Abstract
Background
Crude extracts of Selaginella tamariscina , an oriental medicinal herb, have been evidenced to treat several human diseases. This study investigated the mechanisms by which Selaginella tamariscina inhibits the invasiveness of human oral squamous-cell carcinoma (OSCC) HSC-3 cells.
Methodology/Principal Findings
Herein, we demonstrate that Selaginella tamariscina attenuated HSC-3 cell migration and invasion in a dose-dependent manner. The anti-metastatic activities of Selaginella tamariscina occurred at least partially because of the down-regulation of matrix metalloproteinases (MMP)-2 and MMP-9 gelatinase activity and the down-regulation of protein expression. The expression and function of both MMP-2 and MMP-9 were regulated by Selaginella tamariscina at a transcriptional level, as shown by quantitative real-time PCR and reporter assays. Chromatin immunoprecipitation (ChIP) data further indicated that binding of the cAMP response element-binding (CREB) protein and activating protein-1 (AP-1) to the MMP-2 promoter diminished at the highest dosage level of Selaginella tamariscina . The DNA-binding activity of specificity protein 1 (SP-1) to the MMP-9 promoter was also suppressed at the same concentration. Selaginella tamariscina did not affect the mitogen-activated protein kinase signaling pathway, but did inhibit the effects of gelatinase by reducing the activation of serine–threonine kinase Akt.
Conclusions
These results demonstrate that Selaginella tamariscina may be a potent adjuvant therapeutic agent in the prevention of oral cancer.
Introduction
Head and neck squamous-cell carcinoma accounts for approximately 3% of all cancers in the United States, and oral squamous-cell carcinoma (OSCC) is the most common form of head and neck cancer [1]. The high rate of metastasis to cervical lymph nodes causes the poor survival rate of oral cancer [2]. Cancer cells typically spread by secreting various molecules that degrade the extracellular matrix (ECM), invading the blood vessels, and migrating to distant organs [3]. Matrix metalloproteinases (MMPs) are a major group of enzymes that regulate ECM composition during normal development and pathological responses [4]. Although various MMPs contribute to cancer cell metastasis, the gelatinases MMP-2 and MMP-9 have been most intensively studied [5]. MMP-2, also known as gelatinase A, is a 72-kDa protein expressed in most tissues and cells [6]. In contrast, MMP-9 (Gelatinase B), a 92-kDa protein, is conditionally observed in leukocytes [7]. Elevated MMP-2 and MMP-9 expression have been observed in invasive and metastatic cases of human oral cancer [8–10]. Hence, concentrated efforts have been made to develop MMP inhibitors (MMPIs) to halt the spread of cancer cells [11].
Selaginella tamariscina is an herb traditionally used in oriental medicine that exhibits several therapeutic abilities. First, because Selaginiallatamariscina has been shown to reduce blood sugar and serum lipid peroxide levels, it exhibits potential uses in the treatment of diabetes [12,13]. Second, bioflavonoids isolated from Selaginella tamariscina demonstrated antibacterial and antifungal effects [14–16]. Third, crude extracts from Selaginella tamariscina have inhibited human mesangial cell proliferation, and have decreased interleukin-1beta and tumor necrosis factor-alpha production [17]. Fourth, Selaginella tamariscina could be a potential chemopreventive agent against various human cancer cell lines, such as gastric cancer [18], lung cancer [19], breast cancer [20], and cervical cancer [21]. The aim of this study was to elucidate the effects of Selaginella tamariscina on human OSCC HSC-3 cells. Our results showed that Selaginella tamariscina halted oral cancer cell migration through the down-regulation of MMP-2 and MMP-9 expression and by decreasing DNA-binding activity to promoter elements. In addition, the anti-metastatic effects were associated with the inactivation of serine–threonine kinase Akt.
Materials and Methods
Extract from Selaginella tamariscina
Selaginella tamariscina was purchased from herb stores and dried whole plants (100 g) were extracted twice with 500 ml of 50% ethanol in distilled water. The pooled extracts were filtered and concentrated at 70°C using a rotary evaporator under low pressure. The concentrated crude extract was frozen at −80°C for 2-3 days and then it was freeze-dried in a lyophilizer and stored at −20°C. The extraction yield was 2.8% (w/w) and the chemical profile of Selaginella tamariscina extract (STE) was analyzed by using high-pressure liquid chromatograms (HPLC)-mass spectrometer [19]. Briefly, Selaginella tamariscina were analysed by HPLC-mass spectrometer using a HPLC (Hitachi L-6200 with an L-4500 Diode Array detector) with a PE Sciex Qstar Pulsar ESI-TOF mass spectrometer. Samples (10 µl) were injected onto a Merck LiChrospher 100 RP-18 column (4 x 250 mm). The column was equilibrated in 0.05% acetic acid/water (solution A) and elution of the components was achieved by increasing the concentration of solution B (100% acetonitrile) from 0 to 100% in 30 min at a flow rate of 1 ml/min. Absorbance was monitored at 254 nm. The molecular masses of the peaks were determined from electrospray ionisation mass spectra using multiply-charged ion profile [19]. The extract was dissolved in dimethyl sulfoxide (DMSO) (Sigma Co., USA) and was prepared at different concentrations for the subsequent experiments.
Cell culture and Selaginella tamariscina extract (STE) treatment
HSC-3, a human tongue squamous cell carcinoma cell line obtained from ATCC (Manassas, VA, USA), was cultured in Dulbecco’s modified Eagle’s medium (Life Technologies, Grand Island, NY, USA), 10% fetal bovine serum (Hyclone Laboratories, Logan, UT, USA), 2 mM glutamine, 100 U/mL penicillin, and 100 µg/mL streptomycin. All cell cultures were maintained at 37 oC in a humidified atmosphere of 5% CO2. For STE treatment, appropriate amounts of stock solution of STE were added into culture medium to achieve the indicated concentrations and then incubated with cells for indicated time periods, whereas dimethyl sulfoxide solution without STE was used as blank reagent.
