Abstract
A shared understanding of medical conditions between patients and their health care providers may improve self-care and outcomes. In this study, the concordance between responses to a medical history self-report (MHSR) form and the corresponding provider documentation in electronic health records (EHRs) of 19 select co-morbidities and habits in 230 patients with heart failure were evaluated. Overall concordance was assessed using the κ statistic, and crude, positive, and negative agreement were determined for each condition. Concordance between MHSR and EHR varied widely for cardiovascular conditions (κ = 0.37 to 0.96), noncardiovascular conditions (κ = 0.06 to 1.00), and habits (κ = 0.26 to 0.69). Less than 80% crude agreement was seen for history of arrhythmias (72%), dyslipidemia (74%), and hypertension (79%) among cardiovascular conditions and lung disease (70%) and peripheral arterial disease (78%) for noncardiovascular conditions. Perfect agreement was observed for only 1 of the 19 conditions (human immunodeficiency virus status). Negative agreement >80% was more frequent than >80% positive agreement for a condition (15 of 19 [79%] vs 8 of 19 [42%], respectively, p = 0.02). Only 20% of patients had concordant MSHRs and EHRs for all 7 cardiovascular conditions; in 40% of patients, concordance was observed for ≤5 conditions. For noncardiovascular conditions, only 28% of MSHR-EHR pairs agreed for all 9 conditions; 37% agreed for ≤7 conditions. Cumulatively, 39% of the pairs matched for ≤15 of 19 conditions. In conclusion, there is significant variation in the perceptions of patients with heart failure compared to providers’ records of co-morbidities and habits. The root causes of this variation and its impact on outcomes need further study.
Heart failure (HF) prevalence is growing and primarily affects the elderly.1 The complex array of physiologic, psychological, social, and health care delivery issues that accompany HF make it a difficult chronic disease to manage.2 Optimal self-care behavior is important for achieving the best outcomes for chronic diseases such as HF. For patients to actively participate in their care, however, it is important for them to have a clear understanding of their health-related problems.3 This is particularly critical for patients with HF, as they tend to be older, have a higher co-morbidity burden, and often require complex treatment plans.4 From a provider perspective, medical record documentation of disease states is an essential part of care provision.5 This is especially true in the current era of increasing use of electronic health records (EHRs), as many providers communicate information exclusively through this medium.6 It may be assumed that what is documented in EHRs is the same as patients’ understanding and reporting. However, if this is not true, this discordance may lend itself to poor patient self-care behavior (related to not understanding or not reporting their conditions) or to insufficient medical care (due to misunderstanding by providers). In the current era, whether EHR entries are congruent with patients’ reporting of health-related conditions, and to what extent, is not known. In this study, we sought to assess and compare patient self-report versus EHR documentation of cardiovascular and noncardiovascular conditions and behavioral habits in patients with HF.
Methods
The data for this study were derived from patients enrolled in the Atlanta Cardiomyopathy Consortium. This prospective cohort study is enrolling patients from the Emory University Hospital, Emory University Hospital Midtown, and the Grady Memorial Hospital in Atlanta, Georgia. All patients undergo detailed medical history surveys, electrocardiography, 6-minute walk tests, standardized questionnaires, and collection of blood and urine samples at baseline. Every 6 months, patients are contacted to assess outcomes, including interim medication changes, procedures, new disease diagnoses, and hospitalizations. Mortality data are collected through medical record review, information obtained from family members, and Social Security Death Index query. The institutional review board has approved the study. At the time of this analysis, a total of 238 patients were enrolled; we included 230 of these patients (96.6%), excluding 8 patients who did not complete medical history surveys.
Research nurses abstracted data from the EHRs independently without discussion with the patients or their survey documentation. The main source of EHR data (n = 222 [96.5%]) was Emory Healthcare’s electronic medical record system, which is based on the Cerner Millennium platform (Cerner Corporation, Kansas City, Missouri). The system provides a comprehensive view of clinical data collected across hospitals and clinics. Data on 8 patients (3.5%) were collected from the EHR system at the Grady Memorial Hospital, which is based on the Siemens Medical Solutions (Malvern, Pennsylvania) Health Services platform.
