Abstract
Objective
To identify an alternative inhaled corticosteroid to fluticasone propionate that can be safely coadministered with HIV protease inhibitors (PIs), we assessed the safety and pharmacokinetics of beclomethasone dipropionate (BDP) and its active metabolite, 17-monopropionate (17-BMP), in combination with ritonavir (RTV) and darunavir/ritonavir (DRV/r).
Design
Open label, prospective, randomized pharmacokinetic and pharmacodynamic study in healthy volunteers.
Methods
Thirty healthy volunteers received inhaled BDP 160 mcg BID for 14 days and were then randomized (1:1:1) into three groups: Group 1 (control) remained on BDP alone for 28 days; Group 2 received BDP + RTV 100 mg BID for 28 days, and Group 3 received BDP + DRV/r 600/100 mg BID for 28 days. Pharmacokinetic sampling for 17-BMP was performed on days 14 and 28, and pharmacokinetic parameter values were compared within patients and between groups. Cortisol stimulation testing was also performed on days 1, 14, 28, and 42 and compared within and between groups.
Results
Geometric mean ratios (day 28:day 14) (90% CI) for 17-BMP area under the concentration-time curve in groups 1, 2, and 3, respectively, were 0.93 (0.81–1.06, p=0.27), 2.08 (1.52–2.65; p=0.006), and 0.89 (0.68–1.09; p=0.61). There were no significant reductions in serum cortisol levels within or between groups (p>0.05).
Conclusions
DRV/r did not increase 17-BMP exposure, while RTV alone produced a statistically significant but clinically inconsequential 2-fold increase in 17-BMP exposure. Adrenal suppression was not observed in any of the study groups. These data suggest BDP can be safely coadministered with DRV/r and likely other RTV-boosted PIs.
Keywords: beclomethasone, darunavir, ritonavir, drug interactions, adrenal function
Introduction
The availability of numerous antiretroviral (ARV) medications, including ritonavir (RTV)-boosted protease inhibitor (PI) combinations, has greatly improved the treatment of HIV infection. While effective for the treatment of HIV, most RTV-boosted PIs are substrates for and potent inhibitors of cytochrome P450 3A (CYP3A), resulting in many drug-drug interactions. Darunavir/ritonavir (DRV/r), when administered as part of potent combination antiretroviral therapy (ART), is one of two PI combinations recommended as first-line treatment of HIV infection [1]. DRV/r is also used to treat ARV-experienced patients, as are other RTV-boosted PIs when given as part of ART [2–3].
HIV-infected patients with asthma or seasonal allergies often require inhaled and intranasal corticosteroids. Inhaled and intranasal fluticasone propionate (hereafter referred to as fluticasone) is associated with adrenal suppression and Cushing's syndrome when coadministered with HIV PI combinations [4–13]. In the majority of published reports in adults, discontinuation of inhaled fluticasone without replacement by another inhaled corticosteroid resulted in resolution of cushingoid symptoms [4, 13]. In two cases, fluticasone replacement with inhaled budesonide resulted in only partial resolution of symptoms [5, 7]. In one case, symptoms completely resolved after inhaled fluticasone was replaced by inhaled beclomethasone [10].
Pharmacokinetic parameters of fluticasone have been assessed with and without RTV in healthy volunteers [14–15]. Coadministration of RTV (100 mg twice daily) with intranasal fluticasone increased the fluticasone area under the concentration-time curve (AUC) and maximum plasma concentration (Cmax) by > 350-fold and > 25-fold, respectively. As a result of excessive fluticasone exposure in that study, plasma cortisol decreased by a mean of 86% among the participants [14–15]. The mechanism of the interaction between fluticasone, a CYP3A substrate, and RTV likely involves CYP3A inhibition in the gastrointestinal tract and liver by RTV, thereby reducing fluticasone metabolism, causing excessive exposure, and resulting in adrenal suppression. Because of this interaction, coadministration of fluticasone and PIs is not recommended [14–15].
Inhaled beclomethasone dipropionate (BDP) is an alternative to fluticasone in HIV-infected patients receiving PI-containing ART. To better define this alternative, we assessed the safety and pharmacokinetics of BDP and its active metabolite, 17-monopropionate (17-BMP), in combination with the HIV PIs RTV or DRV/r. BDP is a pro-drug that is rapidly hydrolyzed by esterases to 17-BMP and two inactive metabolites. The glucocorticoid receptor affinity of 17-BMP is 25 times greater than that of BDP, which has weak binding affinity [16]. We chose to study this corticosteroid because of its favorable pharmacokinetic profile that makes it less likely to interact with HIV PIs. The bioavailability of inhaled 17-BMP is 62%, with 36% and 26% absorption from the lung and gut, respectively [16]. As a result, an inhaled BDP dose of 1,000 mcg was shown to produce very low 17-BMP AUC0–24 and Cmax of 3,851 pcg*hr/mL and 944 pcg/mL, respectively [16]. In addition to its limited bioavailability, 17-BMP has a short terminal half-life of 2.7 hours and a mean residence time of 4.1 hours; as a result, it does not significantly accumulate in tissues [16–17]. We chose a lower, clinically relevant dose of 160 mcg twice daily, which we anticipated would be associated with lower systemic exposure and accumulation and a low risk of adrenal insufficiency.