Determination of cell viability (MTT assay)
For cell viability experiment, a microculture tetrazolium (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) colorimetric assay was performed to determine the cytotoxicity of STE. HSC-3 cells were seeded in 24-well plates at a density of 5 x 104 cells/well and treated with STE at a concentration between 0–100 µg/mL at 37 oC for 24 h. After the exposure period, the media was removed, and cells were washed with phosphate buffered saline (PBS) and then incubated with 20 µL MTT (5 mg/mL) (Sigma chemical Co., St. Louis, MO, USA) for 4 h. The viable cell number per dish is directly proportional to the production of formazan, which can be measured spectrophotometrically at 563 nm following solubilization with isopropanol.
In vitro wound closure
HSC-3 cells (1×105 cells/well) were plated in 6-well plates for 24 h, wounded by scratching with a pipette tip, then incubated with DMEM medium containing 0.5% FBS and treated with or without STE (0, 25, 50, 75 and 100 µg/mL) for 0, 12 and 24 h. Cells were photographed using a phase-contrast microscope (×100).
Cell migration and invasion assays
Cell migration and invasion were assayed according to the methods described by Yang et al. [19]. After a treatment with STE (0, 25, 50, 70 and 100 µg/mL) for 24 h, surviving cells were harvested and seeded to Boyden chamber (Neuro Probe, Cabin John, MD, USA) at 104 cells/well in serum free medium and then incubated for 24 hours at 37 oC. For invasion assay, 10 µL Matrigel (25 mg/50 mL; BD Biosciences, MA, USA) was applied to 8 µm pore size polycarbonate membrane filters and the bottom chamber contained standard medium. Filters were then air-dried for 5 h in a laminar flow hood. The invaded cells were fixed with 100% methanol and stained with 5% Giemsa. Cell numbers were counted under a light microscope. The migration assay was carried out as described in the invasion assay with no coating of Matrigel.
Determination of MMP-2 and MMP-9 by gelatin zymography
The activities of MMP-2 in conditional medium were measured by gelatin zymography protease assays. Briefly, collected media of an appropriate volume (adjusted by vital cell number) were prepared with SDS sample buffer without boiling or reduction and subjected to 0.1% gelatin-8% SDS-PAGE electrophoresis. After electrophoresis, gels were washed with 2.5% Triton X-100 and then incubated in reaction buffer (40 mM Tris–HCl, pH 8.0; 10 mM CaCl2 and 0.01% NaN3) for 12 h at 37 oC. Then gel was stained with Coomassie brilliant blue R-250.
Preparation of total cell lysates
For total cell lysates preparation, cells were rinsed with PBS twice and scraped with 0.2 mL of cold RIPA buffer containing protease inhibitors cocktail, and then vortexed at 4 oC for 10 min. Cell lysates were subjected to a centrifugation of 10,000 rpm for 10 min at 4 oC, and the insoluble pellet was discarded. The protein concentration of total cell lysates was determined by Bradford assay.
Western blot analysis
The 20 µg samples of total cell lysates or nuclear fractions were separated by SDS-PAGE on 10% polyacrylamide gels and transferred onto a nitrocellulose membrane using the Mini-Protean Tetra Electrophoresis System as described previously [22]. The blot was subsequently incubated with 5% non-fat milk in Tris-buffered saline (20 mM Tris, 137 mM NaCl, pH 7.6) for 1 h to block non-specific binding and then overnight with polyclonal antibodies against MMP-2, MMP-9, TIMP-1, TIMP-2, three MAPKs (ERK 1/2, JNK 1/2 and p38), or Akt with the specific antibodies for unphosphorylated or phosphorylated forms of the corresponding ERK 1/2, JNK 1/2, p38 and Akt. Blots were then incubated with a horseradish peroxidase goat anti-rabbit or anti-mouse IgG for 1 h. Afterwards, signal was detected by using enhanced chemiluminescence (ECL) commercial kit (Amersham Biosciences) and relative photographic density was quantitated by scanning the photographic negatives on a gel documentation and analysis system (AlphaImager 2000, Alpha Innotech Corporation, San Leandro, CA, USA).
RNA preparation and TaqMan quantitative real-time PCR
Total RNA was isolated from oral cancer cells using Trizol (Life Technologies, Grand Island, NY) according to the manufacturer’s instructions. Quantitative real-time PCR analysis was carried out using Taqman one-step PCR Master Mix (Applied Biosystems). 100 ng of total cDNA was added per 25 µl reaction with MMP-2, MMP-9 or GAPDH primers and Taqman probes. The MMP-2, MMP-9 and GAPDH primers and probes were designed using commercial software (ABI PRISM Sequence Detection System; Applied Biosystems). Quantitative real-time PCR assays were carried out in triplicate on a StepOnePlus sequence detection system. The threshold was set above the non-template control background and within the linear phase of target gene amplification to calculate the cycle number at which the transcript was detected.
Transfection and MMP-2, MMP-9 promoter-driven luciferase assays
HSC-3 cells were seeded at a concentration of 5 x104 cells per well in 6-well cell culture plates. After 24 h of incubation, pGL3-basic (vector), MMP-2 or MMP-9 promoter plasmid were co-transfected with a β-galactosidase expression vector (pCH110) into cells using Turbofect (Fermentas, Carlsbad, CA). After 12 h of transfection, cells were treated with vehicle or STE (0 or 100 µg/mL) for 24 h. The cell lysates were harvested, and luciferase activity was determined using a luciferase assay kit. The value of the luciferase activity was normalized to transfection efficiency and monitored by β-galactosidase expression.
Chromatin immunoprecipitation analysis (ChIP)
Chromatin immunoprecipitation analysis was performed as described previously [23,24]. DNA immunoprecipitated with anti-CREB, anti-SP1 or anti c-fos was purified and extracted using phenol-chloroform. Immunoprecipitated DNA was analyzed by PCR or quantitative PCR by using specific primers as described previously [23].
Statistical analysis
For all of the measurements, analysis of variance followed by Scheffe posteriori comparison was used to assess the differences between control and cells treated with various concentration of STE. A difference at p < 0.05 was considered to be statistically significant and the experiments were repeated three times.
