Summary
All movements are thought to be ‘prepared’ in the brain before initiation [1–3], and preparation can be impaired in motor diseases [4, 5]. However, little is known about what sort of preparation precedes self-initiated, naturally-learned sequences of movements. Here we took advantage of a canonical example of a precisely timed learned motor sequence, adult zebra finch song, to examine motor preparation. We found that the sequences of short vocalizations or introductory notes (INs) preceding song gradually increased in speed and converged on an acoustic end point highly similar across renditions, just before song initiation. The more the initial IN differed acoustically from the final IN, the greater the number of INs produced pre-song. Moreover, the song premotor nucleus HVC exhibited IN-related neural activity that progressed to a distinctive end-point immediately before song. Together, our behavioral and neural data suggest that INs reflect a variable period of preparation during which the brain attains a common ‘ready’ state each time sequence generation is about to begin.
Results
Preparation in the brain before movement [1–3, 6, 7] is believed to be important for movement initiation, and diseases like Parkinson’s disease and speech apraxia include initiation defects [4, 5]. Thus, understanding preparation could provide insight into how movements initiate or fail to do so. Preparation before cue-triggered movements and movement sequences has been investigated extensively in primates. Early studies showed that disrupting preparatory neural activity delayed movements [1, 2], and suggested that this activity was sub-threshold movement-related activity [8–11]. Recent studies propose instead that preparatory activity is best explained as motor circuit dynamics converging onto an internal state required for movement generation, without a clear relationship to movement parameters [12–14].
In addition to triggered movements, there exist naturally-learned movement sequences, like the dives of Olympians or basketball players’ free throws, that are self-initiated without explicit external triggers. These movement sequences are highly stereotyped and almost habit-like as a result of extensive practice. The preparation before such self-initiated, naturally-learned movement sequences remains poorly understood. The songs of adult zebra finches are also precise learned motor sequences, with similarities to human speech [15–17]. Although birds sing to court females, they sing when alone as well, showing that song can begin without external triggers. Thus, adult zebra finch song provides a readily quantifiable example of a self-initiated, naturally-learned movement sequence.
A consistent feature of virtually all zebra finch songs is the production of short introductory notes (INs), prior to singing one or more repetitions of the core learned portion of song (the ‘motif’, Fig. 1A). Here, to test whether INs might represent motor preparation, we analyzed the properties of INs and how they transition to song, as well as the accompanying IN-related neural activity from the song premotor nucleus HVC.
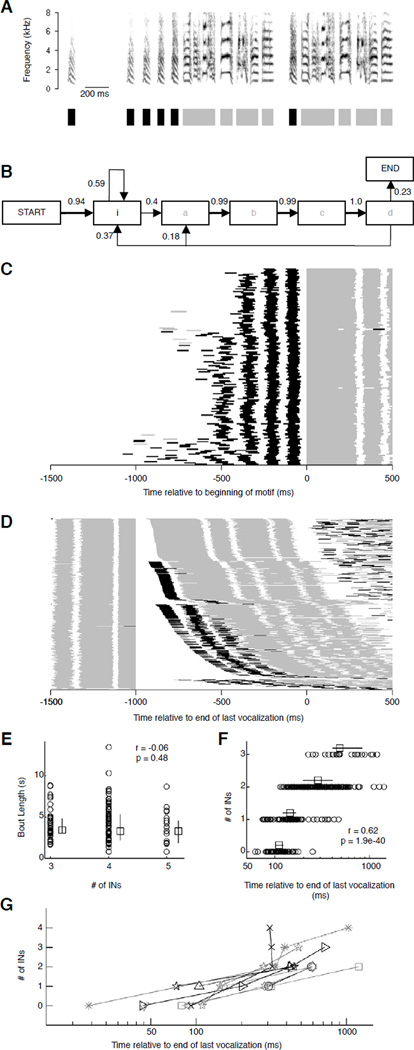
Figure 1.
Number of introductory notes varies and can depend on the time since last vocalization. (A) Spectrogram of one song bout (top) with 5 introductory notes (INs, ‘i’) before the bird's characteristic motif comprising 4 syllables (‘a’, ‘b’, ‘c’ and ‘d’) separated by gaps. To visualize INs across multiple bouts, we generated an IN/Non-IN representation (bottom) where syllables and gaps were replaced with appropriately timed colored bars: black (INs), gray (non-INs), and white (silent periods). (B) Syllable transition diagram generated from 157 song bouts (same bird as in A). Nodes represent syllables, direction and thickness of arrows between nodes represent syllable transitions and associated probabilities (numbers above arrows also represent transition probabilities). Calls have been omitted for clarity, and account for all missing transitions. (C) IN/Non-IN representation of the beginning of 157 song bouts from one bird, each aligned at motif onset (t=0). Bouts are arranged in ascending order of number of INs. (Small gray bars seen in (C) and (D) before IN sequences on some trials are calls that differ from INs in their acoustic structure – see Fig. S1D,E). (D) Representation of 379 motifs aligned to the end of the previous motif (t=0). Motifs are arranged in ascending order of number of INs, and within groups with identical numbers of INs, in ascending order of the silent interval since the last vocalization. (E) Bout length vs. number of INs at bout onset (one bird). Circles represent individual bouts; squares and error bars represent median and inter-quartile range. (F) Number of INs vs. the interval since the last vocalization (same bird as in D). Circles represent individual bouts, squares and error bars represent median values and inter-quartile range. (G) Number of INs vs. interval since the last vocalization (all birds). Symbols represent median values for each bird. Half of the birds are plotted in a different color for clarity. See also Figure S1.
