Abstract
Background
Anxiety is common among patients presenting with suspected coronary artery disease (CAD). In a sample of women with signs and symptoms of ischemia, we examined three anxiety markers as predictors of CAD endpoints including: 1) cardiac symptom indicators; 2) angiographic CAD severity; and 3) healthcare utilization (cardiac hospitalizations & 5-year cardiovascular [CVD] healthcare costs).
Methods
Participants completed a baseline protocol including coronary angiogram, cardiac symptoms, psychosocial measures and a median 5.9-year follow-up to track hospitalizations. We calculated CVD costs based on cardiac hospitalizations, treatment visits, and CVD medications. Anxiety measures included anxiolytic medication use, Spielberger Trait Anxiety Inventory (STAI) scores, and anxiety disorder treatment history.
Results
The sample numbered 514 women with anxiety measure data and covariates (mean age=57.5[11.1]). One in five (20.4%) women reported using anxiolytic agents. Anxiety correlated with cardiac symptom indicators (anxiolytic use with nighttime angina & nitroglycerine use; STAI scores & anxiety disorder treatment history with nighttime angina, shortness of breath, & angina frequency). Anxiety disorder treatment history (but not STAI scores or anxiolytics) predicted less severe CAD. Anxiolytic use (but not STAI scores or anxiety disorder treatment history) predicted hospitalizations for chest pain and coronary catheterization (HR’s=2.0, 95% CI’s=1.1–4.7). Anxiety measures predicted higher 5-year CVD costs (+9.0–42.7%) irrespective of CAD severity.
Conclusions
Among women with signs and symptoms of myocardial ischemia, anxiety measures predict cardiac endpoints ranging from cardiac symptom severity to healthcare utilization. Based on these findings, anxiety may warrant greater consideration among women with suspected CAD.
Keywords: cardiovascular disease, women, prospective, anxiety, depression
Clinical anxiety and depression are often comorbid among patients with obstructive coronary artery disease (CAD). For example, in separate studies of patients with CAD in the U.S. and Europe, authors reported that between 77% and 90% of patients exhibiting either anxiety or depression also showed elevations on the alternative mood dimension (1–3). Despite this evidence of congruence, however, depression has received the majority of empirical attention in studies of patients with CAD. A 2010 review, for instance, yielded 494 independent papers on the subject of depression and cardiovascular disease (CVD) published in 2009 alone (4). Depression similarly earned consideration from the American Heart Association in 2008, with the latter body calling for routine depression screenings among patients with CAD (5). In comparison to these depression standards, anxiety boasts only a fraction of the publication volume and no official recommendations in clinical cardiology guidelines.
In light of the overlap between anxiety and depression, the growing literature linking anxiety symptoms and disorders to higher rates of cardiac risk factors (e.g., 6–7) and an increased risk for premature events (e.g., 8–9), anxiety may warrant greater scrutiny in cardiac settings. Among patients with suspected myocardial ischemia, studies of anxiety have produced seemingly paradoxical findings. For example, prior findings from the Women’s Ischemia Syndrome Evaluation (WISE) indicated a negative relationship between a history of treatment for anxiety disorders and angiographic CAD severity (10) and positive relationships between anxiety symptoms, current anxiolytic use, and CVD events such as stroke, heart attacks, congestive heart failure, and cardiac mortality (e.g., 11–13). Attempts to reconcile the role(s) of anxiety in CAD, therefore, may require a multifaceted approach that includes multiple measures of anxiety and the CAD process.
The current paper evaluated novel relationships between anxiety symptom severity, anxiolytic use, anxiety disorder treatment history (hereafter referred to as anxiety treatment history), and CAD endpoints from the WISE study, including: (1) cardiac symptom indicators; (2) angiographic CAD severity; (3) healthcare utilization in the form of cardiac hospitalization events and CVD healthcare costs. We predicted that elevated anxiety status would correlate positively with cardiac symptom indicators, correlate negatively with angiographic CAD severity, and predict increased healthcare utilization over a median 5.9 year follow-up.
Methods
Participant recruitment and entrance criteria
Women age 18 and over and undergoing a clinically indicated coronary angiogram for suspected myocardial ischemia were recruited for the WISE from four participating study sites (University of Alabama at Birmingham; University of Florida, Gainesville; University of Pittsburgh; and Allegheny General Hospital, Pittsburgh; 14). The WISE was designed to improve the understanding and diagnosis of ischemic heart disease in women. Exclusion criteria included major comorbidity compromising follow-up, pregnancy, contraindication to provocative diagnostic testing, cardiomyopathy, NYHA class IV heart failure, recent myocardial infarction or revascularization procedure, significant valvular or congenital heart disease, and language barrier. Participants provided written informed consent, and all participating sites obtained Institutional Review Board approval. The authors of this manuscript have certified that they comply with the Principles of Ethical Publishing in the International Journal of Cardiology (15).
Angiographic severity & cardiac hospitalization events
The WISE Angiographic Core Laboratory (Rhode Island Hospital, Providence, RI) performed quantitative analysis of coronary angiograms, with investigators blinded to all other subject data (16). Luminal diameter was measured at all stenoses and at nearby reference segments using an electronic cine projector-based “cross-hair” technique (Vanguard Instrument Corporation, Melville, NY). Each participant received a continuous CAD severity score based on angiogram results and a modified Gensini score (hereafter referred to as a CAD severity score; 16). This severity score was developed with points assigned according to the category of severity of the stenosis (0–19, 20–49, 50–69, 70–89, 90–98, 99–100) adjusting for partial and complete collaterals. Scores were further adjusted according to lesion location, with more proximal lesions receiving a higher weighting factor. Obstructive CAD was defined as the presence of a stenosis ≥50% in a major coronary artery (4).