Results
Effects of Selaginella tamariscina on HSC-3 cell viability and motility
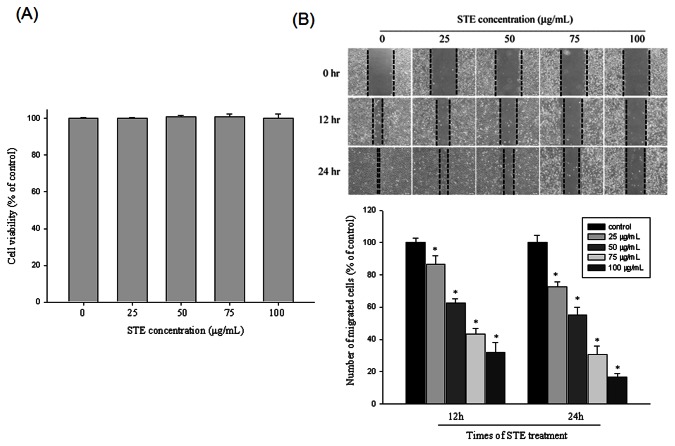
HSC-3 cell viability in the presence of various concentrations (0-100 µg/mL) of Selaginella tamariscina for 24 hours is shown in Figure 1A. Even the highest concentration, 100 µg/mL, did not have a cytotoxic effect on the HSC-3 cells. We used 0-100 µg/mL of Selaginella tamariscina to conduct the following experiments. Figure 1B shows the results of using a scratch-wound assay to calculate the migration ability of HSC-3 cells treated with various concentrations of Selaginella tamariscina . The results demonstrate that Selaginella tamariscina significantly reduced cell motility both time- and dose-dependently (p<0.001) (Figure 1B and 1C).
Figure 1. Effect of Selaginella tamariscina on cell viability and in vitro wound closure in HSC-3 cells.
(A) HSC-3 cells were treated with STE (0, 25, 50, 75 and 100 µg/mL) for 24 h before being subjected to a MTT assay for cell viability. The values represented the means ± SD of at least three independent experiments. (B) HSC-3 cells were wounded and then treated with vehicle (DMSO) or STE (0, 25, 50, 75 and 100 µg/mL) for 0h, 12h and 24 h in 10% FBS-containing medium. At 0, 12 and 24 h, phase-contrast pictures of the wounds at three different locations were taken.
Effects of Selaginella tamariscina on migration and invasion of HSC-3 cells
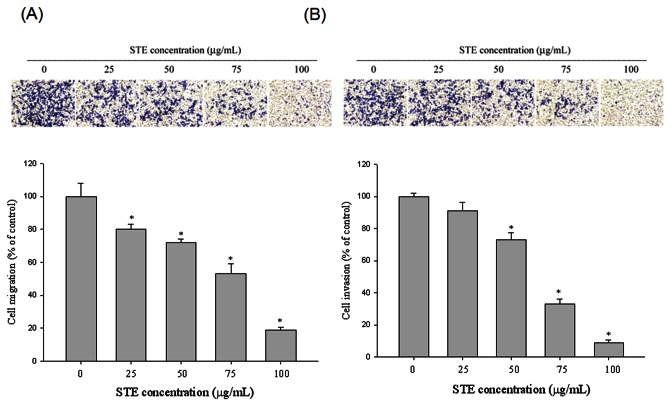
To examine the effects of Selaginella tamariscina on cell migration and invasion, we used a Boyden chamber assay to detect cell motility. Figure 2A shows that Selaginella tamariscina significantly inhibited migration in a concentration-dependent manner for 24 hours. Similarly, Figure 2B indicates that the invasiveness of HSC-3 cells was also reduced after incubation with different concentrations (0-100 µg/mL) of Selaginella tamariscina for 24 hours.
Figure 2. Effect of STE on cell migration and invasion in HSC-3 cells.
(A) The cell migration and (B) cell invasion were measured using a Boyden chamber for 16h and 24 h with polycarbonate filters respectively. The migration and invasion abilities of HSC-3 cells were quantified by counting the number of cells that invaded to the underside of the porous polycarbonate as described in the Materials and Methods section. The values represented the means ± SD of at least three independent experiments. *p < 0.05 as compared with the vehicle group.
Effects of Selaginella tamariscina on MMP-2 and MMP-9 protein expression and enzyme activity
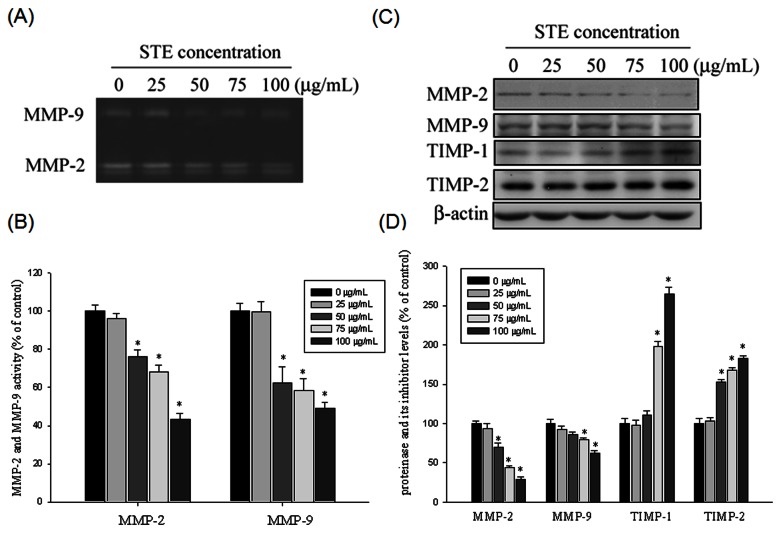
The ability of Selaginella tamariscina to suppress the migratory and invasive abilities of HSC-3 cells by decreasing MMP-2 and MMP-9 expression was evaluated using gelatin zymography. Figure 3A shows that the enzyme activity of MMP-2 and MMP-9 was suppressed by Selaginella tamariscina in a concentration-dependent manner. The highest concentration of Selaginella tamariscina , 100 µg/mL, inhibited MMP-2 and MMP-9 activity by 57% and 51%, respectively (Figure 3B). Selaginella tamariscina also substantially reduced MMP-2 and MMP-9 protein expression when detected using western blotting (Figure 3C). Thus, we suggest that the anti-metastatic ability of Selaginella tamariscina at least partially inhibited MMP-2 and MMP-9 expression. Investigation of the effects of STE on the protein expression of the MMPs endogenous inhibitor, TIMP-1 and TIMP-2, showed that STE induced TIMP-1 and TIMP-2 upregulation in a concentration-dependent manner (Figure 3C and 3D)
Figure 3. Effects of STE on the activity and protein level of MMP-2, MMP-9 and the protein level of the endogenous inhibitor TIMP-2 and TIMP-1.