METHODS
Subjects
Healthy HIV-negative volunteers between the ages of 18 and 60 years, with no significant underlying medical conditions, were eligible to participate in this study. Subjects were not permitted to take any medications within 30 days of study participation. Additional exclusion criteria included baseline morning serum cortisol level < 5 mcg/dL, use of nicotine-containing tobacco products, active drug or alcohol abuse, history of intolerance to any of the study medications, and persistent diarrhea. Females of childbearing potential were required to have a negative pregnancy test and use a non-hormonal method of contraception throughout the study or to practice abstinence. The study was approved by the National Institute of Allergy and Infectious Diseases Institutional Review Board, and written informed consent was obtained from all study participants.
Study design
This study was a single-center, open-label, prospective, randomized, pharmacokinetic and pharmacodynamic study. The study was designed to evaluate the influence of RTV and DRV/r on the steady state pharmacokinetics of 17-BMP in healthy volunteers taking orally inhaled BDP. The study was also designed to determine the influence of these combinations on adrenal function.
Treatment and blood sampling for beclomethasone 17-monopropionate (17-BMP)
The study schema is presented in detail in Figure 1. Briefly, 30 HIV-seronegative volunteers received inhaled BDP (QVAR 80 mcg Inhalation Aerosol; Teva Respiratory, LLC– Horsham, PA) 160 mcg (2 inhalations) twice daily from study days 1 to 14. At study initiation, all subjects received thorough training and demonstrated proficiency with proper inhalation technique. Prior to BDP administration on study days 1 and 14, subjects underwent a low-dose adrenocorticotropic hormone (ACTH) stimulation test between 7 a.m. and 9 a.m. to assess adrenal function. On study day 14, blood samples for the determination of steady-state 17-BMP concentrations were collected immediately prior to and at 0.5, 1, 2, 4, 6, 8, and 12 hours after BDP administration. All samples were collected into sodium-heparin (green top) tubes and centrifuged within 12 hours at 3200 rpm for 10 minutes after which plasma was harvested and stored at −80°C until analysis. On study day 14, subjects were randomized to one of three groups of 10 participants each. All three groups continued BDP at the same dosage from study days 14 to 42. Group 1 (control) received no additional medications. Group 2 added RTV 100 mg twice daily, and Group 3 added DRV/r 600/100 mg twice daily for study days 14 to 42. On study day 28 subjects repeated low-dose ACTH stimulation testing and blood sample collection for 17-BMP determination, and on study day 42 subjects had a final ACTH stimulation test, after which study medications were discontinued. Adherence was assessed by self-report, examination of diary cards, pharmacy refills, and pill counts.
Figure 1.
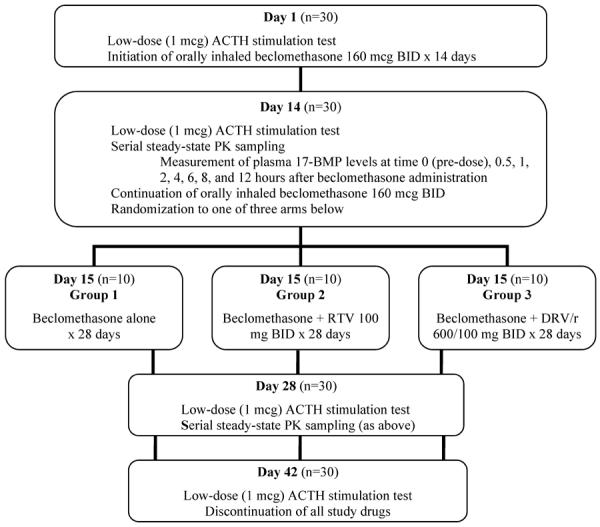
Study Design
Adrenocorticotropic hormone (ACTH) stimulation testing
A low-dose ACTH stimulation test with 1 mcg cosyntropin (prepared with Cortrosyn, Amphastar Pharmaceuticals) was used instead of standard-dose (250 mcg) testing. This dose was chosen because it is less likely to yield a falsely normal cortisol response than the standard dose test [18–19]. Cosyntropin was prepared and administered using previously described methods, including morning testing and the use of minimal or no tubing [20–22]. Serum cortisol was measured before and 20, 30, and 60 minutes after cosyntropin administration for peak cortisol determination. Adrenal insufficiency was defined as a peak cortisol level < 16 mcg/dL. In the event of a peak level of < 16 mcg/dL, subjects received an endocrinology consult and further testing as clinically indicated (e.g., metyrapone stimulation test).