All patients completed a medical history self-report (MHSR) form, which included questions regarding cardiovascular conditions (history of heart attack or myocardial infarction, high blood pressure or hypertension, high cholesterol, heart rhythm problems or arrhythmias, coronary artery bypass graft surgery, coronary stent placement, and implantable cardioverter defibrillator or pacemaker implantation), and noncardiovascular conditions (diabetes mellitus, peripheral arterial disease, pulmonary disease, liver disease, peptic ulcer disease, thyroid disease, cancer, osteoarthritis, and human immunodeficiency virus (HIV) infection). The pulmonary disease question was open ended, allowing patients to manually enter specific diagnoses. Data on history of tobacco, alcohol, and cocaine use were also obtained.
To assess the reliability of EHR data abstraction, data on 10% of the total charts, selected using a random number generator (http://www.random.org), were independently abstracted. Cumulative agreement between the 2 independent EHR data abstractions for all study variables was 93.1%.
EHR data for each condition (yes or no) were compared with the data from MHSR forms (yes or no), and concordance was assessed using the κ statistic. Crude, positive, and negative agreement were calculated to facilitate interpretation of κ values.7 Crude agreement is equal to the number of pairs that agree divided by the number of pairs available for analysis. The number of pairs available differed for each condition because of missing values in patient responses. Positive and negative agreement measures were calculated. The positive agreement measure is the ratio of total concordant positive responses over the average positive responses of patients and EHRs. The negative agreement is the ratio of total concordant negative responses over the average negative responses of patients and EHRs. Kappa statistics were interpreted as follows8: values of 0.93 to 1.00 denote almost perfect agreement, 0.81 to 0.92 very good agreement, 0.61 to 0.80 substantial agreement, 0.41 to 0.60 moderate agreement, 0.21 to 0.40 fair agreement, 0.01 to 0.20 slight agreement, and 0 no agreement. Finally, to summarize agreement by patient, the sum of the number of concordant conditions per participant was calculated. There were 7 cardiovascular and 9 noncardiovascular conditions and 3 habits included in the summary measure. McNemar’s statistic was calculated for paired comparisons. Finally, patients’ responses as “don’t know” to select conditions were captured and compared with EHR data. All analyses were performed using SAS version 9.2 (SAS Institute Inc., Cary, North Carolina).
Results
The baseline patient characteristics and treatment pattern are listed in Table 1. The mean age of patients was 56.6 ± 11.9 years; 64.5% were men, and 55.2% were white. The mean left ventricular ejection fraction was 39.3 ± 14.6%.
Table 1.
Baseline characteristics (n = 230)
| Characteristic | Value |
|---|---|
| Age (years) | 56.6 ± 11.9 |
| Male | 149 (64.8%) |
| White | 127 (55.2%) |
| Education (years) | 14.1 ± 3.1 |
| Living alone | 41 (17.9%) |
| Insured | 212 (92.2%) |
| Married | 143 (62.2%) |
| Ischemic cause of HF | 69 (31.3%) |
| Left ventricular ejection fraction (%) | 39.3 ± 14.6 |
| Systolic blood pressure (mm Hg) | 112 ± 18 |
| Diastolic blood pressure (mm Hg) | 71 ± 11 |
| Heart rate (beats/min) | 72 ± 11 |
| Creatinine (mg/dl) | 1.4 ± 1.1 |
| Sodium (mEq/L) | 138 ± 3 |
| Hemoglobin (g/dl) | 13.3 ± 1.8 |
| Brain natriuretic peptide (ng/L) | 202 (73–664) |
| β-blocker use | 219 (94.8%) |
| Angiotensin-converting enzyme inhibitor or angiotensin receptor blocker use | 197 (85.6%) |
| Defibrillator/pacemaker | 145 (64.5%) |
Data are expressed as mean ± SD, number (percentage), or median (interquartile range).
Table 2 lists the agreement data. There was fair agreement for arrhythmia history and moderate agreement for dyslipidemia and hypertension. The strongest agreement was noted for procedural care, including coronary artery bypass grafting and implantable cardioverter-defibrillator and/or pacemaker implantation. For noncardiovascular conditions, there was only fair agreement for pulmonary disease; of the 47 of 82 patients (57%) who entered specific diagnoses, there was poor agreement for chronic obstructive pulmonary disease, asthma, and sleep apnea (not listed in Table 2). There was very good agreement for cancer and diabetes mellitus and perfect agreement for HIV. For alcohol use, there was fair agreement. In 80% of the patients (12 of 15) in whom there was disagreement, the patients did not report alcohol use when the EHRs suggested histories. There was moderate agreement for cocaine and tobacco use. “Don’t know” responses were uncommon, including 13 for myocardial infarction (12 had no EHR documentation), 6 for stents and 1 for coronary bypass surgery (all with no EHR entries), and 2 for diabetes mellitus (1 had EHR documentation).
