Abstract
Background
The impact of improved nutritional status on health-related quality of life (HRQOL) is unknown for children with cystic fibrosis (CF).
Methods
Associations between nutritional status and HRQOL were examined over 2 years in 95 children, aged 9–19 years, who were followed in the Wisconsin Newborn Screening Project. HRQOL was assessed using the Cystic Fibrosis Questionnaire (CFQ). Associations between height z-score (HtZ), BMI z-score (BMIZ) and seven CFQ dimensions were evaluated.
Results
Mean values of at least 80 were observed for all CFQ dimensions except respiratory symptoms and treatment burden. Treatment burden was significantly worse in patients with meconium ileus (57) compared to pancreatic insufficient (65) and sufficient (78) subjects, p<0.0001. HtZ and BMIZ were positively associated with physical functioning and body image (p<0.05).
Conclusions
Better nutritional status was associated with increased HRQOL scores. Early diagnosis through newborn screening and improved nutrition provides an opportunity to enhance quality of life and body image perception.
1. Background
Achieving optimal growth and maintaining adequate nutritional status are cornerstones of clinical care in cystic fibrosis (CF). Indeed, in recent years there has been an increased focus on nutritional management with early intervention [1, 2]. Although achieving optimal growth is important and is significantly associated with pulmonary health [3, 4], the impact of achieving nutritional goals on improving pediatric patients’ perceptions of well-being is largely unknown.
Investigations of patient-reported outcomes in people with CF, such as health-related quality of life (HRQOL), and functional measures of health [5–17] have primarily focused on respiratory well-being and pulmonary function tests as endpoints in drug trials [11–17], although other reports have had a broader scope [5–10]. Overall, pulmonary measures are consistently associated with self-reported respiratory health. Few studies [6, 9], and only one in children [9], have evaluated nutritional status and HRQOL. These sparse data suggest that weight status is important for supporting both physical dimensions of HRQOL (respiratory and physical) and those related to nutritional issues (body image, eating disturbances). However, to our knowledge, there are no published reports on multiple indicators nutritional status, such as stature, and HRQOL in children with CF who experienced early diagnosis. Thus, the objective of this study is to examine longitudinal associations between nutritional status and HRQOL in children and adolescents with CF.
2. Subjects and Methods
2.1 Study Population
The study population consists of children and adolescents who were enrolled in the Wisconsin Randomized Clinical Trial (RCT) of CF Newborn Screening (NBS). The Wisconsin RCT is a prospective longitudinal investigation [18, 19], designed to assess the benefits and risks of newborn screening for CF. It enrolled 138 infants with CF from 1985–1998 and included quarterly visits through 2011. In 2002–2006, an ancillary study was conducted to evaluate psychosocial outcomes. The current study is composed of subjects aged 9–19 years (N=95) who agreed to participate in the ancillary study and complete psychosocial assessments. Included in these assessments was the administration of a HRQOL questionnaire (described below). The study was approved by Institutional Review Boards of the two participating CF centers, and informed consent was obtained prior to participation.
2.2 Assessment of health-related quality of life
The Cystic Fibrosis Questionnaire (CFQ) [20–22] was administered yearly during three regularly scheduled routine clinic visits. The CFQ assesses multiple dimensions of HRQOL in patients with CF. By design, it was interviewer-administered to 6–11 year olds (8 dimensions, ‘child’ questionnaire, N=31 at first CFQ administration) and self-administered by 12–13 (8 dimensions, ‘child’ questionnaire, N=23 at first CFQ administration) and ≥ 14 year olds (12 dimensions, ‘adolescent’ questionnaire, N=41 at first CFQ administration). Eight dimensions are common to the child and adolescent questionnaire versions, but the dimension “digestive symptoms” had only one question to assess it in the child version, and was not included. The remaining seven dimensions were evaluated: physical functioning, respiratory symptoms, social functioning, emotional functioning, treatment burden, body image and eating disturbances. Scores for each dimension are standardized to a 0–100 point scale, with 100 representing the most favorable HRQOL.
2.3 Categorization of CFQ dimensions
Scores were categorized to minimize the imbalance caused by ceiling effects, which have been reported by others [20, 22]. Categories were also established to assign meaning to the value of the scores. Categories of scores were defined as follows: “Mostly Low” are scores <66.0, which correspond to a combination of the two least favorable responses to questions in a CFQ dimension (e.g. ‘never’ and ‘sometimes’ for frequency responses). Because only two subjects ever gave the lowest response for all questions representing a given dimension, a ‘worst’ category could not be assigned. “Mostly Good” is a score between 66.0–99.9 which corresponds to a combination of the two most favorable responses to questions in a CFQ dimension (e.g. ‘often’ or ‘always’ for frequency responses); “Perfect” is a score equal to 100 which corresponds to all responses for all questions in a given dimension equal to the most favorable response (e.g. ‘always’ for frequency responses). The validity of these categorizations has not been formally evaluated in independent studies.
