Abstract
Objective
To investigate the individual effects of ibuprofen, diclofenac, naproxen, and piroxicam on pregnancy outcome.
Design
Cohort study.
Setting
Norwegian population.
Population
Total of 90 417 women and singleton child pairs.
Methods
Norwegian Mother and Child Cohort Study and Medical Birth Registry of Norway datasets were used.
Main outcome measures
Infant survival, congenital malformations, structural heart defects, neonatal complications, haemorrhage during pregnancy and postpartum, asthma at age of 18 months.
Results
One or more of the four NSAIDs were used by 6511 pregnant women, (7.2%). No effect on infant survival, congenital malformation, or structural heart defect rate was found. Second trimester ibuprofen use was significantly associated with low birth weight (adjusted OR 1.7, 95% CI 1.3 to 2.3) and second and third trimester use was significantly associated with asthma in the 18 month old child (adjusted OR 1.5, 95%CI 1.2 to 1.9 and adjusted OR 1.5, 95%CI 1.1 to 2.1). Second trimester diclofenac use was significantly associated with low birth weight (adjusted OR 3.1, 95% CI 1.1 to 9.0), whereas third trimester use was significantly associated with maternal vaginal bleeding (adjusted OR 1.8, 95% CI 1.1 to 3.0). No associations with other neonatal complications were found.
Conclusions
The lack of associations with congenital malformations is reassuring. The significant association between diclofenac and ibuprofen use late in pregnancy and maternal bleeding and asthma in the child respectively is consistent with their pharmacological effects. The increased risk of low birth weight may partly have been caused by underlying inflammatory conditions and was reassuringly similar to the expected baseline risk of low birth weight.
Keywords: Ibuprofen, diclofenac, naproxen, piroxicam, congenital malformations, haemorrhage, low birth weight, premature delivery, asthma
INTRODUCTION
The frequency of in utero exposure to non-steroidal anti-inflammatory drugs (NSAIDs) ranges between 5 and 20%.1–8 NSAIDs often form the basis of first-line therapy for numerous conditions that also manifest during pregnancy. Exacerbations of headache and migraine often occur during the first few gestational weeks9, 10; inflammatory conditions affecting the musculoskeletal system usually arise later in pregnancy.11, 12 Patients with rheumatologic disorders often continue or initiate NSAID therapy during pregnancy.13
Studies on the safety of NSAID use during pregnancy have so far mainly focused on early exposure and the risk of miscarriage and cardiac defects1, 14–18,19 or late exposure and the risk of premature closure of the ductus arteriosus and decreased neonatal renal function.20–23
As most of the above studies 1, 14–18, 20–23 have evaluated the effects of NSAIDs as a group, data on the impact of individual NSAIDs on pregnancy outcome remain scarce. There are no studies that have examined a possible relationship of individual NSAIDs with maternal, fetal or neonatal haemorrhage, birth weight, or gestational age. Also not yet studied is the possibility of an association between in utero exposure to NSAIDs and neonatal respiratory distress, or asthmatic symptoms in the infant, despite the fact that NSAIDs may cause exacerbations in patients with asthma.24
The aim of our study was to analyse the individual effect of four of the most frequently used NSAIDs, ibuprofen, diclofenac, naproxen, and piroxicam, on pregnancy outcome and complications during and after delivery with particular emphasis on maternal bleeding and haemorrhage, infant survival, malformations, low birth weight, premature delivery, and asthmatic symptoms in the child.
METHODS
Data used in this study were retrieved from the quality-assured Norwegian Mother and Child Cohort Study dataset (version six) released in autumn 2011 and from The Medical Birth Registry of Norway (MBRN) records. The Norwegian Mother and Child Cohort Studyis a nationwide prospective cohort study conducted by the Norwegian Institute of Public Health with the intention to evaluate the effect of several exposures on the course of pregnancy and pregnancy outcome, and the health status of the mother and child during and after pregnancy.25 The participation rate after the initial invitation was 38.5%.26, 27 The MBRN28 comprises all births in Norway and has been prospectively collecting data on all deliveries since 1967.
Information from The Norwegian Mother and Child Cohort Study was acquired from four self-administered questionnaires answered by pregnant women who participated in the study between 1999 and 2006. The questionnaires covered sociodemographic and lifestyle characteristics, maternal medical history, maternal health during pregnancy, drug use, and neonatal and infant health during the first six and eighteen months of age. The first questionnaire, sent together with a postal invitation with an informed consent form prior to the first ultrasound examination, covered the time period between six months prior to pregnancy and gestational week 18. The second questionnaire covered the time period between gestational weeks 19 and 29, the third questionnaire covered the time period up to delivery and the first six months post partum, and the fourth questionnaire covered the time period between six and eighteen months post partum. The response rate was 95% for the first questionnaire, 92% for the second questionnaire, 87% for the third questionnaire, and 77% for the fourth questionnaire among those who agreed to participate in The Norwegian Mother and Child Cohort Study. 27
The MBRN contains detailed medical information regarding the newborn originating from mandatory notification forms completed by midwives, obstetricians, and/or paediatricians at delivery and during the hospital stay.29 In addition, the MBRN contains all information recorded during pregnancy in the woman’s maternity form (a standardised form with medical information from every pregnancy visit for all pregnant women in Norway). The forms encompass maternal sociodemographic and lifestyle characteristics, maternal health prior to and during pregnancy and information on the course of delivery and postpartum complications and interventions.
Data from the Norwegian Mother and Child Cohort Study and MBRN were linked via the women’s unique personal identification number allocated to everyone legally residing in Norway.
Study population
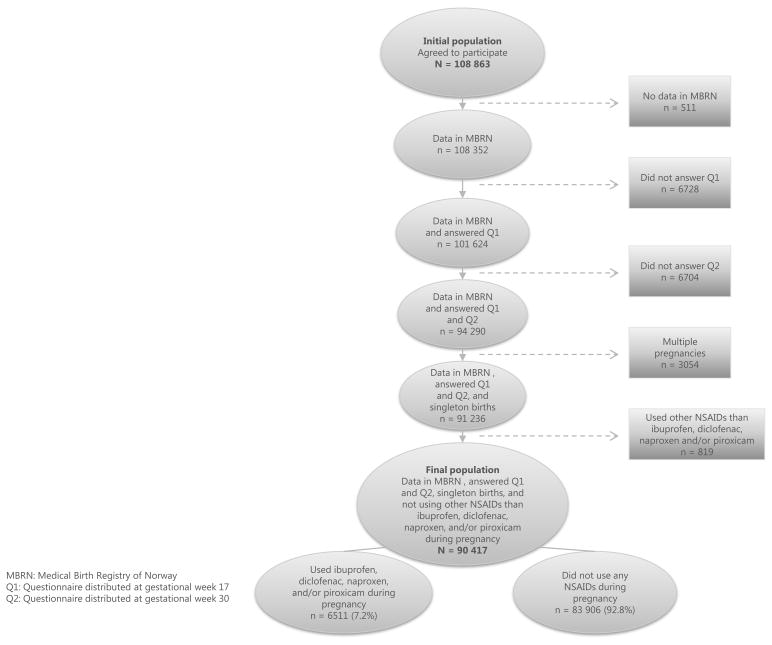
A total of 94 290 pregnant women who had records from the first and second questionnaires of The Norwegian Mother and Child Cohort Study and from the MBRN were eligible for inclusion in our study. This corresponds to 86.6% of the population in the initial quality assured data file consisting of 108 863 subjects. Multiple pregnancies (n = 3,054) were excluded. The exposed group consisted of pregnant women reporting the intake of one or several of the four most frequently used oral NSAIDs ibuprofen, diclofenac, naproxen, or piroxicam. Women who used other NSAIDs (i.e. acetylsalicylic acid, indomethacin, celecoxib, ketoprofen, tolfenamic acid, meloxicam, and nabumetone) (n = 819) were excluded from the study. The final study population therefore consisted of 90 417 women; 6511 exposed and 83 906 non-exposed (figure 1).
