Abstract
The salinity responses of cyanobacteria, anoxygenic phototrophs, sulfate reducers, and methanogens from the laminated endoevaporitic community in the solar salterns of Eilat, Israel, were studied in situ with oxygen microelectrodes and in the laboratory in slurries. The optimum salinity for the sulfate reduction rate in sediment slurries was between 100 and 120‰, and sulfate reduction was strongly inhibited at an in situ salinity of 215‰. Nevertheless, sulfate reduction was an important respiratory process in the crust, and reoxidation of formed sulfide accounted for a major part of the oxygen budget. Methanogens were well adapted to the in situ salinity but contributed little to the anaerobic mineralization in the crust. In slurries with a salinity of 180‰ or less, methanogens were inhibited by increased activity of sulfate-reducing bacteria. Unicellular and filamentous cyanobacteria metabolized at near-optimum rates at the in situ salinity, whereas the optimum salinity for anoxygenic phototrophs was between 100 and 120‰.
Halotolerant and halophilic microorganisms frequently grow to high densities at increased salt concentrations. In addition to the fact that such organisms have great commercial importance, the study of these organisms may improve our understanding of the development of life on Earth and the ultimate environmental limits to its existence on this and other planets (11, 28). Based on the extensive collection of halotolerant and halophilic organisms obtained in culture (16, 18, 19, 21, 25, 28) and on in situ observations for hypersaline environments (1, 2, 22), there seems to be progressive exclusion of physiological groups as the salinity increases. Thus, some physiological groups of organisms (e.g., acetoclastic methanogens and autotrophic nitrite oxidizers) seem to be unable to tolerate salinities much above those in seawater, while a few groups, including oxygenic and anoxygenic phototrophs, aerobically respiring archaea and bacteria, and denitrifiers, are able to live at salt concentrations of more than 300‰ (27). While sulfate-reducing bacteria are quantitatively and qualitatively important in environments with salinities greater than 200‰, isolated strains are poorly adapted to such high salinities (3, 4, 27).
In coastal solar salterns in which seawater is evaporated during production of NaCl, microbial mats are present at salinities up to more than 130‰, and photosynthetic subevaporitic or endoevaporitic stratified bacterial communities associated with precipitated salt crusts (mainly gypsum) develop on the bottom of evaporation basins at salinities greater than 200‰ (8, 29, 36). Members of such natural prokaryotic communities may have salt adaptations that are not reflected in culture collections, and studies of pure cultures should be supplemented with salinity response studies of natural communities in order to fully understand to what extent increased salinity limits prokaryotic growth.
This study was an attempt to understand the salinity tolerance of natural populations belonging to different metabolic groups of an endoevaporitic, stratified, photosynthetic community in the solar salterns of Eilat, Israel (29). The rates of photosynthesis, sulfate reduction, and methanogenesis were measured at different salinities in situ and/or in slurry experiments, and the results were compared with previous studies of related organisms.
MATERIALS AND METHODS
Site description.
The sampling site was situated in pond 200 of the solar saltern plant in Eilat, Israel. The salinity in this pond was 215‰ at the time of sampling, and a gypsum crust had developed on the bottom of the pond. The thickness of this crust varied from a few centimeters to more than 10 cm. Samples were taken in the thicker sections of the crust where the bacterial community was fully developed, and the characteristics resembled those in the descriptions given in previous studies of the same location (29) (Fig. 1). Initially, the crust was examined by phase-contrast microscopy to identify the dominant phototrophs in each layer. The brown layer in the upper 0.5 to 1 cm of the crust was composed mainly of unicellular Halothece-type cyanobacteria. Below this layer was a white layer without photosynthetic organisms, and this was followed by a bright green cyanobacterial layer at a depth of 1 to 1.5 cm. The phototrophs in this second oxygenic layer were straight or helical filaments with cylindrical cells in single trichomes 1 to 10 μm in diameter and resembled Halospirulina and Phormidium-type organisms. Below this layer, at a depth of about 2 cm, was a purple layer in which Chromatium-like anoxygenic phototrophs about 5 μm in diameter with intracellular sulfur granules were abundant, and sometimes below this there was an olive green layer of thin filaments (diameter, about 1 μm) of uncertain affiliation. The filamentous organisms in this olive green layer were strongly red autofluorescing, indicating that they contained chlorophyll a and thus probably were cyanobacteria. However, the occurrence of a deep cyanobacterial layer below a layer of purple phototrophs is unusual, and further characterization is needed in order to confirm the identity and physiology of these green filaments. In total, the photic zone stretched 2 to 3 cm into the crust, below which the color was increasingly dark due to precipitation of metal sulfides. Below the solid crust was a black sediment consisting of sand grains and gypsum crystals.
FIG. 1.
Crust in pond 200 of the solar salterns in Eilat, Israel, showing the laminated microbial community.
Chemical analysis of the pore water.
Pore water samples (1 ml) were collected by using a 5-ml syringe with a long needle that was pressed down into the crust to depths between 1 and 10 cm, and the samples were immediately injected into closed anaerobic 12-ml glass tubes containing 2 ml of 5% (wt/vol) zinc acetate. All tubes were placed in the dark and frozen immediately after they were returned to the lab.