2.4 CF pancreatic phenotype and nutritional status
Pancreatic function was primarily determined by 72-hour fecal fat at approximately 4 years of age [19]. Pancreatic status phenotype was defined according to the presence of meconium ileus (MI), pancreatic insufficiency (PI) without MI, and pancreatic sufficiency (PS).
Nutritional status was represented by multiple indices of growth, including height and body mass index (BMI). Height and weight were measured concurrently at the time of CFQ administration, as part of routine clinical examinations. BMI was calculated [weight in kilograms / (height in meters) 2]. Age- and gender-specific z-scores and percentiles for BMI (BMIZ and BMIP, respectively), weight (WtZ and WtP, respectively), and height (HtZ and HtP, respectively) were computed by using the 2000 Centers for Disease Control’s growth reference (www.cdc.gov/growthcharts) [23].
Nutritional status was classified using both BMIP and HtP according to the following cut-points [24, 25]: short stature was defined as HtP <5th percentile and underweight was defined as BMIP <10th percentile. ‘Below BMI goal’ was defined by a BMIP <50th percentile [2].
2.5 Statistical analysis
The dataset is composed of 265 observations from 95 participants. Data analysis was performed using SAS statistical software (version 9.2; SAS Institute Inc, Cary, NC). Simple summary statistics were computed at each CFQ administration time point (CFQ-1, CFQ-2, CFQ-3). When data are not presented by CFQ time point, all observations were utilized. Chi-square or Fisher Exact Test was used to test for differences in proportions and Cochran-Mantel-Haenszel (CMH) statistics were used when adjusting for other factors.
For longitudinal analysis, generalized estimating equation (GEE) methods with repeated measures were used to examine associations between continuous dimension scores (each dimension as a separate outcome variable) and continuous growth indicators (BMIZ and HtZ), while accounting for CFQ version (child or adolescent version), age, gender, pancreatic function phenotype and mode of diagnosis (screened or conventional diagnosis). HtZ and BMIZ were included in models together in order to account for both stature and relative weight status. Regression analyses involving continuous height and BMI utilized z-score values, but percentile results are presented because the latter are displayed on the CDC growth charts and are commonly used in the clinical setting.
In order to minimize the influence of the ceiling effect in the CFQ dimension scores, logistic regression using GEE was also employed. For these models, dichotomous dimension variables (Mostly Low scores, compared to the combined categories of Mostly Good scores and Perfect scores) were constructed in order to compute the odds of Mostly Low scores for categorical levels of nutritional status indicators (i.e., indicators of short stature, underweight, and below BMI goal). The dichotomous nutritional status indicators, short stature and underweight, were included simultaneously, and all models also included CFQ version, age, gender, phenotype and mode of diagnosis.
3. Results
3.1 Subject characteristics at each CFQ administration
Mean age at first CFQ was 13.5 years (Table 1). Nutritional status, represented by weight, height and BMI percentiles (43 ± 30, 42 ± 29 and 48 ± 26, respectively, at first CFQ), was stable over the 2-year study period (p>0.1 for change over the three time points). On average, over the three time points, 12% of subjects had short stature, 8% were underweight, and 53% were below the BMI goal of being at the 50th percentile recommended by current CFF guidelines [2]. Very few (≤ 2 subjects) exhibited both short stature and underweight.
Table 1.
Nutritional status and CFQ dimension scores at each CFQ.*
| CFQ-1 | CFQ-2 | CFQ-3 | Overall** | |
|---|---|---|---|---|
| N | 94 | 91 | 80 | |
| Age (years), mean ± SD | 13.5 ± 2.8 | 14.5 ± 2.8 | 15.5 ± 2.8 | -- |
| Nutritional Status | ||||
| Weight percentile, mean ± SD | 43 ± 30 | 46 ± 29 | 45 ± 28 | 44 ± 29 |
| Height percentile, mean ± SD | 42 ± 30 | 42 ± 30 | 42 ± 30 | 42 ± 29 |
| BMI percentile, mean ± SD | 47 ± 28 | 51 ± 28 | 50 ± 26 | 48 ± 26 |
| Short stature† [N (%)] | 12 (13%) | 10 (11%) | 10 (13%) | 12% |
| Underweight† [N (%)] | 9 (10%) | 8 (9%) | 5 (6%) | 8% |
| Short and underweight† [N (%)] | 1 (1%) | 2 (2%) | 0 | 1% |
| Below BMI goal† [N (%)] | 53 (56%) | 46 (51%) | 41 (52%) | 53% |
| CFQ Dimensions (mean ± SD) | ||||
| Eating disturbances | 88 ± 19 | 91 ± 16 | 92 ± 16 | 90 ± 13 |
| Physical functioning | 84 ± 18 | 85 ± 18 | 88 ± 16 | 85 ± 16 |
| Emotional functioning | 82 ± 16 | 83 ± 14 | 84 ± 15 | 83 ± 13 |
| Body image | 81 ± 25 | 81 ± 24 | 82 ± 23 | 82 ± 21 |
| Social functioning | 80 ± 14 | 81 ± 16 | 81 ± 16 | 81 ± 13 |
| Respiratory symptoms | 77 ± 18 | 78 ± 16 | 79 ± 14 | 77 ± 14 |
| Treatment burden‡ | 63 ± 19 | 68 ± 21 | 65 ± 18 | 65 ± 15 |
For assessing change over time, nutritional status GEE models (WtZ, HtZ and BMIZ) controlled for age, sex and mode of diagnosis; for CFQ dimensions, GEE models controlled for age, sex, mode of diagnosis and CFQ version
Overall mean values represent the average of means from each subject
Nutritional status indicators are defined as follows: short stature is height <5th percentile; underweight is BMI <10th percentile; below BMI goal is BMI <50th percentile.