(A and B) HSC-3 cells were treated with STE (0-100 µg/mL) for 24 h and then subjected to gelatin zymography to analyze the activity of MMP-2 and MMP-9, respectively. (C) HSC-3 cells were treated with STE (0-100 µg/mL) for 24 h and then subjected to western blotting to analyze the protein levels of MMP-2, MMP-9, TIMP-1 and TIMP-2. (D) Quantitative results of MMP-2, MMP-9, TIMP-1 and TIMP-2 protein levels which were adjusted with β-actin protein level. The values represented the means ± SD of at least three independent experiments. *p < 0.05 as compared with the vehicle group.
Effects of Selaginella tamariscina on MMP-2 and MMP-9 mRNA expression and DNA-binding activity
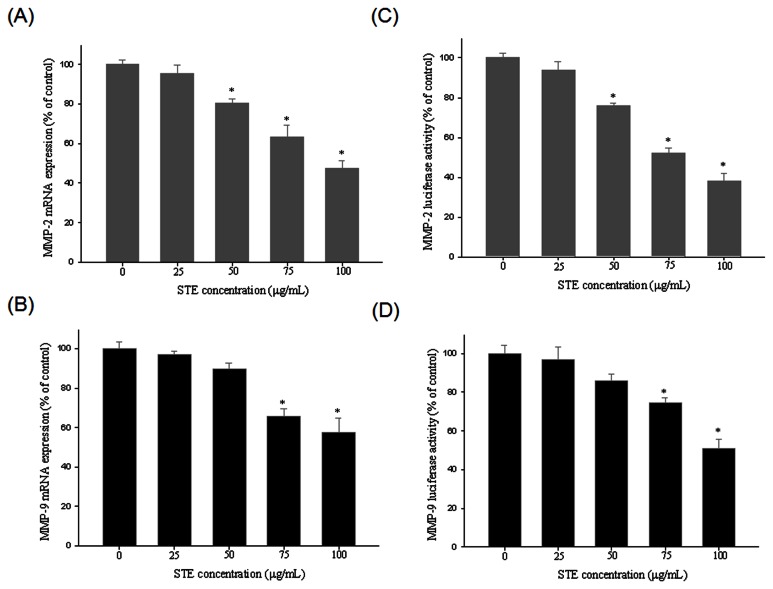
The effects of Selaginella tamariscina on MMP-2 and MMP-9 mRNA expression were also examined. A low level of MMP-2 and MMP-9 mRNA expression was observed at the highest dose of Selaginella tamariscina (100 µg/mL) for 6 hours (Figure 4A and 4B). To further investigate how Selaginella tamariscina regulates the transcriptional activity of MMP-2 and MMP-9, we conducted a luciferase reporter assay in which both Selaginella tamariscina and the control cells were transfected with an MMP-2 and MMP-9 promoter construct. Figure 4C shows that the MMP-2 promoter activity was reduced by Selaginella tamariscina in a dose-dependent manner. Similarly, approximately 50% inhibition of MMP-9 promoter activity was evident at 100 µg/mL of Selaginella tamariscina (Figure 4D). These observations suggest that Selaginella tamariscina regulates MMP-2 and MMP-9 activity at the transcriptional level.
Figure 4. STE suppresses MMP-2 and MMP-9 expression at a transcriptional level.
HSC-3 cells were treated with STE (0, 25, 50, 75 and 100 µg/mL) for 24 h and then subjected to quantitative real-time PCR to analyze the mRNA expression of MMP-2 (A), or MMP-9 (B). (C) MMP-2 or (D) MMP-9 promoter reporter assay to analyze the promoter activity of MMPs. Luciferase activity, determined in triplicates, was normalized to β-galactosidase activity. The values represented the means ± SD of at least three independent experiments. *p < 0.05 as compared with the vehicle group.
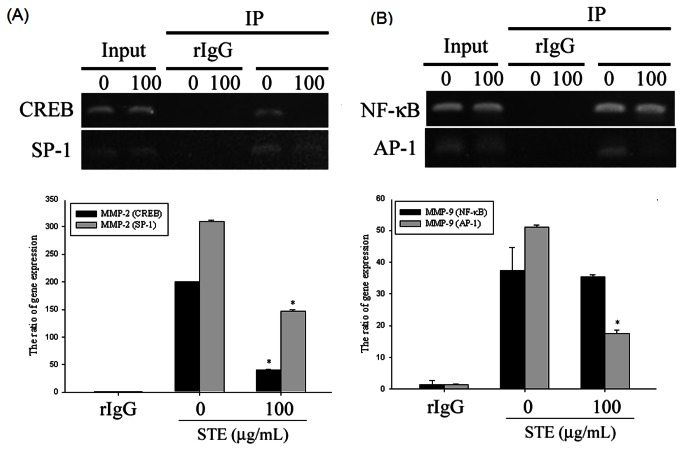
Previous studies have shown that MMP promoters are regulated by several transcription factors, such as AP-1, NFκB, CREB, and SP-1 [23,25,26]. We performed a chromatin immunoprecipitation (ChIP) assay to evaluate the involvement of transcription factors in the inhibitory effects of Selaginella tamariscina on MMP-2 and MMP-9 activity (Figure 5A and 5B). ChIP assay and quantitative real-time PCR showed that Selaginella tamariscina substantially suppressed binding of CREB and SP-1 to the MMP-2 promoter (Figure 5A). Figure 5B indicates that Selaginella tamariscina considerably inhibited AP-1, but not the NF-κB DNA-binding to the MMP-9 promoter. These results indicate that Selaginella tamariscina inhibited MMP-2 and MMP-9 expression by regulating the binding activity of transcription factors on the cis-element of MMP promoters.
