional Cdt toxin requires carriage of the three genes, cdtA, cdtB, and cdtC [27]. The genes are present in the majority of the A. actinomycetemcomitans strains that have been isolated, but the proportion of isolates that lack all or some of the genes varies among the populations studied [17], [22], [34]–[38]. The cdt genes reside in a genomic island of the variable region of the A. actinomycetemcomitans pangenome [7]. The activity of Cdt varies among different A. actinomycetemcomitans strains and an enhanced Cdt-activity (cell growth inhibition >65%) was equally distributed among serotype b and c strains [22], [36]. The presence of a specific immunoreactivity to Cdt has also been studied and assumed to be a marker for presence of Cdt-expressing A. actinomycetemcomitans [39], [40]. Interestingly, while all carriers of A. actinomycetemcomitans exhibit neutralizing antibodies to the leukotoxin, the systemic immunoreactivity to Cdt is not always capable to neutralize the toxin [40], [41]. Despite substantial evidence supporting that Cdt has an ability to function as a virulence factor in pathogens producing the toxin [42], the importance of Cdt in the pathogenesis of periodontal disease remains to be understood [9]. It is unknown if the toxic action of the Cdt may account for a part of the A. actinomycetemcomitans-associated periodontal disease process on-going in young populations, particularly of African descent [43]–[45]. Prior to this study, the occurrence of A. actinomycetemcomitans in West African adolescents and the potential role of the Cdt in relation to periodontal disease has never been undertaken.
The presence of cdt-encoding genes, Cdt-activity and its serotype distribution in A. actinomycetemcomitans isolated from Ghanaian adolescents were determined in the present study. A two-year prospective cohort study was undertaken to evaluate the carrier status and selected characteristics of A. actinomycetemcomitans in relation to progression of attachment loss (AL).
Materials and Methods
Subject Recruitment
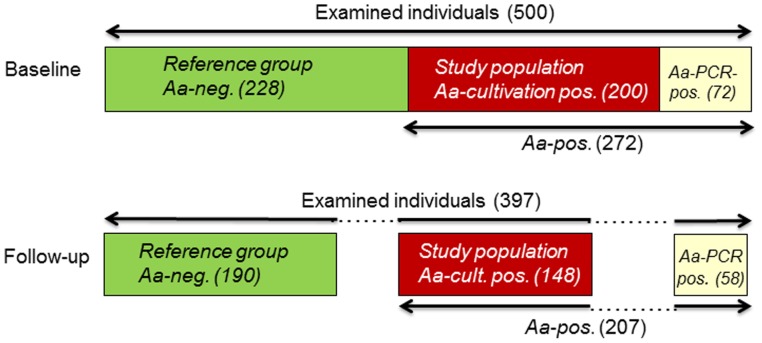
An adolescent West African population, described in details previously, was examined [45]. Briefly, a random cohort of 500 school children (mean age 13.2 years; SD ±1.5) was included in the cross-sectional study, which was performed in Accra, Ghana, in 2009 [45]. In the follow-up study, performed two years later, 397 (79.4%) of these individuals showed up for a periodontal re-examination (Fig. 1). The drop-out individuals consisted of children in families that moved to another area within the follow-up period or school children who dropped out from school. The school system had no information concerning the individuals that had left school.
Figure 1. Flow chart overview of the study population and the detection of A. actinomycetemcomitans (Aa).
At baseline, 500 adolescents were examined for the presence of Aa with cultivation and PCR-based methods. Aa was detected in 272 individuals and the bacterium could be isolated from 200 of them (Aa cultivation-positive group (red)). A second clinical examination (follow-up) was performed after 2 years and included 397 of the 500 individuals, 207 of the initially Aa-positive subjects and 148 of them from whom the bacterium had been isolated at baseline (red). The individuals without detectable Aa with any of the two methods used, were classified as negative for the bacterium (Aa-negative group (green)) and consisted of 228 individuals at the baseline examination and 190 at the follow-up examination. The number of individuals that were positive for Aa by PCR, but not with cultivation (yellow), was 72 at baseline and 58 of them were examined at the follow-up.
Ethical clearance for the study was obtained from the Noguchi Memorial Institute for Medical Research, University of Ghana (IRB 000 1276), and from the local Ethical committee of Umeå University, Sweden (Dnr 2010-188-31M). Signed consents were received from the parents or the guardians of the children before they entered the study.
Clinical Examination
All participants enrolled were given a full-mouth periodontal examination by the same, certified periodontist with identical procedures used at the baseline and at the follow-up examination [45]. Attachment loss (AL) was measured at the buccal aspect of the mesial and distal surfaces of all fully erupted permanent teeth, which gave a potential maximum of 56 sites per individual. AL was defined as the distance from the cemento-enamel junction (CEJ) to the bottom of the periodontal pocket or crevice and was calculated as the difference between two measurements (probing pocket depth and the distance from gingival margin to CEJ). Differences between baseline and follow-up were calculated at site level. The disease status was established using the cut-off point of AL ≥3 mm in one or more sites in the dentition. Individuals were defined as having progressive disease if they showed ≥ one site that had a progression of AL ≥3 mm based on data collected from the baseline and the follow-up examinations. None of the participants had received any periodontal treatment during the two-year follow-up period. Very few individuals had visited a dentist, except for treatment of pain related to the teeth. Traditional periodontal treatment strategies are not possible to introduce today in developing countries due to lack of dentists and resources for funding. Until affordable alternative strategies are available any form of periodontal treatment would be difficult to provide. This topic is a future challenge for the present project.