Analysis of undirected song
We recorded and analyzed self-initiated, ‘undirected’ song bouts from eleven adult male zebra finches (median song bouts/finch:153; range:69–175). Bouts typically began with a variable number of INs preceding the motif (Fig. 1A). Although motif sequence was highly stereotyped, motif initiation could be preceded either by a variable number of INs or by the last syllable of the previous motif (Fig. 1B). Motifs at bout onsets were almost always preceded by INs (Figs. 1C,S1A), but the number of these INs was not correlated with bout length (Fig. 1E) (p>0.05, 9/11 birds). However, the number of INs within bouts was strongly correlated with the duration of silence following the end of the previous motif (Fig. 1 D,F) (p<0.05, 10/11 birds; mean r=0.70, range: 0.37–0.90; Fig 1G), suggesting that the next motif requires more INs if it is sung after a longer silence.
Sequences of INs speed up and reach a similar acoustic end-point across renditions
Because the number of INs before each motif varies, we hypothesized that sequences of INs reach a similar last IN before motif initiation (Fig. 2A). To evaluate this, we characterized IN sequences using two measures: 1) IN sequence timing as measured by the gap between successive INs, and 2) the acoustic properties of individual INs.
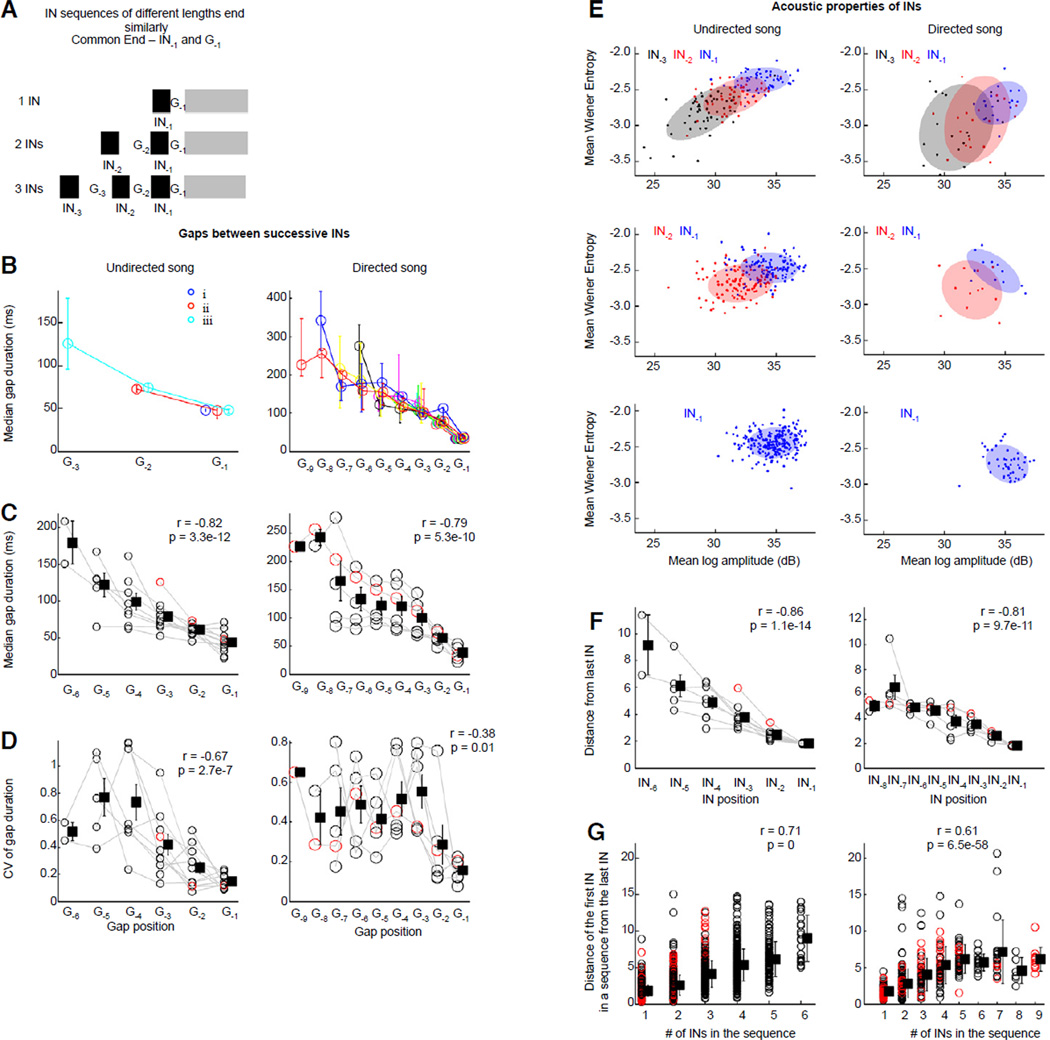
Figure 2.
