Abstract
Neurons rely on their cytoskeleton to give them shape and stability, and on cytoskeletal dynamics for growth and synaptic plasticity. Because drug addiction is increasingly seen as the inappropriate learning of strongly reinforcing stimuli, the role of the cytoskeleton in shaping drug memories has been of increasing interest in recent years. Does the cytoskeleton have an active role in shaping these memories, and to what extent do alterations in the cytoskeleton reflect the acute actions of drug exposure, or homeostatic reactions to the chronic exposure to drugs of abuse? Here we will review recent advances in understanding the role of the cytoskeleton in the development of drug addiction, with a focus on actin filaments, as they have been studied in greater detail.
Introduction
The cytoskeleton, consisting of microtubules, intermediate, and actin filaments is indispensable for any eukaryotic cell. In neurons, these cytoskeletal elements give the cell dynamic structure and are involved in a myriad of processes, including developmental ones like axon pathfinding and synapse formation [1], as well as functional processes like long-term potentiation (LTP) [2,3] or dendritic spine growth and morphology [4]. The latter processes seem to be especially relevant to drug abuse, as ‘inappropriate’ learning of strongly reinforcing cues is a hallmark in the development of addiction [5], and changes in the number of dendritic spines have long been known to occur after repeated exposure to drugs of abuse [6].
Effects of drug exposure on the cytoskeleton
Early findings
Chronic alcohol consumption causes changes in the cytoskeleton of liver cells [7]. For example, tissue samples from patients suffering from alcoholic liver disease revealed a shift from polymerized microtubules to free tubulin [8] and the redistribution of intermediate filaments [9]. Also, long-term ethanol consumption in rats decreased the attachment of their hepatocytes to numerous substrates, a process dependent on the actin cytoskeleton [10]. While these defects have been described consistently, it remained unclear whether they represent a cellular reaction to long-term, toxic ethanol exposure in the liver, the major site of ethanol catabolism, or whether these ethanol-induced cytoskeletal changes are representative of global cytoskeletal dysfunction occurring in other areas of the body, like the central nervous system, that contribute to the development of alcohol addiction.
Effects on cultured CNS cells
Chronic exposure of primary astrocytes to ethanol (30 mM for 7 days) alters the actin cytoskeleton, with a noted reduction of actin stress fibers and an increase in filamentous actin (F-actin) near the plasma membrane [11]. The Rho-family of small GTPases (including RhoA, Rac, and Cdc42) are key regulators of actin dynamics [12]. The ethanol-induced changes in actin are likely due to a chronic ethanol-induced decrease in RhoA activity, since treatment with lysophosphatic acid (LPA), an activator of RhoA [11], or transfection with activated RhoA [13] blocks the ethanol-induced effects. Also, astrocyte cultures treated acutely with ethanol (100 mM for 10 minutes) have reduced stress fibers [13,14], suggesting a rapid change in RhoA activity. One potential mechanism for reduced RhoA activity is via upregulation of p190RhoGAP, a GTPase activating protein (GAP) that converts active RhoA-GTP to inactive RhoA-GDP. Chronic alcohol exposure increases p190RhoGAP activity and redistributes it to the plasma membrane [15], but the precise mechanism(s) remains unclear. Nevertheless, these data suggest that acute ethanol has a negative effect on F-actin stability, and that the long-term increases in plasma membrane actin filaments seen may be a compensatory reaction to prolonged ethanol exposure.
There are fewer studies on the effects of ethanol on the neuronal cytoskeleton; however, there are many neuronal proteins that are directly or indirectly regulated by the F-actin cytoskeleton and/or ethanol. Chronic treatment of the secretory cell line, PC12, with ethanol (100 mM for 4 days) inhibits nicotine and potassium-induced dopamine release and breakdown of F-actin, suggesting that chronic ethanol causes a stabilization of the actin cytoskeleton [16]. These experiments employed a high chronic dose of ethanol, and the effects may reflect a cellular adaptation to the exposure. In agreement with such an interpretation are findings that prolonged EtOH treatment (50 mM for 4 days) of primary hippocampal neurons increases punctate F-actin staining that is apposed by PSD95 puncta, suggesting an increase in synaptic contacts. Acute ethanol directly binds and inhibits NMDA receptors (NMDAR) [17], a key molecule in the induction of LTP and behavioral plasticity [18]. Treating the cultured neurons with the NMDAR antagonist, APV, also increases F-actin/PSD95 puncta, while the addition of the agonist NMDA in the presence of ethanol prevents the increase in F-actin/PSD95 puncta [19]. This suggests that the cells compensate for ethanol’s continued inhibition of the NMDAR with a homeostatic adaptation, which includes increases in F-actin and NMDAR abundance.