Salinity was measured with a hydrometer and was calculated by using the standard conversion factors (http:/www.unisense.com/support/pdf/gas_tables.pdf.). The methane concentration was measured in the headspace of the tubes containing pore water by using an HP 5710A gas chromatograph, and the results were converted to molar concentrations of methane in the samples. The amount of sulfide fixed as zinc sulfide in the same samples was measured by the spectrophotometric method of Cline (9). Pore water sulfate concentrations were measured by using filtered water samples and a Sykam ion chromatograph system equipped with a polystyrene-based LAC A14 column and an S3111 detector (Sykam). Prior to measurements, excess zinc in the samples was removed by precipitation with carbonate, followed by filtration (pore size, 0.2 μm; Osmonics Cameo 25AS), and finally the samples were diluted by adding 30 volumes of 5 mM NaHCO3 buffer.
In situ sulfate reduction rates.
Cores were obtained with Plexiglas tubes (25 by 5 cm) that were knocked through the crust and into the underlying sandy sediment with a hammer. The hard crystalline structure of the crust preserved the lamination of the prokaryotic community inside the cores despite the rough sampling method that was necessary. The cores were obtained in the morning before sunrise, when oxygen was depleted from all but the surface layer of the crust. The samples were then brought to the lab, and sulfate reduction rates were measured by the radiotracer method (12, 17). Initially, 35S-labeled sulfate (0.5 to 1 MBq) was injected with a needle through several small holes in the sides of the Plexiglas tubes and into the top 10 cm of the crust. The cores were incubated for 24 h (the rather long incubation period was necessary because of the large amount of dissolved sulfate in the water) at the in situ temperature (23°C) in the dark and then frozen in a −80°C freezer to terminate the incubation. The frozen cores were sawed into 1-cm slices that were immediately fixed in equal volumes of 20% zinc acetate and stored frozen until further processing. The samples were distilled by the one-step distillation technique of Fossing and Jørgensen (12). After centrifugation and removal of the supernatant from each fixed sample, the pellets were distilled with HCl and Cr2+ to release all reduced sulfur as H2S. The sulfide was trapped in Zn-containing traps, and the amount of radiolabel in the sulfate and the reduced sulfur pool was quantified by liquid scintillation counting (Packard Tri-Carb 2200CA liquid scintillation analyzer) of the supernatant fluids and the traps.
Oxygen profiles.
Microelectrodes were used for measurement of oxygen profiles and photosynthesis rates in the crust. Intact pieces of evaporite were obtained for these experiments by sawing off chunks of the crust covering the bottom of pond 200. The crust samples were kept submerged in pond water while they were transported to the laboratory. The measurement procedure took place on a table on the beach in front of the laboratory of the Moshe Shilo Center for Marine Biogeochemistry, The Interuniversity Institute for Marine Sciences, Eilat, Israel, where the light conditions were the same as those in the ponds (i.e., full sun exposure during daytime). A piece of crust that was about 20 by 20 by 5 cm was placed in a water bath containing water from pond 200. Above the crust, an oxygen needle microelectrode was mounted in a micromanipulator on an electrode stand and connected to a picoamperometer (Unisense, Aarhus, Denmark). Since the crust was impossible to penetrate without breaking the needle electrode, a predrilled hole that was the same size as the electrode needle was used to measure the oxygen profile. In a pilot experiment, we measured oxygen in this way at regular intervals during a period of several days and obtained consistent results. This was important, since the highly heterogeneous structure of the crust meant that all measurements during the salinity manipulations had to be performed in the same hole. The temperature in the water bath was continuously measured and was allowed to increase to 23°C in the morning, which was the water temperature in the ponds, and the temperature was kept stable during the day by adding ice cubes made with pond water. An aquarium pump continuously aerated the water, which maintained atmospheric equilibrium with respect to oxygen and kept the water well mixed.
Rates of oxygen production were measured by the light-dark shift method (30). The electrode was placed at the desired position in the crust and allowed to equilibrate. Light was kept off the electrode setup by covering it with a black bed sheet, and the decrease in oxygen concentration, corresponding to the rate of oxygen production, was monitored. The electrode did not always respond immediately to the light-dark shift, probably due to the use of a predrilled hole, which introduced a micrometer-scale distance between the bacterial community and the electrode tip. This delay made it impossible to obtain a stable decrease in the electrode signal reflecting the photosynthesis in the highly active surface layer, where the oxygen concentrations decreased dramatically within a few seconds of initiation of dark conditions (30, 31, 32). However, the method was successfully applied to the less active green layer below.
Oxygen profiles were measured in the crust at noon after 24 h of incubation at various salinities. Initially, the water bath was filled with water from pond 201, an adjacent pond containing 264‰ salt, a higher salinity than that of pond 200 where the crust was sampled (215‰). The crust was then left overnight in order to saturate it with the high-salinity water, and the oxygen profiles and rates of oxygen production were measured at noon the following day. After the measurements were obtained, the water was exchanged with water having a lower salinity, the crust was left overnight, and the oxygen profile was measured again the following day at noon. This procedure was repeated on the following days; the oxygen concentrations and production rates were always measured at noon and in the same hole. The salinity was checked regularly during incubation and was shown to increase 5 to 10‰ each day due to evaporation from the water bath. The mean salinities during the different incubations were used as the most representative data.