Analytical methods
17-BMP
Beclomethasone dipropionate (BDP) and 17-beclomethasone monopropionate (17-BMP), as well as their respective internal standards beclomethasone dipropionate-d10 (BDP-d10) and 17-beclomethasone monopropionate-d5 (17-BMP-d5), were separated using a newly developed high performance liquid chromatography method and detected by tandem mass spectrometry using multiple reaction monitoring (MRM). Calibration curves for both BDP and 17-BMP were linear from 0.050 ng/ml - 10.0 ng/ml with R2 > 0.998. Percent errors, as a measure of accuracy, were <15%. The inter- and intra-assay coefficients of variation for BDP were 2.88–5.52% and 4.40–7.82%, respectively, at three different drug concentrations, and the inter- and intra-assay coefficients of variation for 17-BMP were 2.84–6.94% and 5.28–9.52%, respectively. The limit of quantitation for both BDP and 17-BMP was 0.050 ng/ml, and the limit of detection was 0.030 ng/ml. During the validation, stability of the drug in plasma was tested after storage at 25°C, 4°C, and -80°C for up to three months. Repeated freezing and thawing (three cycles) of plasma was evaluated at -80°C. The overall recovery of BDP, BDP-d10 (IS), 17-BMP, and 17-BMP-d5 (IS) was >85%.
Cortisol
The National Institutes of Health (NIH) Department of Laboratory Medicine (DLM) measured serum cortisol using a chemiluminescent competitive immunoassay (Siemens, Los Angeles, CA, USA). The inter-assay and intra-assay coefficients of variation (CV) are 11.1 and 7.4%, respectively. The functional detectable value is 1.01 mcg/dL.
ACTH
The NIH DLM analyzed plasma ACTH using a chemiluminescence immunoassay on a Siemens Immulite 2500 analyzer (Siemens, Los Angeles, CA, USA). The respective inter-assay and intra-assay coefficients of variation (CV) are 2.8 and 8.4% at 28.9 pg/mL and 1.7 and 2.3% at 379 pg/mL.
Pharmacokinetic Analysis
Pharmacokinetic parameter values were determined for 17-BMP using noncompartmental methods with WinNonlin Professional computer program (version 5.0; Pharsight Corporation, Mountain View, CA). Maximum plasma concentration (Cmax) and time to reach Cmax (Tmax) for 17-BMP were obtained directly by visual inspection of the plasma concentration vs. time profiles. The apparent elimination rate constant (λZ) was determined by calculating the absolute value of the slope of the log-linear regression of at least 3 points of the plasma concentration-time plot. The elimination half-life (T1/2) was calculated as 0.693/λZ. The area under the concentration-time curve (AUC0–12) was calculated by using the linear trapezoidal rule. The apparent oral clearance (Cl/F) was calculated by dose/AUC.
Statistical analysis
17-BMP
To determine the influence of RTV (group 2) and DRV/r (group 3) on 17-BMP pharmacokinetics, we compared 17-BMP pharmacokinetic profiles within groups between days 14 and 28 using paired t-tests, except for Tmax, which we analyzed using the Wilcoxon signed-rank test. Geometric mean ratios (GMR) with 90% confidence intervals (CI) are reported. A P value of < 0.05 was accepted as statistically significant. SYSTAT Software, version 11 (Richmond, CA, USA) was used for statistical testing.
Adrenocorticotropic hormone (ACTH) stimulation testing
Serum basal and peak cortisol measurements on days 14, 28, and 42 were compared to those on day 1; individual changes in serum cortisol between study days were calculated for each subject along with the arithmetic mean of these values. A paired students t-test was used for within-group comparisons, and Analysis of the Variance (ANOVA) with post-hoc Tukey testing was used for between-group comparisons. A P value less than 0.05 was accepted as statistically significant for all comparisons. JMP Software, version 9.0 (SAS Institute, Cary, NC, USA) was used for statistical testing.
Sample size
A difference in 17-BMP AUC of at least 30% was selected for estimating sample size. A standard deviation of 551 pcg·hr/mL with a mean AUC0–12 of 2,064 pcg·hr/mL was assumed for 17-BMP based on previous data [23]. With α set at 0.05, a sample of 10 subjects per group was deemed necessary to provide 80.2% power to detect a 30% difference in 17-BMP AUC0–12 before and after RTV (group 2) and DRV/r (group 3) coadministration. SYSTAT Software, version 11 (Richmond, CA, USA) was used for sample size determination.
RESULTS
Subjects and dosing
Thirty-six subjects enrolled and completed at least day 1 study procedures. Six subjects were withdrawn from the study prior to Day 15 for the following reasons: ACTH-stimulated peak cortisol < 16 mcg/dL on day 1 (1), ACTH-stimulated peak cortisol < 16 mcg/dL on day 14 (2), and development of an off-study criterion (3). Demographic information for the 30 subjects who completed both pharmacokinetic sampling periods (days 14 and 28) are presented in Table 1. One subject in group 3 was withdrawn from the study after pharmacokinetic sampling on day 28 because of an ACTH-stimulated peak serum cortisol level < 16 mcg/dL (15.7 mcg/dL). BDP was well-tolerated by all subjects with no graded adverse events being directly attributable to BDP administration. Overall, subjects receiving RTV or DRV/r tolerated these medications with minimal and transient side effects, such as diarrhea. Self-reported adherence was excellent among all study participants who completed enrollment. Twenty-five subjects exhibited over 95% adherence, 15 of whom reported 100% adherence. Five subjects demonstrated over 90% adherence and were distributed across all three arms.