Does ethanol also have acute effects on the neuronal actin cytoskeleton? A brief, 30 second, pre-exposure of cultured cerebellar granule cells to ethanol potentiates subsequent NMDAR inhibition by ethanol, even when the pretreatment is applied intracellularly [20•]. Phalloidin, an F-actin stabilizer, prevents this potentiation, while latrunculin A (latA), an actin depolymerizer, mimics the effect [20•]. These findings suggest that acute ethanol leads to F-actin instability, which in turn causes decreases in NMDAR current, a notion also supported by data from Eps8 knock out mice (see below).
It is worth noting that not all findings support an acute negative effect of ethanol on neuronal F-actin, which is followed by a homeostatic increase under chronic exposure (eg. [21]). These discrepancies may reflect the exact exposure conditions (dose and length), and/or the tissue examined (cell type, anatomical location in vivo, and even subcellular location), highlighting the fact that proper function of the cytoskeleton is essential for many different cellular functions.
Mutations in cytoskeleton regulators
Clearly, drug exposure can have diverse effects on the cytoskeleton of exposed CNS cells, including changes in vivo (see below, and [22,23] for examples with ethanol). Recently, findings using organisms with genetic alterations in cytoskeletal organizing proteins have emerged that underscore the in vivo relevance of these processes in drug relevant behaviors. One of the first reports showing that mutations in actin regulators affect behavioral drug responses centered on Drosophila white rabbit mutations [24•]. The white rabbit gene encodes three distinct isoforms of RhoGAP18B, a GTPase activating protein of the Rho-family. One of these isoforms is involved in the extent of ethanol-induced hyperactivity in flies, whereas a second one controls ethanol-induced sedation via Rho1 and/or Rac1 in vivo [24•]. Rac1 works together with the membrane-curvature associated BAR domain protein Arfaptin and another small GTPase, Arf6, to regulate ethanol-induced sedation [25]. Arf6 is an important regulator of the actin cytoskeleton and membrane trafficking at the plasma membrane [26], including endocytosis and recycling of the mu-opioid receptor [27], a well-known receptor molecule involved in addiction to numerous drugs [28,29]. Interestingly, both RhoGAP18B, and Arf6 are required in the adult nervous system, as only adult, but not developmental expression is able to rescue the ethanol-sedation phenotypes [24•,25].
Effects converging on NMDAR
The fly arouser gene encodes the homolog of EPS8. arouser mutants are sensitive to ethanol-induced sedation, and the gene regulates sensitivity downstream of the mitogen-activated protein kinase (MAPK) Erk [30]. Mammalian EPS8 is a known regulator of the actin cytoskeleton, both via growth factor triggered activation of Rac, as well as via its direct actin capping activity. Eps8 knock-out mice are resistant to ethanol-induced loss-of-righting, and they drink more ethanol and show increased ethanol-preference in a 2-bottle choice test [31••]. Upon ethanol exposure, acute cerebellar slices from wild-type mice show a rundown in NMDAR current, while primary cerebellar granule cells from wild-type mice show a decrease in F-actin staining. Both of these responses are blunted in Eps8 knock outs [31••], suggesting that F-actin stability is required for proper NMDAR function and behavioral ethanol responses in vivo.
Actin regulation mutants and cocaine
Kalirin is a guanine nucleotide exchange factor (GEF) for the Rho-family, which has numerous isoforms. One of them, Kalirin-7 (Kal7), activates Rac1 and is highly enriched in the postsynaptic density (PSD) of rat cerebral cortex [32]. In the conditioned place preference paradigm (CPP), cocaine is used as the reinforcing cue for animals to form a place memory. Knock out mice of Kal7, Kal7KO, show reduced CPP [33], possibly as a result of decreased Rac1 levels. The mutants also have reduced levels of the NMDAR subunit NR2B, and the reduction of CPP seen in wild type mice injected with the NR2B-specific blocker ifenprodil is occluded in Kal7KO mice [34•]. These data again underscore the connection between the actin cytoskeleton, NMDAR, and drug-induced behavioral plasticity.