Bacterial Isolates and Growth Conditions
Subgingival plaque samples from the 500 adolescents were collected for microbiological analysis, transported, and analyzed as described earlier [45]. In total, 792 A. actinomycetemcomitans isolates (1–7 per subject) were collected from 200 (40%) of the 500 subjects entered into the study at baseline. The leukotoxin promoter types (JP2/non-JP2) of isolates was determined by PCR technique [46], and the results are described in a previous study [45].
Serotyping of A. actinomycetemcomitans by PCR
Suspensions of the strains were boiled for eight minutes and centrifuged. The supernatants were used as template when the 792 isolates were serotyped by PCR. The primers used in this study and the temperature profiles for amplification of the various genes have previously been described, for serotyping a–e [47], and for serotype f [17]. All isolates could be serotyped by the 6 primer pairs (a-f). No nonserotypable was found, making it irrelevant to consider testing for the recently described serotype g [18].
The PCR products were analysed by agarose (1.2%) gel electrophoresis in a Tris-acetate (40 mM; pH 8.3) buffer containing 1 mM EDTA. The gel was stained with ethidium bromide and photographed under ultraviolet light. Base pair sequences for each of the forward and reversed primers used and gel product size is presented in the supporting information (Table S1).
Cdt Genotyping of A. actinomycetemcomitans by PCR
From the 792 serotyped and leukotoxin promoter-typed isolates, 249 isolates were selected for cdt-genotyping by PCR. One isolate from each of the 200 subjects were used, but when more than one serotype and/or leukotoxin promoter type (JP2 or non-JP2) were identified in the same subject, these additional isolates were included and summed up to 249. For the detection of the three Cdt genes (cdtABC), two PCR-based methods were used [36]. One of the methods was designed for detection of all three genes (cdtABC) and revealed a 2105 base pair (bp) product. When discrepancy between detection of cdtABC and Cdt-activity occurred, the individual cdt genes (A, B and C, respectively) were screened for by the second method [34]. The PCR products are documented as described for serotyping. Primer sequences, gene position, and gene fragment size for the cdt gene analyses, are shown in the supporting information (Table S1).
Cdt-activity Test of A. actinomycetemcomitans in Cell Cultures Examined by Flow Cytometry
The 249 selected isolates were analyzed for Cdt-activity in a cell culture assay [14]. HL-60 cells (human carcinoma leukocyte cell line) were cultured in RPMI-1640 with 10% fetal bovine serum (Sigma-Aldrich). A suspension of OD600 nm 2.0 was centrifuged (10 000×g, 10 min), and the supernatant added to the cultured cells. The HL-60 cells (1 ml 5×105 cells/ml) were transferred to each well in a 24-well cell culture plate (Nunc) and mixed with 1 µl of each of the bacterial supernatants. After 24 h of incubation the cells were transferred to a 2 ml Eppendorf tube and washed with PBS by centrifugation (500×g, 5 min). The cell pellet was solved in 300 µl PBS and 900 µl ice-cold 99% ethanol and fixed for 1 h at 4°C. Cells were washed with PBS by centrifugation and treated with RNase (100 µl, 100 µg/ml, Sigma-Aldrich) for 15 min at 37°C. After the incubation, 400 µl of propidium iodide (Molecular Probes, Eugene, OR, USA) in 3.8 mM sodium citrate in PBS was added and further incubated in darkness for 1–3 h at 4°C. Cdt-activity was determined by the ability of the bacterial supernatants to inhibit proliferation and causing the typical accumulation of the target cells in the G2/M-phase examined and the increased cell size (FSC) by cell cycle analyses with flow cytometry (FACS Calibur, Becton Dikinson; Franklin Lakes, NJ, USA). Bacterial isolates that resulted in ≥50% of the target cell population in the G2/M-phase after 24 h incubation were classified as positive for Cdt-activity.
Statistical Analysis
Data analyses were performed using SPSS 19.0 (SPSS Inc., Chicago, IL, USA) and STATA 8.0 (StataCorp LP., College Station, Texas, USA). In the statistical analyses, the primary outcome was progression of AL ≥3 mm in one or more sites at subject level, based on the collection of data performed at baseline (in November 2009) and at the follow-up (in November 2011). Descriptive statistics were performed using mean and standard deviation for the number of teeth with AL ≥3 mm per individual and group differences assessed using a non-parametric test (Mann-Whitney U test). The Mantel-Haenszel test was used for comparison of the distribution between groups of individuals harboring sites with AL ≥3 mm. The estimated risk associated with progression of AL ≥3 mm during a two-year follow-up period according to the carrier status of A. actinomycetemcomitans, characteristics as serotype and Cdt-activity (negative or positive), was evaluated by calculations of odds ratios (OR). A value of p<0.05 was considered statistically significant. The calculation of OR was repeated after exclusion of JP2-positive individuals, who were defined as carriers of the JP2-genotype based on positive plaque samples by the cultivation technique and/or by the PCR.
Results
A. actinomycetemcomitans was isolated from 200 (40%) of the examined individuals at baseline. From these subjects, a collection of 249 isolates was selected, one isolate from each of the 200 subjects and additional isolates included from subjects where more than one serotype or more than one leukotoxin promoter type could be detected in the same sample. The highly leukotoxic JP2 genotype of A. actinomycetemcomitans was found in isolates from five (2.5%) of the individuals. All five of these individuals carried also the non-JP2 genotypes.