The women were contacted at six weeks post-baseline and annually thereafter for a median of 5.9 years (25th percentile=2.5 years; 75th percentile=6.9 years) to track subsequent coronary catheterization, hospitalization for chest pain, and adverse events (including myocardial infarction, stroke, & congestive heart failure). Follow-up consisted of a scripted telephone interview by an experienced research nurse. This data collection tool was validated previously against medical records (17). We defined cardiac hospitalizations as either receiving cardiac catheterization or admission for chest pain (prior papers [e.g., 11–13] reported relationships with CVD events).
Cardiovascular cost accounting methods
A detailed cost method description was previously published (17). In brief, we used a hybrid cost model (including hospital-specific and published costs as inputs) for cases in which patient bills were not available. In this model, we used extensive prior published reports on procedural and hospital costs as well as cost estimates from national and regional average procedural and hospital charges (adjusted by state-specific cost-charge ratios; 17–18). Hospitalization (for chest pain, myocardial infarction [MI], heart failure) and procedural (for coronary angiography, revascularization, stress cardiac imaging) costs were obtained from published reports (17–21). CVD drug costs were derived from the 2003 Red Book (22). We performed numerous sensitivity analyses using a range of costs for procedures and hospitalizations. We totaled 5-year and annual costs for CVD hospitalizations, coronary revascularization and angiography, outpatient testing, and visits to generalists, specialists, nurse practitioners/physician’s assistants, or community clinics. Summed 5-year costs were considered a measure of direct CVD care costs, and did not include costs for mental health care. Costs were discounted with the use of a 5% annual rate, corrected for inflation by the U.S. medical service sector estimate (city average) of the consumer price index (for urban wage earners and clerical workers [23]). Each patient’s out-of-pocket expenses (i.e., indirect costs) were also collected by self-report (19). Indirect cost data were estimated for hours lost from work for healthcare, estimated reduced productivity hours, transportation costs, and out-of-pocket costs for drugs, medical devices (e.g., glucometer), and alternative therapies (e.g., vitamins).
Cardiac risk factors
All participants completed a baseline evaluation that included a physical examination with blood pressure and physical measurements, clinical interview, and a fasting blood draw for the measurement of lipids and glucose. Major cardiac risk factors in the WISE protocol included smoking status, blood pressure, dyslipidemia, diabetes, and obesity. We assessed smoking based on self-reported current versus not current smoker status. We operationally defined obesity in terms of waist-hip ratio values, with waist circumference measured at the umbilicus. Both hip and waist values were rounded to the nearest inch. For the purpose of subgroup analyses, we dichotomously defined elevated waist-to-hip ratio values ≥ .85 (13). Participants reported their use of angiotensin-converting-enzyme (ACE) inhibitors, angiotensin receptor blockers, diuretics, or vasodilator agents as indicators of antihypertensive treatment.
Cardiac symptom indicators
As part of the baseline protocol, participants completed a detailed assessment of cardiac symptoms. For the purpose of the current study, we assessed the following five cardiac symptom indicators: 1) Use of nitroglycerin for chest pain (yes/no); 2) shortness of breath (yes/no); 3) angina frequency in the prior 6-weeks (ranging from never to multiple times per day); 4) angina with stress or physical exertion (yes/no); and 5) angina at night (yes/no). Cardiac symptom categories were not mutually exclusive. In order to assess the role of participants’ perception of their disease status on results, they responded yes or no to the question “Do you think you have coronary disease?”
Psychosocial & demographic measures
Participants completed the 10-item trait anxiety subscale from the State Trait Anxiety Inventory (STAI; 24) and the 21-item Beck Depression Inventory (BDI; 25) as measures of anxiety and depressive symptom severity, respectively, at study baseline. The STAI and BDI predict objective health outcomes in cardiac populations (6). In the present sample, internal consistency values for the BDI (Cronbach’s α = .88) and STAI (Cronbach’s α = .85) indicated acceptable levels of reliability. Participants reported their use of anxiolytics (medications taken to treat anxiety) and antidepressants (medications taken to treat depression) in the week prior to study entry. Finally, participants reported their history of treatment for an anxiety disorder.
Participants’ self-reported education history served as an estimate of socioeconomic status (SES). Based upon self-reported ethnicity status, we categorized women into Caucasian (i.e., White, not of Hispanic origin) and non-Caucasian groups.
Statistical Analyses
We completed analyses separately by cardiac endpoint, including categories of cardiac symptoms, angiographic disease severity, hospitalization events, and cardiovascular costs. Using descriptive statistics, we reported means, standard deviations, and percentages of the sample across demographic, psychosocial, and CVD risk factors.
To assess the relationship between anxiety and the cardiac symptom indicators, we performed independent t-tests and Spearman/point-biserial correlations. We also used t-tests (anxiolytics, anxiety treatment history) and correlations (STAI scores) to evaluate the relationship between anxiety and angiographic disease severity indicators (maximum stenosis values, CAD severity scores, & rates of obstructive CAD; CAD severity scores were log transformed to correct for skewness) and CVD healthcare costs.
Using Cox regression, we assessed anxiety as a predictor of hospitalizations for chest pain and for cardiac catheterizations in separate models. Covariates in these models included age, ethnicity, education, menopause status, obstructive CAD status, and an ‘anxiety X obstructive CAD status’ interaction term. We also completed models adjusting for BDI scores in order to evaluate anxiety relationships independent of depression severity. All statistical analyses were completed using SPSS software, version 17.0 (SPSS Inc., Chicago, IL, USA), with significance declared at p<.05.