While above studies imply that Rac1 activity is required for CPP, another study reported that cocaine transiently reduces active Rac1 in the nucleus accumbens (NAc), and this inhibition is required for the establishment of cocaine reward in the CPP assay, as transient activation of Rac1 at the time of drug exposure (7.5 mg/kg) actually inhibits formation of CPP [35••]. The transient (15 min) reduction in Rac1 activity after repeated cocaine is accompanied by a longer-lasting decrease (>4 hr) in cofilin phosphorylation (P-cofilin) [35••]. Cofilin is a key downstream effector of active Rac. In its unphosphorylated state, cofilin severs actin filaments and increases actin cytoskeletal dynamics [36]. P-cofilin can be triggered by RhoA/Rho kinase or Rac/Pak/Limk signaling cascades and prevents severing, leading to increased F-actin stability. Expression of either dominant-negative Rac1 or constitutively-active cofilin (which cannot be phosphorylated) facilitates CPP acquisition to a subthreshold dose of cocaine (4 mg/kg) [35••]. These data suggest that a dynamic actin cytoskeleton is needed for conditioning to cocaine. Prior experiments also suggested that actin dynamics are important in cocaine-induced reinstatement [37•]. Rats that were extinguished from cocaine self-administration show enhanced cocaine-induced reinstatement of lever pressing if they are injected with a peptide (derived from cofilin) inhibiting Limk, or with the actin depolymerizer, latA [37•]. Lastly, MEF2 mutant mice have dificits in cocaine-CPP, and the gene targets for this transcription factor include the actin regulators N-WASP, Wave3, and Profilin1 [38•].
A link from drugs and actin to dendritic spines?
The aforementioned experiments support the importance of the actin cytoskeleton in drug-mediated behavioral plasticity, but how are actin and drugs of abuse linked to neuronal and dendritic plasticity? This could occur by means of increasing any of the following: 1) total length and complexity (eg. branching) of the dendrite [39], 2) size and strength of individual spines [4], and/or 3) the number and density of spines along the dendrite [40]. All of these possibilities require the appropriate dynamic regulation of the actin cytoskeleton. Numerous studies have analyzed these changes in the context of drug abuse (see [41] for a recent review), but most have focused on spine density.
Spine density and locomotor sensitization
Repeated administration of stimulants like cocaine or amphetamine leads to sensitization of their locomotor-stimulating effects, i.e. the same drug dose causes greater locomotor activation. Because such drug exposures very often lead to an increase in spine density in the NAc (reviewed in [6]), the two phenomena were initially suggested to be causally linked. However, numerous recent findings with various mutants have raised questions about this link. In Kal7KO mutants, for example, basal spine density in the NAc is normal, but unlike in wild-type mice, repeated cocaine injections (20 mg/kg for 4–8 days) fail to increase spine density in the Kal7KO [33], suggesting a critical role for Kalirin-7 (and Rac1?) in this process. Interestingly, even in the absence of the spine increase in the NAc, the Kal7KO mice show increased locomotor sensitization behavior, suggesting a functional disconnect between cocaine-induced increases in spine density and sensitized behavior. The first hints of such a functional disconnect between spines and behavior came from studies on the MEF2 transcription factors [38•] and cyclin-dependent kinase 5 [42–44], which revealed that experimental conditions that blocked the cocaine-induced increase in NAc spine density result in enhanced locomotor sensitization. Since then, many other molecular manipulations have revealed a similar inverse relationship between spines and behavior, including MeCP2, Dnmt3a, CREB, SynCAM1, and FMRP ([45–49] and unpublished findings (FMRP), LN Smith and CW Cowan). On the surface, this would argue that the drug-induced spine changes are not required for the behavioral plasticity and may be an epiphenomenon. Considering the reported decrease in excitability of prefrontal cortical neurons after chronic drug exposure [50,51], the reduced presynaptic function after chronic cocaine (J Jedynak, MJ Thomas and CW Cowan, unpublished findings), the noted transient decrease in postsynaptic function [52–54] and the emergence of new silent synapses [55], it is interesting to speculate that the chronic cocaine-induced increase in dendritic spine density/size [6], surface AMPA receptors [56], and occlusion of LTP in the NAc [57] represent homeostatic reactions that attempt to normalize/stabilize excitatory synaptic connectivity and circuit function. These changes might promote drug withdrawal behaviors (e.g. craving), limit long-term circuit changes, or be functionally unrelated phenomenon.
Conclusions
Structural plasticity of neurons is undoubtedly a key contributor in the development of addicted behaviors, though it remains to be seen whether we currently have the spatial resolution to isolate and characterize the relevant synaptic connections that mediate the behavioral adaptations. What we do know is that cytoskeletal dynamics are a necessary component of these synaptic changes. In a meta-analysis of nine mouse strains that show significant differences in voluntary ethanol consumption, two of the three most overrepresented genome annotations of the gene transcripts that correlate with drinking were “regulation of the actin cytoskeleton” and “MAPK signaling/ERK pathway”, implying that these pathways might predispose animals towards voluntary ethanol consumption [58]. As discussed here, the cytoskeleton plays a crucial role in neuronal, dendritic, and behavioral plasticity seen in addicted animals. Recent work suggests that the small GTPases RhoA (regulating actin dynamics) and Ras (an upstream activator of MAPK/ERK signaling) are activated locally in dendritic spines upon induction of LTP, and then diffuse out of these spines to facilitate further plasticity in nearby spines micrometers away, and over longer time spans of minutes [59,60]. These findings reiterate the strong connection between learning and memory, synaptic plasticity mediated by cytoskeletal dynamics, and the acquisition of drug addiction.