Study Population and Subject Recruitment
Demographic characteristics from the baseline and the follow-up examination of the studied individuals are shown in Table 1. From the initially 500 examined individuals at baseline, 397 of them (79.4%) could be identified and were available for a two-year clinical follow-up examination (Fig. 1). Descriptive data for the number of individuals with affected sites with AL ≥3 mm and the number of affected teeth (mean) with AL ≥3 mm for the examined individuals, the selected study population of A. actinomycetemcomitans cultivation-positive individuals, and the reference group of individuals tested negative for the presence of this bacterium, are shown in Table 2. The mean age of the A. actinomycetemcomitans-positive individuals at baseline (n = 200) and at follow-up (n = 148) was 13.4 (SD; ±1.48) and 15.2 (SD; ±1.38), respectively, and for the A. actinomycetemcomitans-negative referents at baseline (n = 228) and at follow up (n = 190) the corresponding ages were 12.9 (SD; ±1.43) and 14.7 (SD; ±1.28), respectively. The odds ratios (OR) for having one or more sites with AL ≥3 mm was higher in the A. actinomycetemcomitans-positive group in relation to the A. actinomycetemcomitans-negative reference group at baseline and at follow up (OR = 2.197; 95% CI [1.355–3.561] p = 0.001 and OR = 5.138; 95% CI [3.167–8.334] p<0.001) (Mantel-Haenszel test) (Table 2). Also the number of affected teeth (mean) with AL ≥3 mm was significantly higher among the A. actinomycetemcomitans-positive individuals than among the A. actinomycetemcomitans-negative reference group at both baseline (p≤0.001) and at the follow-up (p≤0.001) (Mann-Whitney U test) (Table 2). None of the examined individuals was cigarette smokers or had diabetes.
Table 1. Descriptive and demographic characteristics of the examined individuals at baseline and at the two-year follow-up examination.
| Study population | Baseline (n = 500) | Follow-up (n = 397) | ||
| Demographics | ||||
| Age (years) | mean | SD | mean | SD |
| 13.2 | 1.53 | 15.0 | 1.39 | |
| Gender | n | % | n | % |
| Male | 232 | 46.4 | 176 | 44.3 |
| Female | 268 | 53.6 | 221 | 55.7 |
SD; standard deviation.
Table 2. Descriptive and clinical characteristics of the examined individuals (all), the study population (Aa-cultivation positive), and the reference group (Aa-negative) at baseline (BL), and at the two-year follow-up (FU) examination.
| Examined individuals | ||||
| Clinical | BL n = 500 | FU n = 397 | ||
| n | % | n | % | |
| N individuals (%) with sites AL ≥3 mm | 107 | 21.4 | 156 | 39.2 |
| mean | SD | mean | SD | |
| N of teeth (mean) with AL ≥3 mm | 0.52 | 1.37 | 1.92 | 3.34 |
| Aa -cultivation-positive | ||||
| BL n = 200 | FU n = 148 | |||
| n | % | n | % | |
| N individuals (%) with sites AL ≥3 mm | 54 | 27.0 | 82 | 54.4 |
| mean | SD | mean | SD | |
| N of teeth (mean) with AL ≥3 mm | 0.67 | 1.44 | 3.01 | 3.90 |
| Reference group ( Aa -negative) | ||||
| BL n = 228 | FU n = 190 | |||
| n | % | n | % | |
| N individuals (%) with sites AL ≥3 mm | 33 | 14.4 | 37 | 19.4 |
| mean | SD | mean | SD | |
| N of teeth (mean) with AL ≥3 mm | 0.34 | 1.28 | 0.89 | 2.63 |
AL; attachment loss; BL, at baseline; FU, at two-year follow-up.
Aa; Aggregatibacter actinomycetemcomitans.
SD; standard deviation.
Serotyping
Serotyping of the 249 A. actinomycetemcomitans isolates showed presence of six different serotypes (a–f) (Fig. 2). Serotype c was the most frequently found and was detected in 104 (42.0%) of the isolates, while the less frequently found serotype e could be detected in only 8 (3.2%) of the isolates. The frequency of serotype a, b, d, and f in the examined isolates was 59 (23.7%), 47 (18.9%), 12 (4.8%), and 19 (7.6%), respectively. More than one serotype could be detected in samples from 45 (22.5%) of the 200 subjects. Only three individuals (1.5%) were poly-infected with three or more serotypes.
Figure 2. Agarose gel electrophoresis of PCR products of amplified cdt-genes in serotypes a through f isolates of A. actinomycetemcomitans, including a serotype a isolate (612), which was negative for detection of the cdtABC cluster, but positive for detection of the individual cdt genes.
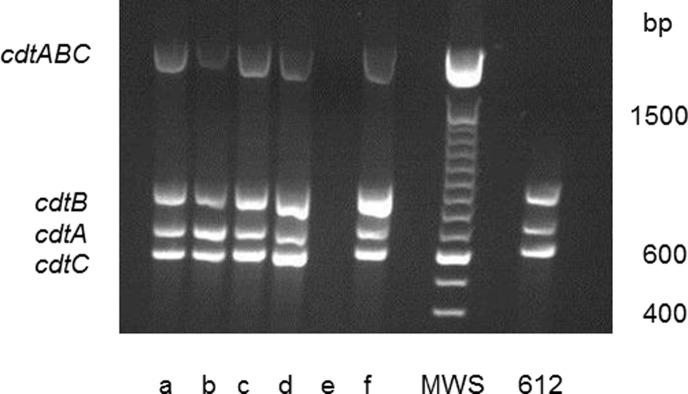
Size of the cdt gene cluster cdtABC, cdtA, cdtB, cdtC: 2105, 693, 862, 592 bp (base pair), respectively.