Table 2.
Agreement for cardiovascular and noncardiovascular conditions
| Co-Morbidity | Number | Yes/Yes | No/No | Yes/No | No/Yes | Crude Agreement | Positive Agreement | Negative Agreement | κ |
|---|---|---|---|---|---|---|---|---|---|
| Cardiovascular | |||||||||
| Arrhythmia | 191 | 103 | 35 | 36 | 17 | 72% | 80% | 57% | 0.37 |
| Dyslipidemia* | 223 | 94 | 71 | 34 | 24 | 74% | 76% | 71% | 0.48 |
| Hypertension | 224 | 127 | 49 | 26 | 22 | 79% | 84% | 67% | 0.51 |
| Stent | 221 | 15 | 185 | 21 | 0 | 90% | 59% | 95% | 0.54 |
| Myocardial infarction | 196 | 50 | 123 | 16 | 7 | 88% | 81% | 91% | 0.73 |
| Coronary bypass surgery | 225 | 39 | 183 | 3 | 0 | 99% | 97% | 99% | 0.95 |
| Defibrillator/pacemaker | 221 | 140 | 77 | 3 | 1 | 98% | 99% | 98% | 0.96 |
| Noncardiovascular | |||||||||
| Peptic ulcer disease | 218 | 1 | 198 | 4 | 15 | 91% | 10% | 95% | 0.06 |
| Peripheral arterial disease | 230 | 4 | 176 | 0 | 50 | 78% | 14% | 88% | 0.11 |
| Lung disease | 203 | 29 | 113 | 39 | 22 | 70% | 49% | 79% | 0.28 |
| Osteoarthritis | 215 | 10 | 174 | 23 | 8 | 86% | 39% | 92% | 0.32 |
| Liver disease | 225 | 3 | 212 | 2 | 8 | 96% | 38% | 98% | 0.36 |
| Thyroid | 214 | 26 | 174 | 7 | 7 | 93% | 79% | 96% | 0.75 |
| Cancer | 229 | 34 | 185 | 8 | 2 | 96% | 87% | 97% | 0.85 |
| Diabetes mellitus | 228 | 69 | 144 | 6 | 9 | 93% | 90% | 95% | 0.85 |
| HIV infection | 182 | 1 | 181 | 0 | 0 | 100% | 100% | 100% | 1.00 |
| Behavioral | |||||||||
| Excess alcohol use | 218 | 3 | 200 | 3 | 12 | 93% | 29% | 96% | 0.26 |
| Cocaine abuse | 224 | 11 | 198 | 14 | 1 | 93% | 59% | 96% | 0.56 |
| Tobacco | 203 | 64 | 108 | 31 | 0 | 85% | 81% | 87% | 0.69 |
Kappa p values <0.001 for all conditions except peripheral arterial disease (p = 0.006) and peptic ulcer disease (p = 0.27). All yes and no citations are by patient report first, followed by EHR documentation.
Use of lipid-lowering medications or fulfilling the National Cholesterol Education Program Adult Treatment Panel III criteria.
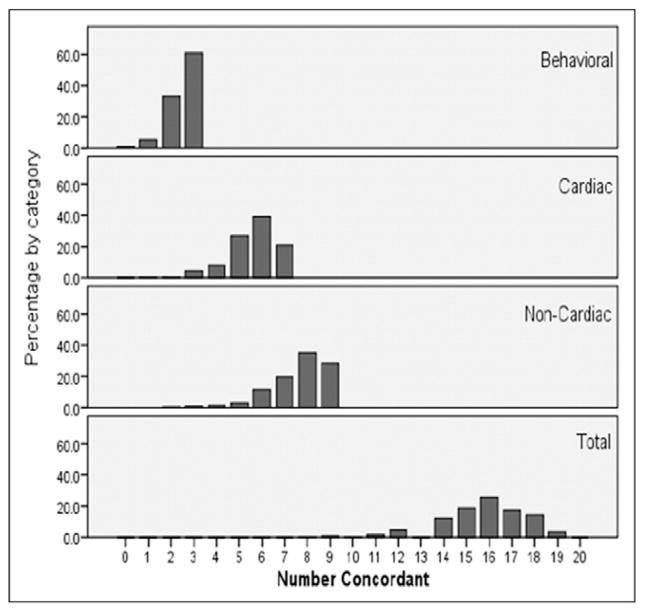
A summary measure of patient versus EHR agreement is shown in Figure 1. Of the 7 cardiovascular conditions, only 20% of patients had concordant MHSR responses and EHR entries for all conditions, and 40% agreed for ≤5 conditions. For noncardiovascular conditions, 28% agreed for all 9 conditions, and 37% agreed for ≤7 conditions. Cumulatively, 39% of the pairs matched for ≤15 of 19 co-morbidities.