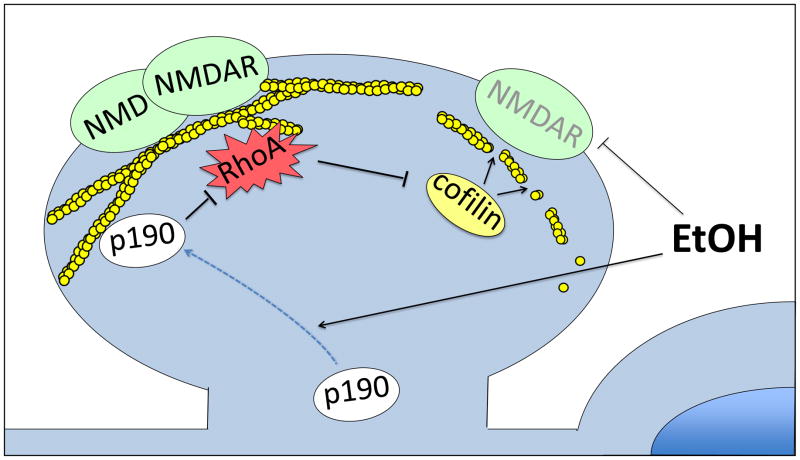
Figure 1.
Model of acute ethanol effects on NMDAR in dendritic spines. Ethanol directly inhibits NMDAR at high doses, and at lower doses leads to relocation of p190RhoGAP to the plasma membrane, which causes RhoA inactivation, cofilin activation, loss of filamentous actin, and a decrease in NMDAR at the plasma membrane.
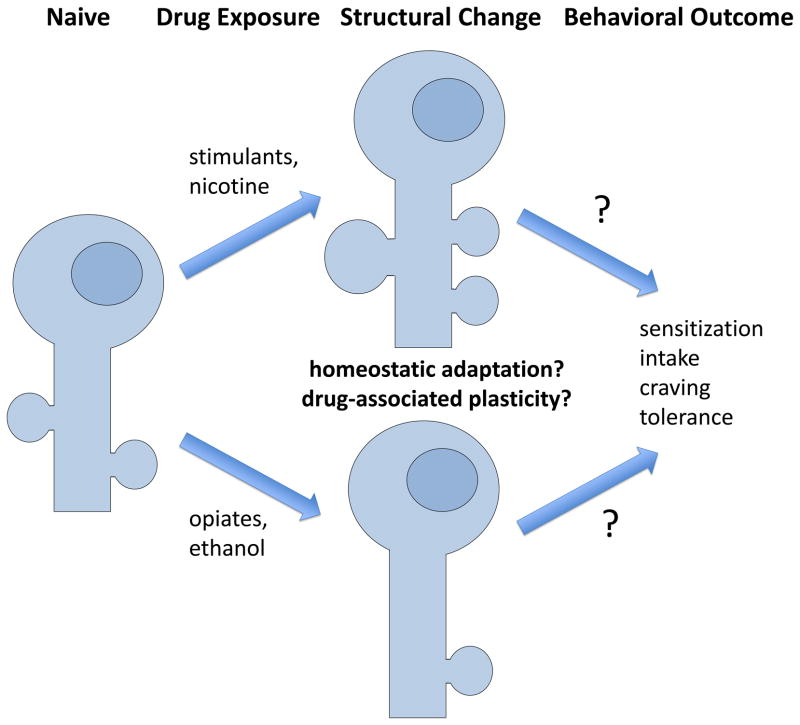
Figure 2.
Drug-induced structural plasticity in NAc medium spiny neurons. Many reports have found diverse effects that depend on variables such as anatomical region (core, or shell), neuronal subtype (D1, versus D2 dopamine receptor), duration of drug exposure (acute, chronic, or chronic followed by withdrawal) and others. The exact behavioral consequence of the structural changes observed remains elusive.
Highlights.
Exposure to drugs of abuse causes changes in the cytoskeleton and neuronal structure.
The explanations for, and consequences of the changes remain elusive.