cdt-genotype and Cdt-activity
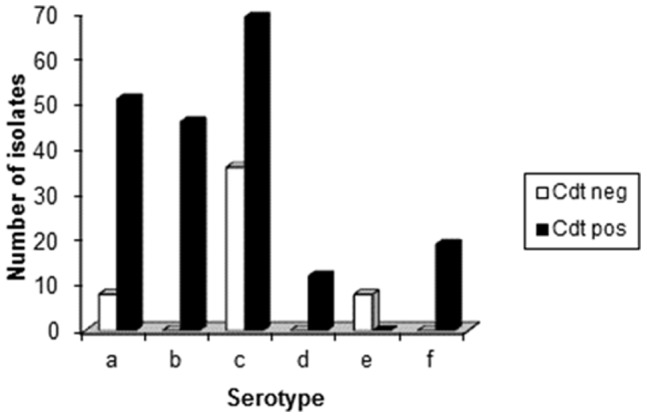
The activity of Cdt was examined in a cell culture-based assay by its ability to induce an accumulation of enlarged cells in the G2/M phase of growth (Fig. 3). Cdt-activity was detected in 196 (79%) of the 249 examined isolates. These 196 isolates with Cdt-activity also harboured all three cdt-genes (A, B and C) when examined by PCR and afterwards visualized in an agarose gel (Fig. 4). However, in one isolate the cdt-genes were found only by the method used for detection of the individual three cdt-genes. It may be possible that the method for amplifying the cdt-genes as a complete 2100 base pair product was not optimal for this isolate or, e.g. that a single base mutation had occurred resulting in unsuccessful annealing of the primers (amplification of the genes). The distribution of isolates with Cdt-activity and presence of all three cdt-encoding genes varied among the different serotypes of A. actinomycetemcomitans. All serotype b (n = 47), d (n = 12), and f (n = 19) isolates were positive for Cdt-activity and the cdt-genes, while all serotype e isolates (n = 8) lacked Cdt-activity and intact cdt-encoding genes (Fig. 5). A total of 35 (33.7%) of the serotype c isolates were tested negative for Cdt-activity, and for serotype a the number of negative isolates was 8 (13.6%). Taken together, these results showed a significant serotype-dependent pattern of cdt-genotypes in A. actinomycetemcomitans isolated from this population (Fig. 5). Isolates with the presence of all three cdt-encoding genes and a substantial Cdt-activity were considered as Cdt-positive, while the other isolates were counted as Cdt-negative.
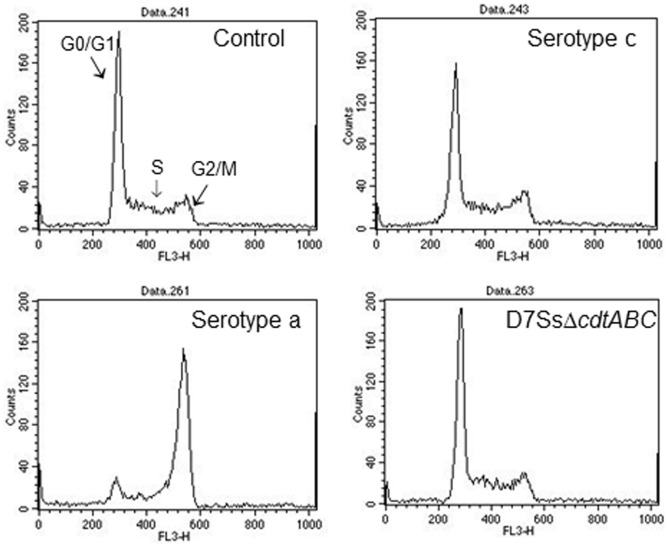
Figure 3. Histogram from flow cytometric analyses of leukocytes (HL-60 cells) exposed for 24 h to different extracts from various isolates of A. actinomycetemcomitans.
The DNA-content of each exposed HL-60 cell were determined with PI-staining and indicate cell cycle phase of the analysed cell. The upper left panel (control) was exposed for 0.1% isoton NaCl, the upper right panel for 0.1% NaCl extract from an isolate with non-complete cdt-genome, the lower left panel for 0.1% NaCl extract from an isolate with intact cdt-genome and the lower right panel exposed to 0.1% NaCl extract from a cdt knockout mutant strain (D7SSΔcdtABC).
Figure 4. Agarose gel electrophoresis of PCR products amplified from serotypes a through f strains of A. actinomycetemcomitans isolated from a young Ghanaian population.
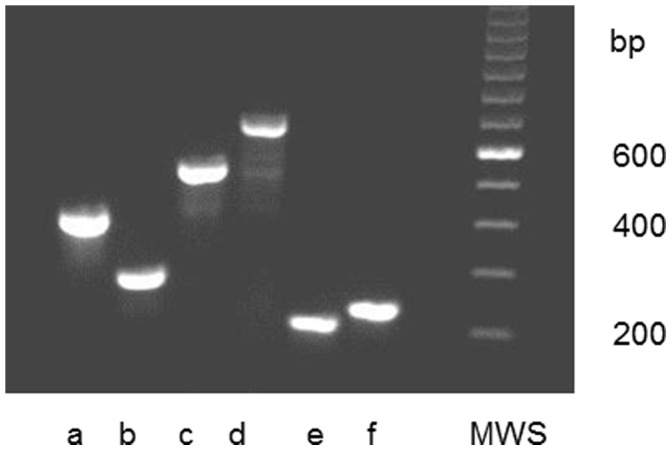
Size of the serotype–associated gel fragments (a through f, bp (base pair): 428, 298, 559, 690, 211, 231.
Figure 5. Distribution of isolates with Cdt-activity among the various serotypes of A. actinomycetemcomitans.
Carrier Status of A. actinomycetemcomitans and Progression of AL
The progression of AL ≥3 mm at one or more sites over a two-year follow-up period was examined in relation to the carrier status of A. actinomycetemcomitans. There was a significantly increased progression of AL in the A. actinomycetemcomitans-positive individuals (OR = 5.126, 95% CI [2.994–8.779] p<0.001) in relation to that of the A. actinomycetemcomitans-negative reference individuals (Table 3). Exclusions of individuals with presence of the JP2-genotype, detected by cultivation and/or PCR, resulted in for the A. actinomycetemcomitans positive individuals (n = 130) an OR of 4.323 (95% CI [2.482–7.530], p≤0.001).