Intervals between INs become shorter and INs converge on similar acoustic structure across trials. (A) Gap and IN position (white and black bars) in each sequence was represented relative to a common end-point, the gap between the last IN and the motif, called G−1, and the last IN before motif onset, called IN−1. (B) Median gap duration (one bird) vs. gap position across IN sequences of different lengths; undirected, left; n=11 birds, and directed song, right; n=6 birds. Each color represents IN sequences of a particular length, and circles and bars represent median values and inter-quartile range. Values have been slightly offset along the x-axis for clarity. (C, D) Median gap duration (C) and variability of gap duration (D) are negatively correlated with gap position for both undirected, left and directed song, right. Squares and error bars represent mean and s.e.m. Black circles and gray lines represent individual birds, red circles represent bird in B. (E) Two acoustic features (mean log amplitude vs. mean wiener entropy) of INs illustrate the convergence on a similar end state for IN sequences of different lengths (data from one bird; undirected, left, directed song, right). Blue, red, and black symbols represent IN−1, IN−2, IN−3, respectively, and ellipses represent 1 std. from the mean. (F) Acoustic distance (Mahalanobis distance) from the last IN is negatively correlated with IN position, for both undirected (left) and directed song (right). Conventions as in C,D; bird in E shown as red circles here and in G. (G) Acoustic distance of the first IN in a sequence from the last IN was positively correlated with the number of INs in that sequence (undirected, left; directed song, right). Black circles represent individual sequences from all birds, squares represent mean and 1 std across all birds.
Intervals between successive INs become shorter and more stereotyped close to motif onset
We found that gap duration within each song sequence decreased as the sequence progressed towards the last gap, which we called G−1 (Fig, 2A, B, left; p < 0.05 for 10/11 birds, Wilcoxon sign-rank test; mean decrement across consecutive gaps: 23.8%; range: 11.4%–46.7%). Moreover, in each bird, despite a variable number of INs before individual motifs, the gap at a particular position relative to G−1 was highly similar in duration (Fig. 2B, left). Across all birds, median gap duration became shorter closer to motif onset, and variability of gap duration across renditions decreased (Fig. 2 C,D left).
Acoustic properties of INs converge onto a highly similar last IN across motif renditions
We next asked whether the acoustic properties of INs changed as each IN sequence progressed to the last IN, which we called IN−1 (Fig. 2A). To characterize acoustic properties, we used four different features ([18]; see Supplemental Experimental Procedures): duration, log amplitude, entropy and mean frequency. Across all birds, INs became shorter, louder, higher in mean frequency, and changed their entropy (p<0.05 for 9–11/11 birds for all features, Fig.2E) as IN sequences progressed towards motif onset.
To examine whether these changes converged on an IN−1 with similar acoustic properties across renditions, we carried out all further analysis in the 4-dimensional space formed by these features. Regardless of the number of INs in a sequence, we found that acoustic properties were similar for INs at a given position relative to IN−1 (Fig. 2E, left; one bird). We quantified this using the mean Mahalanobis distance of all INs at a given position from the centroid of the cluster of all last INs for each bird. Across all birds, this distance was strongly correlated with IN position (Fig. 2F, left). Conversely, the further the acoustic distance of the first IN was from the cluster of all last INs, the greater the number of INs before motif onset (Fig. 2G, left).
These findings show that despite different initial conditions, variable numbers of INs ensured that motifs always started from a common pre-motif state, suggesting that IN sequences reflect ‘preparation’ before motif initiation.
Analysis of similarity between INs and song
In addition to being motif preparation, INs might influence the quality of subsequent song. To test this, we used the acoustic distance between pairs of syllables as a similarity measure, and asked if similar last INs preceded similar song. In all birds, we found significant, albeit weak, correlations between the similarity of last IN pairs and the similarity of subsequent first motif syllables (n=11 birds, p<0.05, mean r=0.25+/−0.04; range:0.1–0.4). Thus, while INs are not obligatory for motif production, as evidenced by motifs without INs within bouts, when present they not only prepare for singing, but may also influence subsequent song. Further support for this idea will require experiments disrupting the last IN state.
Analysis of INs during courtship song
To test further our hypothesis that IN sequences represent preparation, we asked if the large number of INs seen before female-directed song (Fig. S1C) [19] might reflect more preparation. We analyzed courtship song for 6/11 birds used for undirected song analysis. Similar to undirected song, the gaps between successive INs in directed song became shorter and more stereotyped (Fig. 2B-D, right). However, gap durations were longer at the beginning of directed IN sequences compared to undirected IN sequences, consistent with the idea that directed song requires more preparation. Acoustic properties of directed song INs also converged on a similar pre-motif state across renditions (Fig. 2E, right, one bird). Similar to undirected song, the acoustic distance from the last IN was strongly correlated with IN position (Fig. 2F, right) and the acoustic distance of the first IN from the mean last IN was correlated with the number of INs produced (Fig. 2G, right). Together, these data suggest that the large number of INs before directed song, triggered by the unexpected appearance of a female, reflects a requirement for more preparation for convergence on a common pre-motif state.