Mutants in cytoskeletal regulatory genes alter drug-induced behavior.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
- 1.Shen K, Cowan CW. Guidance molecules in synapse formation and plasticity. Cold Spring Harb Perspect Biol. 2010;2:a001842. doi: 10.1101/cshperspect.a001842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rex CS, Chen LY, Sharma A, Liu J, Babayan AH, Gall CM, Lynch G. Different Rho GTPase-dependent signaling pathways initiate sequential steps in the consolidation of long-term potentiation. J Cell Biol. 2009;186:85–97. doi: 10.1083/jcb.200901084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Craddock TJA, Tuszynski JA, Hameroff S. Cytoskeletal signaling: is memory encoded in microtubule lattices by CaMKII phosphorylation? PLoS Comput Biol. 2012;8:e1002421. doi: 10.1371/journal.pcbi.1002421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bosch M, Hayashi Y. Structural plasticity of dendritic spines. Curr Opin Neurobiol. 2012;22:383–388. doi: 10.1016/j.conb.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grueter BA, Rothwell PE, Malenka RC. Integrating synaptic plasticity and striatal circuit function in addiction. Curr Opin Neurobiol. 2012;22:545–551. doi: 10.1016/j.conb.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robinson TE, Kolb B. Structural plasticity associated with exposure to drugs of abuse. Neuropharmacology. 2004;47 (Suppl 1):33–46. doi: 10.1016/j.neuropharm.2004.06.025. [DOI] [PubMed] [Google Scholar]
- 7.French SW, Katsuma Y, Ray MB, Swierenga SH. Cytoskeletal pathology induced by ethanol. Ann N Y Acad Sci. 1987;492:262–276. doi: 10.1111/j.1749-6632.1987.tb48680.x. [DOI] [PubMed] [Google Scholar]
- 8.Matsuda Y, Takada A, Kanayama R, Takase S. Changes of hepatic microtubules and secretory proteins in human alcoholic liver disease. Pharmacol Biochem Behav. 1983;18 (Suppl 1):479–482. doi: 10.1016/0091-3057(83)90221-6. [DOI] [PubMed] [Google Scholar]
- 9.Ray MB. Distribution patterns of cytokeratin antigen determinants in alcoholic and nonalcoholic liver diseases. Hum Pathol. 1987;18:61–66. doi: 10.1016/s0046-8177(87)80195-8. [DOI] [PubMed] [Google Scholar]
- 10.Xu D, Sorrell MF, Casey CA, Clemens DL, Tuma DJ. Long-term ethanol feeding selectively impairs the attachment of rat perivenous hepatocytes to extracellular matrix substrates. Gastroenterology. 1994;106:473–479. doi: 10.1016/0016-5085(94)90607-6. [DOI] [PubMed] [Google Scholar]
- 11.Tomás M, Lázaro-Diéguez F, Durán JM, Marín P, Renau-Piqueras J, Egea G. Protective effects of lysophosphatidic acid (LPA) on chronic ethanol-induced injuries to the cytoskeleton and on glucose uptake in rat astrocytes. J Neurochem. 2003;87:220–229. doi: 10.1046/j.1471-4159.2003.01993.x. [DOI] [PubMed] [Google Scholar]
- 12.Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature. 2002;420:629–635. doi: 10.1038/nature01148. [DOI] [PubMed] [Google Scholar]
- 13.Guasch RM, Tomas M, Miñambres R, Valles S, Renau-Piqueras J, Guerri C. RhoA and lysophosphatidic acid are involved in the actin cytoskeleton reorganization of astrocytes exposed to ethanol. J Neurosci Res. 2003;72:487–502. doi: 10.1002/jnr.10594. [DOI] [PubMed] [Google Scholar]
- 14.Allansson L, Khatibi S, Olsson T, Hansson E. Acute ethanol exposure induces [Ca2+]i transients, cell swelling and transformation of actin cytoskeleton in astroglial primary cultures. J Neurochem. 2001;76:472–479. doi: 10.1046/j.1471-4159.2001.00097.x. [DOI] [PubMed] [Google Scholar]
- 15.Selva J, Egea G. Ethanol increases p190RhoGAP activity, leading to actin cytoskeleton rearrangements. J Neurochem. 2011;119:1306–1316. doi: 10.1111/j.1471-4159.2011.07522.x. [DOI] [PubMed] [Google Scholar]
- 16.Funk CK, Dohrman DP. Chronic ethanol exposure inhibits dopamine release via effects on the presynaptic actin cytoskeleton in PC12 cells. Brain Research. 2007;1185:86–94. doi: 10.1016/j.brainres.2007.09.069. [DOI] [PubMed] [Google Scholar]