Figure 1.
Explanatory variables
Information on the type and timing of NSAID use was available from the three study questionnaires at gestational week 17 and 30 and 6 months after delivery. The questionnaires can be accessed via the following links: http://www.fhi.no/dokumenter/1f32a49514.pdf http://www.fhi.no/dokumenter/7b6b32b0cd.pdf http://www.fhi.no/dokumenter/9ecca1c459.pdf http://www.fhi.no/dokumenter/2640dd4bcc.pdf. Several indications relevant for NSAIDs use such as pelvic girdle pain, back pain, neck and shoulder pain, arthritis, sciatica, and fever were specifically named to increase the reporting of these medications. One or several medications could be reported for each indication. The respondent could specify five exposure windows for each indication; in the first questionnaire: 6 months before pregnancy, gestational weeks 0–4, 5–8, 9–12, and 13 + (until completion of the first questionnaire), in the second questionnaire five exposure weeks could be specified: weeks 13–16, 17–20, 21–24, 25–28, 29+ (until completion of the second questionnaire) and in the third questionnaire it was use towards the end of pregnancy (from gestational week 30 until birth). When two or more medications were reported for one indication, with several timings crossed out, we assumed that the medications have been used during all the time periods specified. No data on dosage were available. Data on the duration of treatment were incomplete and therefore not used. Drug exposure was classified according to the Anatomical Therapeutic Classification (ATC) system.30 We defined NSAIDs exposure as exposure to a drug belonging to the ATC code M01AE01 for ibuprofen, M01AB05 for diclofenac, M01AE02 for naproxen, and M01AC01 for piroxicam.
The effect of each of the four individual NSAIDs on the course of pregnancy and pregnancy outcome was analysed according to the timing of therapy:
“use during pregnancy (total)” (yes/no),
“use during the first trimester (gestational weeks 1 to 12)” (yes/no),
“use during the second trimester (gestational weeks 13 to 28)” (yes/no), and
“use during the third trimester (gestational week 29 until delivery)” (yes/no).
Outcome variables
The choice of outcome variables was based on current knowledge of NSAID pharmacology and results from prior studies. Information on outcome variables was obtained mainly from MBRN records. All diagnoses from the MBRN records are based on the International Classification of Diseases, 10th Revision (ICD-10).31 The chosen outcomes from the MBRN were infant survival (live birth), any congenital malformations, major congenital malformations, patent ductus arteriosus, birth weight < 2500g, gestational age < 37 weeks, Apgar score < 7 at 5 minutes, neonatal respiratory depression, intracranial haemorrhage, intraventricular haemorrhage, vaginal bleeding during pregnancy (including bleeding during the first and second and/or third trimesters), and postpartum haemorrhage >500ml. Two pregnancy outcomes, namely structural heart defect and asthma symptoms, were based upon the mother’s recordings in the fourth questionnaire at 18 months after delivery where she was specifically asked whether the child has been diagnosed with a congenital cardiac defect or had been referred to a specialist for asthma investigation. The outcome variables were dichotomised into yes or no categories.
Potential confounding factors
The confounding factors that we adjusted for are listed in Appendix S1. These included sociodemographic, lifestyle, and medical characteristics, concomitant drug use (this information was derived from the questionnaires of The Norwegian Mother and Child Cohort Study), factors related to delivery (this information was derived from the MBRN), and lifestyle factors and medical characteristics postpartum (this information was derived from the questionnaires of The Norwegian Mother and Child Cohort Study).
Statistical analysis
Significant associations between each of the four individual NSAIDs and pregnancy complications and outcome were measured using logistic regression. Risk ratio estimates are given as adjusted odds ratios (ORs) with 95% confidence intervals (CIs). For ORs significant at the 5 % level 99 % CIs are also presented due to the large number of comparisons made. The following analysis strategy was followed:
chi-square tests were used to assess the relationships between explanatory and outcome variables (all were categorical) and those with a p-value < 0.25 plus all that were clinically significant were selected for inclusion in the initial model
logistic regression was run on all the variables selected in step i) and variables with a high p-value (p > 0.5) were removed one by one (the coefficient change was checked after each removal so that it should not exceed 20%)
when only those variables with low p-values (p < 0.05) and those that lead to a significant (>20%) coefficient change were present in the model, all the variables not selected in step i) were inserted into the model and those that were significant were retained
a list of clinically plausible interactions among the variables in the model after step iii) was prepared
the interactions to be included in the final model were selected following steps i) and ii); however the required p-value for the interaction to be retained in the model was 0.05
the Hosmer and Lemeshow goodness-of-fit test value of > 0.05 had to be satisfied.
Potential multicollinearity among the independent variables was identified using multiple regression analysis. All statistical analyses were performed using the SPSS 19.0.0 for Windows (SPSS Inc., Chicago, IL, USA).
RESULTS
Of the 90 417 pregnant women included in the study (figure 1), 6511 (7.2%) reported using one of the NSAIDs ibuprofen, diclofenac, naproxen, or piroxicam, or a combination thereof during pregnancy (the exposed group), whereas 83 906 (92.8%) did not use any NSAIDs during pregnancy (the unexposed group). Table 1 shows the frequency of use of the individual NSAIDs anytime in pregnancy and during the specific trimesters.
Table 1.
Frequency of use of non-steroidal anti-inflammatory drugs (NSAIDs) by trimester in the study population (n = 90,417).
| NSAID | Anytime during pregnancy (total) | First trimester | Second trimester | Third trimester | ||||
|---|---|---|---|---|---|---|---|---|
| No. | % of n | No. | % of n | No. | % of n | No. | % of n | |
| Ibuprofen | 5325 | 5.9% | 3034 | 3.3% | 2126 | 2.3% | 882 | 1.0% |
| Diclofenac | 491 | 0.5% | 192 | 0.2% | 108 | 0.1% | 122 | 0.1% |
| Naproxen | 354 | 0.4% | 168 | 0.2% | 76 | 0.1% | 85 | 0.1% |
| Piroxicam | 150 | 0.2% | 82 | 0.1% | 23 | 0.0% | 22 | 0.0% |
| Several* | 191 | 0.2% | 53 | 0.1% | 30 | 0.0% | 29 | 0.0% |
| Total sum | 6511 | 7.2% | 3529 | 3.9% | 2363 | 2.6% | 1140 | 1.3% |
Women could report taking the drugs in more than one trimester.
A combination of any two or more of the four NSAIDs.