Salinity experiments with sulfate reducers and methanogens.
To measure the influence of changing salinity on the activity of sulfate-reducing bacteria and methanogens in the crust, a series of slurry experiments were performed in which samples of the black, sulfidic sediment below the crust were mixed with degassed and dithionite-reduced pond water (total volume, 30 ml) diluted to obtain various salinities in 60-ml glass bottles. The slurries were incubated at room temperature on a shaking table. The accumulation of methane in the gas phase was measured by gas chromatography. Sulfate reduction rates were measured by injecting [35S]sulfate (0.5 MBq) into slurries, fixing each slurry with 5 ml of 20% (wt/vol) zinc acetate after 24 h of incubation, and finally quantifying the amount of radiolabel in the reduced sulfur and sulfate pools as described above. By measuring the methane in the gas phase before and after the [35S]sulfate incubation, the rates of methane formation and sulfate reduction could be directly compared. The effect of molybdate addition on the rate of methanogenesis was examined by using similar slurries with various concentrations of salt and molybdate.
Salinity experiments with phototrophic slurries.
The salinity responses of various populations of phototrophs were measured by determining [14C]bicarbonate uptake during incubation at a range of salinities. The photosynthetic crust was dissected into three fractions: the surface layer consisting mainly of unicellular cyanobacteria, the bright green layer consisting of filamentous cyanobacteria, and the purple anoxic layer. The two cyanobacterial layers were placed into a 200‰ NaCl solution, from which 1-ml subsamples were transferred to NaCl solutions of various salinities in 10-ml glass tubes. The final salt concentrations in the tubes ranged from 60 to 280‰. Samples of anoxygenic phototrophs were prepared in the same way but with anoxic water having final salinities ranging from 60 to 260‰. The tubes were flushed with N2, and the preparations were reduced with sulfide (final concentration, 0.5 mM). Prior to incubation, [14C]carbonate (10kBq; Risø, Roskilde, Denmark) was injected into each tube. For each layer, one series of glass tubes was closed with rubber lids and incubated in the light at different intensities for 24 h, while another series was incubated in the dark and used to correct for dark carbon fixation. Following incubation, 1 ml of 2 M NaOH was injected into each glass tube to fix all radiolabeled carbon in the liquid phase. The samples were introduced into a distillation setup, in which nitrogen was bubbled through the samples and subsequently through two sets of CO2 traps, each containing 10 ml of 1 M NaOH. Distillation was initiated by adding 1 ml of 4 M HCl to each sample and was stopped after 30 min of constant N2 flow through the system. The amount of radioactivity in each trap was determined by scintillation counting, as was the amount of remaining radioactivity in the distilled sample. The photosynthetic activity was calculated by determining the ratio of the counts in the distilled sample to the counts in the traps and was corrected for the contribution from dark fixation.
Diffusion coefficients.
The diffusive transport of different ions and gases in the crust can be calculated from the concentration profiles by using Fick's first law of diffusion: F = −Φ × Dc × dC/dz, where F is the vertical flux, Φ is the porosity of the crust, Dc is the diffusion coefficient in the crust, which can be calculated from the diffusion coefficient in free solution by correcting for tortuosity, and dC/dz is the vertical concentration gradient. Thus, the diffusion coefficient in free solution, the porosity, and the tortuosity must be known for such calculations. Furthermore, the possibility of convection has to be considered.
The photosynthetic layers were rich in exopolymeric substances that stabilized the pore water and limited convective transport. The porosity of the crust was between 60 and 70%. The tortuosity of the crust was difficult to assess, but the crystals were mainly vertically oriented and continuous for several centimeters. We therefore ignored tortuosity effects. The resulting diffusion coefficients are summarized in Table 1.
TABLE 1.
Diffusion coefficients used in this studya
| Salinity (‰, wt/vol) | O2Dw (10−5 cm2 s−1) | O2 Φ × Dc (10−5 cm2 s−1) | CH4Dc (10−5 cm2 s−1) |
|---|---|---|---|
| 264 | 1.46 | 0.95 | 0.81 |
| 228 | 1.56 | 1.01 | 0.86 |
| 215 | 1.60 | 1.04 | 0.88 |
| 205 | 1.63 | 1.06 | 0.90 |
| 198 | 1.65 | 1.07 | 0.91 |
| 164 | 1.76 | 1.14 | 0.97 |
| 128 | 1.89 | 1.23 | 1.04 |
Data are available at http:/www.unisense.com/support/pdf/gas_tables.pdf. Dw, diffusion coefficient in free solution; Dc, diffusion constant in the crust.
RESULTS
Sulfide and methane.