Neural correlates of ‘preparation’ in premotor nucleus HVC
To examine neural correlates of the apparent preparation during INs, we recorded extracellular activity (n=46 single-units, mean SNR=6.84+/−0.17; range 4.93- 9.92; and n=23 multi-unit sites from 6 birds) in the premotor nucleus HVC of adult male zebra finches during undirected singing (Fig. 3A). We recorded from HVC because it is required for normal motif production [20–22], and asked how its activity related to the progression of INs pre-song.
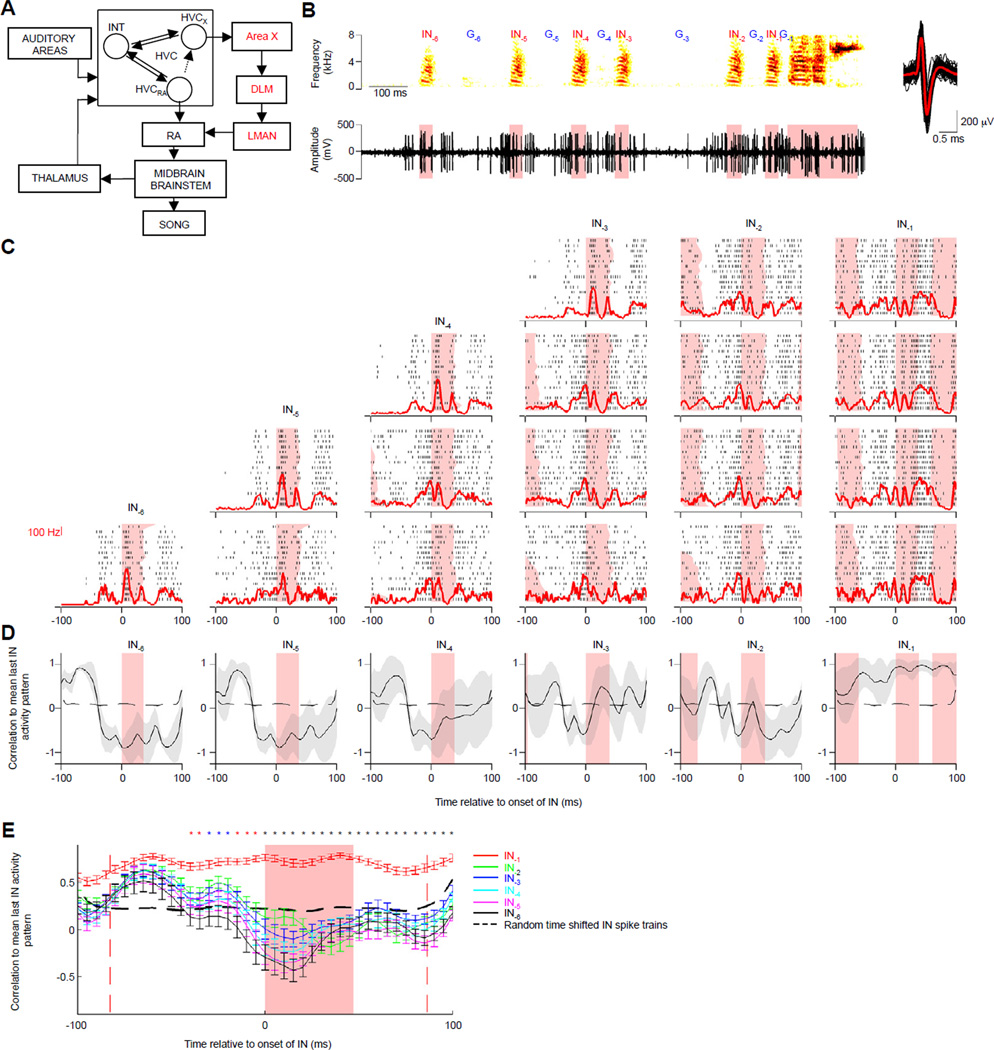
Figure 3.