Figure 5. Critical role of transcription factor in STE-induced transcriptional inhibition of MMP-2 and MMP-9 in HSC-3 cells.
HSC-3 cells were treated with STE 100 µg/mL for 24 h and then the nuclear fraction was prepared as described in "Materials and Methods". ChIP analysis of the association of various transcription factors with the MMP-2 (A) or MMP-9 (B) promoter region in HSC-3 cells. The values represented the means ± SD of at least three independent experiments. *p < 0.05 as compared with the vehicle group.
Effects of Selaginella tamariscina on MAPK and Akt pathways
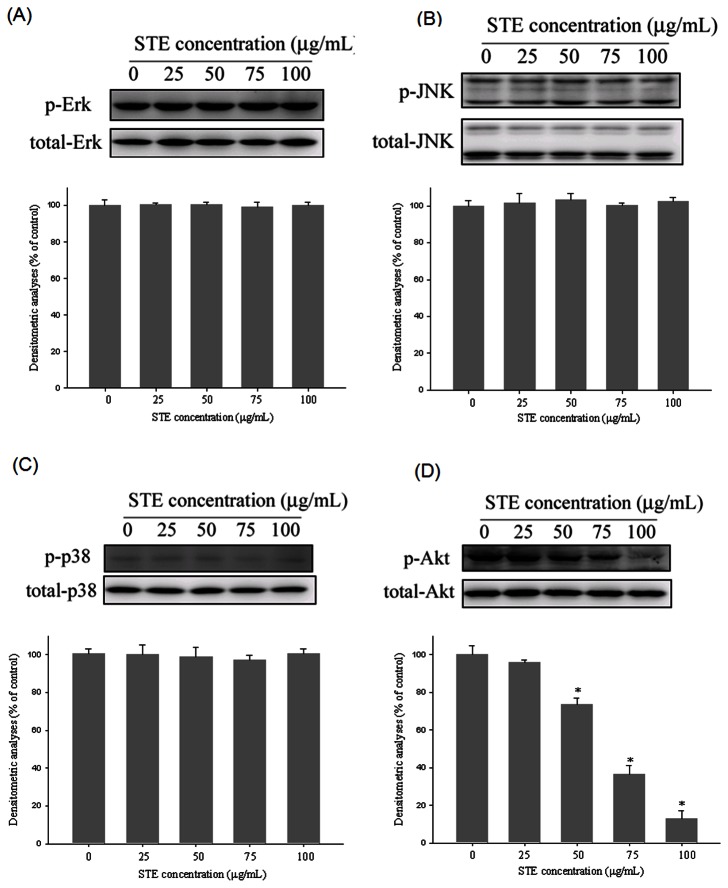
To further investigate the underlying mechanisms of the upstream signaling pathways of MMP-2 and MMP-9, we used western blotting to evaluate the effects of Selaginella tamariscina on the MAPK and Akt pathways. Figure 6A–6C reveal that the MAPK pathway, which includes ERK, JNK, and p38 protein kinases, was not notably inhibited. However, Selaginella tamariscina reduced phosphorylation of Akt in a dose-dependent manner (Figure 6D). Thus, we suggest that the activation of the Akt signaling pathway is required for Selaginella tamariscina to suppress MMP-2 and MMP-9.
Figure 6. Effects of STE on the MAPKs pathway and Akt signalings.
HSC-3 cells were cultured in various concentrations of STE (0, 25, 50, 75 and 100 µg/mL) for 24 hours, and then the cell lysates were subjected to SDS–PAGE followed by western blots with (A) anti-ERK1/2, (B) anti-JNK, (C) anti-p38 and (D) anti-Akt (total and phosphorylated) antibodies as described in Materials and Methods. Determined activities of these proteins were subsequently quantified by densitometric analyses with that of control being 100% as shown just below the gel data. The values represented the means ± SD of at least 3 independent experiments. *p< 0.05 as compared with the vehicle group.
Discussion
Numerous medicinal plants have been studied for anticancer applications, such as Dioscorea nipponica Makino [23] and Terminalia Catappa [26]. Over the past decade, Selaginella tamariscina has become a traditional treatment for various diseases [14,15,18,19]. In this study, we suggest that Selaginella tamariscina exhibits beneficial effects on oral cancer cell treatment by (1) inhibiting HSC-3 oral cancer cell migration and invasion, (2) reducing MMP-2 and MMP-9 gene expression and enzyme activity, (3) inhibiting phosphorylation of AKT, (4) decreasing nuclear translocation of CREB and SP-1 to an MMP-2 promoter, and (5) decreasing nuclear translocation of AP-1 to an MMP-9 promoter. Numerous flavonoids are found in the crude extracts of Selaginella tamariscina that exhibit various pharmacological effects. Amentoflavone markedly arrested cell cycles and induced apoptosis of human breast and cervical cancer cells [21,27,28]. In addition, sumaflavone exerted anti-inflammatory effects by blocking iNOS expression through AP-1 inhibition [29]. Moreover, Mirzoeva et al showed that apigenin exhibits antiangiogenic potential in prostate carcinoma cells by inhibiting Smad2/3 and Src/FAK/Akt pathways [30]. The previous studies have suggested that flavonoids play a critical role in the anti-metastatic effects of Selaginella tamariscina , but the underlying mechanisms of this process require further explanation.