Figure 1.
Summary measures of patient versus EHR concordance for co-morbidities. Suboptimal proportional concordance was noted for cardiovascular and noncardiovascular co-morbidities and for the behavioral habits assessed.
For cardiovascular conditions, positive agreement ranged from 59% (stent placement) to 99% (implantable cardioverter-defibrillator and/or pacemaker implantation). Negative agreement ranged from 57% (arrhythmias) to 99% (coronary bypass surgery). For noncardiovascular conditions, positive agreement ranged from 9.5% (peptic ulcer disease) to 100% (HIV infection), whereas negative agreement ranged from 79% (pulmonary disease) to 100% (HIV infection). Positive agreement for habits ranged from 29% for alcohol use to 81% for tobacco use, and negative agreement ranged from 87% for tobacco to 96% for alcohol and cocaine use. Negative agreement of >80% was more frequent than positive agreement (15 of 19 [79%] vs 8 of 19 [42%] conditions, respectively, p = 0.02). Approximately 40% of pairs were discordant for ≥4 conditions.
Discussion
In this study, we observed considerable variability in patient report versus EHR entry of a range of medical conditions and habits, including many conditions for which optimization of care and outcomes requires participation on behalf of patients. Agreement was expectedly better for conditions involving interventions (e.g., defibrillator implantation, coronary bypass surgery). We found better negative agreement between MHSR and EHRs (i.e., when the condition was absent) than positive (i.e., when the condition was present). These results provide insights into an understudied area of health care delivery that may influence outcomes. The accurate capture of patient information by EHRs depends on patient awareness and the documentation practices of providers; however, the effectiveness with which patient information is captured is unknown. O’Malley et al9 surveyed physicians with EHR experience, chief medical officers of EHR vendors, and thought leaders and showed a significant gap between policy makers’ expectations and clinicians’ assessments of EHR as a tool to improve care coordination.
A large portion of health care costs and hospitalizations in HF are related not only to worsening HF but also to the high burden of co-morbidities seen in these patients. Braunstein et al10 showed that 39% of patients with HF had ≥5 noncardiac co-morbidities, and only 4% had none. Importantly, patients with HF with ≥5 co-morbidities accounted for 81% of total inpatient days. Given the burgeoning cost and poor outcomes for patients with HF, increasing emphasis is being placed on nonpharmacologic care, including self-care, as a mechanism for improving outcomes.11 Patients’ recognition of their medical conditions is critical to effective self-care. Our study showed that 37% of MHSR-EHR pairs exhibited less than moderate agreement, underscoring a problem as well as an opportunity for improving care. Many conditions had agreement of <80%. There could be multiple explanations for these results (e.g., patients may not fully understand the terminology or the significance of a disease or meaning of their symptoms). Alternatively, providers may not be documenting or asking patients pertinent questions. We also observed a high discordance for history of lipid abnormalities, peripheral arterial disease, and hypertension; these co-morbidities commonly accompany HF, and patient participation is important for optimal treatment.12,13 Similarly, coexisting pulmonary disease may exacerbate or be confused with HF symptoms and affects HF prognosis and treatment options.14 Many instances of cocaine and tobacco use were not mentioned in the medical records. The inability to identify these behaviors naturally leads to inadequate patient counseling on the importance of cessation. Also, a significant segment of patients denied alcohol use that was nevertheless documented in their EHRs. Interestingly, we noted better negative (absence of disease) as opposed to positive (presence of disease) agreement. Whether this is related to perceptual challenges on behalf of patients, lack of documentation by providers, incomplete co-morbidity classification, or the variable prevalence of different disease states needs further study.
This study was limited by its size and by the fact that data were collected at a single academic medical center.
Acknowledgments
Funding: This project was funded by the Emory University Heart and Vascular Board grant titled “The Atlanta Cardiomyopathy Consortium,” and supported in part by PHS grant (UL1 RR025008, KL2 RR025009 or TL1 RR025010) from the Clinical and Translational Science Award program, National Institutes of Health, National Center for Research Resources.