Neural activity of HVC interneurons progresses towards a distinct common state before the last IN. (A) Schematic of the song neural pathways, including a motor pathway (black), and a basal ganglia-thalamus-‘cortex’ loop (red) specialized for song. HVC contains at least 3 different types of interconnected neurons: HVCRA and HVCX projection neurons, and interneurons. Other important feedback loops through HVC are also shown. (B) Activity of a single HVC interneuron during the production of an IN sequence. Top: spectrogram, Bottom: extracellular voltage trace, Right: 100 example spike waveforms (black), average spike waveform (red). Signal-to-noise ratio: 6.89. Syllable durations highlighted in pink. (C) Raster plots of spiking activity for interneuron in B during IN sequences of different lengths. The 200ms analysis window was centered on IN onset (t=0, IN durations in pink). Each row of raster plots represents activity during INs from an IN sequence of a specific length, with peri-IN time histograms of neural activity in red overlaid on each plot. (D) Correlation to mean last IN firing pattern as a function of IN position for the neuron in C. Black traces and gray shading represent mean and 1 standard deviation for the correlations at a particular IN position. The black dashed line represents a measure of random correlation, IN durations are in pink. (E) Correlation to mean last IN firing pattern as a function of time within the 200ms analysis window is shown for different IN positions for all neurons. Each trace represents the mean and s.e.m of the correlation between the firing pattern of INs at a particular position and the mean firing pattern across all last INs. Different colors represent different IN positions as shown in the legend. The red dashed lines before IN onset and after IN offset represent respectively the mean offset time of IN−2, and the mean onset time of the first motif syllable on all last IN trials. For INs at all other positions, the 200ms analysis window typically did not include the offset of the previous syllable nor the onset of the next IN. The black dashed line represents the mean random correlation across all neurons. Because the change in IN firing pattern across INs was progressive in some neurons, the last IN similarity was not different at all time points from every other IN-last IN comparison: different color asterisks represent time-points where the last IN correlation was significantly different from 2 (*) , 3 or more (*) or all (*) of the other IN curves (p < 0.005, Kruskal Wallis analysis of variance, post-hoc Tukey Kramer criterion). See also Figure S2.
One class of HVC neurons projects to motor nucleus RA (HVCRA neurons), and is critical to motif production. These neurons are difficult to record, and only a small fraction are active during IN production [23, 24], so our data set did not include HVCRA recordings with IN-related activity. Instead we analyzed recordings from single neurons projecting to Area X (HVCX neurons; n=30/46), and from putative interneurons (n=16/46; see Supplemental Experimental Procedures), which are thought to represent a population read-out of the activity of HVCRA neurons. Multi-unit sites (n=23), which are likely to be dominated by high interneuron firing rates, were included with single interneurons.
HVC Interneurons
Interneurons were active during IN sequences (Fig. 3B), with a significantly higher mean firing rate than baseline before an IN (Fig. S2B, p<0.05, Kruskal-Wallis analysis of variance, post-hoc Tukey-Kramer criterion).
We examined the progression of neural activity (in a 200ms window centered on IN onset) over the course of IN sequences (Fig. 3C). We first calculated the similarity between activity patterns of all pairs of last INs, and found that they were strongly correlated with each other (r-value;mean+/−s.e.m=0.68+/−0.02; 39 sites; correlation significantly different than that expected by chance: r-value; mean+/−s.e.m=0.01+/−0.01; p<0.05, M-W test for each neuron). Neuronal activity patterns during earlier INs at a given position relative to IN−1 were also strongly correlated with each other (r-value;mean+/−s.e.m=0.68+/−0.03; 39 sites; p<0.05). Thus, neural activity patterns are stereotyped at each position, including a highly similar state during each last IN, regardless of the number of preceding INs.
We then asked how neuronal activity during earlier INs compared to activity during last INs, by calculating the similarity between the firing pattern during each IN and the mean firing pattern during all last INs (see Supplemental Experimental Procedures). Starting 40ms before IN onset (approximate HVC premotor latency), the firing pattern during the last IN was significantly different from the firing patterns at earlier INs (Fig. 3D,E; p<0.005, Kruskal-Wallis analysis of variance, post-hoc Tukey-Kramer criterion; see Supplemental Experimental Procedures for details of statistics used). Thus, for many HVC interneurons, activity reached a distinct state for the last IN and was highly similar across renditions.
HVCX neurons
HVCX neurons typically burst only at specific times during song ([23–26]) and are thought to provide an efference copy of motor commands to song basal ganglia (Area X). Given the importance of mammalian basal ganglia for movement initiation [27], we asked if the firing of HVCX neurons (n=30; 12 antidromically identified, 18 putative; see Supplemental Experimental Procedures) provided information about IN sequence progression and motif initiation.