Table 3. Presence of A. actinomyctemcomitans (Aa) with different serotypes, cdt-genotype and disease progression.
| OR | 95% CI | p-value | Total number | Individuals (%) with progression of AL ≥3 mm | |
| Aa -pos | 5.126 | 2.994–8.779 | <0.001 | 148 | 63 (42.6) |
| Aa-serotype a | 6.917 | 2.869–16.673 | <0.001 | 26 | 13 (50.0) |
| Aa-serotype b | 7.685 | 2.835–20.830 | <0.001 | 19 | 10 (52.6) |
| Aa-serotype c | 3.365 | 1.659–6.826 | <0.001 | 55 | 18 (32.7) |
| Aa-Cdt-pos | 5.237 | 3.000–9.143 | <0.001 | 123 | 53 (43.1) |
| Aa-Cdt-neg | 4.611 | 1.861–11.426 | = 0.001 | 25 | 10 (40.0) |
| Aa -neg * | 1.000 | reference | – | 190 | 24 (12.6) |
Odds ratio (OR) showing the association of progression of attachment loss (AL) ≥3 mm after a two-year follow-up period in relation to the carrier status of Aa.
OR, odds ratio; CI, confidence interval; AL, attachment loss. Significance (p<0.05).
The reference group. Reference is the progression of AL in individuals that were Aa-negative.
Serotype and Progression of AL
The most prevalent serotypes of A. actinomycetemcomitans isolated from the present study population were a, b, and c. In order to examine for the importance of the serotype of the bacterium for the progression of AL ≥3 mm, the individuals tested positive for one of these three serotypes were selected from the study population. In each group of individuals positive for any of the three selected serotypes, the OR for progression of AL compared to that of the A. actinomycetemcomitans-negative referents was high (Table 3). The highest OR for progression was associated with presence by the b serotype (OR = 7.685; 95% CI [2.835–20.830], p<0.001), closely followed by the a serotype (OR = 6.917; 95% CI [2.869–16.673], p<0.001), while the OR for progression was lower, however still significant, in the individuals positive for the c serotype (OR = 3.365; 95% CI [1.659–6.826], p<0.001). Due to the low number of individuals carrying A. actinomycetemcomitans of serotypes d, e, and f, these were not included in the analyses. The corresponding results for serotypes b and c after exclusion for the presence of the JP2-genotype strains were calculated. We also had to consider some individuals positive for serotype c as these were co-infected with serotype b JP2-genotype strains. For the individuals with the presence of the b serotype A. actinomycetemcomitans (n = 16), the OR was 6.917 (95% CI [2.374–20.152], p≤0.001), and for the individuals with the c serotype (n = 50), the OR was 2.964 (95% CI [1.413–6.219], p = 0.004). None of the individuals with the presence of the a serotype of the bacterium were in addition co-infected with the JP2-genotype.
cdt-genotype and Progression of AL
Presence of Cdt-negative A. actinomycetemcomitans, as well as the presence of Cdt-positive bacteria showed a significant association with an increased progression of AL compared with that of the A. actinomycetemcomitans-negative referents (Table 3). The results for the A. actinomycetemcomitans-positive individuals (n = 148), for the individuals (n = 123) with Cdt-positive bacteria, and for the individuals (n = 25) with Cdt-negative bacteria were OR = 5.126 (95% CI [2.994–8.779], p≤0.001); OR = 5.237 (95% CI [3.000–9.143], p≤0.001), and OR = 4.611 (95% CI [1.861–11.426], p = 0.001), respectively. The OR after exclusion of individuals with presence of JP2 genotype bacteria was OR = 4.402 (95% CI [2.472–7.837], p≤0.001) for individuals (n = 108) with Cdt-positive bacteria and OR = 3.952 (95% CI [1.501–10.409], p = 0.005) for individuals (n = 22) with Cdt-negative bacteria.
Discussion
In the present study, we have examined a collection of A. actinomycetemcomitans isolated from individuals included as a part of a prospective cohort study carried out in Accra, Ghana [45]. The proportion of individuals (79.4%) identified for the follow-up examination after two years was considerably high in the population chosen for this study, represented at baseline by a group of 500 medically healthy adolescents. This is the first microbiological study of A. actinomycetemcomitans from West-Africa, and neither cdt-genotype and activity nor serotypes have been reported on before based on such a collection of West-African A. actinomycetemcomitans strains.
Six different serotypes (a-e) were detected in the collection of 249 isolates from the 200 Ghanaian individuals. In line with earlier studies, examining isolates from Asian or American populations, the serotype c was the most prevalent (42.0%), followed by serotype a (23.7%), and serotype b (18.9%) [22]–[24]. The other three detected serotypes d, e and f were present in relatively low proportions being 4.8%, 7.6%, and 3.2%, respectively. This is also in accordance with previous studies [16], [21], [23].
The distribution of cdt-genotypes among the analysed isolates showed a serotype-dependent pattern that was unique for the present collection in relation to results from previous studies [19]–[24]. The present collection of A. actinomycetemcomitans is from a representative adolescent population consisting of otherwise healthy subjects, all living in the same geographic area located in West-Africa. Most comparable studies have dealt with isolates from patient cohorts with a history of periodontal disease [16], [19]–[23].