p=0.045
3.2 CFQ dimension scores at each time point
Table 1 also displays the mean of each of the 7 CFQ dimensions at each time point, as well as the overall mean score. Dimension scores were typically high, with mean scores of at least 80 observed for eating disturbances, physical functioning, emotional functioning, body image, and social functioning. Respiratory symptoms and treatment burden had the lowest scores indicating more frequent issues with pulmonary health and the intrusion of medical therapy in daily activities. In general, CFQ scores did not change appreciably over the two years of observations, i.e., there were no trends discernible in the longitudinal data.
3.3 Nutritional status and QOL dimension scores according to phenotype
Nutritional status indicators, CFQ dimension scores, and distribution of score categories, according to pancreatic function phenotype, are shown in Table 2. PI was present in 63% of subjects (N=59), while 21% (n=20) and 16% (n=15) of subjects had MI and PS, respectively. Mean HtP was highest in PS subjects (60 ± 19), followed by PI (43 ± 30) and MI (27 ± 27), p=0.002. BMIP did not differ significantly among the 3 groups (p=0.21), though a similar pattern to HtP was observed.
Table 2.
Nutritional status and CFQ scores according to phenotype*.
| Meconium Ileus | Pancreatic Insufficient | Pancreatic Sufficient | Overall p-value | |
|---|---|---|---|---|
| No. subjects (No. observations) | 21 (59) | 59 (163) | 15 (43) | |
| Height percentile, mean ± SD | 27 ± 27a | 43 ± 30b | 60 ± 19c | 0.002† |
| BMI percentile, mean ± SD | 42 ± 20 | 48 ± 26 | 60 ± 31 | 0.21† |
| Eating disturbances, mean ± SD | 89 ± 15 | 90 ± 13 | 92 ± 10 | 0.68† |
| Score distribution [n (%)] | 0.71‡ | |||
| Mostly Low (<66) | 5 ( 9%) | 15 ( 9%) | 3 ( 7%) | |
| Mostly Good (66–99) | 20 (34%) | 48 (29%) | 12 (28%) | |
| Perfect (100) | 33 (57%) | 100 (61%) | 28 (65%) | |
| Physical functioning, mean ± SD | 78 ± 18 | 87 ± 15 | 88 ± 14 | 0.08† |
| Score distribution [n (%)] | 0.04‡ | |||
| Mostly Low (<66) | 15 (25%) | 15( 9%) | 3 ( 7%) | |
| Mostly Good (66–99) | 25 (42%) | 82 (50%) | 24 (56%) | |
| Perfect (100) | 19 (32%) | 66 (40%) | 16 (37%) | |
| Emotional functioning, mean ± SD | 81 ± 14 | 83 ± 13 | 84 ± 10 | 0.42† |
| Score distribution [n (%)] | 0.70‡ | |||
| Mostly Low (<66) | 11 (19%) | 21 (13%) | 2 ( 5%) | |
| Mostly Good (66–99) | 34 (58%) | 107 (66%) | 36 (84%) | |
| Perfect (100) | 14 (24%) | 35 (21%) | 5 (11%) | |
| Body image, mean ± SD | 80 ± 27 | 83 ± 19 | 82 ± 20 | 0.87† |
| Score distribution [n (%)] | 0.99‡ | |||
| Mostly Low (<66) | 13 (22%) | 27 (17%) | 9 (21%) | |
| Mostly Good (66–99) | 18 (31%) | 66 (40%) | 15 (35%) | |
| Perfect (100) | 27 (47%) | 70 (43%) | 19 (44%) | |
| Social functioning, mean ± SD | 80 ± 14 | 80 ± 13 | 87 ± 9 | 0.20 † |
| Score distribution [n (%)] | 0.46‡ | |||
| Mostly Low (<66) | 13 (22%) | 30 (18%) | 2 ( 5%) | |
| Mostly Good (66–99) | 33 (57%) | 107 (66%) | 34 (79%) | |
| Perfect (100) | 12 (21%) | 26 (16%) | 7 (16%) | |
| Respiratory symptoms, mean ± SD | 71 ± 18 | 78 ± 12 | 82 ± 12 | 0.13† |
| Score distribution [n (%)] | 0.03‡ | |||
| Mostly Low (<66) | 19 (32%) | 21 (13%) | 5 (12%) | |
| Mostly Good (66–99) | 35 (59%) | 132 (81%) | 33 (77%) | |
| Perfect (100) | 5 ( 8%) | 10 ( 6%) | 5 (12%) | |
| Treatment burden, mean ± SD | 57 ± 16a | 65 ± 14b | 78 ± 14c | 0.002† |
| Score distribution [n (%)] | <0.0001‡ | |||
| Mostly Low (<66) | 34 (59%) | 69 (42%) | 4 (10%) | |
| Mostly Good (66–99) | 21 (36%) | 86 (53%) | 26 (63%) | |
| Perfect (100) | 3 ( 5%) | 8 ( 5%) | 11 (27%) |
mean values represent average of mean scores from each subject
values with different superscripts within the same row were statistically different
Continuous outcomes: for WtZ, HtZ and BMIZ, GEE models also included age, sex and mode of diagnosis; for domain scores, the GEE models included CFQ version (child or adolescent), age, sex and mode of diagnosis