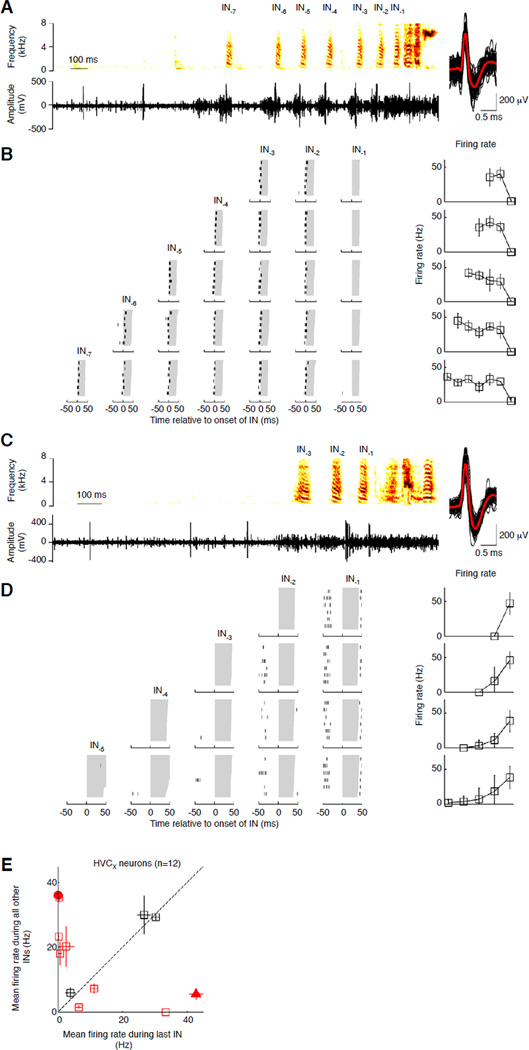
A subset of our HVCX neurons (n=12/30; 4 identified, 8 putative) produced single spikes and/or sparse high-frequency bursts during INs. Since these neurons only changed their firing rate without changing burst location, we used a window large enough to include the burst (a 100ms window centered on IN onset) to analyze firing rate. HVCX neurons showed large firing rate changes for the last IN (Figs. 4A-D). Across all neurons we found that the firing rate for the last IN was significantly different from the distribution of firing rates for all other preceding INs for 9/12 neurons (Fig. 4E, red symbols; p<0.05, Kruskal-Wallis analysis of variance). Further, in 4/9 neurons, the firing rate for the last IN was significantly different from the firing rate at each preceding IN position (p<0.05, Kruskal-Wallis analysis of variance, post-hoc Tukey-Kramer criterion), reflecting a unique representation for the last IN. For the other 5 neurons the firing rate during the last IN was significantly different from the firing rate at a subset of preceding IN positions (p<0.05). Finally, a smaller subset of HVCX neurons (n=3/12) had equal activity for all INs irrespective of position and sequence length (Fig. S3A, Fig. 4E, black squares). Thus, a majority of the neurons that we recorded represented the last IN differently from all other INs, providing song basal ganglia with a signal about a key behavioral transition from INs to song, independent of the number of INs. Similarly, recent work in Bengalese finches suggests that HVCX neuron firing rates can encode motif sequence-related information [26].
Figure 4.
HVCX projecting neurons fire differently for the last IN. (A, C) Activity of a putative HVCX neuron (A) and an antidromically identified HVCX neuron (C) during the production of one IN sequence. Conventions as in Fig. 3B. Signal-to-noise ratio: 5.63 (A); 8.00 (C). (B, D) Raster plots (left) and mean firing rates (right) for the HVCX neurons shown in A and C respectively during the production of IN sequences of different lengths. Activity was characterized in a 100ms window centered on IN onset; IN durations in gray. Each row of raster plots represents activity during INs from an IN sequence of a specific length, with the mean firing rate as a function of IN position (right). Error bars represent 1 std. (E) Comparison of mean firing rate (+/− s.e.m.) for the last IN vs. all other INs for all HVCX neurons (n=12). Red symbols represent neurons with significant differences (p < 0.05, Kruskal-Wallis analysis of variance), black symbols non-significant differences. The neuron in 4A,B is the red circle, and the neuron in 4C,D the red triangle. See also Figure S3.
Discussion
Here we show that IN sequences preceding the motif of an adult finch progress towards a common final acoustic state across motif renditions, with more INs if the initial IN is more acoustically distant from the final state. IN-related neural activity in a song premotor nucleus also reached a distinct common final state before the last IN. These findings suggest that INs reflect preparation before motif initiation, ensuring that motifs always begin in the same state, regardless of initial conditions. Consistent with the preparation hypothesis, the greater number of INs before courtship song, sung in response to the unexpected appearance of a female, was associated with an initial state farther away from the pre-motif state and a slower process of converging on the final state. While neural preparation before movements has been shown in many organisms [28, 29], this preparation remains poorly understood. Recent primate studies suggest that it reflects neural dynamics during the progression from resting to movement initiation state [12, 13]. While this has been inferred from neural activity, our results extend these findings to show that both behavioral and neural properties converge on a highly similar state across renditions before initiation of naturally-learned motor sequences. Unlike the hypothesized neural trajectory through space to a ready point [12, 13], the repetitive nature of INs suggests that preparation can involve repeating certain motor gestures until the state is ready.
What neural mechanisms might underlie ‘readiness’? Complex motor behaviors require the orderly and coordinated firing of multiple brain areas, within and across hemispheres. The songbird’s repeated introductory vocalizations may be a mechanism for achieving this coordination before song, by repeatedly activating loops between HVC and respiratory or auditory centers (Fig. 3A), and across hemispheres. Consistent with this idea, both deafening and disruptions of inter-hemispheric coordination result in a marked increase in the number of INs, with failure to progress to song when bilateral synchronization is impeded [30–34].
Regardless of mechanism, songbirds, with their strings of repeated notes before a complex learned motor sequence, provide a highly tractable system for studying motor preparation. By analogy to our birds, the ball-bouncing of basketball players before their free throws may represent not irrelevant habit or superstition, but a useful set of movements by which the brain prepares to execute a practiced motor skill.