Results
Among 936 total participants enrolled in WISE, the current study consisted of 514 providing data regarding their anxiolytic use, STAI scores, anxiety treatment history status, and study outcome variables. Due to delayed implementation of the psychosocial questionnaires into the WISE protocol, the initial 400+ women did not complete the STAI. Relative to participants without STAI scores, the current study sample was significantly younger (57.5 vs. 59.2 years, p=.02) and had less severe CAD severity scores (13.2 vs. 16.2 p=.003). Obstructive CAD was present in 32.9% of the 514 women. Slightly more than half the sample (50.3%) reported a belief in having CAD. Angiographic evidence of CAD, however, correlated only moderately with participants belief in having CAD (r=.39, p<.001). Table 1 displays CAD risk factor and psychosocial data. Approximately 10% of women underwent a revascularization procedure during follow-up, with percutaneous coronary interventions comprising the majority (75%).
Table 1.
Descriptive summary of coronary risk factor and psychosocial variables.
| Variable | Study sample (N=514) |
|---|---|
| Age (mean[standard deviation]) | 57.5(11.1) |
| Education (%>high school) | 18.9 |
| Ethnicity (% non-Caucasian) | 17.1 |
| STAI* scores | 18.9(5.8) |
| Beck Depression Inventory scores | 10.5(8.3) |
| Anxiolytic use (% yes) | 20.4 |
| Antidepressant use (% yes) | 18.7 |
| Antihypertensive use (% yes) | 75.7 |
| % Current smoker | 19.1 |
| % Diabetic | 22.5 |
| % Dyslipidemia | 64.2 |
| % Hypertensive | 57.2 |
| % Elevated waist-hip ratio (>.85) | 41.5 |
| % History of heart disease** | 29.6 |
| % Obstructive CADΨ | 32.9 |
| % Belief in having CAD | 50.3 |
| % Revascularization during follow-upΩ | 9.9 |
STAI=Spielberger Trait Anxiety Inventory
Defined as history of myocardial infarction, coronary angioplasty, or coronary bypass surgery
Defined as ≥50% occlusion in at least one coronary artery on angiogram
Included coronary artery bypass surgery, percutaneous coronary intervention and other vascular procedures
Table 2 presents correlations among the anxiety and depression measures. Overall, the magnitude of the correlations between the three anxiety measures were small to moderate (r’s=.15–.31). A comparison of pre versus post-menopausal women (26) showed that pre-menopausal women reported higher trait anxiety scores (means 20.4[5.9] vs. 18.7[5.7), respectively, p=.01), but no differences in anxiolytic use or anxiety treatment history.
Table 2.
A summary of associations among anxiety and depression measures (N=514).
| Anxiolytic use | Anxiety treatment history | STAI scoresΨ | Antidepressant use | BDI scoresΩ | |
|---|---|---|---|---|---|
| Anxiolytic use | ----- | r=.19* | r=.15* | r=.23* | r=.15* |
| Anxiety treatment history | ----- | r=.31* | r=.26* | r=.36* | |
| STAI scores** | ----- | r=.17* | r=.69* | ||
| Antidepressant use | ----- | r=.26* | |||
| BDI scoresΩ | ----- |
p<.01
STAI=Spielberger Trait Anxiety Inventory
BDI=Beck Depression Inventory
Anxiety, cardiac symptom indicators, and angiographic disease severity
As shown in Table 3, higher anxiety as indicated by each of the three anxiety measures predicted greater cardiac symptom indicators in bivariate analyses. A positive (vs. negative) anxiety treatment history and STAI measures each predicted greater angina frequency, nighttime angina, and higher rates of shortness of breath, whereas anxiolytic use (vs. non-use) predicted higher rates of nighttime angina and nitroglycerine use.
Table 3.
Anxiety measures, cardiac symptoms, and angiographic disease severity indicators. Relationships include: 1) Spearman correlations between STAI scores, cardiac symptoms, & CAD severity; and 2) frequencies and t-tests of cardiac symptoms and CAD severity between a) anxiolytic users vs. non-users and b) those with vs. without a history of anxiety treatment.
| Cardiac symptoms | CAD severity | |||||
|---|---|---|---|---|---|---|
| Anxiety measure | Nighttime angina | Nitroglycerine use | Shortness of breath | Daily angina frequency | CAD severity score | % with obstructive CAD |
| Anxiolytic use (n=105) | 49.4%* | 56.7%* | 58.4% | 35.0% | 14.6 (13.6) | 35.6 |
| No use of anxiolytics (n=409) | 41.4% | 41.5% | 59.0% | 42.9% | 14.9 (15.0) | 32.2 |
| Anxiety treatment history (n=54) | 58.5%* | 50.9% | 72.2%* | 51.9%* | 9.5** (10.6) | 20.4* |
| No anxiety treatment history (n=456) | 38.9% | 49.7% | 57.0% | 34.7% | 14.0 (13.4) | 34.7 |
| STAI scoresΨ (n=514) | r = 0.15** | r=.04 | r = 0.16** | r = 0.10* | r= −.004 | r= −.006 |
p<.05
p<.01
STAI=Spielberger Trait Anxiety Inventory
CAD=Coronary Artery Disease
Angiographic CAD status or severity did not relate to anxiety when measured in the form of anxiolytic use or STAI scores. Relative to women without an anxiety treatment history, however, those with an anxiety treatment history showed less severe CAD in the form of CAD severity scores (9.5[10.6] vs. 14.0[13.4]) and rates of obstructive CAD (20.4% vs. 34.7%, p’s<.05). Women believing they had CAD (versus those that did not) showed significantly higher rates of anxiolytic use (27.4% vs. 13.6%, respectively, p<.001) and higher STAI symptom scores (19.6[6.0] vs. 18.2[5.5], respectively, p=.005).