Tables 2 and 3 show maternal sociodemographic and medical characteristics. More NSAID users were overweight (body mass index > 25.0 kg/m2) prior to pregnancy, were on sick leave during pregnancy, smoked throughout pregnancy, and consumed alcohol once a week or more during pregnancy (table 2). Table 3 shows that women using NSAIDs were also more likely to suffer from various conditions and medical complications prior to and during pregnancy. Musculoskeletal pain, headache or migraine, and fever were particularly common in the exposed group and may be suggestive of the indication for NSAID use. Consequently, concomitant drug use was also more frequent in the exposed group. For example, paracetamol was used by 75.1% in the NSAID group compared to 45.3% in the unexposed group. Opioid analgesics were used by 7.1% vs. 1.9%, antidepressants by 2.4% vs. 1.2%, anxiolytics by 1.4% vs. 0.5%, hypnotics by 1.0% vs. 0.4%, and systemic corticosteroids by 1.3% vs. 0.6%, respectively.
Table 2.
Maternal characteristics of the study population.
| Women who used ibuprofen, diclofenac, naproxen, and/or piroxicam anytime during pregnancy (n = 6511) | Women who did not use any NSAID during pregnancy (n = 83,906) | |||
|---|---|---|---|---|
| No. | % of n | No. | % of n | |
| Maternal age (years) | ||||
| < 20 | 119 | 1.8% | 1673 | 2.0% |
| 20 – 29 | 2840 | 43.6% | 36,158 | 43.1% |
| 30 – 39 | 3474 | 53.4% | 42,292 | 54.0% |
| ≥ 40 | 78 | 1.2% | 783 | 0.9% |
| Parity | ||||
| 0 | 3157 | 48.5% | 37,932 | 45.2% |
| ≥1 | 3354 | 51.5%* | 45,974 | 54.8% |
| Mother tongue | ||||
| Norwegian | 5939 | 91.2% | 75,037 | 89.4% |
| Other | 572 | 8.8%* | 8869 | 10.6% |
| Marital status | ||||
| Married/ cohabiting | 6308 | 96.9% | 81,945 | 97.7% |
| Other | 203 | 3.1%* | 1961 | 2.3% |
| Education | ||||
| Primary | 139 | 2.1% | 1627 | 1.9% |
| Secondary | 2092 | 32.1% | 24,103 | 28.7% |
| Tertiary | 4151 | 63.8%* | 56,434 | 67.3% |
| BMI prior to pregnancy (kg/m2) | ||||
| < 18.5 | 178 | 2.7% | 2500 | 3.0% |
| 18.5–25.0 | 3848 | 59.1% | 54,726 | 65.2% |
| > 25.0 | 2345 | 36.0%* | 24,482 | 29.2% |
| Daily folic acid intake prior to pregnancy | 2076 | 31.9%* | 30,549 | 36.4% |
| In vitro fertilisation | 86 | 1.3%* | 1739 | 2.1% |
| Sick leave lasting longer than 2 weeks during pregnancy | 2563 | 39.4%* | 27,907 | 33.3% |
| Smoking daily at gestational week 30 | 393 | 6.0%* | 2909 | 3.5% |
| Alcohol intake | ||||
| Never | 5270 | 80.9%† | 66,707 | 79.5% |
| Once a week | 349 | 5.4%* | 3274 | 3.9% |
| Up to three times a week | 109 | 1.7%* | 913 | 1.1% |
Some data do not add up to the total due to missing values.
BMI = body mass index
Pearson’s χ2 test p < 0.001 when compared with the unexposed control group.
Pearson’s χ2 test p < 0.05 when compared with the unexposed control group.
Table 3.
Self-reported maternal health and pregnancy complications of the study population.
| Women who used ibuprofen, diclofenac, naproxen, and/or piroxicam anytime during pregnancy (n = 6511) | Women who did not use any NSAID during pregnancy (n = 83,906) | |||
|---|---|---|---|---|
| No. | % of n | No. | % of n | |
| MATERNAL HEALTH DURING PREGNANCY | ||||
| Musculoskeletal pain | 6206 | 95.3%* | 76,429 | 91.1% |
| Headache including migraine | 4289 | 65.9%* | 27,543 | 32.8% |
| Temperature> 38.5°C | 1342 | 21.8%* | 14,172 | 16.9% |
| Proteinuria | 890 | 13.7%* | 9714 | 11.6% |
| Urinary tract infection and/or pyelonephritis | 464 | 7.1%† | 5291 | 6.3% |
| Hospitalisation‡ | 417 | 6.4%* | 3958 | 4.7% |
| Involved in an accident | 293 | 4.5%* | 2839 | 3.4% |
| Preeclampsia and/or eclampsia | 294 | 4.5%* | 3061 | 3.6% |
| High blood pressure during the first trimester § | 284 | 4.4%† | 2998 | 3.6% |
| MATERNAL HEALTH PRIOR TO PREGNANCY | ||||
| Musculoskeletal pain | 3701 | 56.8%* | 40,565 | 48.3% |
| Rheumatoid arthritis/systemic lupus erythematosus/fibromyalgia | 551 | 8.5%* | 2614 | 3.1% |
| Depression | 637 | 9.8%* | 5120 | 6.1% |
| Asthma | 517 | 7.9% | 6184 | 7.4% |
| Cardiac disease | 62 | 1.0% | 741 | 0.9% |
| Thyroid disorder | 191 | 2.9% | 2173 | 2.6% |
| Diabetes (type I or II) | 32 | 0.5% | 387 | 0.5% |
Pearson’s χ2 test p < 0.001 when compared with the unexposed control group.
Pearson’s χ2 test p < 0.01 when compared with the unexposed control group.
Excluding hospitalisation due to vaginal bleeding and high blood pressure.
Defined as systolic blood pressure ≥ 140mmHg.
Associations between the use of the four NSAIDs and congenital malformations are shown in table 4. No significant difference in the survival, overall congenital malformation, major congenital malformation, or structural heart defect rate was found when comparing first trimester use with the unexposed group. There was a borderline association between ibuprofen use during the first trimester and structural heart defects detected in the infant during the first 18 months of life (adjusted OR 1.2, 95% CI 1.0 to 1.6).
Table 4.
Adjusted Odds Ratios (OR) for congenital malformations detected at birth in children of women who used ibuprofen, diclofenac, naproxen, or piroxicam during pregnancy compared with the unexposed control group.