Categorical outcomes: CMH models utilized all observations and accounted for CFQ version and age.
Average QOL dimension score was borderline significantly different for physical functioning (p=0.08) with lowest scores (78 ± 18) observed in MI subjects compared to PI (87 ± 15) and PS (88 ± 14). MI subjects had significantly greater prevalence of Mostly Low scores (score <66) for physical functioning (25% compared to 9% in PI and 7% in PS, p=0.04). Similarly, low scores for respiratory symptoms were more prevalent among MI subjects (32%) compared to PI (13%) and PS (12%), p=0.03. Scores for treatment burden differed significantly, with the highest observed in PS subjects (78 ± 14), followed by PI (65 ± 14) and MI (57 ± 16), p=0.002. Mostly Low scores for treatment burden were approximately 6 times more prevalent in MI (59%) compared to PS (10%) subjects, with a prevalence of 42% in PI, p<0.0001. No significant CFQ dimension score differences were observed among MI, PI and PS for eating disturbances, body image, emotional functioning and social functioning.
3.4 CFQ dimension scores according to nutritional status
Figure 1 shows mean CFQ dimension scores according to HtP and BMIP. P-values are from GEE models where both the CFQ dimension scores and nutritional status indicators (HtZ and BMIZ) are continuous variables. HtZ (p=0.07) and BMIZ (p=0.09) exhibited a borderline positive association with eating disturbances and no association with emotional functioning, social functioning, respiratory symptoms or treatment burden. However, HtZ and BMIZ were positively associated with physical functioning (p=0.02 and p=0.02, respectively) and body image (p=0.02 and p=0.02, respectively). A threshold effect appeared evident for HtP in that the lowest physical functioning and body image scores were observed for HtP less than the 10th percentile, with an approximately 20 point increase that leveled off for HtP greater than the 10th percentile. BMIP showed less pronounced differences, but somewhat more linear relationship with physical functioning and body image. Scores were approximately 10 points lower for BMIP less than the 25th percentile compared to higher BMIP categories. Large standard deviations indicate that there was high variability in dimension scores, particularly at the low end of nutritional status. Associations with WtZ followed patterns similar to those observed with BMIZ. Specifically, WtZ was significantly and positively associated with physical functioning (p=0.02) and body image (p=0.009), and showed a positive, borderline significance with eating disturbances (p=0.054).
Figure 1.
Mean quality of life scores according to height and BMI percentiles. P-values are from separate GEE models whereby the outcome (CFQ dimension) and nutritional status indicators (HtZ, BMIZ) are continuous variables. Covariates included version of CFQ, age, sex, phenotype and mode of diagnosis.
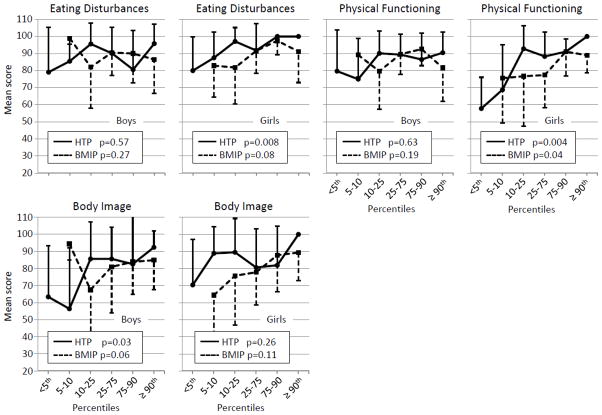
Figure 2 shows CFQ dimension scores according to nutritional status separately in boys and girls. Although there were no gender differences in mean CFQ dimension scores (data not presented), three dimensions showed gender-specific associations with nutritional status: eating disturbances, physical functioning and body image. HtZ (p=0.008) showed a positive association with eating disturbances in girls only, and the association was borderline significant with BMIZ (p=0.08) in girls. Similarly, positive associations between physical functioning and HtZ (p=0.004) and BMIZ (p=0.04) were observed in girls, but not boys. In girls, physical functioning was low (<60) in the lowest category of HtP, and increased incrementally until reaching 100 in the highest HtP group. Body image was positively associated with HtZ (p=0.03) and BMIZ (borderline p=0.06) in boys, but not girls. The HtP threshold effect observed for body image with all subjects (Figure 1) was more pronounced in boys (Figure 2).