Anxiety and cardiac hospitalizations
Among 514 participants, 19.8% reported at least one cardiac hospitalization for chest pain over the median 5.9 years of follow-up and 21.4% reported receiving at least one coronary catheterization. The hospitalization categories correlated significantly (r=.58, p<.001). At the bivariate level, anxiolytic users (vs. non-users) were significantly more likely to experience hospitalization for either chest pain (28% vs. 19%, p=.03) or cardiac catheterization (27% vs. 19%, p=.03). There was no statistical relationship at the bivariate level between STAI scores or anxiety treatment history with cardiac hospitalization.
Table 4 displays the results of Cox regression models using anxiety variables to predict cardiac hospitalization events. The same pattern of findings emerged for each hospitalization outcome: anxiolytic users showed a significantly greater propensity towards hospitalization outcomes in covariate-adjusted analyses, whereas STAI scores and anxiety treatment history showed no relationship. There was no evidence of an anxiety x obstructive CAD interaction. The anxiolytic use hazard ratios for coronary catheterization (HR=2.1, 95% CI=1.4–3.3) and chest pain hospitalizations (HR=2.0, 95% CI=1.3–3.1) remained significant after BDI adjustment.
Table 4.
Cox regression results (hazard ratios and 95% confidence intervals) predicting cardiac hospitalization over a median 5.9 years of follow-up for chest pain and coronary catheterization (N=514)*.
| a. Hospitalization for chest pain | b. Hospitalization for coronary catheterization | |
|---|---|---|
|
| ||
| Model 1: Anxiolytic use* | ||
| a. Obstructive CAD status** | 2.5 (1.5–4.0) | 3.0 (1.9–4.8) |
| b. Anxiolytic use | 2.0 (1.1–3.6) | 2.0 (1.1–4.7) |
| c. Anxiolytic use X CAD | 1.2 (.50–2.7) | 1.1 (.50–2.6) |
|
| ||
| Model 2: STAI scores* | ||
| a. Obstructive CAD status | 3.8 (1.3–11.7) | 2.5 (.87–7.1) |
| b. STAIΩ scores | 1.0 (.97–1.1) | 1.0 (.96–1.03) |
| c. STAI scores X CAD | .78 (.39–1.6) | 1.2 (.60–2.2) |
|
| ||
| Model 3: Anxiety tx history* | ||
| a. Obstructive CAD status | 2.8 (1.8–4.2) | 3.2 (2.2–4.9) |
| b. Anxiety tx history | 1.4 (.65–3.0) | 1.2 (.60–2.8) |
| c. Anxiety tx history X CAD | .34 (.04–2.9) | .29 (.04–2.4) |
All models included demographic covariates (age, education history, ethnicity, & menopause status)
CAD=Coronary artery disease, with non-obstructive CAD participants as the reference group;
STAI=Spielberger Trait Anxiety Inventory
Anxiety and cardiovascular costs
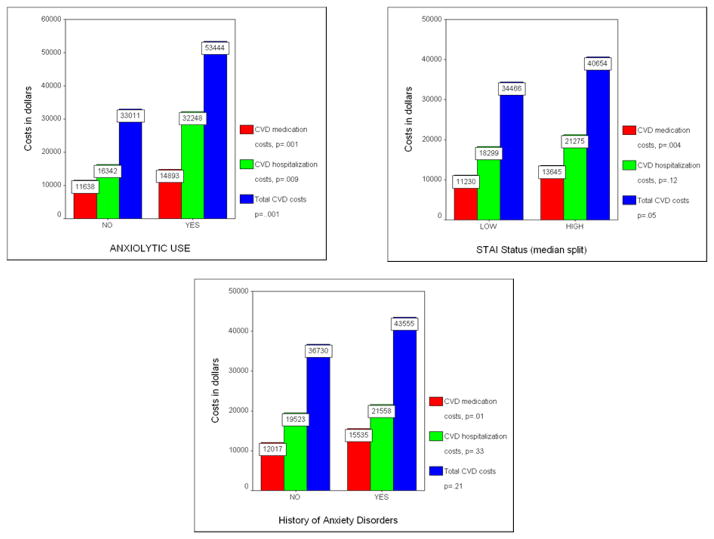
Figure 1 illustrates cardiovascular costs among women dichotomized by anxiety variables (using a median STAI value of 18.0). We further divided costs into categories of CVD medication costs, CVD hospitalization costs, and total CVD costs. At the bivariate level, anxiolytic-using women incurred significantly higher healthcare costs across all three categories relative to non-users. STAI analyses showed a similar pattern, with higher scorers showing significantly higher CVD medication costs and total CVD costs, but not CVD hospitalization costs. Anxiety treatment history predicted only higher CVD medications costs. An anxiety by CAD status interaction term was not significant. Adjusting for BDI scores had no substantial effect on the relationship between anxiolytic use and cardiovascular costs, with users remaining significantly higher (p’s<.05) across all cost categories. In contrast, relationships significant at the bivariate level between STAI scores or anxiety treatment history with cardiovascular costs were no longer significant after BDI adjustment.
Figure 1.
Five-year drug, hospital, and total cardiovascular costs for women categorized by anxiety status variables.