| Any congenital malformation* | ||||||||
|---|---|---|---|---|---|---|---|---|
| Use during pregnancy (total) | Use during the first trimester | |||||||
| No./n | % of n | OR | 95%CI | No./n | % of n | OR | 95%CI | |
| Women who did not use any NSAIDs during pregnancy. | 4010/83,906 | 4.8% | Ref. | Ref. | 4010/83,906 | 4.8% | Ref. | Ref. |
|
| ||||||||
| Ibuprofen | 247/5325 | 4.6% | 1.0 | 0.8 – 1.1 | 140/3034 | 4.6% | 1.0 | 0.8 – 1.1 |
| Diclofenac | 17/491 | 3.5% | 0.7 | 0.4 – 1.2 | 5/192 | 2.6% | 0.5 | 0.2 – 1.3 |
| Naproxen | 14/354 | 4.0% | 0.8 | 0.5 – 1.4 | 9/168 | 5.4% | 1.1 | 0.6 – 2.2 |
| Piroxicam | 6/150 | 4.0% | 0.8 | 0.4 – 1.9 | 4/82 | 4.9% | 1.0 | 0.4 – 2.8 |
| Major congenital malformation* | ||||||||
|---|---|---|---|---|---|---|---|---|
| Use during pregnancy (total) | Use during the first trimester | |||||||
| No./n | % of n | OR | 95%CI | No./n | % of n | OR | 95%CI | |
| Women who did not use any NSAIDs during pregnancy. | 2203/83,906 | 2.6% | Ref. | Ref. | 2203/83,906 | 2.6% | Ref. | Ref. |
|
| ||||||||
| Ibuprofen | 130/5325 | 2.4% | 0.9 | 0.8 – 1.1 | 73/3034 | 2.4% | 0.9 | 0.7 – 1.2 |
| Diclofenac | 12/491 | 2.4% | 0.9 | 0.5 – 1.6 | 2/192 | 1.0% | 0.4 | 0.1 – 1.5 |
| Naproxen | 9/354 | 2.5% | 1.0 | 0.5 – 1.9 | 7/168 | 4.2% | 1.6 | 0.7 – 3.5 |
| Piroxicam | 3/150 | 2.0% | 0.7 | 0.2 – 2.3 | 2/82 | 2.4% | 0.9 | 0.2 – 3.8 |
| Structural heart defect detected during the first 18 months of age† | ||||||||
|---|---|---|---|---|---|---|---|---|
| Use during pregnancy (total) | Use during the first trimester | |||||||
| Women who did not use any NSAIDs during pregnancy. | No./n | % of n | OR | 95%CI | No./n | % of n | OR | 95%CI |
| 1001/83,906 | 1.2% | Ref. | Ref. | 1001/83,906 | 1.2% | Ref. | Ref. | |
|
| ||||||||
| Ibuprofen | 65/5325 | 1.2% | 1.0 | 0.8 – 1.3 | 44/3034 | 1.5% | 1.2 | 1.0 – 1.6 |
| Diclofenac | 9/491 | 1.8% | 1.4 | 0.7 – 2.8 | 2/192 | 1.0% | 0.8 | 0.2 – 3.5 |
| Naproxen | 2/354 | 0.6% | 0.9 | 0.5 – 1.9 | 1/168 | 0.6% | - | - |
| Piroxicam | 4/150 | 2.7% | 2.3 | 0.8 – 6.2 | 2/82 | 2.4% | 2.3 | 0.5 – 9.3 |
Data registered in the Medical Birth Registry of Norway.
Data self-reported by mother. Only infants referred to a specialist were included.
Associations between the use of the four NSAIDs and maternal bleeding are shown in table 5. An increased likelihood of vaginal bleeding in the second and/or third trimesters and postpartum haemorrhage was found to be associated with diclofenac use towards the end of pregnancy. Compared with the unexposed group, the associations were 13.1% vs. 7.1% (adjusted OR 1.8, 95% CI 1.1 to 3.0); and 27.8% vs. 15.3% (adjusted OR 1.9, 95%CI 1.2 to 2.9), respectively. The associations did not remain significant at the one per cent level (adjusted OR 1.8, 99% CI 0.9 to 3.6 and adjusted OR 1.9, 99% CI 1.0 to 3.3, respectively).
Table 5.
Adjusted Odds Ratios (OR) for maternal bleeding associated with ibuprofen, diclofenac, naproxen, or piroxicam use during pregnancy.
| Bleeding disorder | Women who used ibuprofen, diclofenac, naproxen, or piroxicam during pregnancy. | Women who did not use any NSAIDs during pregnancy. (n = 83,906) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Use during pregnancy (total) | Use during the first trimester | Use during the second trimester | Use during the third trimester | |||||||||||||||
| No. | % of n | OR | 95%CI | No. | % of n | OR | 95%CI | No. | % of n | OR | 95%CI | No. | % of n | OR | 95%CI | No. | % of n | |
| Vaginal bleeding during the first trimester* | 13,129 | 15.6% | ||||||||||||||||
| Ibuprofen | 517 | 17.0% | 1.1 | 1.0–1.2 | Ref. | Ref. | ||||||||||||
| Diclofenac | 24 | 12.5% | 0.7 | 0.5–1.1 | Ref. | Ref. | ||||||||||||
| Naproxen | 34 | 20.2% | 1.3 | 0.9–1.9 | Ref. | Ref. | ||||||||||||
| Piroxicam | 15 | 18.3% | 1.2 | 0.7–2.1 | Ref. | Ref. | ||||||||||||
| Vaginal bleeding during the second and/or third trimesters* | 5999 | 7.1% | ||||||||||||||||
| Ibuprofen | 442 | 8.3% | 1.1 | 1.0–1.2 | 249 | 8.2% | 1.1 | 1.0–1.3 | 181 | 8.5% | 1.1 | 1.0–1.3 | 76 | 8.6% | 1.1 | 0.9–1.4 | Ref. | Ref. |
| Diclofenac | 43 | 8.8% | 1.2 | 0.8–1.6 | 11 | 5.7% | 0.7 | 0.4–1.4 | 8 | 7.4% | 1.0 | 0.5–2.0 | 16 | 13.1% | 1.8 | 1.1–3.0 | Ref. | Ref. |
| Naproxen | 20 | 5.6% | 0.7 | 0.4–1.1 | 11 | 6.5% | 0.8 | 0.5–1.6 | 6 | 7.9% | 1.0 | 0.4–2.3 | 3 | 3.5% | 0.4 | 0.1–1.4 | Ref. | Ref. |
| Piroxicam | 14 | 9.3% | 1.3 | 0.7–2.2 | 9 | 11.0% | 1.6 | 0.8–3.2 | 1 | 4.3% | - | - | 1 | 4.5% | - | - | Ref. | Ref. |
| Postpartum haemorrhage† | 12,849 | 15.3% | ||||||||||||||||
| Ibuprofen | 822 | 15.4% | 1.0 | 0.9–1.1 | 466 | 15.4% | 1.0 | 0.9–1.1 | 305 | 14.3% | 0.9 | 0.8–1.0 | 132 | 15.0% | 0.9 | 0.8–1.1 | Ref. | Ref. |
| Diclofenac | 100 | 20.4% | 1.2 | 1.0–1.5 | 37 | 19.3% | 1.2 | 0.8–1.7 | 30 | 27.8% | 1.9 | 1.2–2.9 | 24 | 19.7% | 1.0 | 0.6–1.6 | Ref. | Ref. |
| Naproxen | 62 | 17.5% | 1.1 | 0.8–1.4 | 27 | 16.1% | 0.9 | 0.6–1.4 | 21 | 27.6% | 1.1 | 1.0–2.8 | 19 | 22.4% | 1.6 | 0.9–2.7 | Ref. | Ref. |
| Piroxicam | 19 | 12.7% | 0.8 | 0.5–1.3 | 7 | 8.5% | 0.5 | 0.2–1.1 | 1 | 4.3% | - | - | 3 | 13.6% | 1.1 | 0.3–3.6 | Ref. | Ref. |
Data from both the Medical Birth Registry of Norway and self-reported vaginal bleeding lasting more than 1 day or of an amount exceeding a trace or two or more episodes of bleeding or vaginal bleeding which led to hospitalisation.Second and third trimester data on bleeding had to be evaluated together because trimester-specific data were not available.
Haemorrhage > 500ml (MBRN).