Table 1.
Baseline Characteristics of Subjects
| Group 1 (n=10) (BDP alone) | Group 2 (n=10) (BDP + RTV) | Group 3 (n=10) (BDP + DRV/r) | |
|---|---|---|---|
| Age, years – mean (SD) | 35 (9) | 28 (6) | 36 (10) |
| Sex – no. (%) | |||
| Male | 5 (50%) | 7 (70%) | 8 (80%) |
| Female | 5 (50%) | 3 (30%) | 2 (20%) |
| Race or ethnic group – no. (%) | |||
| White | 8 (80%) | 7 (70%) | 4 (40%) |
| Black | 1 (10%) | 1 (10%) | 4 (40%) |
| Hispanic | 1 (10% | 2 (20%) | 1 (10%) |
| Asian | 0 (0%) | 0 (0%) | 1 (10%) |
BDP = inhaled beclomethasone dipropionate
RTV = ritonavir
DRV/r = darunavir/ritonavir
Pharmacokinetics
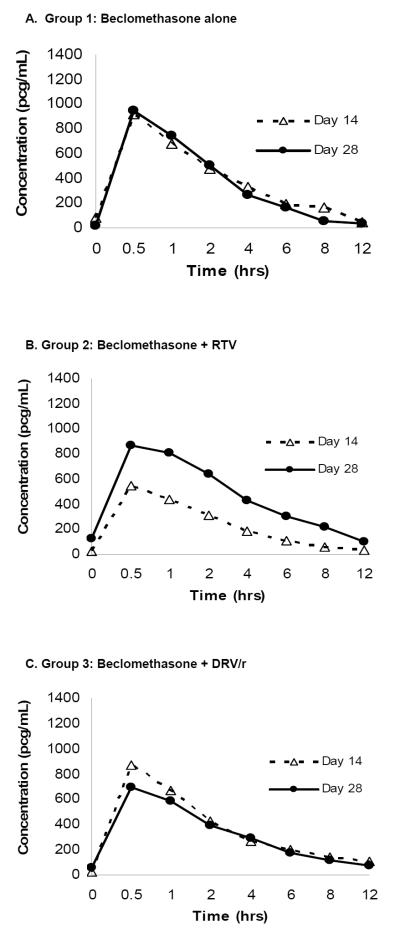
Geometric mean pharmacokinetic parameter values and GMRs for 17-BMP are provided in Table 2. Concentration vs. time profiles for 17-BMP on days 14 and 28 are shown in Figure 2. Group 1 received no additional medications, and accordingly, 17-BMP GMRs did not differ significantly for any of the pharmacokinetic parameters between sampling periods. With the addition of RTV 100 mg twice daily for 14 days, group 2 experienced a statistically significant increase in 17-BMP GMR (90% CI) for AUC0–12 (2.08 [1.52–2.65]; P=0.006) and for Cmax (1.67 [1.33–2.01] P=0.006). Similar to group 1, GMRs for all pharmacokinetic parameter values did not significantly differ in group 3 (DRV/r 600/100 mg twice daily for 14 days) between pharmacokinetic sampling periods.
Table 2.