Cdt-activity was detected in 79% in the collection of the 249 selected isolates from 200 individuals. Previous studies, aimed at examining the expression of Cdt, has mainly focused on the presence of cdt-genes in bacteria isolated from periodontally diseased individuals [22], [23], [36], [40]. The results from these previous studies showed a substantial variation in the proportion of isolates that contained all three cdt-encoding genes. This may be explained by the different geographic origins and periodontal status of the examined individuals, but can also involve differences in the methodology for the detection of the cdt-genes. Two studies based on PCR-data from plaque samples have indicated a relatively low proportion of detectable cdt-genes in the A. actinomycetemcomitans-positive sites [21], [35]. However, variations in the methodology may explain the relatively low detection frequency of cdt-genes in these studies. Further, the cdt-genes are located in the variable region of the A. actinomycetemcomitans pangenome, which can explain the variations in the detection frequency of these genes in A. actinomycetemcomitans isolated from individuals of various origin [7]. Results from the present study showed that all isolates with Cdt-activity contained the three cdt-genes, while all the isolates without Cdt-activity lacked some of the cdt-encoding genes.
In the present study, we applied a cell culture-based method for determination of Cdt-activity that quantified cell cycle phase of the Cdt-exposed cells [14]. This method is more specific than the other commonly used method that demonstrates a general inhibition of the cell proliferation or effects on cell morphology and viability [22], [36], [38]. Examination of the accumulation of cells in the G2/M phase of the cell cycle is a specific marker for Cdt-intoxication and therefore a relevant marker for detection of Cdt-activity [13]. The complete correlation between the presence of an intact cdt-genome and a substantial activity of the toxin might depend on the specific methodology selected for the Cdt-activity analyses.
Despite substantial evidence supporting that Cdt has an ability to function as a true virulence factor in many pathogens producing the toxin [27], [42], the importance of Cdt in the pathogenesis of periodontal disease remains to be evaluated [9]. In our study, the presence of A. actinomycetemcomitans was significantly associated (p≤0.001) with progression of AL over a two-year observation period. This is in line with previous longitudinal studies examining the relation between the presence of A. actinomycetemcomitans and progression of AL in adolescent individuals [43], [48], [49]. In the present study, both individuals colonized with Cdt-positive or Cdt-negative bacteria were significantly more often included among the subjects that showed progression of AL (≥ 3 mm at one or more periodontal site) than the A. actinomycetemcomitans-negative individuals. As particularly the JP2 genotype has been strongly associated with progression of AL, it was important for us to eliminate this genotype from the analyses and afterwards repeat the data analyses. Therefore, exclusion of the JP2 genotype-positive individuals from the study population was done, but this did not change the results substantially. Thus, data suggests that the expression of Cdt might be of low importance for the pathogenesis of periodontitis, although further studies are needed to clarify whether this toxin can act and how to as a true virulence factor.
In the present cohort of Ghanaian adolescents, the progression of AL was examined in relation to the presence of serotype a, b, or c of A. actinomycetemcomitans. Using the progression of AL in the A. actinomycetemcomitans-negative individuals as the reference group, the presence of each of these three serotypes showed a significant association with progression of AL. Exclusion of the JP2-genotype positive individuals did not substantially change the estimates. Finding that the serotype b strains have the strongest relation to disease progression (OR = 7.685), this is in line with previous studies indicating an increased virulence in A. actinomycetemcomitans from serotype b [25], [26]. Although, the presence of A. actinomycetemcomitans as an important etiological factor was studied in this cohort of Ghanaian adolescents, an effect of other periodontal pathogens present concomitantly in the subgingival biofilm cannot be excluded.
In conclusion, the distribution of serotypes, cdt-genotypes and Cdt-activity of A. actinomycetemcomitans isolated from Ghanaian adolescents, showed a pattern that was comparable with results found in other populations. Progression of AL is mainly associated with the presence of A. actinomycetemcomitans and appears weakly associated with the cdt-genotype. All serotypes of A. actinomycetemcomitans studied were related to the progression of AL, serotype b showing the strongest association with disease progression.
Supporting Information
Primer sequences, gene position, and gene fragment size for the serotype and cdt -genotype PCR analyses.
(DOC)
Acknowledgments
We thank Dr Gunnar Dahlén at Gotheburg University, Sweden, for valuable support during the preparation of this manuscript.
Funding Statement
This study was supported by the Swedish National Graduate School in Odontological Science, the Research Fund (TUA), County of Västerbotten, Sweden, the Swedish Dental Association, Sweden, Ingeborg and Leo Dannins Foundation, Aarhus University Research Foundation (F-2009-SUN-1-57), funds from the Danish Dental Association (KOF and CALCIN and the dentists Kai O. Mehlsen and Espen Leth Esbensens Foundation). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Pihlstrom BC, Michalowicz BS, Johnson DW (2005) Periodontal diseases. Lancet 366: 1809–1820. [DOI] [PubMed] [Google Scholar]