The effects of the four individual NSAIDs on other pregnancy outcomes are shown in table 6. An increased risk of low birth weight (< 2500g) after ibuprofen exposure in the second trimester (4.1% vs. 2.5%; adjusted OR 1.7, 95% CI 1.3 to 2.3) was detected, however, within a normal baseline risk range. This association remained significant at the one per cent level (adjusted OR 1.7, 99% CI 1.2 to 2.5). Diclofenac use during the second trimester was also associated with low birth weight (6.5 % vs. 2.5%; adjusted OR 3.1, 95% CI 1.1 to 9.0) but this association did not remain significant at the one per cent level (adjusted OR 3.1, 99% CI 0.8 to 12.5). No significant associations with patent ductus arteriosus or intraventricular haemorrhage were found when logistic regression analyses were performed. However, there were three cases of patent ductus arteriosus when diclofenac was used during the third trimester (1.3% vs. 0.3%). Similarly, there was one case of intraventricular haemorrhage when diclofenac had been used during the third trimester (4.8% vs. 0.0%).
Table 6.
Adjusted Odds Ratios (OR) for pregnancy outcomes associated with ibuprofen, diclofenac, naproxen, or piroxicam exposure during pregnancy.
| Pregnancy outcome | Women who used ibuprofen, diclofenac, naproxen, or piroxicam during pregnancy. | Women who did not use any NSAIDs during pregnancy. (n = 83,906) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Use during pregnancy (total) | Use during the first trimester | Use during the second trimester | Use during the third trimester | |||||||||||||||
| No. | % of n | OR | 95%CI | No. | % of n | OR | 95%CI | No. | % of n | OR | 95%CI | No. | % of n | OR | 95%CI | No. | % of n | |
| Birth weight < 2500g | 2066 | 2.5% | ||||||||||||||||
| Ibuprofen | 178 | 3.3% | 1.4 | 1.1–1.6 | 97 | 3.2% | 1.2 | 0.9–1.6 | 88 | 4.1% | 1.7 | 1.3–2.3 | 27 | 3.1% | 1.1 | 0.7–1.8 | Ref. | Ref. |
| Diclofenac | 24 | 4.9% | 1.6 | 0.9–2.9 | 10 | 5.2% | 2.4 | 1.0–5.6 | 7 | 6.5% | 3.1 | 1.1–9.0 | 8 | 6.6% | 1.3 | 0.5–3.7 | Ref. | Ref. |
| Naproxen | 12 | 3.4% | 1.5 | 0.8–3.0 | 6 | 3.6% | 1.4 | 0.5–3.6 | 2 | 2.6% | 0.8 | 0.1–4.1 | 1 | 1.2% | - | - | Ref. | Ref. |
| Piroxicam | 4 | 2.7% | 1.0 | 0.3–3.5 | 2 | 2.4% | 1.1 | 0.2–6.4 | 0 | 0.0% | - | - | 1 | 4.5% | - | - | Ref. | Ref. |
| Gestational age < 37 weeks | 3651 | 4.4% | ||||||||||||||||
| Ibuprofen | 260 | 4.9% | 1.1 | 1.0–1.3 | 155 | 5.1% | 1.2 | 1.0–1.4 | 112 | 5.3% | 1.2 | 0.9–1.4 | 42 | 4.8% | 1.0 | 0.8–1.4 | Ref. | Ref. |
| Diclofenac | 32 | 6.5% | 1.3 | 0.9–1.9 | 10 | 5.2% | 1.1 | 0.6–2.1 | 6 | 5.6% | 1.2 | 0.5–2.7 | 12 | 9.8% | 1.7 | 0.9–3.3 | Ref. | Ref. |
| Naproxen | 19 | 5.4% | 1.2 | 0.7–1.9 | 11 | 6.5% | 1.6 | 0.8–2.7 | 4 | 5.3% | 0.9 | 0.3–2.5 | 2 | 2.4% | 0.5 | 0.1–2.0 | Ref. | Ref. |
| Piroxicam | 7 | 4.7% | 1.1 | 0.5–2.3 | 3 | 3.7% | 0.8 | 0.3–2.7 | 0 | 0.0% | - | - | 1 | 4.5% | - | - | Ref. | Ref. |
| Apgar score < 7 at 5 min | 1021 | 1.2% | ||||||||||||||||
| Ibuprofen | 59 | 1.1% | 0.8 | 0.6–1.1 | 31 | 1.0% | 0.7 | 0.5–1.1 | 23 | 1.1% | 0.9 | 0.5–1.4 | 8 | 0.9% | 0.9 | 0.4–1.7 | Ref. | Ref. |
| Diclofenac | 4 | 0.8% | 0.4 | 0.1–1.1 | 2 | 1.0% | 0.4 | 0.1–1.9 | 1 | 0.9% | - | - | 1 | 0.8% | - | - | Ref. | Ref. |
| Naproxen | 4 | 1.1% | 0.7 | 0.2–2.2 | 2 | 1.2% | 1.0 | 0.2–4.6 | 1 | 1.3% | - | - | 1 | 1.2% | - | - | Ref. | Ref. |
| Piroxicam | 2 | 1.3% | 0.8 | 0.2–3.8 | 2 | 2.4% | 1.7 | 0.3–9.3 | 0 | 0.0% | - | - | 0 | 0.0% | - | - | Ref. | Ref. |
| Neonatal respiratory depression | 666 | 0.8% | ||||||||||||||||
| Ibuprofen | 45 | 0.8% | 0.9 | 0.7–1.3 | 21 | 0.7% | 0.8 | 0.5–1.2 | 19 | 0.9% | 0.8 | 0.5–1.4 | 2 | 0.2% | 0.2 | 0.1–1.0 | Ref. | Ref. |
| Diclofenac | 4 | 0.8% | 0.5 | 0.2–1.6 | 3 | 1.6% | 1.3 | 0.4–4.7 | 3 | 2.8% | 2.1 | 0.6–8.0 | 1 | 0.8% | - | - | Ref. | Ref. |
| Naproxen | 3 | 0.8% | 1.0 | 0.3–3.3 | 2 | 1.2% | 1.3 | 0.3–5.7 | 0 | 0.0% | - | - | 1 | 1.2% | - | - | Ref. | Ref. |
| Piroxicam | 0 | 0.0% | - | - | 0 | 0.0% | - | - | 0 | 0.0% | - | - | 0 | 0.0% | - | - | Ref. | Ref. |
| Referral to specialist for asthmatic symptoms in the infant during the first 18 months of age* | 2009 | 2.4% | ||||||||||||||||
| Ibuprofen | 182 | 3.4% | 1.3 | 1.1–1.5 | 96 | 3.2% | 1.2 | 1.0–1.5 | 86 | 4.0% | 1.5 | 1.2–1.9 | 40 | 4.5% | 1.5 | 1.1–2.1 | Ref. | Ref. |
| Diclofenac | 19 | 3.9% | 1.3 | 0.8–2.1 | 8 | 4.2% | 1.4 | 0.7–2.9 | 6 | 5.6% | 1.9 | 0.8–1.3 | 5 | 4.1% | 1.4 | 0.6–3.4 | Ref. | Ref. |
| Naproxen | 7 | 2.0% | 0.7 | 0.3–1.5 | 4 | 2.4% | 0.8 | 0.3–2.3 | 2 | 2.6% | 0.9 | 0.2–3.7 | 2 | 2.4% | 0.8 | 0.2–3.4 | Ref. | Ref. |
| Piroxicam | 3 | 2.0% | 0.7 | 0.2–2.3 | 1 | 1.2% | - | - | 0 | 0.0% | - | - | 0 | 0.0% | - | - | Ref. | Ref. |
Data self-reported by mother. Only infants referred to a specialist for asthma diagnosis were included.