References
- 1.Lloyd-Jones D, Adams R, Brown T, Carnethon M, Dai S, De Simone G, Ferguson T, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho P, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott M, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger V, Rosamond W, Sacco R, Sorlie P, Stafford R, Thom T, Wasserthiel-Smoller S, Wong N, Wylie-Rosett J. Heart disease and stroke statistics—2010 update: a report from the American Heart Association. Circulation. 2010;121:e46– e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 2.Liu L. Changes in cardiovascular hospitalization and comorbidity of heart failure in the United States: findings from the National Hospital Discharge Surveys 1980–2006. Int J Cardiol. doi: 10.1016/j.ijcard.2009.11.037. In press. [DOI] [PubMed] [Google Scholar]
- 3.Epstein R, Alper B, Quill T. Communicating evidence for participatory decision making. JAMA. 2004;291:2359–2366. doi: 10.1001/jama.291.19.2359. [DOI] [PubMed] [Google Scholar]
- 4.Baker D, Asch S, Keesey J, Brown J, Chan K, Joyce G, Keeler E. Differences in education, knowledge, self-management activities, and health outcomes for patients with heart failure cared for under the chronic disease model: the improving chronic illness care evaluation. J Card Fail. 2005;11:405–413. doi: 10.1016/j.cardfail.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 5.Bayliss EA, Ellis JL, Steiner JF. Subjective assessments of comorbidity correlate with quality of life health outcomes: initial validation of a comorbidity assessment instrument. Health Qual Life Outcomes. 2005;3:51. doi: 10.1186/1477-7525-3-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith P, Araya-Guerra R, Bublitz C, Parnes B, Dickinson L, Van Vorst R, Westfall J, Pace W. Missing clinical information during primary care visits. JAMA. 2005;293:565–571. doi: 10.1001/jama.293.5.565. [DOI] [PubMed] [Google Scholar]
- 7.Cicchetti DV, Feinstein AR. High agreement but low kappa: II. Resolving the paradoxes. J Clin Epidemiol. 1990;43:551–558. doi: 10.1016/0895-4356(90)90159-m. [DOI] [PubMed] [Google Scholar]
- 8.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- 9.O’Malley AS, Grossman JM, Cohen GR, Kemper NM, Pham HH. Are electronic medical records helpful for care coordination? Experiences of physician practices. J Gen Intern Med. 2010;25:177–185. doi: 10.1007/s11606-009-1195-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Braunstein JB, Anderson GF, Gerstenblith G, Weller W, Niefeld M, Herbert R, Wu AW. Noncardiac comorbidity increases preventable hospitalizations and mortality among Medicare beneficiaries with chronic heart failure. J Am Coll Cardiol. 2003;42:1226–1233. doi: 10.1016/s0735-1097(03)00947-1. [DOI] [PubMed] [Google Scholar]
- 11.Riegel B, Moser DK, Anker SD, Appel LJ, Dunbar SB, Grady KL, Gurvitz MZ, Havranek EP, Lee CS, Lindenfeld J, Peterson PN, Pressler SJ, Schocken DD, Whellan DJ. State of the science: promoting self-care in persons with heart failure: a scientific statement from the American Heart Association. Circulation. 2009;120:1141–1163. doi: 10.1161/CIRCULATIONAHA.109.192628. [DOI] [PubMed] [Google Scholar]
- 12.Velagaleti RS, Massaro J, Vasan RS, Robins SJ, Kannel WB, Levy D. Relations of lipid concentrations to heart failure incidence: the Framingham Heart Study. Circulation. 2009;120:2345–2351. doi: 10.1161/CIRCULATIONAHA.109.830984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kostis JB, Davis BR, Cutler J, Grimm RH, Jr, Berge KG, Cohen JD, Lacy CR, Perry HM, Jr, Blaufox MD, Wassertheil-Smoller S, Black HR, Schron E, Berkson DM, Curb JD, Smith WM, McDonald R, Applegate WB SHEP Cooperative Research Group. Prevention of heart failure by antihypertensive drug treatment in older persons with isolated systolic hypertension. JAMA. 1997;278:212–216. [PubMed] [Google Scholar]
- 14.Iversen KK, Kjaergaard J, Akkan D, Kober L, Torp-Pedersen C, Hassager C, Vestbo J, Kjoller E. The prognostic importance of lung function in patients admitted with heart failure. Eur J Heart Fail. 2010;12:685–691. doi: 10.1093/eurjhf/hfq050. [DOI] [PubMed] [Google Scholar]