- 17.Dopico AM, Lovinger DM. Acute alcohol action and desensitization of ligand-gated ion channels. Pharmacol Rev. 2009;61:98–114. doi: 10.1124/pr.108.000430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yashiro K, Philpot BD. Regulation of NMDA receptor subunit expression and its implications for LTD, LTP, and metaplasticity. Neuropharmacology. 2008;55:1081–1094. doi: 10.1016/j.neuropharm.2008.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carpenter-Hyland EP, Chandler LJ. Homeostatic plasticity during alcohol exposure promotes enlargement of dendritic spines. Eur J Neurosci. 2006;24:3496–3506. doi: 10.1111/j.1460-9568.2006.05247.x. [DOI] [PubMed] [Google Scholar]
- 20•.Popp RL, Dertien JS. Actin depolymerization contributes to ethanol inhibition of NMDA receptors in primary cultured cerebellar granule cells. Alcohol. 2008;42:525–539. doi: 10.1016/j.alcohol.2008.06.006. Shows the negative effects that ethanol can have on NMDAR function via its action on intracellular actin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Romero AM, Esteban-Pretel G, Marín MP, Ponsoda X, Ballestín R, Canales JJ, Renau-Piqueras J. Chronic ethanol exposure alters the levels, assembly, and cellular organization of the actin cytoskeleton and microtubules in hippocampal neurons in primary culture. Toxicol Sci. 2010;118:602–612. doi: 10.1093/toxsci/kfq260. [DOI] [PubMed] [Google Scholar]
- 22.Zhou FC, Anthony B, Dunn KW, Lindquist WB, Xu ZC, Deng P. Chronic alcohol drinking alters neuronal dendritic spines in the brain reward center nucleus accumbens. Brain Research. 2007;1134:148–161. doi: 10.1016/j.brainres.2006.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Whitcher LT, Klintsova AY. Postnatal binge-like alcohol exposure reduces spine density without affecting dendritic morphology in rat mPFC. Synapse. 2008;62:566–573. doi: 10.1002/syn.20532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24•.Rothenfluh A, Threlkeld R, Bainton RJ, Tsai L, Lasek A, Heberlein U. Distinct Behavioral Responses to Ethanol Are Regulated by Alternate RhoGAP18B Isoforms. Cell. 2006;127:199–211. doi: 10.1016/j.cell.2006.09.010. One of the first reports establishing the in vivo relevance of Rho-family GTPases in behavioral drug responses. [DOI] [PubMed] [Google Scholar]
- 25.Peru y Colon de Portugal RL, Acevedo SF, Rodan AR, Chang LY, Eaton BA, Rothenfluh A. Adult Neuronal Arf6 Controls Ethanol-Induced Behavior With Arfaptin Downstream of Rac1 and RhoGAP18B. J Neurosci. 2012;32:17706–17713. doi: 10.1523/JNEUROSCI.1944-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jaworski J. ARF6 in the nervous system. Eur J Cell Biol. 2007;86:513–524. doi: 10.1016/j.ejcb.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 27.Rankovic M, Jacob L, Rankovic V, Brandenburg L-O, Schröder H, Höllt V, Koch T. ADP-ribosylation factor 6 regulates mu-opioid receptor trafficking and signaling via activation of phospholipase D2. Cellular Signalling. 2009;21:1784–1793. doi: 10.1016/j.cellsig.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 28.Simmons D, Self DW. Role of mu- and delta-opioid receptors in the nucleus accumbens in cocaine-seeking behavior. Neuropsychopharmacology. 2009;34:1946–1957. doi: 10.1038/npp.2009.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chamorro A-J, Marcos M, Mirón-Canelo J-A, Pastor I, González-Sarmiento R, Laso F-J. Association of μ-opioid receptor (OPRM1) gene polymorphism with response to naltrexone in alcohol dependence: a systematic review and meta-analysis. Addict Biol. 2012;17:505–512. doi: 10.1111/j.1369-1600.2012.00442.x. [DOI] [PubMed] [Google Scholar]
- 30.Eddison M, Guarnieri DJ, Cheng L, Liu C-H, Moffat KG, Davis G, Heberlein U. arouser reveals a role for synapse number in the regulation of ethanol sensitivity. Neuron. 2011;70:979–990. doi: 10.1016/j.neuron.2011.03.030. [DOI] [PubMed] [Google Scholar]
- 31••.Offenhauser N, Castelletti D, Mapelli L, Soppo BE, Regondi MC, Rossi P, D’Angelo E, Frassoni C, Amadeo A, Tocchetti A, et al. Increased ethanol resistance and consumption in Eps8 knockout mice correlates with altered actin dynamics. Cell. 2006;127:213–226. doi: 10.1016/j.cell.2006.09.011. A pioneering paper linking in vivo ethanol-induced F-actin lability with NMDAR current rundown, and showing these effects are reduced in the EPS8 knockout-mice, which also drink more alcohol. [DOI] [PubMed] [Google Scholar]