Discussion
Anxiety and depressive conditions correlate strongly among patients with CAD, yet depression continues to gather the majority of interest among psychosocial features of CAD. Based upon this disparity, we proposed that the clinical value of anxiety in CAD might be underappreciated. In order to explore this hypothesis, we examined relationships between three measures of anxiety and multiple CAD endpoints. Our findings supported the presence of relationships between anxiety and cardiac symptom indicators (e.g., nighttime angina, nitroglycerine use, shortness of breath, & angina frequency) and increased healthcare utilization (cardiac hospitalizations & CVD healthcare costs). Women’s beliefs about their CAD status were more strongly associated with anxiety symptoms than was their objective CAD status. These findings suggest that measures of anxiety can provide clinically valuable information concerning women presenting with suspected CAD.
Several prior publications from WISE described anxiety relationships with CAD variables different from those reported here. These prior publications reported relationships between antidepressant and anxiolytic medication use with CVD events (congestive heart failure, myocardial infarction, stroke, & total mortality; 12); independent and interactive relationships between anxiety (STAI scores) and depression (BDI scores) in the prediction of CVD events and mortality (11); a study of anxiety as part of a factor analytic effort to combine multiple psychosocial measures in predicting CVD events (13); and finally a paper reporting relationships between depression and higher CVD healthcare costs (27).
Some reviews of anxiety and CAD suggested that anxiety is less consistent as a predictor of cardiovascular outcomes compared to depression (e.g., 28). Studies, however, providing analyses specific to women reliably indicate relationships between higher levels of anxiety and adverse event rates. Results from the Nurse’s Health Study (29) and Women’s Health Initiative (30), for example, each reported increased event rates among women with higher anxiety scores. The most recent studies and meta-analyses support associations between anxiety and CAD incidence and events in both genders (9, 31–32).
Although prior studies have linked anxiety conditions to elevated healthcare costs (e.g., 33) we are unaware of prior published relationships specifically between anxiety and CVD costs. The CVD distinction is important because the increased costs reported in this paper were separate from mental health care costs, which would be predictably higher among those with higher anxiety. The direction of and mechanisms between anxiety-CAD relationships remain open to speculation. These relationships, for example, may result from cardiac symptom-induced anxiety, increased symptom sensitivity among more anxious patients (34), direct physiological changes resulting from anxiety (32), or increased treatment seeking behavior by more anxious women (35). We suspect that anxiolytic use was the most consistent anxiety predictor as it may represent a marker for greater involvement in medical care and because anxiolytics may have been prescribed in at least some cases to assist with cardiac symptoms.
Rates of anxiety disorders among women exceed the rates diagnosed in similar populations of men (36), raising concerns that anxiety symptoms may introduce biases into the process of diagnosing and treating CAD in women. Understanding how anxiety may affect the diagnosis and treatment of CAD, however, is complex and controversial. For example, some findings indicate that higher levels of anxiety among women may result in less aggressive treatment for women (e.g., 37–38), whereas others have suggested from similar patterns of data that the lower average levels of anxiety present among men may instead result in overly aggressive treatment of men (39).
Perhaps the most novel feature of the current study was the combination of anxiety measures. Although any one measure contained important limitations, the combination offered a broad assessment of anxiety indicators in a CAD context. The anxiety measures intercorrelated modestly, suggesting that they were not redundant markers. In the prediction of CAD endpoints, the anxiety measures showed both similarities (e.g., predicting similar cardiac symptom indicators & increased CVD healthcare costs) and differences (CAD severity relationships & hospitalization outcomes).
STAI scores in WISE consisted of trait anxiety items only (i.e., no state anxiety scores) because state anxiety was suspected to be inflated by women’s concerns regarding their CAD symptoms and status. STAI anxiety is not the same as phobic anxiety (e.g., 31), the latter capturing anxiety related to specific objects or situations (e.g., heights, public places). STAI trait items have content similarity with the BDI (e.g., self-confidence, self-satisfaction, feeling like a failure are themes assessed in both), explaining the high observed STAI-BDI correlation. Anxiolytic use (current treatment), surprisingly, correlated only moderately with anxiety treatment history (past treatment) and these anxiety measures showed distinct relationship patterns with the CAD variables. These findings offer insight into the practical questions of whether and how to measure anxiety in CAD contexts. Based on our results, it is possible to assess anxiety in CAD settings using any of several brief measures, and anxiety assessment can predict important aspects of both current and future CAD function. The relative choice of measure appears to depend upon the CAD outcome(s) of interest.
Limitations
Although the WISE protocol included a wealth of psychosocial data, the comprehensive measurement of mental health variables was not a study objective. For example, a substantial number of WISE participants did not complete the STAI questionnaires due to the absence of the psychosocial measures during the initial months of the study. Only at baseline were the psychological questionnaires administered, offering no means of assessing changes in the symptoms over time. We have no specific information concerning the type, dosage, or duration of the anxiolytic agents used by participants, or their effectiveness on anxiety symptoms over follow-up. The absence of more detailed anxiety treatment information leaves our findings open to different interpretations (e.g., was anxiolytic use associated with cardiac hospitalizations because it captured women with higher anxiety severity, because anxiety mimics CAD symptoms or drives treatment-seeking behavior, or because anxiety influences CAD progression?). Our methods permit only correlative, not causative conclusions and the results do not imply that anxiety interventions among patients with CAD would reverse the relationships observed. Finally, Our CAD symptom indicators did not measure functional impact as captured in measures such as the Seattle Angina Questionnaire (40).
The recruitment methodology in WISE also had a potential effect on our findings. Our sample was comprised exclusively of clinically symptomatic women, among whom about 40% showed evidence of obstructive CAD (14). These rates raise caution in comparing our results to studies with a higher prevalence of CAD or with substantial percentages of men.