Metastasis, which causes approximately 90% of cancer deaths, is the process by which cancer cells spread from the original tumor site to distant organs [31]. The degradation of the ECM components and the basement membrane is a critical step in metastasis. There are multiple types of proteases that control ECM degradation and remodeling. MMP-2 and MMP-9 are the most extensively studied of the MMP family because of their high association with cancer migration and invasion [5]. Several previous studies have indicated that natural products inhibit cancer metastasis by inhibiting MMP-2 and MMP-9 expression [23,26]. Our results indicate that Selaginella tamariscina inhibited MMP-2 and MMP-9 enzyme activity, as well as protein expression. A decrease in migration and invasion abilities resulting from the suppression of MMP-2 and MMP-9 activity has been suggested. The results are similar to our previous study, in which the anti-metastatic effects of Selaginella tamariscina on lung cancer cells occurred through reduced gelatinase expression [19]. Numerous reports indicate that MMP gene expression was specifically regulated by mitogen-activated protein kinases (MAPKs), a family of serine/threonine kinases including ERKs, JNKs, and p38 [31–33]. However, our study results indicated that no observable effects on the MAPK signaling pathway resulted from the regulation of MMP production by Selaginella tamariscina . In addition, the involvement of the phosphoinositide-3 kinase (PI3K)/AKT signal transduction pathway in MMP gene expression and cell migration has been adequately studied. Wang et al revealed that isoliquiritigenin inhibited the expression and gelatinolytic activity of MMP-2 and MMP-9 by regulating the upstream AKT signaling pathways in breast cancer MDA-MB-231 cells [34]. Another study concluded that berberine, an isoquinoline alkaloid, inhibited breast cancer cell metastasis by modulating the AKT pathway [35]. Our data also suggested that the PI3K/AKT signaling pathway is involved as an upstream trigger of MMP-2 and MMP-9 regulation.
The expression of MMPs can be regulated at multiple levels, including transcription, post-transcription, translation, proenzyme-activation, and repression levels, by specific inhibitors [36]. It is suggested that Selaginella tamariscina regulated MMP-2 and MMP-9 at the transcriptional level because promoter activity and mRNA expression were inhibited. MMP promoters have several cis-elements that can be transactivated by several transcription factors, such as NF-κB, AP-1, CREB, and SP-1. Previous studies have indicated that the AKT/SP-1 pathway regulated MMP-2 promoter activity and affected the migration ability of cancer cells [24,37]. Satpathy et al showed that tissue transglutaminase 2 modulates CREB activation and MMP-2 transcription in ovarian cancer [38]. The upstream promoter sequence of the MMP-9 gene contains AP-1 and NF-κB sites. Epigallocatechin Gallate (EGCG) exerts its anti-invasive effect by suppressing AP-1 activation in human gastric cancer cells [39]. In addition, NF-κB regulates the expression of MMP-9 in various cancers [33,40,41]. Although MMP-9 mRNA expression was regulated by Selaginella tamariscina , we did not observe a notable effect on the NF-κB DNA-binding activities. Our study demonstrates that MMP-2 expression was regulated by CREB and SP-1 DNA-binding activities when affected by Selaginella tamariscina , and AP-1 site were necessary for the inhibition of MMP-9 expression.
The results of this study show that Selaginella tamariscina reduced oral cancer migration and invasion by inhibiting MMP-2 and MMP-9 gene expression, and enzyme activity. These anti-tumor effects on OSCC are associated with the suppression of AKT and the repression of DNA-binding activities on MMP-2 and MMP-9 promoters. OSCC invasion and metastasis are a major obstacle for cancer treatment. Therefore, the inhibition of metastasis by Selaginella tamariscina could provide vital preventive and therapeutic benefits for the treatment of oral cancer.
Funding Statement
This study was supported by a research grant from National Science Council, Taiwan (NSC100-2632-B-040-001-MY3). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Mao L, Hong WK, Papadimitrakopoulou VA (2004) Focus on head and neck cancer. Cancer Cell 5: 311-316. doi:10.1016/S1535-6108(04)00090-X. PubMed: 15093538. [DOI] [PubMed] [Google Scholar]
- 2. Massano J, Regateiro FS, Januário G, Ferreira A (2006) Oral squamous cell carcinoma: review of prognostic and predictive factors. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 102: 67-76. doi:10.1016/j.tripleo.2005.07.038. PubMed: 16831675. [DOI] [PubMed] [Google Scholar]
- 3. Ribatti D, Vacca A (2008) The role of microenvironment in tumor angiogenesis. Genes Nutr 3: 29-34. doi:10.1007/s12263-008-0076-3. PubMed: 18850197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chakraborti S, Mandal M, Das S, Mandal A, Chakraborti T (2003) Regulation of matrix metalloproteinases: an overview. Mol Cell Biochem 253: 269-285. doi:10.1023/A:1026028303196. PubMed: 14619979. [DOI] [PubMed] [Google Scholar]