Figure 2.
Mean quality of life scores according to height and BMI percentiles in boys (N=54) and girls (N=41). P-values are from separate GEE models whereby the outcome (CFQ dimension) and nutritional status indicators (HtZ, BMIZ) are continuous variables. Covariates included version of CFQ, age, phenotype and mode of diagnosis. Only the CFQ dimension scores that showed a different association with nutritional status between boys and girls are presented.
3.5 Odds of Mostly Low CFQ dimension scores according to nutritional status characteristics
Odds ratios of selected Mostly Low CFQ dimension scores (<66) according to nutritional status are shown in Table 3. Short stature was associated with a greater odds of Mostly Low scores for eating disturbances (OR=4.08, 95% CI=1.20–13.79, p=0.02). Underweight was not associated with any CFQ dimension. Below BMI goal was associated with an increased odds of Mostly Low body image scores (OR=2.67, 95% CI=1.19–6.01, p=0.02).
Table 3.
Odds ratios (95% confidence intervals) of selected Mostly Low CFQ dimension scores according to nutritional status characteristics*.
| Eating Disturbances | Physical Functioning | Body Image | Respiratory Symptoms | |||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Mostly Low score | Good to Perfect score | Mostly Low score | Good to Perfect score | Mostly Low score | Good to Perfect score | Mostly Low score | Good to Perfect score | |
| SHORT STATURE** | ||||||||
| No [N (%)] | 16 (7%) | 215 (93%) | 19 (8%) | 213 (92%) | 43 (18%) | 198 (82%) | 34 (15%) | 198 (85%) |
| Yes [N (%)] | 7 (22%) | 25 (78%) | 14 (44%) | 18 (56%) | 6 (27%) | 16 (73%) | 11 (34%) | 21 (66%) |
| OR (95% CI) (‘yes’ versus ‘no’) | 4.08 (1.20, 13.79)† | 3.59 (0.81, 15.94) | 2.31 (0.62, 8.57) | 1.40 (0.60, 3.28) | ||||
| UNDERWEIGHT** | ||||||||
| No [N (%)] | 21 (9%) | 220 (91%) | 29 (12%) | 213 (88%) | 43 (18%) | 198 (82%) | 40 (17%) | 202 (83%) |
| Yes [N (%)] | 2 (9%) | 20 (91%) | 4 (18%) | 18 (82%) | 6 (27%) | 16 (73%) | 5 (23%) | 17 (77%) |
| OR (95% CI) (‘yes’ versus ‘no’) | 5.12 (0.82, 31.82) | 1.46 (0.22, 9.73) | 1.15 (0.37, 3.58) | 1.93 (0.53, 7.11) | ||||
| BELOW BMI GOAL** | ||||||||
| No [N (%)] | 11 (9%) | 112 (91%) | 10 (8%) | 114 (92%) | 12 (10%) | 111 (90%) | 15 (12%) | 109 (88%) |
| Yes [N (%)] | 12 (9%) | 128 (91%) | 23 (16%) | 117 (84%) | 37 (26%) | 103 (74%) | 30 (21%) | 110 (79%) |
| OR (95% CI) (‘yes’ versus ‘no’) | 0.74 (0.28, 1.93) | 1.69 (0.68, 4.20) | 2.67 (1.19, 6.01)† | 1.98 (0.74, 5.33) | ||||
Separate model for each CFQ dimension. Covariates: version of CFQ, age, sex, phenotype, and mode of diagnosis. Short stature and underweight in models simultaneously.
Short stature is height < 5th percentile; Underweight is BMI < 10th percentile; Below BMI goal is BMI <50th percentile.
p<0.05
4. Discussion
To our knowledge, this is the first report to describe associations between multiple indicators of nutritional status and HRQOL as assessed by the CFQ, particularly in CF patients who experienced early diagnosis and aggressive nutritional management. In this population of older children and adolescents, nutritional status had a measurable impact on self-assessments of eating disturbances, physical functioning and body image over two years. Results provide strong evidence that achieving optimal growth over the short-term (i.e., BMI) and long-term (i.e., stature) is beneficial for HRQOL. Nutritional status was not significantly associated with emotional functioning, social functioning, respiratory symptoms and treatment burden indicating that not all aspects of HRQOL respond to long-term nutrition intervention, particularly those of the emotional and social domains. Given the association between nutrition and pulmonary outcome measures [3, 4], and our previously reported association between objective lung disease surrogates and CFQ respiratory health [5], it is notable that the qualitative aspects of pulmonary health were not related to nutritional status. On the other hand, treatment burden was strongly associated with pancreatic status phenotype.