17-BMP Pharmacokinetic Parameters
| Geometric Mean Values (90% CI) | Geometric Mean Ratio (90% CI) | |||
|---|---|---|---|---|
| Day 14 | Day 28 | Day 28/Day 14 | p-value* | |
| Group 1 | BDP Alone | BDP Alone | ||
| AUC0–12 (pcg*h/mL) | 3342 (2988 – 3695) | 3116 (2846 – 3386) | 0.93 (0.81 – 1.06) | 0.27 |
| Cmax (pcg/mL) | 935 (823 – 1048) | 1043 (909 – 1178) | 1.12 (0.83 – 1.40) | 0.38 |
| Tmax (h) | 0.5 (0.5 – 1.0)† | 0.5 (0.5 – 1.0)† | – | 0.59 |
| T1/2 (h) | 3.2 (2.8 – 3.7) | 2.6 (2.3 – 2.9) | 0.81 (0.61 – 1.02) | 0.07 |
| Clss/F | 47.9 (41.2 – 54.6) | 51.4 (47.2 – 55.5) | 1.07 (0.96 – 1.18) | 0.54 |
| Group 2 | BDP Alone | BDP + RTV | ||
| AUC0–12 (pcg*h/mL) | 2126 (1662 – 2590) | 4426 (3117 – 5734) | 2.08 (1.52 – 2.65) | 0.006 |
| Cmax (pcg/mL) | 576 (434 – 719) | 963 (728 – 1198) | 1.67 (1.33 – 2.01) | 0.006 |
| Tmax (h) | 0.5 (0.5 – 1.0)† | 1.0 (0.5 – 4.0)† | – | 0.20 |
| T1/2 (h) | 3.7 (2.7 – 4.6) | 3.7 (3.2 – 4.3) | 1.02 (0.7 – 1.3) | 0.82 |
| Clss/F | 75.2 (36.3 – 114.2) | 35.6 (17.5 – 53.8) | 0.47 (0.37 – 0.57) | 0.01 |
| Group 3 | BDP Alone | BDP + DRV/r | ||
| AUC0–12 (pcg*h/mL) | 3202 (2695 – 3708) | 2840 (2247 – 3433) | 0.89 (0.68 – 1.09) | 0.61 |
| Cmax (pcg/mL) | 889 (736 – 1043) | 716 (559 – 873) | 0.81 (0.69 – 0.92) | 0.06 |
| Tmax (h) | 0.5 (0 – 1.0)† | 0.5 (0.5 – 1.0)† | – | 0.71 |
| T1/2 (h) | 3.9 (3.5 – 4.4) | 3.6 (3.0 – 4.1) | 0.9 (0.7 – 1.2) | 0.59 |
| Clss/F | 49.5 (41.2 – 57.8) | 55.5 (25.7 – 85.2) | 1.12 (0.80 – 1.45) | 0.36 |
AUC0–12 = area under the concentration vs. time curve, Cmax = maximum concentration, Tmax = time to maximum concentration, T1/2 = half-life, Clss/F = apparent inhalation clearance, BDP = inhaled beclomethasone dipropionate, RTV = ritonavir, DRV/r = darunavir/ritonavir
Student's paired, two-tailed t-test was used for all statistical comparisons, except for Tmax (Wilcoxon signed rank).
Median values (ranges)
Figure 2.
Plasma 17-BMP Concentration vs. Time Curves on Day 14 and Day 28
In many subjects the concentrations of BDP were below the limit of quantitation. Hence, it was not possible to accurately generate PK parameter values for these individuals. As a result, it was not possible to compare conversion rates of BDP to BMP among the treatment arms.
Adrenocorticotropic hormone (ACTH) stimulation testing
All but one subject completed all four ACTH stimulation tests; one subject completed three. For each of the ACTH sampling days (1, 14, 28, and 42), all but two subjects achieved peak cortisol levels > 16 mcg/dL. The aforementioned subject in group 3 and a subject in group 1 had a peak cortisol level of 15.7 mcg/dL on day 28 and day 42, respectively. Both were in fact not adrenally suppressed based on an endocrinology evaluation, which included a metyrapone stimulation test in one patient and a random serum cortisol level of 13.0 mcg/dL in the other patient. Although the subject in group 3 was withdrawn from the study on day 28, ACTH stimulation results were carried forward from day 28 to day 42 to avoid an artificial increase in the mean peak cortisol measurement for day 42 in group 3.
Mean basal and peak cortisol levels for each group at all four study visits are displayed in Table 3. From day 1 to day 42, subjects in groups 1, 2, and 3, respectively, had a mean change in basal cortisol level of −1.4, 0.8, and 0.8 mcg/dL and a mean change in peak cortisol level of −1.1, 0.4, and 0.6 mcg/dL. There were no statistically significant reductions in basal or peak cortisol levels at day 14, 28, or 42 in any group and no significant differences between groups at any time-point (P > 0.05 for all comparisons).
Table 3.
Mean (± SD) Cortisol Levels at Baseline, after 14 Days of Beclomethasone Alone, and after 14 and 28 Days of Additional Beclomethasone Alone or Beclomethasone Plus Ritonavir or Darunavir/Ritonavir
| Basal Cortisol (mcg/dL) (pre-ACTH) | Peak Cortisol (mcg/dL) (ACTH stimulated) | |
|---|---|---|
| Day 1 | ||
| Group 1 | 11.3 (3.9) | 21.9 (1.7) |
| Group 2 | 13.9 (2.5) | 21.8 (3.2) |
| Group 3 | 10.7 (3.6) | 21.3 (2.5) |
| Day 14 | ||
| Group 1 | 10.5 (5.6) | 20.4 (2.5) |
| Group 2 | 13.0 (3.6) | 21.5 (2.0) |
| Group 3 | 12.5 (3.6) | 20.6 (2.8) |
| Day 28 | ||
| Group 1 | 11.6 (5.3) | 21.2 (4.0) |
| Group 2 | 11.7 (4.8) | 20.6 (2.5) |
| Group 3 | 11.5 (5.0) | 19.7 (3.3) |
| Day 42 | ||
| Group 1 | 9.8 (4.6) | 20.8 (3.4) |
| Group 2 | 14.7 (4.3) | 22.2 (4.0) |
| Group 3 | 12.6 (2.2) | 22.5 (5.0) |
Group 1: beclomethasone alone
Group 2: beclomethasone + ritonavir
Group 3: beclomethasone + darunavir/ritonavir
DISCUSSION
A clinically significant pharmacokinetic and pharmacodynamic drug interaction between fluticasone and HIV PIs, particularly when enhanced with RTV, is well established [4–15]. In an attempt to identify an inhaled corticosteroid that can be safely coadministered with HIV PIs, we assessed the pharmacokinetics and pharmacodynamics of 17-BMP in subjects receiving inhaled BDP and concurrent RTV or DRV/r. In the absence of a safety concern, substituting BDP for fluticasone is unlikely to be of therapeutic consequence since BDP is safe and effective for the treatment of asthma [24–25].