- 32.Penzes P, Johnson RC, Alam MR, Kambampati V, Mains RE, Eipper BA. An isoform of kalirin, a brain-specific GDP/GTP exchange factor, is enriched in the postsynaptic density fraction. J Biol Chem. 2000;275:6395–6403. doi: 10.1074/jbc.275.9.6395. [DOI] [PubMed] [Google Scholar]
- 33.Kiraly DD, Ma X-M, Mazzone CM, Xin X, Mains RE, Eipper BA. Behavioral and morphological responses to cocaine require kalirin7. Biol Psychiatry. 2010;68:249–255. doi: 10.1016/j.biopsych.2010.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34•.Kiraly DD, Lemtiri-Chlieh F, Levine ES, Mains RE, Eipper BA. Kalirin binds the NR2B subunit of the NMDA receptor, altering its synaptic localization and function. J Neurosci. 2011;31:12554–12565. doi: 10.1523/JNEUROSCI.3143-11.2011. Phenotypic characterization of the Rac activator Kalirin7 knock out. Shows a decrease in cocaine-conditioned place preference, an increase in locomotor sensitization to cocaine, yet no change in NAc spine density upon cocaine exposure. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35••.Dietz DM, Sun H, Lobo MK, Cahill ME, Chadwick B, Gao V, Koo JW, Mazei-Robison MS, Dias C, Maze I, et al. Rac1 is essential in cocaine-induced structural plasticity of nucleus accumbens neurons. Nat Neurosci. 2012 doi: 10.1038/nn.3094. This paper shows that decreased Rac1 activity is required for both cocaine-mediated spine plasticity and conditioned place preference. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bernstein BW, Bamburg JR. ADF/cofilin: a functional node in cell biology. Trends Cell Biol. 2010;20:187–195. doi: 10.1016/j.tcb.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37•.Toda S, Shen H-W, Peters J, Cagle S, Kalivas PW. Cocaine increases actin cycling: effects in the reinstatement model of drug seeking. J Neurosci. 2006;26:1579–1587. doi: 10.1523/JNEUROSCI.4132-05.2006. Shows complex effects of various cocaine regimens on F-actin and cofilin, but establishes in vivo relevance of cofilin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38•.Pulipparacharuvil S, Renthal W, Hale CF, Taniguchi M, Xiao G, Kumar A, Russo SJ, Sikder D, Dewey CM, Davis MM, et al. Cocaine regulates MEF2 to control synaptic and behavioral plasticity. Neuron. 2008;59:621–633. doi: 10.1016/j.neuron.2008.06.020. One of the first reports to show dissociation between dendritic spine density and behavioral sensitization. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Simó S, Cooper JA. Regulation of dendritic branching by Cdc42 GAPs. Genes Dev. 2012;26:1653–1658. doi: 10.1101/gad.199034.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kessels MM, Schwintzer L, Schlobinski D, Qualmann B. Controlling actin cytoskeletal organization and dynamics during neuronal morphogenesis. Eur J Cell Biol. 2011;90:926–933. doi: 10.1016/j.ejcb.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 41.Golden SA, Russo SJ. Mechanisms of psychostimulant-induced structural plasticity. Cold Spring Harb Perspect Med. 2012:2. doi: 10.1101/cshperspect.a011957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Norrholm SD, Bibb JA, Nestler EJ, Ouimet CC, Taylor JR, Greengard P. Cocaine-induced proliferation of dendritic spines in nucleus accumbens is dependent on the activity of cyclin-dependent kinase-5. Neuroscience. 2003;116:19–22. doi: 10.1016/s0306-4522(02)00560-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Taylor JR, Lynch WJ, Sanchez H, Olausson P, Nestler EJ, Bibb JA. Inhibition of Cdk5 in the nucleus accumbens enhances the locomotor-activating and incentive-motivational effects of cocaine. PNAS. 2007;104:4147–4152. doi: 10.1073/pnas.0610288104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Benavides DR, Quinn JJ, Zhong P, Hawasli AH, DiLeone RJ, Kansy JW, Olausson P, Yan Z, Taylor JR, Bibb JA. Cdk5 modulates cocaine reward, motivation, and striatal neuron excitability. J Neurosci. 