Summary
In this study of women with suspected myocardial ischemia, anxiety (anxiety symptom severity, anxiety treatment history, & anxiolytic use) was associated with cardiac symptom indicators (all anxiety measures), less severe CAD (anxiety treatment history), an increased risk of cardiac hospitalizations over a median 5.9 year follow-up (anxiolytic use), and higher 5-year CVD healthcare costs (all anxiety measures). Observed relationships did not vary by CAD severity. These findings are consistent with recent anxiety-focused papers describing anxiety-event relationships in samples of women with CAD and highlight some of the specific ways in which clinicians’ attention to psychosocial aspects of their cardiac patients could potentially improve healthcare outcomes.
Acknowledgments
This work was supported by contracts from the National Heart, Lung and Blood Institutes, nos. N01-HV-68161, N01-HV-68162, N01-HV-68163, N01-HV-68164, grants U0164829, U01 HL649141, U01 HL649241, T32HL69751, 1R03AG032631 from the National Institute on Aging, GCRC grant MO1-RR00425 from the National Center for Research Resources and grants from the Gustavus and Louis Pfeiffer Research Foundation, Danville, NJ, The Women’s Guild of Cedars-Sinai Medical Center, Los Angeles, CA, The Ladies Hospital Aid Society of Western Pennsylvania, Pittsburgh, PA, and QMED, Inc., Laurence Harbor, NJ, the Edythe L. Broad Women’s Heart Research Fellowship, Cedars-Sinai Medical Center, Los Angeles, California, the Barbra Streisand Women’s Cardiovascular Research and Education Program, Cedars-Sinai Medical Center, Los Angeles and The Society for Women’s Health Research (SWHR), Washington, D.C.
Footnotes
The listed authors have no financial disclosures or conflicts of interest with the findings in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Frasure-Smith N, Lesperance F. Depression and anxiety as predictors of 2-year cardiac events in patients with stable coronary artery disease. Arch Gen Psychiatry. 2008;65:62–71. doi: 10.1001/archgenpsychiatry.2007.4. [DOI] [PubMed] [Google Scholar]
- 2.Denollet J, Strik JJ, Lousberg R, Honig A. Recognizing increased risk of depressive comorbidity after myocardial infarction: looking for 4 symptoms of anxiety-depression. Psychother Psychosom. 2006;75:346–52. doi: 10.1159/000095440. [DOI] [PubMed] [Google Scholar]
- 3.Suls J, Bunde J. Anger, anxiety, and depression as risk factors for cardiovascular disease: the problems and implications of overlapping affective dispositions. Psychol Bull. 2005 Mar;131(2):260–300. doi: 10.1037/0033-2909.131.2.260. [DOI] [PubMed] [Google Scholar]
- 4.Davidson KW, Korin MR. Depression and cardiovascular disease: selected findings, controversies, and clinical implications from 2009. Cleve Clin J Med. 2010;77 (Suppl 3):S20–6. doi: 10.3949/ccjm.77.s3.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lichtman JH, Bigger JT, Jr, Blumenthal JA, Frasure-Smith N, Kaufmann PG, Lespérance F, Mark DB, Sheps DS, Taylor CB, Froelicher ES. Depression and coronary heart disease: recommendations for screening, referral, and treatment: a science advisory from the American Heart Association Prevention Committee of the Council on Cardiovascular Nursing, Council on Clinical Cardiology, Council on Epidemiology and Prevention, and Interdisciplinary Council on Quality of Care and Outcomes Research: endorsed by the American Psychiatric Association. Circulation. 2008;118:1768–1775. doi: 10.1161/CIRCULATIONAHA.108.190769. [DOI] [PubMed] [Google Scholar]
- 6.Pajak A, Jankowski P, Kotseva K, Heidrich J, de Smedt D, De Bacquer D. Depression, anxiety, and risk factor control in patients after hospitalization for coronary heart disease: the EUROASPIRE III Study. Eur J Prev Cardiol. 2012 Mar 6; doi: 10.1177/2047487312441724. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 7.Olafiranye O, Jean-Louis G, Zizi F, Nunes J, Vincent M. Anxiety and cardiovascular risk: Review of Epidemiological and Clinical Evidence. Mind Brain. 2011;2:32–37. [PMC free article] [PubMed] [Google Scholar]
- 8.Moser DK, McKinley S, Riegel B, Doering LV, Meischke H, Pelter M, Davidson P, Baker H, Dracup K. Relationship of persistent symptoms of anxiety to morbidity and mortality outcomes in patients with coronary heart disease. Psychosom Med. 2011;73:803–9. doi: 10.1097/PSY.0b013e3182364992. [DOI] [PubMed] [Google Scholar]
- 9.Roest AM, Martens EJ, de Jonge P, Denollet J. Anxiety and risk of incident coronary heart disease: a meta-analysis. J Am Coll Cardiol. 2010;56:38–46. doi: 10.1016/j.jacc.2010.03.034. [DOI] [PubMed] [Google Scholar]