As expected, 17-BMP exposure did not change between study days 14 and 28 of the control arm (BDP alone). This observation is consistent with the fact that 17-BMP does not accumulate with repeated dosing. Similarly, 17-BMP pharmacokinetics also remained unchanged after 14 days of inhaled BDP and DRV/r coadministration. These results suggest that the combination of DRV/r does not alter the metabolism, transport, or formation of 17-BMP.
In contrast to the influence of DRV/r on 17-BMP pharmacokinetics, 14 days of inhaled BDP with RTV 100 mg twice daily resulted in an increased 17-BMP exposure by 2-fold (P=0.006). Although statistically significant, this increase in 17-BMP AUC is minimal compared to the 350-fold increase in fluticasone AUC reported with the same dose of RTV [14–15]. Although the routes of administration were different for BDP and fluticasone in these studies (oral inhalation vs. nasal inhalation, respectively), it unlikely contributed to the disparate results between investigations. The reason for the greater impact of RTV on fluticasone versus BDP pharmacokinetics is likely due to differences in metabolism between the two drugs. Fluticasone, whether swallowed or systemically absorbed through the vasculature in the lung, is highly dependent upon CYP3A for its intestinal and hepatic biotransformation [14, 26]. Conversely, BDP undergoes rapid biotransformation in the cytosol of lung cells to the pharmacologically active 17-BMP metabolite via esterase enzymes. The 17-BMP metabolite is further converted to fatty acid esters and inactive polar metabolites [27–28]. According to the manufacturer of QVAR, the metabolism of BDP and 17-BMP is catalyzed by CYP3A [17]. While CYP3A may be capable of catalyzing these reactions in vitro, several studies of BDP metabolism, including this one, suggest that esterase enzymes and not CYP3A are primarily responsible for the metabolism of BDP and 17-BMP when BDP is administered via inhalation [27–28].
Based on what is known about 17-BMP metabolism, there is no clear explanation for why 17-BMP exposure was significantly elevated by RTV alone and not by DRV/r. One possibility is that DRV and RTV produced opposing effects on metabolism, transport, or formation of 17-BMP, resulting in no net change in 17-BMP exposure. Since the independent influence of DRV on various metabolic and transport pathways is largely unknown, this theory remains speculative.
The differing effect of RTV and DRV/r on 17-BMP exposure implies that results from drug interaction studies using RTV alone cannot be routinely applied to all PI combinations. To this end, we cannot say with certainty that results from this investigation can be applied to all PI combinations given in conjunction with inhaled BDP. However, considering that RTV tends to modulate a large number of metabolic and transport proteins and is involved in more drug interactions than other HIV PIs, it seems unlikely that other RTV-boosted PI regimens would increase 17-BMP concentrations beyond the clinically insignificant 2-fold increase observed with lone ritonavir administration. Further investigation is necessary to confirm this hypothesis.
In contrast to 17-BMP concentrations, there was no significant impact of DRV/r or RTV on serum cortisol measurements in any study participant. One individual receiving DRV/r met criteria for adrenal suppression when he was noted to have a peak serum cortisol concentration below 16 mcg/mL (15.7 mcg/mL) on study day 28; however, a metyrapone stimulation test later indicated that this individual was not in fact adrenally suppressed. The absence of a pharmacodynamic interaction suggests that the 2-fold increase in 17-BMP exposure observed with concurrent RTV administration is not clinically relevant. Conversely, the clinical relevance of a 350-fold increase in systemic fluticasone exposure by RTV is evident by the number and severity of case reports of adrenal insufficiency [4–13]. While we did not study prolonged (> 28 days) coadministration of BDP with RTV and DRV/r, it is unlikely that coadministration of inhaled BDP with HIV protease inhibitors beyond 28 days would produce adrenal insufficiency given the limited absorption and short half-life of 17-BMP.
In addition to an orally inhaled formulation, BDP is also available as an intranasal formulation. Although we did not study intranasal BDP in the current investigation, we believe our results with inhaled BDP can be extrapolated to include intranasal administration. When administered alone in the same subject, the respective systemic bioavailabilities of intranasal and inhaled 17-BMP are shown to be comparable at 44% and 62%, respectively [16]. For both routes of BDP administration, an overlapping site of systemic absorption is the gut. In fact, the gut is the only significant site of 17-BMP absorption when the drug is administered intranasally, as absorption from the lung is irrelevant and absorption from the nasal cavity is < 1% [16]. Based on these data, if an interaction between intranasal BDP and RTV were to occur, the gut would be the most probable site of the interaction. Since 17-BMP absorption from the gut is reasonably comparable between intranasal and inhaled BDP (43% and 26%, respectively), and we did not observe a clinically significant interaction between inhaled BDP and HIV PIs in the current study, it is unlikely that such an interaction would manifest with intranasal BDP [16].