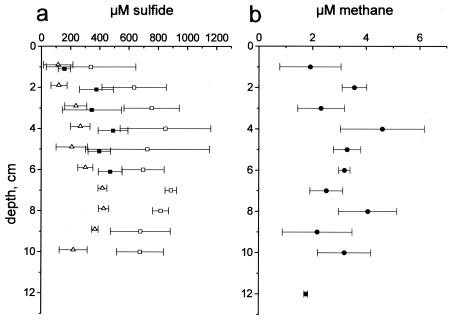
Sulfide profiles were measured at sunrise, at noon, and at sunset. The sulfide concentrations were highest in the morning at sunrise (320 to 840 μM) and lowest at noon (100 to 440 μM). At sunset sulfide had started to accumulate in the crust again (Fig. 2a). Sulfide was present in all samples from the crust, and the concentrations varied with the time of day down to a depth of at least 10 cm. The presence of several hundred micromoles of sulfide per liter in the surface at a depth of 0 to 2 cm during the daytime was probably the result of the poor depth resolution of the sampling protocol. As samples were sucked into the syringe, pore water was directed along the vertically oriented gypsum crystals, which mixed water from different layers in the crust. Thus, the values in Fig. 2 should be considered averages for several centimeters rather than exact values at each depth. This vertical mixing of water may also explain in part the large differences in the morning and noon sulfide concentrations down to a depth of 10 cm. The variation in the deep part of the crust is difficult to explain if it was not a consequence of mixing with water from the surface, where sulfide accumulated only during the night.
FIG. 2.
Sulfide and methane profiles. (a) Sulfide concentrations measured at sunrise (□), at noon (▵), and at sunset (▪). (b) Methane profiles, which did not change during the day. Data points are means of triplicate measurements, and the error bars indicate the standard deviations.
The methane concentrations were about 2 orders of magnitude lower than the sulfide concentrations, and the profiles showed little variation with the time of day. Figure 2b shows the results for the pooled morning, noon, and evening measurements. The mean concentration was between 2 and 5 μM except in the upper 1 cm of the crust and near the crust-sediment interface at a depth of about 12 cm, where the mean concentration was less. The coarse depth resolution and the large variation in the measured concentrations did not allow detailed calculations of the diffusive methane flux in the crust, nor was it possible to identify specific zones of production. However, a rough estimate of the total methane production could be obtained by assuming that there was a linear gradient between a depth of 2 cm and the surface of the crust (i.e., throughout the zone dominated by cyanobacteria). This was reasonable for methane since with a few exceptions (35), aerobic methanotrophs are absent in hypersaline systems and since oxygen in the cyanobacterial layers probably excludes methanogens (references 10 and 27 and references therein). Under this assumption, the methane gradient was 1.8 nmol cm−4, which corresponded to a flux of 1.6 × 10−5 nmol cm−2 s−1. In a previous study of the gypsum crust in the solar salterns of Eilat, the methane diffusion across the crust-water interface was found to be between 1.1 × 10−4 and 1.9 × 10−4 nmol cm−2 s−1 (i.e., about 1 order of magnitude larger). These measurements, however, were obtained for a pond with a lower salinity (138‰), and it seems likely that the high salinity depressed the methanogenesis in the crust of pond 200. Similar estimation of the sulfide formation rate in the crust was not possible as sulfide is subject to rapid cycling in oxic-anoxic interfaces and may form even in aerobic surface layers of benthic photosynthetic systems (5).
Sulfate reduction rate.
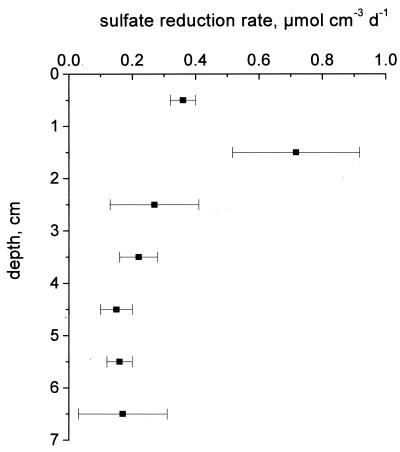
The sulfate reduction rates measured in the dark (Fig. 3) peaked at depths between 1 and 2 cm, which was above the permanently anoxic zone. The rate of sulfate reduction in this layer was between 0.36 ± 0.04 and 0.72 ± 0.20 μmol cm−3 day−1, and the rate decreased rapidly in the crust below to less than 0.2 μmol cm−3 day−1. Integration of the sulfate reduction rates in the upper 7 cm of the crust yielded a depth-integrated rate of 1.9 μmol cm−2 day−1 or 0.022 nmol cm−2 s−1.
FIG. 3.
Sulfate reduction rates in the crust measured in triplicate by using [35S]sulfate and 24 h of incubation in the dark. The error bars indicate standard deviations.