2007;27:12967–12976. doi: 10.1523/JNEUROSCI.4061-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.LaPlant Q, Vialou V, Covington HE, Dumitriu D, Feng J, Warren BL, Maze I, Dietz DM, Watts EL, Iñiguez SD, et al. Dnmt3a regulates emotional behavior and spine plasticity in the nucleus accumbens. Nat Neurosci. 2010;13:1137–1143. doi: 10.1038/nn.2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Deng JV, Rodriguiz RM, Hutchinson AN, Kim I-H, Wetsel WC, West AE. MeCP2 in the nucleus accumbens contributes to neural and behavioral responses to psychostimulants. Nat Neurosci. 2010;13:1128–1136. doi: 10.1038/nn.2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vialou V, Feng J, Robison AJ, Ku SM, Ferguson D, Scobie KN, Mazei-Robison MS, Mouzon E, Nestler EJ. Serum response factor and cAMP response element binding protein are both required for cocaine induction of ΔFosB. J Neurosci. 2012;32:7577–7584. doi: 10.1523/JNEUROSCI.1381-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brown TE, Lee BR, Mu P, Ferguson D, Dietz D, Ohnishi YN, Lin Y, Suska A, Ishikawa M, Huang YH, et al. A silent synapse-based mechanism for cocaine-induced locomotor sensitization. J Neurosci. 2011;31:8163–8174. doi: 10.1523/JNEUROSCI.0016-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Giza JI, Jung Y, Jeffrey RA, Neugebauer NM, Picciotto MR, Biederer T. The Synaptic Adhesion Molecule SynCAM 1 Contributes to Cocaine Effects on Synapse Structure and Psychostimulant Behavior. Neuropsychopharmacology. 2012 doi: 10.1038/npp.2012.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jentsch JD, Taylor JR. Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli. Psychopharm. 1999;146:373–390. doi: 10.1007/pl00005483. [DOI] [PubMed] [Google Scholar]
- 51.Volkow ND, Fowler JS, Wang G-J. The addicted human brain: insights from imaging studies. J Clin Invest. 2003;111:1444–1451. doi: 10.1172/JCI18533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thomas MJ, Beurrier C, Bonci A, Malenka RC. Long-term depression in the nucleus accumbens: a neural correlate of behavioral sensitization to cocaine. Nat Neurosci. 2001;4:1217–1223. doi: 10.1038/nn757. [DOI] [PubMed] [Google Scholar]
- 53.Kourrich S, Rothwell PE, Klug JR, Thomas MJ. Cocaine experience controls bidirectional synaptic plasticity in the nucleus accumbens. J Neurosci. 2007;27:7921–7928. doi: 10.1523/JNEUROSCI.1859-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kourrich S, Thomas MJ. Similar neurons, opposite adaptations: psychostimulant experience differentially alters firing properties in accumbens core versus shell. J Neurosci. 2009;29:12275–12283. doi: 10.1523/JNEUROSCI.3028-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Huang YH, Lin Y, Mu P, Lee BR, Brown TE, Wayman G, Marie H, Liu W, Yan Z, Sorg BA, et al. In vivo cocaine experience generates silent synapses. Neuron. 2009;63:40–47. doi: 10.1016/j.neuron.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Boudreau AC, Wolf ME. Behavioral sensitization to cocaine is associated with increased AMPA receptor surface expression in the nucleus accumbens. J Neurosci. 2005;25:9144–9151. doi: 10.1523/JNEUROSCI.2252-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pascoli V, Turiault M, Lüscher C. Reversal of cocaine-evoked synaptic potentiation resets drug-induced adaptive behaviour. Nature. 2012;481:71–75. doi: 10.1038/nature10709. [DOI] [PubMed] [Google Scholar]
- 58.Mulligan MK, Ponomarev I, Hitzemann RJ, Belknap JK, Tabakoff B, Harris RA, Crabbe JC, Blednov YA, Grahame NJ, Phillips TJ, et al. Toward understanding the genetics of alcohol drinking through transcriptome meta-analysis. PNAS. 2006;103:6368–6373. doi: 10.1073/pnas.0510188103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Harvey CD, Yasuda R, Zhong H, Svoboda K. The spread of Ras activity triggered by activation of a single dendritic spine. Science. 2008;321:136–140. doi: 10.1126/science.1159675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Murakoshi H, Wang H, Yasuda R. Local, persistent activation of Rho GTPases during plasticity of single dendritic spines. Nature. 2011;472:100–104. doi: 10.1038/nature09823. [DOI] [PMC free article] [PubMed] [Google Scholar]