- 10.Rutledge T, Reis SE, Olson M, Owens J, Kelsey SF, Pepine CJ, Reichek N, Rogers WJ, Merz CN, Sopko G, Cornell CE, Sharaf B, Matthews KA. History of anxiety disorders is associated with a decreased likelihood of angiographic coronary artery disease in women with chest pain: the WISE study. J Am Coll Cardiol. 2001;37:780–5. doi: 10.1016/s0735-1097(00)01163-3. [DOI] [PubMed] [Google Scholar]
- 11.Rutledge T, Linke SE, Krantz DS, Johnson BD, Bittner V, Eastwood JA, Eteiba W, Pepine CJ, Vaccarino V, Francis J, Vido DA, Merz CN. Comorbid depression and anxiety symptoms as predictors of cardiovascular events: results from the NHLBI-sponsored Women’s Ischemia Syndrome Evaluation (WISE) study. Psychosom Med. 2009;71:958–64. doi: 10.1097/PSY.0b013e3181bd6062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krantz DS, Whittaker KS, Francis JL, Rutledge T, Johnson BD, Barrow G, McClure C, Sheps DS, York K, Cornell C, Bittner V, Vaccarino V, Eteiba W, Parashar S, Vido DA, Merz CN. Psychotropic medication use and risk of adverse cardiovascular events in women with suspected coronary artery disease: outcomes from the Women’s Ischemia Syndrome Evaluation (WISE) study. Heart. 2009;95:1901–6. doi: 10.1136/hrt.2009.176040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Whittaker KS, Krantz DS, Rutledge T, Johnson BD, Wawrzyniak AJ, Bittner V, Eastwood JA, Eteiba W, Cornell CE, Pepine CJ, Vido DA, Handberg E, Merz CN. Combining Psychosocial Data to Improve Prediction of Cardiovascular Disease Risk Factors and Events: The National Heart, Lung, and Blood Institute-Sponsored Women’s Ischemia Syndrome Evaluation Study. Psychosom Med. 2012;74:263–70. doi: 10.1097/PSY.0b013e31824a58ff. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bairey Merz CN, Kelsey SF, Pepine CJ, Reichek N, Reis SE, Rogers WJ, Sharaf BL, Sopko G. The Women’s Ischemia Syndrome Evaluation (WISE) study: protocol design, methodology, and feasibility report. J Am Coll Cardiol. 1999;33:1453–1461. doi: 10.1016/s0735-1097(99)00082-0. [DOI] [PubMed] [Google Scholar]
- 15.Coats AJS, Shewan LG. Statement on Authorship and Publishing Ethics in the International Journal of Cardiology. Int J Cardiol. 2011;153:239–40. doi: 10.1016/j.ijcard.2011.10.119. [DOI] [PubMed] [Google Scholar]
- 16.Sharaf BL, Pepine CJ, Kerensky RA, Reis SE, Reichek N, Rogers WJ, Sopko G, Kelsey SF, Holubkov R, Olson M, Miele NJ, Williams DO, Bairey Merz CN. Detailed angiographic analysis of women with suspected ischemic chest pain (pilot phase data from the NHLBI-sponsored Women’s Ischemia Syndrome Evaluation [WISE] study angiographic core laboratory) Am J Cardiol. 2001;87:937–941. doi: 10.1016/s0002-9149(01)01424-2. [DOI] [PubMed] [Google Scholar]
- 17.Mahoney EM, Jurkovitz CT, Chu H, Becker ER, Culler S, Kosinski AS, Robertson DH, Alexander C, Nag S, Cook JR, Demopoulos LA, DiBattiste PM, Cannon CP, Weintraub WS. Cost and cost effectiveness of an early invasive vs. conservative strategy for the treatment of unstable angina and non-ST-segment elevation myocardial infarction. JAMA. 2002;288:1851–1858. doi: 10.1001/jama.288.15.1851. [DOI] [PubMed] [Google Scholar]
- 18.Shaw LJ, Merz CN, Pepine CJ, Reis SE, Bittner V, Kip KE, Kelsey SF, Olson M, Johnson BD, Mankad S, Sharaf BL, Rogers WJ, Pohost GM, Sopko G Women’s Ischemia Syndrome Evaluation (WISE) Investigators. The economic burden of angina in women with suspected ischemic heart disease: results from the National Institutes of Health--National Heart, Lung, and Blood Institute--sponsored Women’s Ischemia Syndrome Evaluation. Circulation. 2006;114:894–904. doi: 10.1161/CIRCULATIONAHA.105.609990. [DOI] [PubMed] [Google Scholar]
- 19.Shaw LJ, Lewis JF, Hlatky MA, Hsueh WA, Kelsey SF, Klein R, Manolio TA, Sharrett AR, Tracy RP. Women’s Ischemia Syndrome Evaluation, Current Status and Future Research Directions: Report of the National Heart, Lung, and Blood Institute Workshop October 2–4, 2002: Gender-Related Risk Factors for Ischemic Heart Disease. Circulation. 2004;109:e56–e58. doi: 10.1161/01.CIR.0000116210.70548.2A. [DOI] [PubMed] [Google Scholar]
- 20.Mark DB, Hlatky MA. Medical economics in the assessment of value in cardiovascular medicine, part I. Circulation. 2002;106:516–520. doi: 10.1161/01.cir.0000021407.93752.7b. [DOI] [PubMed] [Google Scholar]
- 21.Mark DB, Hlatky MA. Medical economics in the assessment of value in cardiovascular medicine, part II. Circulation. 2002;106:626–630.30. doi: 10.1161/01.cir.0000021408.40925.63. [DOI] [PubMed] [Google Scholar]
- 22.Thomson Healthcare. [Accessed July 4, 2004];PDR bookstore. Available at: http://www.pdrbookstore.com.
- 23.US Department of Labor, Bureau of Labor Statistics. [Accessed August 16, 2006];Consumer Price Index home page. Available at: http://www.bls.gov/cpi/home.htm#data.