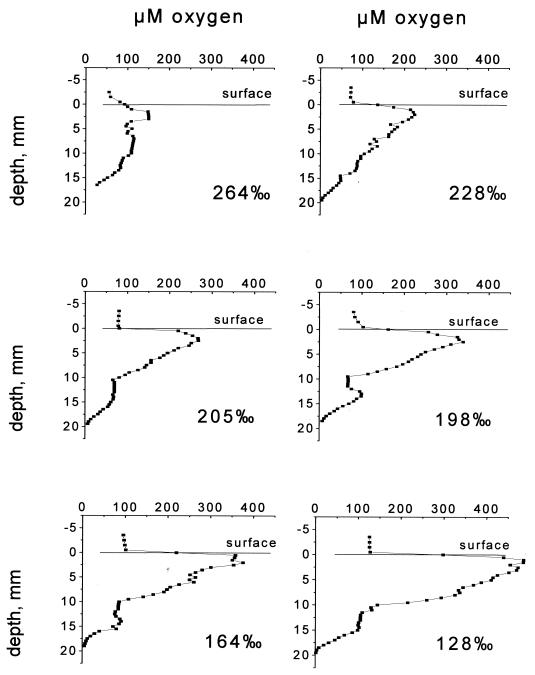
Oxygen profiles in the crust at different salinities.
The results of the oxygen measurement experiments are shown in Fig. 4. The increasing steepness of the gradients indicates that the rates of both formation and consumption of oxygen increased as the salinity decreased. On day 4, when the salinity had decreased to 198‰, growth of phototrophic organisms on top of the crust was evident as an increasingly intense brown layer. It is not known if there was similar growth in the layers below.
FIG. 4.
Oxygen profiles measured at noon in the crust after incubation overnight at different salinities.
Primary production.
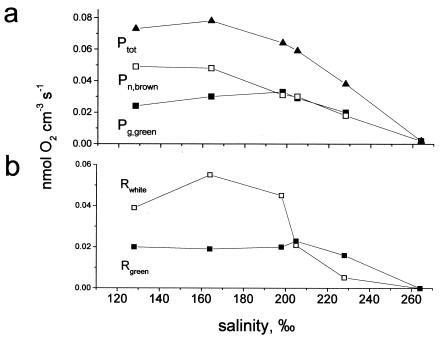
Without direct measurement of gross photosynthesis (see Materials and Methods), the net rate of formation of oxygen in the brown surface layer was calculated by determining the sum of the diffusive fluxes across the water-crust interface and the downward flux to the white layer below estimated from the oxygen profiles (Fig. 5a). The rates determined in this way were net rates and to some (unknown) degree underestimated of the true photosynthesis rates as they did not include oxygen used in respiration in the brown layer.
FIG. 5.
(a) Gross photosynthesis rate in the green layer dominated by filamentous cyanobacteria (Pg,green) (▪) and the net photosynthesis rate in the brown layer (Pn,brown) (□). The photosynthesis rates in the green layer were measured by using the light-dark shift method, whereas the rates in the surface layers were estimated by determining the sum of the O2 fluxes calculated from the oxygen profiles in Fig. 4 by using the diffusion coefficients in Table 1. Ptot is the sum of the two rates (▴). (b) Oxygen consumption rates in the crust at different salinities in the green layer (Rgreen) (▪) and the white nonphototrophic layer between the cyanobacterial layers (Rwhite) (□).
In the green layer, photosynthesis was measured by using the light-dark shift method (Fig. 5a). The photosynthesis rates measured in this way ranged from 0 to 0.033 nmol cm−3 s−1; no oxygen was formed at a salinity of 264‰, and the optimum salinity was 198‰. It is not known whether bacterial growth during the experiment affected the photosynthetic rates, as was the case in the upper brown layer. The decreasing gross photosynthesis rates at salinities below 198‰ may have been an artifact caused by light limitation as the Halothece population above became denser, or they could reflect the actual salinity response of the community. Finally, the total oxygenic photosynthetic rates of the crust at the different salinities were estimated by determining the sum of the gross photosynthesis rate in the green layer and the net photosynthesis in the brown layer. The results of these calculations are shown in Fig. 5a.
During the initial experiments, we observed little change in the oxygen profiles in the crust during the period from 10 a.m. until noon (i.e., during the 2 h preceding measurement), indicating that the concentration gradients reflected the metabolic rates of the various layers. Consequently, the net diffusional fluxes calculated based on the profile together with the rates of gross photosynthesis in the green layer enabled us to calculate the oxygen consumption rates within the white layer and the green layer at the time of measurement. These rates are plotted in Fig. 5b.
Slurry experiments with sulfate reducers and methanogens.
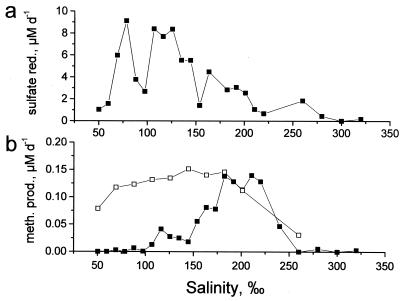
Methane accumulation and sulfate reduction rates were measured in slurries of black sediment incubated in water from pond 200 at different dilutions. The results are shown in Fig. 6. Due to gypsum dissolution after inoculation, the sulfate concentrations in the slurries were less variable than the overall salinities, varying from 49 mM at a salinity of 50‰ to 103 mM at a salinity of 220‰ or more. Considering the dilution factor, the sulfate reduction rates in slurries with water having the in situ salinity were 5 to 10 times lower than the in situ sulfate reduction rates in the deep part of the crust where samples were obtained. This was probably due to the absence of a supply of organic material from the primary producers above. Nevertheless, the sulfate reduction rate peaked between 80 and 130‰ salinity and was strongly inhibited at the in situ salinity, 215‰. The optimum salinity for methane production measured in the same slurries was between 180 and 220‰; at salinities above and below these values the rates decreased rapidly (Fig. 6b).