Experimental Procedures
Experiments were performed in accordance with NIH guidelines and were approved by the UCSF IACUC.
Supplementary Material
Highlights.
Sequences of introductory notes (INs) converge on a pre-song state across renditions
Acoustic distance of the first IN from pre-song state is correlated with IN number
The final pre-song state has a distinctive representation in premotor nucleus HVC
INs that precede zebra finch song may reflect ‘preparation’ for song initiation
Acknowledgments
We thank Michael Brainard, Philip Sabes, Upinder Bhalla, Loren Frank, Steve Lisberger, Kris Bouchard, Alex Kozhevnikov, members of the Doupe lab and three anonymous reviewers for their helpful discussion and comments on the manuscript. This study was supported by an HFSP Long Term Fellowship (LT00759/2007-L) to RR, and NIH grant MH55987 to AJD.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplemental Information
Supplemental information includes Supplemental Figures, Figure Legends and Experimental Procedures.
References
- 1.Day BL, Rothwell JC, Thompson PD, De Noordhout AM, Nakashima K, Shannon K, Marsden CD. Delay In The Execution Of Voluntary Movement By Electrical Or Magnetic Brain Stimulation In Intact Man Evicence For The Storage Of Motor Programs In The Brain. Brain. 1989;112:649–663. doi: 10.1093/brain/112.3.649. [DOI] [PubMed] [Google Scholar]
- 2.Ghez C, Hening W, Gordon J. Organization of voluntary movement. Curr. Opin. Neurobiol. 1991;1:664–671. doi: 10.1016/s0959-4388(05)80046-7. [DOI] [PubMed] [Google Scholar]
- 3.Ackermann H, Ziegler W. Brain Mechanisms Underlying Speech Motor Control. In: Hardcastle WJ, Laver J, Gibbon FE, editors. The Handbook of Phonetic Sciences. Blackwell Publishing Ltd.; [Accessed April 12, 2012]. pp. 202–250. Available at: http://onlinelibrary.wiley.com/doi/10.1002/9781444317251.ch6/summary. [Google Scholar]
- 4.Cunnington R, Iansek R, Johnson KA, Bradshaw JL. Movement-related potentials in Parkinson’s disease. Motor imagery and movement preparation. Brain. 1997;120:1339–1353. doi: 10.1093/brain/120.8.1339. [DOI] [PubMed] [Google Scholar]
- 5.Kent RD. Research on speech motor control and its disorders: a review and prospective. Journal of Communication disorders. 2000;33:391–428. doi: 10.1016/s0021-9924(00)00023-x. [DOI] [PubMed] [Google Scholar]
- 6.Kutas M, Donchin E. Preparation to respond as manifested by movement-related brain potentials. Brain research. 1980;202:95–115. [PubMed] [Google Scholar]
- 7.Rosenbaum DA. Human movement initiation: Specification of arm, direction, and extent. Journal of Experimental Psychology: General; Journal of Experimental Psychology: General. 1980;109:444. doi: 10.1037//0096-3445.109.4.444. [DOI] [PubMed] [Google Scholar]
- 8.Tanji J, Evarts EV. Anticipatory activity of motor cortex neurons in relation to direction of an intended movement. Journal of Neurophysiology. 1976;39:1062–1068. doi: 10.1152/jn.1976.39.5.1062. [DOI] [PubMed] [Google Scholar]
- 9.Riehle A, Requin J. Monkey primary motor and premotor cortex: single-cell activity related to prior information about direction and extent of an intended movement. Journal of Neurophysiology. 1989;61:534–549. doi: 10.1152/jn.1989.61.3.534. [DOI] [PubMed] [Google Scholar]
- 10.Messier J, Kalaska JF. Covariation of primate dorsal premotor cell activity with direction and amplitude during a memorized-delay reaching task. Journal of Neurophysiology. 2000;84:152–165. doi: 10.1152/jn.2000.84.1.152. [DOI] [PubMed] [Google Scholar]
- 11.Churchland MM, Santhanam G, Shenoy KV. Preparatory activity in premotor and motor cortex reflects the speed of the upcoming reach. J Neurophysiol. 2006;96:3130–3146. doi: 10.1152/jn.00307.2006. [DOI] [PubMed] [Google Scholar]
- 12.Churchland MM, Cunningham JP, Kaufman MT, Ryu SI, Shenoy KV. Cortical preparatory activity: representation of movement or first cog in a dynamical machine? Neuron. 2010;68:387–400. doi: 10.1016/j.neuron.2010.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shenoy KV, Kaufman MT, Sahani M, Churchland MM. A dynamical systems view of motor preparation: implications for neural prosthetic system design. Prog Brain Res. 2011;192:33–58. doi: 10.1016/B978-0-444-53355-5.00003-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Afshar A, Santhanam G, Yu BM, Ryu SI, Sahani M, Shenoy KV. Single-trial neural correlates of arm movement preparation. Neuron. 2011;71:555–564. doi: 10.1016/j.neuron.2011.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zann RA, Bamford M. The zebra finch: a synthesis of field and laboratory studies (Oxford University Press Oxford:) 1996 [Google Scholar]