An association between ibuprofen use during the second and third trimesters and an increased risk of asthma detected in the infant at 18 months of age was found (4.0% and 4.5% respectively vs. 1.2%; adjusted OR 1.5 (95% CI 1.2 to 1.9) and 1.5 (1.1 to 2.1), respectively). The association with second trimester use remained significant at the one per cent level (adjusted OR, 1.5 99% CI 1.2 to 2.0) but not with third trimester use (adjusted OR 1.5, 99% CI 1.0 to 2.3). To explore this finding further we performed stratified analyses and found no effect modification by underlying medical conditions most commonly associated with NSAID use (musculoskeletal pain and headache and/or migraine (results not presented).
DISCUSSION
The principal finding in the present study is that there were no associations between the use of any of the four NSAIDs and congenital malformations. There was an increased risk of maternal vaginal bleeding associated with diclofenac use late in pregnancy. Finally there was a significant association between asthma in the child and ibuprofen use late in pregnancy.
Strengths and limitations
This study has several weaknesses that need to be taken into consideration. The participation rate in the Norwegian Mother and Child Cohort Study was 38.5%, and this may have caused selection bias, especially with regard to prevalence estimates (women under the age of 25, without a partner, multipara, smokers, and stillbirth and neonatal death cases were all underreported whereas folic acid and multivitamin users were overrepresented). However, two previous studies have concluded that only minor differences were found (under 2% in absolute differences in sociodemographic variables) between the Norwegian Mother and Child Cohort Study participants and the general Norwegian population of pregnant women, and no differences in the estimates of association measures were reported between the participants and the general population of pregnant women.26, 27 As shown in figure 1, 13% of the women were excluded from the study because they did not complete questionnaire Q1 and/or Q2. We have no reason to believe that these women differed from those who completed these questionnaires, but even so, we consider that although it might have affected the prevalence of medication use, it is unlikely that this factor has affected the associations between NSAID exposure and pregnancy outcomes. Notwithstanding, the risk of misclassification errors due to possible underreporting of NSAID use during pregnancy might bias the effect estimates towards one. On the other hand, some misclassification in the opposite direction may also have occurred due to the way multiple medications were coded when used in multiple time periods. We could not assess dosage and duration of NSAID use in detail; data which would have given us information about possible dose-response effects. The higher frequency of concomitant medication among NSAID users also merits attention; however, comedication with potential teratogens and other medications with possible effects on certain pregnancy outcomes and commonly used by the women have been controlled for in the analyses. Despite the large sample size, we lacked study power to detect a possible increase in specific congenital anomalies (and rare pregnancy outcomes). Finally, due to the large number of analyses performed we cannot exclude the possibility that the associations we did find were caused by mass significance, although some of these associations were significant also at the one per cent level. Our results must be interpreted with these limitations in mind.
On the other hand, the fact that our study included such a large number of participants made it possible to analyse the effect of individual NSAIDs on pregnancy outcome instead of evaluating the group effect of these drugs. Few studies have achieved this so far. We included both over-the-counter and prescription NSAID use in our study, and the control group consisted of women who did not use any NSAIDs during pregnancy. Previous studies have used data from prescription registries, so that only the prescribed NSAID use could be taken into account. In addition, in their control groups, women using over-the-counter NSAIDs were not excluded. 14, 15, 19 In the present study, we were able to avoid these potential limitations. Due to the vast amount of data in The Norwegian Mother and Child Cohort Study and the MBRN, many important confounding factors, in particular underlying medical conditions, pregnancy complications and lifestyle and medical characteristics of the infant were adjusted for in our study. This rigorous control for confounding factors has not been achieved in previous studies. Another strength of our study was the accuracy of registration of major congenital malformations, which is confirmed to be high.32, 33 Finally, recall bias was avoided by the prospective collection of the majority of the data.
Interpretation of results
This study, like previous ones 2–8, has shown that NSAID use, and ibuprofen use in particular, was quite common during pregnancy. We found that approximately 1 out of 14 women reported such use. Furthermore, a relatively high proportion of NSAID users was on sick leave, suffered from medical complications during pregnancy and used other analgesics concomitantly. Despite the fact that NSAID use from gestational week 28 is essentially contraindicated34, 1.3% used any of the four NSAIDs during the third trimester and more than half of these initiated NSAID therapy during this period. It is not unlikely that these women needed NSAID therapy during the third trimester as exacerbations of musculoskeletal conditions may occur during this period. It has also been shown that many women are still not sufficiently informed about the safety of NSAIDs during pregnancy35, and there is a possibility that at least some of the women continued taking these drugs during the third trimester without their physician’s knowledge.
In regards to congenital malformations, the results from this large prospective cohort study are generally reassuring. No significant associations were found between the use of ibuprofen, diclofenac, naproxen, or piroxicam during the first trimester of pregnancy and an increased risk of overall or major congenital malformation rates. This is in accordance with the findings of other studies.14, 17, 18 In addition, and contrary to the results presented by two previous studies15, 16, no significant association was found between first trimester use of either of the four NSAIDs and an increased risk of structural heart defect. This result is of particular interest given the nature and number of confounding factors we have been able to adjust for in our analyses (Appendix S1).
In relation to vaginal bleeding and postpartum haemorrhage, there was some association with diclofenac use towards the end of pregnancy. Even though diclofenac has a relatively high selectivity for the cyclooxygenase-2 (COX-2) enzyme, 70% of COX-1, the enzyme associated with platelet aggregation, is also inhibited at therapeutic levels of the drug.36 In addition, diclofenac has been shown to decrease uterine contractility in vitro37, a factor implicated in postpartum haemorrhage. It should be noted that diclofenac use in the second trimester only, and not in the third trimester, was associated with postpartum haemorrhage. This last association thus seems pharmacologically implausible, and, taking Hill’s criteria for causality38 into consideration, may indicate that the association could be a coincidence and possibly subject to residual confounding.
We found a significantly increased risk of low birth weight after second trimester exposure to ibuprofen and diclofenac, but not after exposure to naproxen or piroxicam. This pregnancy outcome could at least in part be a result of underlying disease: for example, studies evaluating the effect of rheumatoid arthritis on pregnancy outcome have found an association with low birth weight.39–41 Nevertheless, we adjusted for maternal musculoskeletal disease in our analyses, so an independent effect of these drugs on low birth weight can not be ruled out. On the other hand, it was not possible to adjust for the severity of the diseases, so a confounding effect might still exist, and because of this we cannot state with certainty that there is a direct causative effect of the two drugs.38
An association was found between ibuprofen use during the second and third trimester and infant asthma at 18 months. The association remained significant even after adjustment for several potentially important confounding factors including preterm delivery, smoking during pregnancy, in utero exposure to paracetamol, maternal asthma, breastfeeding, infant exposure to cigarette smoke, and post-partum infant exposure to ibuprofen and paracetamol, both directly and concurrently with breastfeeding. Terminal bronchioles are not developed before gestational week 28, and as postulated in one of the studies on the effect of paracetamol exposure in utero on the development of asthma in children42–44, fetal lungs are more likely to be influenced by external factors later in pregnancy from the end of second trimester and onwards.45 It has also been shown that it is COX-1 that is the prevailing isoform in the airways and its inhibition leads to bronchoconstriction.46 Ibuprofen inhibits 90% of COX-1 at maximum plasma concentration.47 In addition, bronchial hyper responsiveness at four weeks of age is associated with asthma in the six and 11 year old child.48 All these factors support a possible causal relationship between ibuprofen use late in pregnancy and asthma in the 18 month old child. Based upon a common mechanism of action among all NSAIDs, it is interesting that exposure to the other three drugs was not associated with asthma. This difference could be explained by a lack of power for the other drugs, as the numbers of exposed subjects were considerably lower for these. This finding warrants further investigation.