- 5. Björklund M, Koivunen E (2005) Gelatinase-mediated migration and invasion of cancer cells. Biochim Biophys Acta 1755: 37-69. PubMed: 15907591. [DOI] [PubMed] [Google Scholar]
- 6. Vartio T, Vaheri A (1981) A gelatin-binding 70,000-dalton glycoprotein synthesized distinctly from fibronectin by normal and malignant adherent cells. J Biol Chem 256: 13085-13090. PubMed: 7309753. [PubMed] [Google Scholar]
- 7. Sopata I, Wize J (1979) A latent gelatin specific proteinase of human leucocytes and its activation. Biochim Biophys Acta 571: 305-312. doi:10.1016/0005-2744(79)90100-1. PubMed: 508769. [DOI] [PubMed] [Google Scholar]
- 8. Juarez J, Clayman G, Nakajima M, Tanabe KK, Saya H et al. (1993) Role and regulation of expression of 92-kDa type-IV collagenase (MMP-9) in 2 invasive squamous-cell-carcinoma cell lines of the oral cavity. Int J Cancer 55: 10-18. doi:10.1002/ijc.2910550104. PubMed: 7688350. [DOI] [PubMed] [Google Scholar]
- 9. Chiang YY, Tsai MH, Lin TY, Chiang IP (2008) Expression profile of metastasis-related genes in invasive oral cancers. Histol Histopathol 23: 1213-1222. PubMed: 18712673. [DOI] [PubMed] [Google Scholar]
- 10. Fullár A, Kovalszky I, Bitsche M, Romani A, Schartinger VH et al. (2012) Tumor cell and carcinoma-associated fibroblast interaction regulates matrix metalloproteinases and their inhibitors in oral squamous cell carcinoma. Exp Cell Res 318: 1517-1527. doi:10.1016/j.yexcr.2012.03.023. PubMed: 22516051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Overall CM, López-Otín C (2002) Strategies for MMP inhibition in cancer: innovations for the post-trial era. Nat Rev Cancer 2: 657-672. doi:10.1038/nrc884. PubMed: 12209155. [DOI] [PubMed] [Google Scholar]
- 12. Miao N, Tao H, Tong C, Xuan H, Zhamg G (1996) [The Selaginella tamariscina (Beauv.) Spring complex in the treatment of experimental diabetes and its effect on blood rheology]. Zhongguo Zhong Yao Za Zhi 21: 493-495, 512. PubMed: 9642416. [PubMed] [Google Scholar]
- 13. Zheng XK, Zhang L, Wang WW, Wu YY, Zhang QB et al. (2011) Anti-diabetic activity and potential mechanism of total flavonoids of Selaginella tamariscina (Beauv.) Spring in rats induced by high fat diet and low dose STZ. J Ethnopharmacol 137: 662-668. doi:10.1016/j.jep.2011.06.018. PubMed: 21718776. [DOI] [PubMed] [Google Scholar]
- 14. Jung HJ, Sung WS, Yeo SH, Kim HS, Lee IS et al. (2006) Antifungal effect of amentoflavone derived from Selaginella tamariscina. Arch Pharm Res 29: 746-751. doi:10.1007/BF02974074. PubMed: 17024847. [DOI] [PubMed] [Google Scholar]
- 15. Lee J, Choi Y, Woo ER, Lee DG (2009) Antibacterial and synergistic activity of isocryptomerin isolated from Selaginella tamariscina. J Microbiol Biotechnol 19: 204-207. doi:10.4014/jmb.0810.566. PubMed: 19307771. [DOI] [PubMed] [Google Scholar]
- 16. Hwang IS, Lee J, Jin HG, Woo ER, Lee DG (2012) Amentoflavone stimulates mitochondrial dysfunction and induces apoptotic cell death in Candida albicans. Mycopathologia 173: 207-218. doi:10.1007/s11046-011-9503-x. PubMed: 22210020. [DOI] [PubMed] [Google Scholar]
- 17. Kuo YC, Sun CM, Tsai WJ, Ou JC, Chen WP et al. (1998) Chinese herbs as modulators of human mesangial cell proliferation: preliminary studies. J Lab Clin Med 132: 76-85. doi:10.1016/S0022-2143(98)90029-4. PubMed: 9665376. [DOI] [PubMed] [Google Scholar]
- 18. Lee IS, Nishikawa A, Furukawa F, Kasahara K, Kim SU (1999) Effects of Selaginella tamariscina on in vitro tumor cell growth, p53 expression, G1 arrest and in vivo gastric cell proliferation. Cancer Lett 144: 93-99. doi:10.1016/S0304-3835(99)00202-5. PubMed: 10503882. [DOI] [PubMed] [Google Scholar]
- 19. Yang SF, Chu SC, Liu SJ, Chen YC, Chang YZ et al. (2007) Antimetastatic activities of Selaginella tamariscina (Beauv.) on lung cancer cells in vitro and in vivo. J Ethnopharmacol 110: 483-489. doi:10.1016/j.jep.2006.10.010. PubMed: 17113737. [DOI] [PubMed] [Google Scholar]
- 20. Lee JS, Lee MS, Oh WK, Sul JY (2009) Fatty acid synthase inhibition by amentoflavone induces apoptosis and antiproliferation in human breast cancer cells. Biol Pharm Bull 32: 1427-1432. doi:10.1248/bpb.32.1427. PubMed: 19652385. [DOI] [PubMed] [Google Scholar]
- 21. Lee S, Kim H, Kang JW, Kim JH, Lee DH et al. (2011) The biflavonoid amentoflavone induces apoptosis via suppressing E7 expression, cell cycle arrest at sub-G₁ phase, and mitochondria-emanated intrinsic pathways in human cervical cancer cells. J Med Food 14: 808-816. doi:10.1089/jmf.2010.1428. PubMed: 21663495. [DOI] [PubMed] [Google Scholar]
- 22. Yu YL, Yu SL, Su KJ, Wei CW, Jian MH et al. (2010) Extended O6-methylguanine methyltransferase promoter hypermethylation following n-butylidenephthalide combined with 1,3-bis(2-chloroethyl)-1-nitrosourea (BCNU) on inhibition of human hepatocellular carcinoma cell growth. J Agric Food Chem 58: 1630-1638. doi:10.1021/jf903043r. PubMed: 20043672. [DOI] [PubMed] [Google Scholar]
- 23. Chien MH, Ying TH, Hsieh YS, Chang YC, Yeh CM et al. (2012) Dioscorea nipponica Makino inhibits migration and invasion of human oral cancer HSC-3 cells by transcriptional inhibition of matrix metalloproteinase-2 through modulation of CREB and AP-1 activity. Food Chem Toxicol 50: 558-566. doi:10.1016/j.fct.2011.12.016. PubMed: 22210353. [DOI] [PubMed] [Google Scholar]