FIG. 6.
Sulfate reduction rates (a) and rates of methanogenesis (b) in slurry experiments with (□) and without (▪) molybdate addition. Slurries were made with 25 ml of diluted pond water and 5 ml of a pond water-sediment mixture (2:1), resulting in a final 18-fold dilution of the sample, and were incubated at room temperature on a shaking table. sulfate red., sulfate reduction; meth. prod., methane production.
The correlation between decreasing sulfate reduction and increasing methanogenesis in the salinity range from 150 to 170‰ suggests that competition between the two groups of microorganisms inhibited the methanogens at the lower salinities. To investigate this, a subsequent experiment with slurries containing 110‰ salt and molybdate (a specific inhibitor of sulfate reduction [26]) at concentrations between 10 and 90 mM was performed. Methanogenesis was progressively stimulated in slurries containing molybdate at a concentration of 40 mM or higher and reached the maximum value at 70 mM molybdate. This indicates that there was increasing inhibition of sulfate-reducing bacteria competing with the methanogens for substrate. There was no sulfide accumulation, and sulfate reduction could not be detected by the radiotracer method in slurries containing molybdate. The increasing rates of methane formation with increasing amounts of molybdate added indicated that sulfate reducers were not entirely inhibited in all the slurries, and the apparent absence of sulfide formation was probably due to formation of molybdate-sulfide complexes, which removed sulfide from solution.
Finally, to explore the salinity response of the methanogens in the absence of competition by sulfate-reducing bacteria, a series of slurries were prepared in which molybdate was added to a final concentration of 100 mM. Methane accumulation in the slurries was monitored for 2 days of incubation in the dark at room temperature, and the methanogens showed a much broader salinity tolerance, with maximum rates of methanogenesis at salinities between 80 and 200‰ (Fig. 6b). The maximum rate of methanogenesis was not affected by molybdate.
Salinity response of phototrophs in slurries.
The phototrophic layers were separately incubated in NaCl solutions having various salinities, and the carbon fixation was recorded. In general, the dark 14C fixation during incubation was less than 5% of the carbon fixation in the light, and the counts were close to the background level. The results of the light 14C experiments are summarized in Fig. 7.
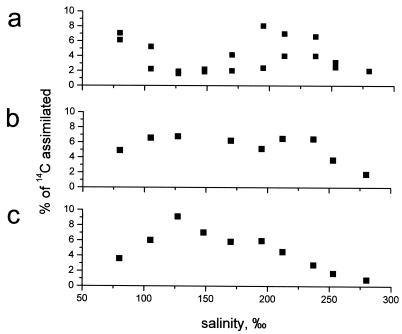
FIG. 7.
Salinity response curves of slurries with the three major phototrophic layers in the crust. The values on y axis are the fraction of [14C]bicarbonate tracer that was assimilated during incubation. Each point represents a single measurement. (a) Surface Halothece-dominated layer; (b) Halospirulina-Phormidium-dominated layer; (c) purple Chromatium-dominated layer.
The brown, Halothece-dominated layer had two salinity optima, one at <100‰ salinity and the other in the range between 180 and 240‰ salinity. This experiment was carried out in duplicate, and the results are shown in Fig. 7a. The deviations in the measurements were quite large, but the two salinity optima were significant compared to the low carbon fixation rates observed at salinities between 120 and 150‰. The green layer of predominantly filamentous cyanobacteria had a broad salinity tolerance, with similar carbon fixation rates at salinities between 80 and 230‰ (Fig. 7b), whereas the optimum salinity for the purple layer with anoxygenic phototrophs was between 120 and 140‰ (Fig. 7c).
DISCUSSION
The observed salinity responses are discussed below. Table 2 summarizes the results of this study, and the results are compared with previously described results.
TABLE 2.
Observed optimum salinities for phototrophs in various layers and for the sulfate-reducing bacteria and methanogenic bacteria in the crust and optimum salinities for representative organisms of each group as described previously
| Layer | Dominant organisms
|
Representative organism
|
Reference | ||
|---|---|---|---|---|---|
| Taxon | Optimum salinity (‰)a | Taxon | Optimum salinity (‰) | ||
| Brown | Unicellular cyanobacteria | 180-240 | Halothece cluster | 15-250b | 14 |
| Green | Filamentous cyanobacteria | 80-230 | Oscillatoria limnetica | 6-15b | 15 |
| Halospirulina cluster | 35-200b | 24 | |||
| Purple | Chromatiaceae | 120 | Halochromatium salexigens | 100-120b | 7 |
| Sulfidic sediment | Sulfate reducers | 80-120 | Desulfocella halophila | 40-50a | 3 |
| Natural population from Great Salt Lake | 120a | 4 | |||
| Methanogens | 140-180 | Methanohalobium evestigatum | 250b | 38 | |
Based on metabolic rates.