There were no significant gender differences in mean CFQ dimension scores or in nutritional status indicators (data not presented), but it is noteworthy that the strength of associations between nutritional status and CFQ differed between boys and girls for some dimensions. The differences between girls and boys were most striking in those who were short, particularly for physical functioning in girls and body image in boys. Additionally, BMI was important for physical functioning in girls only. These gender-specific results provide insight into which domains are more closely related to nutritional status in girls and boys.
The CFQ dimension scores reported here are generally similar to others [9, 20]. Recently, Quittner et al. [26] provided normative values of CFQ dimension scores for the revised version of the CFQ, which reports values according to well and sick visits, pulmonary disease severity and gender. This is an important resource and will provide needed reference data. Analyzing results separately for the child and adolescent CFQ instruments and relying on weight for age as the nutritional status indicator, Sawicki et al. [9] reported improvements in respiratory symptoms and eating disturbances in both children and adolescents, and in physical functioning and body image in adolescents in response to increases in weight for age. Although we combined CFQ scores for analysis and utilized more accurate and direct indicators of nutritional status, overall our results are consistent with what they observed in children and adolescents for eating disturbances, and in adolescents for physical functioning and body image.
In our study population, nutritional status was stable over the 2 years of HRQOL assessment, as were the multiple dimensions of CFQ. One growth channel changes were only observed in 16% for height (N=4 decrease, N=11 increase) and 14% for BMI (N=4 decrease, N=9 increase) over the 2-year study period. Our study population of older children would of course include those at varying stages of pubertal development and it is worthwhile to note that age was not associated with changes in nutritional status. Thus, although our analyses modeled changes in both nutritional status and CFQ scores to assess longitudinal associations, the results reflect associations found in a population with generally stable nutritional status and stable CFQ scores. Further, this study population was in relatively good health. Seventy-four percent of participants exhibited normal FEV-1 values and 14% had mild pulmonary disease (previously reported by Tluczek [5]), and prevalence of short stature (about 12%) and underweight (about 8%) were low, although more than half were below the BMI goal of the 50th percentile. On an individual level, it is possible that acute changes in nutritional status may affect other dimensions of HRQOL not observed in this study. Overall, in this relatively stable population, the potential to show improvement in HRQOL is likely diminished [27], suggesting that the associations that did emerge with eating disturbances, physical functioning and body image, are important with regard to the maintenance of adequate nutritional status.
The interpretation of CFQ scores can be challenging because there are no definitions for poor or excellent HRQOL. Only the respiratory symptom dimension has been evaluated for use in clinical trials, and a change of 4 points has been recommended as the minimal clinically important difference (MCID) [28]. In this study, differences greater than 4 points were observed for respiratory symptoms between those with low and high percentiles of height and BMI, but were not statistically significant. To evaluate CFQ scores in the context of assigning meaning to them, and to also offset imbalances caused by ceiling effects, poor CFQ scores were distinguished from normal or excellent by categorizing the scores according to corresponding responses on CFQ items. Thus, “Mostly Low” in this study corresponds to scores <66, which reflects a combination of the two least favorable responses to questions in a CFQ dimension, such as ‘never’ and ‘sometimes’ for frequency responses. Results showed that those who exhibited short stature were more likely to report low scores for eating disturbances, and those who were below the BMI goal of being at or above the 50th percentile had a greater odds of having Mostly Low scores for body image, consistent with results from analysis of continuous variables. Underweight was not associated with Mostly Low scores for any dimension.
Although not statistically significant when adjusting for other factors, it is noteworthy that among those with short stature, the percent with Mostly Low scores was more than twice as much compared to the percent with Mostly Low scores who were not short, for physical functioning (44% versus 8%) and respiratory symptoms (34% versus 15%). A similar pattern was observed for being below the BMI goal, whereby a doubling of the percentage of Mostly Low scores for physical functioning and social functioning was observed (16% versus 8% and 22% versus 11%, respectively).
Evaluation of Mostly Low scores according to pancreatic phenotype was revealing. MI subjects had a significantly greater prevalence of Mostly Low scores for physical functioning (25%), respiratory symptoms (32%) and treatment burden (59%). Conversely, for dimension scores not significantly associated with pancreatic function phenotype, MI subjects reported a similar prevalence of Perfect scores (all items that constitute a CFQ dimension have best response) for eating disturbances, body image and social functioning compared to PI and PS phenotypes. Worse HRQOL scores in the physical and treatment domains, combined with significantly lower HtZ scores, underscores the greater negative impact on well-being CF has on those with MI.