In summary, DRV/r did not significantly increase 17-BMP exposure, while RTV alone produced a statistically significant but clinically inconsequential 2-fold increase in 17-BMP exposure. The mechanism by which RTV increased the systemic exposure of 17-BMP is not clear. DRV/r and RTV alone did not result in adrenal suppression when coadministered with inhaled BDP for 28 days. These results are in contrast to the significant pharmacokinetic and pharmacodynamic interaction between intranasal and inhaled fluticasone and HIV PIs. To this end, inhaled and likely intranasal BDP is preferable to fluticasone and appears to be relatively safe when coadministered with DRV/r and possibly other PI combinations in patients with HIV infection.
Acknowledgements
Funding Sources: Intramural research programs of the National Institutes of Health Clinical Center, the National Institute of Allergy and Infectious Diseases, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and the Frederick National Laboratory for Cancer Research, National Institutes of Health, under Contract No. HHSN261200800001E. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Source of Funding: Supported in part by the intramural research programs of the National Institutes of Health Clinical Center, the National Institute of Allergy and Infectious Diseases, the Eunice Kennedy Shriver National Institute of Child Health and Human Development, and the Frederick National Laboratory for Cancer Research, National Institutes of Health, under Contract No. HHSN261200800001E
The procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2000.
Footnotes
Conflicts of Interest None of the authors have conflicts of interest to report.
Previous Presentation: Data contained in this manuscript were previously presented in abstract form at the Conference on Retroviruses and Opportunistic Infections, March 2012; Seattle, Washington, USA.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Panel on Antiretroviral Guidelines for Adults and Adolescents . Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services; [Accessed June 22]. Available at http://www.aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf. [Google Scholar]
- 2.Arastéh K, Yeni P, Pozniak A, et al. Efficacy and safety of darunavir/ritonavir in treatment-experienced HIV type-1 patients in the POWER 1, 2 and 3 trials at week 96. Antivir Ther. 2009;14:859–864. doi: 10.3851/IMP1301. [DOI] [PubMed] [Google Scholar]
- 3.Youle M. Overview of boosted protease inhibitors in treatment-experienced HIV-infected patients. J Antimicrob Chemother. 2007;60:1195–1205. doi: 10.1093/jac/dkm364. [DOI] [PubMed] [Google Scholar]
- 4.Foisy MM, Yakiwchuk EM, Chiu I, et al. Adrenal suppression and Cushing's syndrome secondary to an interaction between ritonavir and fluticasone: a review of the literature. HIV Med. 2008;9:389–396. doi: 10.1111/j.1468-1293.2008.00579.x. [DOI] [PubMed] [Google Scholar]
- 5.Clevenbergh P, Corcostegui M, Gérard D, et al. Iatrogenic Cushing's syndrome in an HIV-infected patient treated with inhaled corticosteroids (fluticasone propionate) and low dose ritonavir enhanced PI containing regimen. J Infect. 2002;44:194–195. doi: 10.1053/jinf.2001.0928. [DOI] [PubMed] [Google Scholar]
- 6.Rouanet I, Peyrière H, Mauboussin JM, et al. Cushing's syndrome in a patient treated by ritonavir/lopinavir and inhaled fluticasone. HIV Med. 2003;4:149–150. doi: 10.1046/j.1468-1293.2003.00149.x. [DOI] [PubMed] [Google Scholar]
- 7.Soldatos G, Sztal-Mazer S, Woolley I, et al. Exogenous glucocorticoid excess as a result of ritonavir-fluticasone interaction. Intern Med J. 2005;35:67–68. doi: 10.1111/j.1445-5994.2004.00723.x. [DOI] [PubMed] [Google Scholar]
- 8.Gillett MJ, Cameron PU, Nguyen HV, et al. Iatrogenic Cushing's syndrome in an HIV-infected patient treated with ritonavir and inhaled fluticasone. AIDS. 2005;19:740–741. doi: 10.1097/01.aids.0000166102.21391.81. [DOI] [PubMed] [Google Scholar]
- 9.Samaras K, Pett S, Gowers A, et al. Iatrogenic Cushing's syndrome with osteoporosis and secondary adrenal failure in human immunodeficiency virus-infected patients receiving inhaled corticosteroids and ritonavir/-boosted protease inhibitors: six cases. J Clin Endocrinol Metab. 2005;90:4394–4398. doi: 10.1210/jc.2005-0036. [DOI] [PubMed] [Google Scholar]
- 10.Nocent C, Raherison C, Dupon M, et al. Unexpected effects of inhaled fluticasone in an HIV patient with asthma. J Asthma. 2004;41:793–795. doi: 10.1081/jas-200038366. [DOI] [PubMed] [Google Scholar]
- 11.Valin N, De Castro N, Garrait V, et al. Iatrogenic Cushing's syndrome in HIV-infected patients receiving ritonavir and inhaled fluticasone: description of 4 new cases and review of the literature. J Int Assoc Physicians AIDS Care (Chic) 2009;8:113–121. doi: 10.1177/1545109709332019. [DOI] [PubMed] [Google Scholar]
- 12.Canalejo E, Pacheco MS. Cushing syndrome due to ritonavir-fluticasone interaction. CMAJ. 2012;184:1714. doi: 10.1503/cmaj.111315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mahlab-Guri K, Asher I, Gradstein S, et al. Inhaled fluticasone causes iatrogenic cushing's syndrome in patients treated with Ritonavir. J Asthma. 2011;48:860–863. doi: 10.3109/02770903.2011.606580. [DOI] [PubMed] [Google Scholar]
- 14.Flovent HFA [package insert, GlaxoSmithKline] Nov, 2011.