- 2. Darveau RP (2010) Periodontitis: a polymicrobial disruption of host homeostasis. Nature Rev Microbiol 8: 481–490. [DOI] [PubMed] [Google Scholar]
- 3. Nishihara T, Koseki T (2004) Microbial aetiology of periodontitis. Periodontol 2000 36: 14–26. [DOI] [PubMed] [Google Scholar]
- 4. Paster BJ, Olsen I, Aas JA, Dewhirst FE (2006) The breadth of bacterial diversity in the human periodontal pocket and other oral sites. Periodontol 2000 42: 80–87. [DOI] [PubMed] [Google Scholar]
- 5. Zambon JJ (1985) Actinobacillus actinomycetemcomitans in human periodontal disease. J Clin Periodontol 12: 1–20. [DOI] [PubMed] [Google Scholar]
- 6. Kilian M, Frandsen EV, Haubek D, Poulsen K (2006) The etiology of periodontal disease revisited by population genetic analysis. Periodontol 2000 42: 158–179. [DOI] [PubMed] [Google Scholar]
- 7. Kittichotirat W, Bumgarner RE, Asikainen S, Chen C (2011) Identification of the Pangenome and Its Components in 14 Distinct Aggregatibacter actinomycetemcomitans Strains by Comparative Genomic Analysis. PLoS One 6: e22420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pinheiro ET, Kawamoto D, Ota-Tsuzuki C, Almeida RR, Nunes AC, et al. (2011) Analysis of genotypic variation in genes associated with virulence in Aggregatibacter actinomycetemcomitans clinical isolates. J Periodontal Res 46: 310–317. [DOI] [PubMed] [Google Scholar]
- 9. Henderson B, Ward JM, Ready D (2010) Aggregatibacter (Actinobacillus) actinomycetemcomitans: a triple A* periopathogen? Periodontol 2000 54: 78–105. [DOI] [PubMed] [Google Scholar]
- 10. Johansson A (2011) Aggregatibacter actinomycetemcomitans Leukotoxin: A Powerful Tool with Capacity to Cause Imbalance in the Host Inflammatory Response. Toxins (Basel) 3: 242–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Haubek D (2010) The highly leukotoxic JP2 clone of Aggregatibacter actinomycetemcomitans: evolutionary aspects, epidemiology and etiological role in aggressive periodontitis. APMIS 130: 1–53. [DOI] [PubMed] [Google Scholar]
- 12. Haubek D, Poulsen K, Kilian M (2007) Microevolution and patterns of dissemination of the JP2 clone of Aggregatibacter (Actinobacillus) actinomycetemcomitans . Infect Immun 75: 3080–3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lara-Tejero M, Galán JE (2000) A bacterial toxin that controls cell cycle progression as a deoxyribonuclease I-like protein. Science 13: 354–357. [DOI] [PubMed] [Google Scholar]
- 14. Belibasakis GN, Mattsson A, Wang Y, Chen C, Johansson A (2004) Cell cycle arrest of human gingival fibroblasts and periodontal ligament cells by Actinobacillus actinomycetemcomitans: involvement of the cytolethal distending toxin. APMIS 112: 674–685. [DOI] [PubMed] [Google Scholar]
- 15. Poulsen K, Theilade E, Lally ET, Demuth DR, Kilian M (1994) Population structure of Actinobacillus actinomycetemcomitans: a framework for studies of disease-associated properties. Microbiol 140: 2049–2060. [DOI] [PubMed] [Google Scholar]
- 16. Haubek D, Poulsen K, Asikainen S, Kilian M (1995) Evidence for absence in northern Europe of especially virulent clonal types of Actinobacillus actinomycetemcomitans . J Clin Microbiol 33: 395–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kaplan J, Perry MB, Maclean LL, Furgang D, Wilson ME, et al. (2002) Structural and genetic analysis of O polysaccharide from Actinobacillus actinomycetemcomitans serotype f. Infect Immun 69: 5375–5384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Takada K, Saito M, Tsuzukibashi O, Kawashima Y, Ishida S, et al. (2010) Characterization of a new serotype g isolate of Aggregatibacter actinomycetemcomitans . Mol Oral Microbiol 25: 200–206. [DOI] [PubMed] [Google Scholar]
- 19. Saarela M, Asikainen S, Alaluusua S, Pyhälä L, Lai CH, et al. (1992) Frequency and stability of mono- or poly-infection by Actinobacillus actinomycetemcomitans serotypes a, b, c, d or e. Oral Microbiol Immunol 7: 277–279. [DOI] [PubMed] [Google Scholar]
- 20. Lakio L, Kuula H, Dogan B, Asikainen S (2002) Actinobacillus actinomycetemcomitans proportion of subgingival bacterial flora in relation to its clonal type. Eur J Oral Sci 110: 212–217. [DOI] [PubMed] [Google Scholar]
- 21. Jentsch H, Cachovan G, Guentsch A, Eickholz P, Pfister W, et al. (2012) Characterization of Aggregatibacter actinomycetemcomitans strains in periodontitis patients in Germany. Clin Oral Invest 16: 1589–1597. [DOI] [PubMed] [Google Scholar]
- 22. Kawamoto D, Ando ES, Longo PL, Nunes AC, Wikström M, et al. (2009) Genetic diversity and toxic activity of Aggregatibacter actinomycetemcomitans isolates. Oral Microbiol Immunol 24: 493–501. [DOI] [PubMed] [Google Scholar]
- 23. Kim TS, Frank P, Eickholz P, Eick S, Kim CK (2009) Serotypes of Aggregatibacter actinomycetemcomitans in patients with different ethnic backgrounds. J Periodontol 80: 2020–2027. [DOI] [PubMed] [Google Scholar]
- 24. Chen C, Wang T, Chen W (2010) Occurrence of Aggregatibacter actinomycetemcomitans serotypes in subgingival plaque from United States subjects. Mol Oral Microbiol 25: 207–214. [DOI] [PubMed] [Google Scholar]
- 25. Zambon JJ, Slots J, Genco RJ (1983) Serology of oral Actinobacillus actinomycetemcomitans and serotype distribution in human periodontal disease. Infect Immun 41: 19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yang HW, Asikainen S, Doğan B, Suda R, Lai CH (2004) Relationship of Actinobacillus actinomycetemcomitans serotype b to aggressive periodontitis: frequency in pure cultured isolates. J Periodontol 75: 592–599. [DOI] [PubMed] [Google Scholar]