In conclusion, the results presented here provide further evidence of the importance of optimizing CF nutritional status throughout childhood and the teenage years. This study describes the novel findings that concurrent measures of height and BMI were positively associated with eating disturbances, physical functioning and body image. Our finding that better nutritional status is related to fewer issues within these domains of well-being in a population that had high scores overall provides rationale and support for a greater focus on the integration of measurable clinical outcomes and the enhancement of patients’ quality of life.
Acknowledgments
We thank the families who participated in this project and gratefully acknowledge Dr. Rebecca Koscik for her early contributions and Kathleen Zaremba for her assistance with data collection. We also deeply appreciate guidance from our distinguished consultant, Dr. Alexandra L. Quittner, who facilitated initiation of this project and helped review/interpret the results. In addition, we remain grateful to the entire Wisconsin Neonatal CF Screening Project team in Madison and Milwaukee.
This work was supported by grants from the Cystic Fibrosis Foundation (A001-5-01) and National Institutes of Health (R01 DK72126, DK 34108 and M01 RR03186). Neither funding agency had any role in the concept, design, or conduct of the study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Borowitz D, Robinson KA, Rosenfeld R, et al. Cystic Fibrosis Foundation evidence-based guidelines for management of infants with cystic fibrosis. J Pediatr. 2009;155:S73–93. doi: 10.1016/j.jpeds.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stallings VA, Stark LJ, Robinson KA, Feranchak AP, Quinton HB. Evidence-based practice recommendations for nutrition-related management of children and adults with cystic fibrosis and pancreatic insufficiency: results of a systematic review. J Am Diet Assoc. 2008;108:832–9. doi: 10.1016/j.jada.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 3.Milla CE. Association of nutritional status and pulmonary function in children with cystic fibrosis. Curr Opin Pulm Med. 2004;10:505–9. doi: 10.1097/01.mcp.0000138995.08494.69. [DOI] [PubMed] [Google Scholar]
- 4.Zemel BS, Jawad AF, FitzSimmons S, Stallings V. Longitudinal relationship among growth, nutritional status, and pulmonary function in children with cystic fibrosis: Analysis of the Cystic Fibrosis Foundation National CF Patient Registry. J Pediatr. 2000;137:374–380. doi: 10.1067/mpd.2000.107891. [DOI] [PubMed] [Google Scholar]
- 5.Tluczek A, Becker T, Laxova A, et al. Relationships among health-related quality of life, pulmonary health and newborn screening for cystic fibrosis. Chest. 2011;140:170–77. doi: 10.1378/chest.10-1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gee L, Abbott J, Hart A, Conway SP, Etherington C, Webb AK. Associations between clinical variables and quality of life in adults with cystic fibrosis. J Cystic Fibrosis. 2005;4:59–66. doi: 10.1016/j.jcf.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 7.Gee L, Abbott J, Conway SP, Etherington C, Webb AK. Quality of life in cystic fibrosis: the impact of gender, general health perceptions and disease severity. J Cystic Fibrosis. 2003;2:206–13. doi: 10.1016/S1569-1993(03)00093-6. [DOI] [PubMed] [Google Scholar]
- 8.Sawicki GS, Sellers DE, Robinson WM. Associations between illness perceptions and health-related quality of life in adults with cystic fibrosis. J Psychosomatic Res. 2011;70:161–7. doi: 10.1016/j.jpsychores.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sawicki GS, Rasouliyan L, McMullen AH, et al. Longitudinal assessment of health-related quality of life in an observational cohort of patients with cystic fibrosis. Pediatr Pulmonol. 2011;46:36–44. doi: 10.1002/ppul.21325. [DOI] [PubMed] [Google Scholar]
- 10.Simon SL, Duncan CL, Horky SC, Nick TG, Castro MM, Riekert KA. Body satisfaction, nutritional adherence, and quality of life in youth with cystic fibrosis. Pediatr Pulmonol. 2011;46:1085–92. doi: 10.1002/ppul.21477. [DOI] [PubMed] [Google Scholar]