Based on growth rates.
Brown layer.
The experiments with crust incubated in water having different salinities showed that oxygen production increased rapidly as the salinity decreased. This was a combined effect due to stimulated photosynthesis rates and growth of phototrophs, and it was not possible to distinguish between the two. However, the slurry experiment indicated that the original population was well adapted to the hypersaline conditions and was growing near its optimal salinity in the ponds at 215‰ salinity. Thus, the bacteria that appeared on the crust surface at lower salinities could have been responsible for the second peak of carbon fixation that appeared at low salinities (<100‰) in the slurry experiments. These microorganisms probably originated from the seawater fed to the saltern plant or from a pond having a lower salinity at an earlier stage in the evaporation process.
Generally, the optimum salinities for growth of members of the Halothece cluster are between 50 and 240‰ in culture (14). The observed optimum salinities for the carbon fixation rate, between 180 and 240‰, were within this range. However, at salinities below 180‰, carbon fixation decreased much faster with salinity than expected from culture studies. A similar pattern was observed in samples of evaporite containing Halothece-like bacteria from Guerrero Negro, Mexico (33), except that the minimum 14C uptake occurred at a salinity around 230‰. The discrepancy between studies with pure cultures in which growth rates were monitored and studies with natural populations in which carbon uptake was measured may be due to differences in what is measured. Apart from growth, fixed carbon is used for cell maintenance and synthesis of compatible solutes. At high salinities, like those in crusts, synthesis of compatible compounds accounts for a substantial part of the total cell metabolism (27). When inocula were transferred from an in situ salinity of 215‰ to a medium with a lower salinity, there was an excess of intracellular compatible solutes. Thus, the low carbon uptake values for slurries with salinities below 180‰ may not reflect salt inhibition but rather may reflect the fact that the carbon demand for growth and cell maintenance was already covered by excess compatible solutes.
Green layer.
Filamentous cyanobacteria belonging to the genus Halospirulina thrive at salinities up to more than 200‰ (24). Unlike Halospirulina, Phormidium-like filaments do not form a well-defined phylogenetic group of cyanobacteria. Several Phormidium-type moderately halophilic bacteria have been isolated, including Oscillatoria limnetica, which grows at salinities up to 200‰, and evidence from other salterns also suggests that organisms having the Phormidium morphotype are among the dominatant phototrophs at high salinities (15, 23).
In the present study, the salinity tolerance of the green layer was measured in the crust, as well as in slurry experiments. Both approaches showed that there was strong salt inhibition at salinities above 240‰; however, whereas the slurry experiment indicated that there was a broad range of optimum salinities (80 to 230‰), oxygen formation in the microelectrode experiment peaked at a salinity of 198‰ and decreased slightly at lower salinities. The discrepancy was probably caused by growth in the green layer as the salinity was decreased from 228 to 198‰, followed by increasing light limitation as the overlying Halothece layer became denser.
Purple layer.
The purple layer in the crust in Eilat obtained its coloration mainly from bacteria resembling Chromatium sp. The carbon fixation was optimal at a salinity of 120‰, and the rate was about 50% of the maximum rate at the in situ salinity. The optimum salinity, 120‰, is close to the optimum salinity for growth (100 to 120‰) measured in pure cultures of Halochromatium salexigens isolated from the gypsum-depositing ponds at Salin-de-Giraud (7). Thus, the phototrophs of the purple layer displayed no unusual salt tolerance compared with isolated strains of anoxygenic bacteria.
Oxygen consumption.
As indicated in Fig. 5b, the rates of oxygen consumption in the crust estimated from flux calculations and measurements of oxygen production increased rapidly as the salinity decreased from 264 to 205‰. Potentially, this may have been a direct salinity response of the heterotrophic community in the crust and/or a result of changing organic substrate availability as the rates of primary production changed. Oxygen availability probably did not limit the rate of consumption of O2 at noon as the concentration was always more than 60 μM. Oxygen consumption increased in the white layer along with the photosynthesis rate in the brown surface layer as salinity decreased, whereas in the green layer, where the photosynthetic rates changed little, the respiration rates were more or less unchanged. The different salinity responses of oxygen respiration rates in these two layers indicate that salinity does not directly control the rate of oxygen consumption. Rather, the oxygen consumption rate is controlled by the availability of organic substrates for aerobic respiration or for sulfate reduction followed by sulfide oxidation.
Sulfate-reducing bacteria.