- 16.Doupe AJ, Kuhl PK. Birdsong and human speech: common themes and mechanisms. Annu. Rev. Neurosci. 1999;22:567–631. doi: 10.1146/annurev.neuro.22.1.567. [DOI] [PubMed] [Google Scholar]
- 17.Bolhuis JJ, Okanoya K, Scharff C. Twitter evolution: converging mechanisms in birdsong and human speech. Nat. Rev. Neurosci. 2010;11:747–759. doi: 10.1038/nrn2931. [DOI] [PubMed] [Google Scholar]
- 18.Tchernichovski Nottebohm, Ho Pesaran, Mitra A procedure for an automated measurement of song similarity. Anim Behav. 2000;59:1167–1176. doi: 10.1006/anbe.1999.1416. [DOI] [PubMed] [Google Scholar]
- 19.Sossinka R, Böhner J. Song Types in the Zebra Finch Poephila guttata castanotis1. Zeitschrift für Tierpsychologie. 1980;53:123–132. [Google Scholar]
- 20.Nottebohm F, Stokes TM, Leonard CM. Central control of song in the canary, Serinus canarius. J. Comp. Neurol. 1976;165:457–486. doi: 10.1002/cne.901650405. [DOI] [PubMed] [Google Scholar]
- 21.Simpson HB, Vicario DS. Brain pathways for learned and unlearned vocalizations differ in zebra finches. J. Neurosci. 1990;10:1541–1556. doi: 10.1523/JNEUROSCI.10-05-01541.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aronov D, Andalman AS, Fee MS. A specialized forebrain circuit for vocal babbling in the juvenile songbird. Science. 2008;320:630–634. doi: 10.1126/science.1155140. [DOI] [PubMed] [Google Scholar]
- 23.Hahnloser RHR, Kozhevnikov AA, Fee MS. An ultra-sparse code underlies the generation of neural sequences in a songbird. Nature. 2002;419:65–70. doi: 10.1038/nature00974. [DOI] [PubMed] [Google Scholar]
- 24.Kozhevnikov AA, Fee MS. Singing-related activity of identified HVC neurons in the zebra finch. J. Neurophysiol. 2007;97:4271–4283. doi: 10.1152/jn.00952.2006. [DOI] [PubMed] [Google Scholar]
- 25.Prather JF, Peters S, Nowicki S, Mooney R. Precise auditory-vocal mirroring in neurons for learned vocal communication. Nature. 2008;451:305–310. doi: 10.1038/nature06492. [DOI] [PubMed] [Google Scholar]
- 26.Fujimoto H, Hasegawa T, Watanabe D. Neural coding of syntactic structure in learned vocalizations in the songbird. The Journal of Neuroscience. 2011;31:10023–10033. doi: 10.1523/JNEUROSCI.1606-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.DeLong MR, Georgopoulos AP. Motor Functions of the Basal Ganglia. In: Terjung R, editor. Comprehensive Physiology. Hoboken, NJ, USA: John Wiley & Sons, Inc.; 2011. [Accessed November, 5, 2012]. Available at: http://www.comprehensivephysiology.com/WileyCDA/CompPhysArticle/refIdcp010221.html. [Google Scholar]
- 28.Kornhuber HH, Deecke L. Hirnpotentialänderungen bei Willkürbewegungen und passiven Bewegungen des Menschen: Bereitschaftspotential und reafferente Potentiale. Pflügers Archiv European Journal of Physiology. 1965;284:1–17. [PubMed] [Google Scholar]
- 29.Kagaya K, Takahata M. Readiness discharge for spontaneous initiation of walking in crayfish. The Journal of Neuroscience. 2010;30:1348–1362. doi: 10.1523/JNEUROSCI.4885-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Williams H, Vicario DS. Temporal patterning of song production: participation of nucleus uvaeformis of the thalamus. Journal of neurobiology. 2004;24:903–912. doi: 10.1002/neu.480240704. [DOI] [PubMed] [Google Scholar]
- 31.Coleman MJ, Vu ET. Recovery of impaired songs following unilateral but not bilateral lesions of nucleus uvaeformis of adult zebra finches. J. Neurobiol. 2005;63:70–89. doi: 10.1002/neu.20122. [DOI] [PubMed] [Google Scholar]
- 32.Horita H, Wada K, Jarvis ED. Early onset of deafening-induced song deterioration and differential requirements of the pallial-basal ganglia vocal pathway. European Journal of Neuroscience. 2008;28:2519–2532. doi: 10.1111/j.1460-9568.2008.06535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ashmore RC, Bourjaily M, Schmidt MF. Hemispheric coordination is necessary for song production in adult birds: implications for a dual role for forebrain nuclei in vocal motor control. J. Neurophysiol. 2008;99:373–385. doi: 10.1152/jn.00830.2007. [DOI] [PubMed] [Google Scholar]
- 34.Naie K, Hahnloser RHR. Regulation of learned vocal behavior by an auditory motor cortical nucleus in juvenile zebra finches. J Neurophysiol. 2011;106:291–300. doi: 10.1152/jn.01035.2010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.