- 11.Wolter J, Seeney S, Bell S, Bowler S, Masel P, McCormack J. Effect of long term treatment with azithromycin on disease parameters in cystic fibrosis: a randomized tiral. Thorax. 2002;57:212–216. doi: 10.1136/thorax.57.3.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saiman L, Marshall BC, Mayer-Hamblett N, et al. Azithromycin in patients with cystic fibrosis chronically infected with Pseudomonas aeruginosa: a randomized controlled trial. JAMA. 2003;290:1749–56. doi: 10.1001/jama.290.13.1749. [DOI] [PubMed] [Google Scholar]
- 13.Elkins MR, Robinson M, Rose BR, et al. A controlled trial of long-term inhaled hypertonic saline in patients with cystic fibrosis. N Engl J Med. 2006;354:229–40. doi: 10.1056/NEJMoa043900. [DOI] [PubMed] [Google Scholar]
- 14.Donaldson SH, Bennett WD, Zeman KL, Knowles MR, Tarran R, Boucher RC. Mucus clearance and lung function in cystic fibrosis with hypertonic saline. N Engl J Med. 2006;354:241–50. doi: 10.1056/NEJMoa043891. [DOI] [PubMed] [Google Scholar]
- 15.Accurso FJ, Rowe SM, Clancy JP, et al. Effect of VX-770 in persons with cystic fibrosis and the G551D-CFTR mutation. N Engl J Med. 2010;363:1991–2003. doi: 10.1056/NEJMoa0909825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Modi AC, Lim CS, Driscoll KA, Piazza-Waggoner C, Quittner AL, Wooldridge J. Changes in pediatric health-related quality of life in cystic fibrosis after IV antibiotic treatment for pulmonary exacerbations. J Clin Psychol Med Settings. 2010;17:49–55. doi: 10.1007/s10880-009-9179-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oermann CM, Retsch-Bogart GZ, Quittner AL, et al. An 18-month study of the safety and efficacy of repeated courses of inhaled aztreonam lysine in cystic fibrosis. Pediatr Pulmonol. 2010;45:1121–34. doi: 10.1002/ppul.21301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fost NC, Farrell PM. A prospective randomized trial of early diagnosis and treatment of cystic fibrosis: a unique ethical dilemma. Clin Res. 1989;37:495–500. [PubMed] [Google Scholar]
- 19.Farrell PM, Kosorok MR, Laxova A, Shen G, Koscik RE, Bruns WT, Splaingard M, Mischler EH. Nutritional benefits of neonatal screening for cystic fibrosis. Wisconsin Cystic Fibrosis Neonatal Screening Study Group. N Engl J Med. 1997;337:963–9. doi: 10.1056/NEJM199710023371403. [DOI] [PubMed] [Google Scholar]
- 20.Quittner AL, Buu A, Messer MA, Modi AC, Watrous M. Development and validation of the Cystic Fibrosis Questionnaire in the United States: a health-related quality-of-life measure for cystic fibrosis. Chest. 2005;128:2347–54. doi: 10.1378/chest.128.4.2347. [DOI] [PubMed] [Google Scholar]
- 21.Quittner AL, Buu A, Watrous M, Davis MA. The Cystic Fibrosis Questionnaire: User’s Manual. Washington, DC: Cystic Fibrosis Foundation; 2000. [Google Scholar]
- 22.Modi AC, Quittner AL. Validation of a disease-specific measure of health-related quality of life for children with cystic fibrosis. J Pediatr Psychol. 2003;28:535–45. doi: 10.1093/jpepsy/jsg044. [DOI] [PubMed] [Google Scholar]
- 23.Kuczmaarski RJ, Ogden CL, Guo SS, et al. CDC growth charts for the United States: methods and development. Vital Health Stat. 2000;11:iii–x. 1–190. [PubMed] [Google Scholar]
- 24.Lai HJ, Shoff SM. Classification of malnutrition in cystic fibrosis: implications for evaluating and benchmarking clinical practice performance. Am J Clin Nutr. 2008;88:161–6. doi: 10.1093/ajcn/88.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Borowitz D, Baker RD, Stallings V. Consensus report on nutrition for pediatric patients with cystic fibrosis. J Pediatr Gastroenterol Nutr. 2002;35:246–59. doi: 10.1097/00005176-200209000-00004. [DOI] [PubMed] [Google Scholar]
- 26.Quittner AL, Sawicki GX, McMullen A, Rasouliyan L, Pasta DJ, Yegin A, Konstan MW. Psychometric evaluation of the Cystic Fibrosis Questionnaire-Revised in a national, US sample. Qual Life Res. 2012 doi: 10.1007/s11136-011-0036-z. published online January 13. [DOI] [PubMed] [Google Scholar]
- 27.Abbott J, Hart A, Havermans T, et al. Measuring health-related quality of life in clinical trials in cystic fibrosis. J Cystic Fibrosis. 2011;10 (Suppl 2):S82–S85. doi: 10.1016/S1569-1993(11)60013-1. [DOI] [PubMed] [Google Scholar]
- 28.Quittner AL, Modi AC, Wainwright C, Otto K, Kirihara J, Montgomery AB. Determination of the minimal clinically important difference scores for the Cystic Fibrosis Questionnaire-Revised respiratory symptom scale in two populations of patients with cystic fibrosis and chronic Pseudomonas aeruginosa airway infection. Chest. 2009;135:1610–18. doi: 10.1378/chest.08-1190. [DOI] [PMC free article] [PubMed] [Google Scholar]