- 24.Spielberger CD, Gorsuch RC, Lushene RE. Manual for the State Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1970. [Google Scholar]
- 25.Beck AT. Depression inventory. Philadelphia: Center for Cognitive Therapy; 1978. [Google Scholar]
- 26.Johnson BD, Merz CN, Braunstein GD, Berga SL, Bittner V, Hodgson TK, Gierach GL, Reis SE, Vido DA, Sharaf BL, Smith KM, Sopko G, Kelsey SF. Determination of menopausal status in women: the NHLBI-sponsored Women’s Ischemia Syndrome Evaluation (WISE) Study. J Womens Health (Larchmt) 2004;13:872–87. doi: 10.1089/jwh.2004.13.872. [DOI] [PubMed] [Google Scholar]
- 27.Rutledge T, Vaccarino V, Johnson BD, Bittner V, Olson MB, Linke SE, Cornell CE, Eteiba W, Sheps DS, Francis J, Krantz DS, Bairey Merz CN, Parashar S, Handberg E, Vido DA, Shaw LJ. Depression and cardiovascular health care costs among women with suspected myocardial ischemia: prospective results from the WISE (Women’s Ischemia Syndrome Evaluation) Study. J Am Coll Cardiol. 2009;53:176–83. doi: 10.1016/j.jacc.2008.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suls J, Bunde J. Anger, anxiety, and depression as risk factors for cardiovascular disease: the problems and implications of overlapping affective dispositions. Psychol Bull. 2005;131:260–300. doi: 10.1037/0033-2909.131.2.260. [DOI] [PubMed] [Google Scholar]
- 29.Albert CM, Chae CU, Rexrode KM, Manson JE, Kawachi I. Phobic anxiety and risk of coronary heart disease and sudden cardiac death among women. Circulation. 2005;111(4):480–7. doi: 10.1161/01.CIR.0000153813.64165.5D. [DOI] [PubMed] [Google Scholar]
- 30.Smoller JW, Pollack MH, Wassertheil-Smoller S, Jackson RD, Oberman A, Wong ND, Sheps D. Panic attacks and risk of incident cardiovascular events among postmenopausal women in the Women’s Health Initiative Observational Study. Arch Gen Psych. 2007;64:1153–1160. doi: 10.1001/archpsyc.64.10.1153. [DOI] [PubMed] [Google Scholar]
- 31.Watkins LL, Blumenthal JA, Babyak MA, Davidson JR, McCants CB, Jr, O’Connor C, Sketch MH., Jr Phobic anxiety and increased risk of mortality in coronary heart disease. Psychosom Med. 2010;72:664–71. doi: 10.1097/PSY.0b013e3181e9f357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roest AM, Martens EJ, Denollet J, de Jonge P. Prognostic association of anxiety post myocardial infarction with mortality and new cardiac events: a meta-analysis. Psychosom Med. 2010;72:563–9. doi: 10.1097/PSY.0b013e3181dbff97. [DOI] [PubMed] [Google Scholar]
- 33.Vasiliadis HM, Dionne PA, Préville M, Gentil L, Berbiche D, Latimer E. The Excess Healthcare Costs Associated With Depression and Anxiety in Elderly Living in the Community. Am J Geriatr Psychiatry. 2012 Apr 10; doi: 10.1016/j.jagp.2012.12.016. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 34.Valeriani M, Sestito A, Le Pera D, De Armas L, Infusino F, Maiese T, Sgueglia GA, Tonali PA, Crea F, Restuccia D, Lanza GA. Abnormal cortical pain processing in patients with cardiac syndrome X. Eur Heart J. 2005;26:975–82. doi: 10.1093/eurheartj/ehi229. [DOI] [PubMed] [Google Scholar]
- 35.Asbury EA, Creed F, Collins P. Distinct psychosocial differences between women with coronary heart disease and cardiac syndrome X. Eur Heart J. 2004 Oct;25(19):1695–701. doi: 10.1016/j.ehj.2004.07.035. [DOI] [PubMed] [Google Scholar]
- 36.Gater R, Tansella M, Korten A, Tiemens BG, Mavreas VG, Olatawura MO. Sex differences in the prevalence and detection of depressive and anxiety disorders in general health care settings: report from the World Health Organization Collaborative Study on Psychological Problems in General Health Care. Arch Gen Psychiatry. 1998;55:405–13. doi: 10.1001/archpsyc.55.5.405. [DOI] [PubMed] [Google Scholar]
- 37.Chiaramonte GR, Friend R. Medical students’ and residents’ gender bias in the diagnosis, treatment, and interpretation of coronary heart disease symptoms. Health Psychol. 2006;25:255–66. doi: 10.1037/0278-6133.25.3.255. [DOI] [PubMed] [Google Scholar]
- 38.Steingart RM, Packer M, Hamm P, Coglianese ME, Gersh B, Geltman EM, Sollano J, Katz S, Moyé L, Basta LL, Lewis SJ, Gottlieb SS, Bernstein V, McEwan P, Jacobsen K, Brown EJ, Kukin ML, Kantrowitz NE, Pfeffe MA. Sex differences in the management of coronary artery disease. Survival and Ventricular Enlargement Investigators. N Engl J Med. 1991;325:226–30. doi: 10.1056/NEJM199107253250402. [DOI] [PubMed] [Google Scholar]
- 39.Bösner S, Haasenritter J, Hani MA, Keller H, Sönnichsen AC, Karatolios K, Schaefer JR, Baum E, Donner-Banzhoff N. Gender bias revisited: new insights on the differential management of chest pain. BMC Fam Pract. 2011;12:45. doi: 10.1186/1471-2296-12-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Spertus JA, Winder JA, Dewhurst TA, Deyo RA, Prodzinski J, McDonell M, Fihn SD. Development and evaluation of the Seattle Angina Questionnaire: a new functional status measure for coronary artery disease. J Am Coll Cardiol. 1995;25:333–41. doi: 10.1016/0735-1097(94)00397-9. [DOI] [PubMed] [Google Scholar]