- 24. Yu YL, Chou RH, Chen LT, Shyu WC, Hsieh SC et al. (2011) EZH2 regulates neuronal differentiation of mesenchymal stem cells through PIP5K1C-dependent calcium signaling. J Biol Chem 286: 9657-9667. doi:10.1074/jbc.M110.185124. PubMed: 21216957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sze KM, Wong KL, Chu GK, Lee JM, Yau TO et al. (2011) Loss of phosphatase and tensin homolog enhances cell invasion and migration through AKT/Sp-1 transcription factor/matrix metalloproteinase 2 activation in hepatocellular carcinoma and has clinicopathologic significance. Hepatology 53: 1558-1569. doi:10.1002/hep.24232. PubMed: 21520171. [DOI] [PubMed] [Google Scholar]
- 26. Yeh CB, Hsieh MJ, Hsieh YS, Chien MH, Lin PY et al. (2012) Terminalia Catappa Exerts Antimetastatic Effects on Hepatocellular Carcinoma through Transcriptional Inhibition of Matrix Metalloproteinase-9 by Modulating NF-kappaB and AP-1 Activity. Evid Based Complement Alternat Med: 2012: 595292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jung HJ, Park K, Lee IS, Kim HS, Yeo SH et al. (2007) S-phase accumulation of Candida albicans by anticandidal effect of amentoflavone isolated from Selaginella tamariscina. Biol Pharm Bull 30: 1969-1971. doi:10.1248/bpb.30.1969. PubMed: 17917274. [DOI] [PubMed] [Google Scholar]
- 28. Pei JS, Liu CC, Hsu YN, Lin LL, Wang SC et al. (2012) Amentoflavone Induces Cell-cycle Arrest and Apoptosis in MCF-7 Human Breast Cancer Cells via Mitochondria-dependent Pathway. In Vivo 26: 963-970. PubMed: 23160679. [PubMed] [Google Scholar]
- 29. Yang JW, Pokharel YR, Kim MR, Woo ER, Choi HK et al. (2006) Inhibition of inducible nitric oxide synthase by sumaflavone isolated from Selaginella tamariscina. J Ethnopharmacol 105: 107-113. doi:10.1016/j.jep.2005.10.001. PubMed: 16289413. [DOI] [PubMed] [Google Scholar]
- 30. Mirzoeva S, Franzen CA, Pelling JC (2013) Apigenin inhibits TGF-beta-induced VEGF expression in human prostate carcinoma cells via a Smad2/3- and Src-dependent mechanism. Mol: Carcinog. [DOI] [PubMed] [Google Scholar]
- 31. Chen PN, Hsieh YS, Chiang CL, Chiou HL, Yang SF et al. (2006) Silibinin inhibits invasion of oral cancer cells by suppressing the MAPK pathway. J Dent Res 85: 220-225. doi:10.1177/154405910608500303. PubMed: 16498067. [DOI] [PubMed] [Google Scholar]
- 32. Hsieh YS, Chu SC, Yang SF, Chen PN, Liu YC et al. (2007) Silibinin suppresses human osteosarcoma MG-63 cell invasion by inhibiting the ERK-dependent c-Jun/AP-1 induction of MMP-2. Carcinogenesis 28: 977-987. PubMed: 17116726. [DOI] [PubMed] [Google Scholar]
- 33. Weng CJ, Chau CF, Hsieh YS, Yang SF, Yen GC (2008) Lucidenic acid inhibits PMA-induced invasion of human hepatoma cells through inactivating MAPK/ERK signal transduction pathway and reducing binding activities of NF-kappaB and AP-1. Carcinogenesis 29: 147-156. PubMed: 18024477. [DOI] [PubMed] [Google Scholar]
- 34. Wang KL, Hsia SM, Chan CJ, Chang FY, Huang CY et al. (2013) Inhibitory effects of isoliquiritigenin on the migration and invasion of human breast cancer cells. Expert Opin Ther Targets, 17: 337–49. PubMed: 23327692. [DOI] [PubMed] [Google Scholar]
- 35. Kuo HP, Chuang TC, Tsai SC, Tseng HH, Hsu SC et al. (2012) Berberine, an isoquinoline alkaloid, inhibits the metastatic potential of breast cancer cells via Akt pathway modulation. J Agric Food Chem 60: 9649-9658. doi:10.1021/jf302832n. PubMed: 22950834. [DOI] [PubMed] [Google Scholar]
- 36. Sternlicht MD, Werb Z (2001) How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol 17: 463-516. doi:10.1146/annurev.cellbio.17.1.463. PubMed: 11687497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tang SW, Yang TC, Lin WC, Chang WH, Wang CC et al. (2011) Nicotinamide N-methyltransferase induces cellular invasion through activating matrix metalloproteinase-2 expression in clear cell renal cell carcinoma cells. Carcinogenesis 32: 138-145. doi:10.1093/carcin/bgq225. PubMed: 21045016. [DOI] [PubMed] [Google Scholar]
- 38. Satpathy M, Shao M, Emerson R, Donner DB, Matei D (2009) Tissue transglutaminase regulates matrix metalloproteinase-2 in ovarian cancer by modulating cAMP-response element-binding protein activity. J Biol Chem 284: 15390-15399. doi:10.1074/jbc.M808331200. PubMed: 19324884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kim HS, Kim MH, Jeong M, Hwang YS, Lim SH et al. (2004) EGCG blocks tumor promoter-induced MMP-9 expression via suppression of MAPK and AP-1 activation in human gastric AGS cells. Anticancer Res 24: 747-753. PubMed: 15161022. [PubMed] [Google Scholar]
- 40. Park SY, Kim YH, Kim Y, Lee SJ (2012) Frondoside A has an anti-invasive effect by inhibiting TPA-induced MMP-9 activation via NF-κB and AP-1 signaling in human breast cancer cells. Int J Oncol 41: 933-940. PubMed: 22710811. [DOI] [PubMed] [Google Scholar]
- 41. Lu KW, Chen JC, Lai TY, Yang JS, Weng SW et al. (2011) Gypenosides inhibits migration and invasion of human oral cancer SAS cells through the inhibition of matrix metalloproteinase-2 -9 and urokinase-plasminogen by ERK1/2 and NF-kappa B signaling pathways. Hum Exp Toxicol 30: 406-415. doi:10.1177/0960327110372405. PubMed: 20511288. [DOI] [PubMed] [Google Scholar]