- 15.Norvir [package insert, Abbott] Feb, 2012.
- 16.Daley-Yates PT, Price AC, Sisson JR, et al. Beclomethasone dipropionate: absolute bioavailability, pharmacokinetics and metabolism following intravenous, oral, intranasal and inhaled administration in man. Br J Clin Pharmacol. 2001;51:400–409. doi: 10.1046/j.0306-5251.2001.01374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.QVAR [package insert, Teva Global] Aug, 2008.
- 18.Kazlauskaite R, Evans AT, Villabona CV, et al. Corticotropin tests for hypothalamic-pituitary-adrenal insufficiency: a metaanalysis. J Clin Endocrinol Metab. 2008;93:4245–4253. doi: 10.1210/jc.2008-0710. [DOI] [PubMed] [Google Scholar]
- 19.Dorin RI, Qualls CR, Crapo LM. Diagnosis of adrenal insufficiency. Ann Intern Med. 2003;139:194–204. doi: 10.7326/0003-4819-139-3-200308050-00009. [DOI] [PubMed] [Google Scholar]
- 20.Park YJ, Park KS, Kim JH, et al. Reproducibility of the cortisol response to stimulation with the low dose (1 microg) of ACTH. Clin Endocrinol (Oxf) 1999;51:153–158. doi: 10.1046/j.1365-2265.1999.00773.x. [DOI] [PubMed] [Google Scholar]
- 21.Dickstein G, Shechner C, Nicholson WE, et al. Adrenocorticotropin stimulation test: effects of basal cortisol level, time of day, and suggested new sensitive low dose test. J Clin Endocrinol Metab. 1991;72:773–778. doi: 10.1210/jcem-72-4-773. [DOI] [PubMed] [Google Scholar]
- 22.Wade M, Baid S, Calis K, et al. Technical details influence the diagnostic accuracy of the 1 microg ACTH stimulation test. Eur J Endocrinol. 2010;162:109–113. doi: 10.1530/EJE-09-0746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harrison LI, Soria I, Cline AC, et al. Pharmacokinetic differences between chlorofluorocarbon and chlorofluorocarbon-free metered-dose inhalers of beclomethasone dipropionate in adult asthmatics. J Pharm Pharmacol. 1999;51:1235–1240. doi: 10.1211/0022357991776967. [DOI] [PubMed] [Google Scholar]
- 24.Lage MJ, Gross GN, Brewster C, et al. Outcomes and costs of patients with persistent asthma treated with beclomethasone dipropionate hydrofluoroalkane or fluticasone propionate. Adv Ther. 2009;26:762–775. doi: 10.1007/s12325-009-0056-z. [DOI] [PubMed] [Google Scholar]
- 25.Aubier M, Wettenger R, Gans SJ. Efficacy of HFA-beclomethasone dipropionate extra-fine aerosol (800 microg day(−1)) versus HFA-fluticasone propionate (1000 microg day(−1)) in patients with asthma. Respir Med. 2001;95:212–220. doi: 10.1053/rmed.2000.1025. [DOI] [PubMed] [Google Scholar]
- 26.Pearce RE, Leeder JS, Kearns GL. Biotransformation of fluticasone: in vitro characterization. Drug Metab Dispos. 2006;34:1035–1040. doi: 10.1124/dmd.105.009043. [DOI] [PubMed] [Google Scholar]
- 27.Nave R, Fisher R, McCracken N. In vitro metabolism of beclomethasone dipropionate, budesonide, ciclesonide, and fluticasone propionate in human lung, precision-cut tissue slices. Respir Res. 2007;8:65. doi: 10.1186/1465-9921-8-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wurthwein G, Rohdewald P. Activation of beclomethasone dipropionate by hydrolysis to beclomethasone-17-monopropionate. Biopharm Drug Dispos. 1990;11:381–394. doi: 10.1002/bdd.2510110503. [DOI] [PubMed] [Google Scholar]