CONCLUSION
The lack of associations with congenital malformations in the present study is particularly reassuring, despite the borderline association found between first trimester ibuprofen use and congenital heart defects. Maternal vaginal bleeding during pregnancy and postpartum was associated with diclofenac use. In addition, asthmatic symptoms in the child at 18 months were associated with ibuprofen use, and this could be a result of the mechanism of action of these drugs. The increased risk of low birth weight associated with both ibuprofen and diclofenac may, at least in part, be attributable to underlying inflammatory conditions, and was, reassuringly, within the normal baseline risk range of low birth weight.
Supplementary Material
Acknowledgments
The Norwegian Mother and Child Cohort Study is supported by the Norwegian Ministry of Health and the Ministry of Education and Research, NIH/NIEHS (contract no NO-ES-75558), NIH/NINDS (grant No.1 UO1 NS 047537-01), and the Norwegian Research Council/FUGE (grant no.151918/S10). We are grateful to all the participants and their families for taking part in this study.
Footnotes
Disclosure of Interests
None declared.
Contribution to Authorship
KNH performed the statistical analysis and wrote the article. KNH, OS, and HN planned the study, interpreted the results, revised the article and approved the final draft of the article. HN had the overall study responsibility.
Details of Ethics Approval
The study was approved by the Regional Committee for Ethics in Medical Research, Region South, and the Norwegian Data Inspectorate.
References
- 1.Li D, Liu L, Odouli R. Exposure to non-steroidal anti-inflammatory drugs during pregnancy and risk of miscarriage: population based cohort study. BMJ. 2003;327:368–72. doi: 10.1136/bmj.327.7411.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Olesen C, Steffensen F, Nielsen G, de Jong-van den Berg L, Olsen J. Drug use in first pregnancy and lactation: a population-based survey among Danish women. Eur J Clin Pharmacol. 1999;55:139–44. doi: 10.1007/s002280050608. [DOI] [PubMed] [Google Scholar]
- 3.Werler M, Mitchell A, Hernandez-Diaz S, Honein M the National Birth Defects Prevention Study. . Use of over-the-counter medications during pregnancy. Am J Obstet Gynecol. 2005;193:771–7. doi: 10.1016/j.ajog.2005.02.100. [DOI] [PubMed] [Google Scholar]
- 4.Bonati M, Bortulus R, Marchetti F, Romero M, Tognoni G. Drug use in pregnancy: an overview of epidemiological (drug utilization) studies. Eur J Clin Pharmacol. 1990;38:325–8. doi: 10.1007/BF00315569. [DOI] [PubMed] [Google Scholar]
- 5.Nordeng H, Eskild A, Nesheim B, Aursnes I, Jacobsen G. Drug use during early pregnancy. Eur J Clin Pharmacol. 2001;57:529–63. doi: 10.1007/s002280100304. [DOI] [PubMed] [Google Scholar]
- 6.Nezvalová-Henriksen, Spigset O, Nordeng H. Maternal characteristics and migraine pharmacotherapy during pregnancy: Cross sectional analysis of data from a large cohort study. Cephalalgia. 2009;29:1267–76. doi: 10.1111/j.1468-2982.2009.01869.x. [DOI] [PubMed] [Google Scholar]
- 7.Nezvalová-Henriksen K, Spigset O, Nordeng H. Triptan exposure during pregnancy and the risk of major congenital malformations and adverse pregnancy outcomes: results from the Norwegian Mother and Child Cohort Study. Headache. 2010;50:563–75. doi: 10.1111/j.1526-4610.2010.01619.x. [DOI] [PubMed] [Google Scholar]
- 8.Nezvalová-Henriksen K, Spigset O, Nordeng H. Effects of codeine on pregnancy outcome: results from a large population-based cohort study. Eur J Clin Pharmacol. 2011;67:1253–62. doi: 10.1007/s00228-011-1069-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ertresvåg J, Zwart J-A, Helde G, Johnsen H-J, Bovim G. Headache and transient focal neurological symptoms during pregnancy, a prospective cohort. Acta Neurol Scand. 2005;111:233–7. doi: 10.1111/j.1600-0404.2005.00350.x. [DOI] [PubMed] [Google Scholar]
- 10.Marcus D, Scharff L, Turk D. Longitudinal prospective study of headache during pregnancy and postpartum. Headache. 1999;39:625–32. doi: 10.1046/j.1526-4610.1999.3909625.x. [DOI] [PubMed] [Google Scholar]
- 11.Fast A, Shapiro D, Ducommun E, Friedmann L, Bouklas T, Floman Y. Low-back pain in pregnancy. Spine. 1987;12:368–71. doi: 10.1097/00007632-198705000-00011. [DOI] [PubMed] [Google Scholar]
- 12.Mantle M, Greenwood R, Currey H. Backache in pregnancy. Rheumatol Rehabil. 1977;16:95–101. doi: 10.1093/rheumatology/16.2.95. [DOI] [PubMed] [Google Scholar]
- 13.Østensen M. Prescribing in pregnancy - Rheumatological disorders. Best Pract Res Clin Obstet Gynecol. 2001;15:953–69. doi: 10.1053/beog.2001.0240. [DOI] [PubMed] [Google Scholar]
- 14.Nielsen G, Sørensen H, Larsen H, Pedersen L. Risk of adverse birth outcome and miscarriage in pregnant users of non-steroidal anti-inflammatory drugs: population based observational study and case-control study. BMJ. 2001;322:266–70. doi: 10.1136/bmj.322.7281.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ofori B, Oraichi D, Blais L, Rey E, Berard A. Risk of congenital anomalies in pregnant users of non-steroidal anti-inflammatory drugs: A nested case-control study. Birth Defects Res B Dev Reprod Toxicol. 2006;77:268–79. doi: 10.1002/bdrb.20085. [DOI] [PubMed] [Google Scholar]
- 16.Ericson A, Kallen B. Nonsteroidal anti-inflammatory drugs in early pregnancy. Reprod Toxicol. 2001;15:371–5. doi: 10.1016/s0890-6238(01)00137-x. [DOI] [PubMed] [Google Scholar]
- 17.Kallen B, Olausson P. Maternal drug use in early pregnancy and infant cardiovascular defect. Reprod Toxicol. 2003;17:255–61. doi: 10.1016/s0890-6238(03)00012-1. [DOI] [PubMed] [Google Scholar]
- 18.Cleves M, Savell V, Raj S. Maternal use of acetaminophen and nonsteroidal anti-inflammatory drugs (NSAIDs), and muscular ventricular septal defects. Birth Defects Res A. 2004;70:107–13. doi: 10.1002/bdra.20005. [DOI] [PubMed] [Google Scholar]