- 27. Jinadasa RN, Bloom SE, Weiss RS, Duhamel GE (2011) Cytolethal distending toxin: a conserved bacterial genotoxin that blocks cell cycle progression, leading to apoptosis of a broad range of mammalian cell lineages. Microbiol 157: 1851–1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Elwell CA, Dreyfus LA (2000) DNase I homologous residues in CdtB are critical for cytolethal distending toxin-mediated cell cycle arrest. Mol Microbiol 37: 952–963. [DOI] [PubMed] [Google Scholar]
- 29. Sugai M, Kawamoto T, Pérès SY, Ueno Y, Komatsuzawa H, et al. (1998) The cell cycle-specific growth-inhibitory factor produced by Actinobacillus actinomycetemcomitans is a cytolethal distending toxin. Infect Immun 66: 5008–5019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Belibasakis GN, Johansson A, Wang Y, Chen C, Kalfas S, et al. (2005) The cytolethal distending toxin of Actinobacillus actinomycetemcomitans induces RANKL expression by human gingival fibroblasts and periodontal ligament cells. Infect Immun 73: 342–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Belibasakis GN, Brage M, Lagergård T, Johansson A (2008) The cytolethal distending toxin up-regulates RANKL expression in Jurkat T-cells. APMIS 116: 499–506. [DOI] [PubMed] [Google Scholar]
- 32. Schenkein HA (2006) Host responses in maintaining periodontal health and determining periodontal disease. Periodontol 2000 40: 77–93. [DOI] [PubMed] [Google Scholar]
- 33. Damek-Poprawa M, Haris M, Volgina A, Korostoff J, DiRienzo JM (2011) Cytolethal distending toxin damages the oral epithelium of gingival explants. J Dent Res 90: 874–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ahmed HJ, Svensson LA, Cope LD, Latimer JL, Hansen EJ, et al. (2001) Prevalence of cdtABC genes encoding cytolethal distending toxin among Haemophilus ducreyi and Actinobacillus actinomycetemcomitans strains. J Med Microbiol 50: 860–864. [DOI] [PubMed] [Google Scholar]
- 35. Tan KS, Song KP, Ong G (2002) Cytolethal distending toxin of Actinobacillus actinomycetemcomitans. Occurrence and association with periodontal disease. J Periodontal Res 37: 268–272. [DOI] [PubMed] [Google Scholar]
- 36. Fabris AS, DiRienzo JM, Wïkstrom M, Mayer MP (2002) Detection of cytolethal distending toxin activity and cdt genes in Actinobacillus actinomycetemcomitans isolates from geographically diverse populations. Oral Microbiol Immunol 17: 231–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Leung WK, Ngai VK, Yau JY, Cheung BP, Tsang PW, et al. (2005) Characterization of Actinobacillus actinomycetemcomitans isolated from young Chinese aggressive periodontitis patients. J Periodontal Res 40: 258–268. [DOI] [PubMed] [Google Scholar]
- 38. Yamano R, Ohara M, Nishikubo S, Fujiwara T, Kawamoto T, et al. (2003) Prevalence of cytolethal distending toxin production in periodontopathogenic bacteria. J Clin Microbiol 41: 1391–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Johansson A, Buhlin K, Koski R, Gustafsson A (2005) The immunoreactivity of systemic antibodies to Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis in adult periodontitis. Eur J Oral Sci 113: 197–202. [DOI] [PubMed] [Google Scholar]
- 40. Ando ES, De-Gennaro LA, Faveri M, Feres M, DiRienzo JM, et al. (2010) Immune response to cytolethal distending toxin of Aggregatibacter actinomycetemcomitans in periodontitis patients. J Periodontal Res 45: 471–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Brage M, Holmlund A, Johansson A (2011) Humoral immune response to Aggregatibacter actinomycetemcomitans leukotoxin. J Periodontal Res 46: 170–175. [DOI] [PubMed] [Google Scholar]
- 42. Smith JL, Bayles DO (2006) The contribution of cytolethal distending toxin to bacterial pathogenesis. Crit Rev Microbiol 32: 227–248. [DOI] [PubMed] [Google Scholar]
- 43. Haubek D, Ennibi OK, Poulsen K, Vaeth M, Poulsen S, et al. (2008) Risk of aggressive periodontitis in adolescent carriers of the JP2 clone of Aggregatibacter (Actinobacillus) actinomycetemcomitans in Morocco: a prospective longitudinal cohort study. Lancet 371: 237–242. [DOI] [PubMed] [Google Scholar]
- 44. Elamin AM, Skaug N, Ali RW, Bakken V, Albandar JM (2010) Ethnic disparities in the prevalence of periodontitis among high school students in Sudan. J Periodontol 81: 891–896. [DOI] [PubMed] [Google Scholar]
- 45. Höglund Åberg C, Kwamin F, Claesson R, Johansson A, Haubek D (2012) Presence of JP2 and non-JP2 genotypes of Aggregatibacter actinomycetemcomitans and periodontal attachment loss in adolescents in Ghana. J Periodontol 83: 1520–1528. [DOI] [PubMed] [Google Scholar]
- 46. Poulsen K, Ennibi O-K, Haubek D (2003) Improved PCR for detection of the highly leukotoxic JP2 clone of Actinobacillus actinomycetemcomitans in subgingival plaque samples. J Clin Microbiol 41: 4829–4832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Suzuki N, Nakano Y, Yoshida Y, Ikeda D, Koga T (2001) Identification of Actinobacillus actinomycetemcomitans serotypes by multiplex PCR. J Clin Microbiol 39: 2002–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Van der Velden U, Abbas F, Armand S, Loos BG, Timmerman MF, et al. (2006) Java project on periodontal diseases. The natural development of periodontitis: risk factors, risk predictors and risk determinants. J Clin Periodontol 33: 540–548. [DOI] [PubMed] [Google Scholar]
- 49. Fine DH, Markowitz K, Furgang D, Fairlie K, Ferrandiz J, et al. (2007) Aggregatibacter actinomycetemcomitans and its relationship to initiation of localized aggressive periodontitis: Longitudinal cohort study of initially healthy adolescents. J Clin Microbiol 45: 3859–3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Primer sequences, gene position, and gene fragment size for the serotype and cdt -genotype PCR analyses.
(DOC)