- 19.Daniel S, Matok I, Gorodischer R, et al. Major Malformations Following Exposure to Nonsteroidal Antiinflammatory Drugs During the First Trimester of Pregnancy. The Journal of Rheumatology. 2012;39:2163–9. doi: 10.3899/jrheum.120453. [DOI] [PubMed] [Google Scholar]
- 20.Koren G, Florescu A, Costei A, Boskovic R, Moretti M. Nonsteroidal antiinflammatory drugs during third trimester and the risk of premature closure of the ductus arteriosus: a meta-analysis. Ann Pharmacother. 2006;40:824–9. doi: 10.1345/aph.1G428. [DOI] [PubMed] [Google Scholar]
- 21.Ostensen M, Skomsvoll J. Anti-inflammatory pharmacotherapy during pregnancy. Expert Opin Pharmacother. 2004;5:571–80. doi: 10.1517/14656566.5.3.571. [DOI] [PubMed] [Google Scholar]
- 22.Alano M, Ngougmna E, Ostrea E, Konduri G. Analysis of Nonsteroidal Antiinflammatory Drugs in Meconium and Its Relation to Persistent Pulmonary Hypertension of the Newborn. Pediatrics. 2001;107:519–23. doi: 10.1542/peds.107.3.519. [DOI] [PubMed] [Google Scholar]
- 23.Cuzzolin L, Dal Cere M, Fanos V. NSAID-induced nephrotoxicity from the fetus to the child. Drug Saf. 2001;24:9–18. doi: 10.2165/00002018-200124010-00002. [DOI] [PubMed] [Google Scholar]
- 24.Keaney N. Drug-induced lung disease. In: Walker R, Edwards C, editors. Clinical Pharmacy and Therapeutics. 3. London: Churchill Livingstone; 2003. pp. 414–5. [Google Scholar]
- 25.Norwegian Institute of Public Health. The Norwegian Mother and Child Cohort Study. Oslo, Norway: 2010. [Google Scholar]
- 26.Nilsen R, Vollset S, Gjessing H, et al. Self-selection and bias in a large prospective pregnancy cohort in Norway. Paediatr Perinat Epidemiol. 2009;23:597–608. doi: 10.1111/j.1365-3016.2009.01062.x. [DOI] [PubMed] [Google Scholar]
- 27.Magnus P, Irgens L, Haug K, et al. Cohort profile: The Norwegian mother and child cohort study (MoBa) Int J Epidemiol. 2006;35:1146–50. doi: 10.1093/ije/dyl170. [DOI] [PubMed] [Google Scholar]
- 28.Norwegian Institute of Public Health. Medisinsk fødselsregister. Oslo, Norway: 2010. [Google Scholar]
- 29.Irgens LM. The Medical Birth Registry of Norway. Epidemiological research and surveillance throughout 30 years. Acta Obstetricia et Gynecologica Scandinavica. 2000;79 (6):435–9. [PubMed] [Google Scholar]
- 30.WHO. ATC/DDD Index 2008. 2009. Collaborating Centre for Drug Statistics Methodology. [Google Scholar]
- 31.WHO. http://www.who.int/classifications/icd/en/
- 32.Melve K, Lie R, Skjærven R, et al. Registration of Down syndrome in the Medical Birth Registry of Norway: validity and time trends. Acta Obstet Gynecol Scand. 2008;87:824–30. doi: 10.1080/00016340802217184. [DOI] [PubMed] [Google Scholar]
- 33.Kubon C, Sivertsen A, Vindenes H, Abyholm F, Wilcox A, Lie R. Completeness of registration of oral clefts in a medical birth registry: a population-based study. Acta Obstet Gynecol Scand. 2007;86:1453–7. doi: 10.1080/08037050701645090. [DOI] [PubMed] [Google Scholar]
- 34.Schaefer C, Peters P, Miller RK. Drugs during pregnancy and lactation. Amsterdam, The Netherlands: Elsevier; 2007. [Google Scholar]
- 35.Damase-Michel C, Christaud J, Berrebi A, Lacroix I, Montastruc J-L. What do pregnant women know about non-steroidal anti-inflammatory drugs? Pharmacoepidemiol Drug Saf. 2009;18:1034–8. doi: 10.1002/pds.1817. [DOI] [PubMed] [Google Scholar]
- 36.Gan TJ. Diclofenac: an update on its mechanism of action and safety profile. CMRO. 2010;26:1715–31. doi: 10.1185/03007995.2010.486301. [DOI] [PubMed] [Google Scholar]
- 37.Slattery M, Friel A, Healy D, Morrison J. Uterine relaxant effects of cyclooxygenase-2 inhibitors in vitro. Obstet Gynecol. 2001;98:563–9. doi: 10.1016/s0029-7844(01)01522-8. [DOI] [PubMed] [Google Scholar]
- 38.Hill A. The environment and disease: association or causation? Proc R Soc Med. 1965;58 :295–300. doi: 10.1177/003591576505800503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nørgaard M, Larsson H, Pedersen L, et al. Rheumatoid arthritis and birth outcomes: a Danish and Swedish nationwide prevalence study. J Intern Med. 2010;268:329–37. doi: 10.1111/j.1365-2796.2010.02239.x. [DOI] [PubMed] [Google Scholar]
- 40.de Man Y, Hazes J, van der Heide H, et al. Association of higher rheumatoid arthritis disease activity during pregnancy with lower birth weight: results of a national prospective study. Arthritis Rheum. 2009;60:3196–206. doi: 10.1002/art.24914. [DOI] [PubMed] [Google Scholar]
- 41.Minagar A, editor. Neurological disorders and pregnancy. 1. London: Elsevier; 2011. [Google Scholar]
- 42.Shaheen S, Newson R, Sherriff A, et al. Paracetamol use in pregnancy and wheezing in early childhood. Thorax. 2002;57:958–63. doi: 10.1136/thorax.57.11.958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shaheen S, Newson R, Henderson A, et al. Prenatal paracetamol exposure and risk of asthma and elevated immunoglobulin E in childhood. Clin Exp Allergy. 2005;35:700–2. doi: 10.1111/j.1365-2222.2005.02151.x. [DOI] [PubMed] [Google Scholar]
- 44.Persky V, Piorkowski J, Hernandez E, et al. Prenatal exposure to acetaminophen and respiratory symptoms in the first year of life. Ann Allergy Asthma Immunol. 2008;101:271–8. doi: 10.1016/S1081-1206(10)60492-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Post M, Copland M. Overview of lung development. Acta Pharmacol Sin. 2002;23:4–7. [Google Scholar]
- 46.Harrington L, Lucas R, McMaster S, et al. COX-1, and not COX-2 activity, regulates airway function: relevance to aspirin-sensitive asthma. FASEB. 2008;22:4005–10. doi: 10.1096/fj.08-107979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Masso Gonzalez EL, Patrignani P, Tacconelli S, LAGR Variability among nonsteroidal antiinflammatory drugs in risk of upper gastrointestinal bleeding. Arthritis and Rheumatism. 2010;62:1592–601. doi: 10.1002/art.27412. [DOI] [PubMed] [Google Scholar]
- 48.Turner SW, Young S, Goldblatt J, Landau LI, Le Souëf PN. Childhood Asthma and Increased Airway Responsiveness. American Journal of Respiratory and Critical Care Medicine. 2009;179:98–104. doi: 10.1164/rccm.200805-804OC. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.