Different moderately halophilic strains of sulfate reducers have been isolated. Desulfovibrio halophilus, for example, grows at salinities ranging from 30 to 170‰, Desulfovibrio oxyclinae has a maximum salt tolerance of 225‰, Desulfohalobium retbaense tolerates salinities up to 240‰, and Desulfocella halophila grows in the salinity range from 20 to 190‰ (3, 6, 20, 25). Growth rates rather than metabolic rates were used as an indicator of salinity responses in these studies, and the bacterial strains all displayed maximum growth rates at a salinity of 100‰ or less. Few studies have measured the responses of natural populations of sulfate reducers to changes in salinity by quantifying the metabolic rates. Brandt et al. (4) measured sulfate reduction in slurries of sediments from Great Salt Lake and found a salinity response almost identical to that found in slurries from pond 200 at Eilat. Thus, the salinity responses for the growth rates of isolated strains of sulfate reducers and for metabolic rates of natural populations both indicate that in general, sulfate-reducing bacteria are inhibited by salt concentrations greater than 120‰. Nevertheless, the sulfate reduction rate measured in this study was 0.022 nmol cm−2 s−1; compared with the photosynthesis rate at noon (0.08 nmol of O2 cm−2 s−1), this shows that sulfate reduction is one of the major mineralization processes in the crust and that sulfate-reducing bacteria compete successfully with aerobically respiring bacteria even when they are metabolizing at suboptimal cell-specific rates.
Apart from carbon fixation, sulfate reduction is the most ancient metabolic process documented in the geologic record (34). Thus, isotope evidence indicates that sulfate-reducing organisms were present 3.4 billion years ago in CaSO4-depositing environments. It is remarkable that in spite of such a long evolutionary history in hypersaline environments, modern sulfate-reducing prokaryotes are not better adapted to high salinities.
Methanogens.
The ability of methanogens to tolerate high salt concentrations is well documented. A number of methanogens have been isolated from hypersaline environments, including the extreme halophile Methanohalobium evestigatum, which grows optimally at a salinity of 250‰ (13, 37). The highest metabolic rates in the present study were measured in slurries having salinities between 80 and 220‰, and the methanogens appeared to grow at the near-optimum salinity in the crust. However, the in situ concentrations of methane were low compared to the sulfide concentrations, and the total rate of methanogenesis estimated from diffusion at the top of the crust was 1.6 × 10−5 nmol cm−2 s−1 or <0.1% of the sulfate reduction rate in the crust. Thus, although methanogens were well adapted to the high salinity, they contributed only a minor fraction of the total anaerobic mineralization.
Competitive relationship between sulfate reducers and methanogens.
The increased rates of methanogenesis in slurries to which molybdate was added (Fig. 6) suggested that the methanogenic community in the sediment below the crust was competing with the sulfate reducers for substrates at salinities below 180‰. The maximum rates of methanogenesis in the slurries were 0.12 to 0.14 μM day−1 both with and without molybdate, whereas the uninhibited sulfate reduction rates at the same salinities were in the range from 2.0 to 9.5 μM day−1 or between 15 and 73 times higher. Thus, only a minor fraction of the electron donors used for sulfate reduction could potentially be used in methanogenesis. At salinities below 180‰ this relatively small pool of methanogenic substrate was used by the sulfate-reducing bacteria, but at the in situ salinity sulfate reduction was sufficiently inhibited that the methanogens could successfully compete for the substrate.
Summary.
The cyanobacteria, anoxygenic phototrophs, methanogens, and sulfate reducers from the endoevaporitic community of the solar salterns in Eilat, Israel, did not show any unusual salinity adaptations when they were compared to isolated strains of related organisms. Cyanobacteria metabolized at optimal or nearly optimal rates at the in situ salinity, whereas the optimum salinities for the anoxygenic phototrophs and sulfate reducers were lower. Nevertheless, the sulfate-reducing population in the crust competed successfully with aerobic bacteria. Methanogens were well adapted to the high salinity and survived in the crust by metabolizing a minor pool of the degradable organic material which was unsuitable for the sulfate reducers at the high salinity.
Acknowledgments
We thank the Interuniversity Institute for Marine Sciences in Eilat, Israel, and the Moshe Shilo Minerva Center for Marine Biogeochemistry for logistic support and the Israel Salt Company for allowing access to the saltern ponds.
This study was supported by the Danish Basic Research Foundation (Grundforskningsfonden) and by the Israel Science Foundation, founded by the Israel Academy of Sciences and Humanities.
REFERENCES
- 1.Anton, J., E. Llobet-Brossa, F. Rodriguez-Valera, and R. Amann. 1999. Fluorescence in situ hybridization analysis of the prokaryotic community inhabiting crystallizer ponds. Environ. Microbiol. 1:517-523. [DOI] [PubMed] [Google Scholar]
- 2.Benlloch, S., A. López-López, E. O. Casamayor, L. Øvreås, V. Goddard, F. L. Daae, G. Smerdon, R. Massana, I. Joint, I., F. Thingstad, C. Pedrós-Alió, and F. Rodríguez-Valera. 2002. Prokaryotic genetic diversity throughout the salinity gradient of a coastal solar saltern. Environ. Microbiol. 4:349-360. [DOI] [PubMed] [Google Scholar]
- 3.Brandt, K. K., B. K. Patel, and K. Ingvorsen. 1999. Desulfocella halophila gen. nov., sp. nov., a halophilic, fatty-acid-oxidizing, sulfate-reducing bacterium isolated from sediments of the Great Salt Lake. Int. J. Syst. Bacteriol. 49:193-200. [DOI] [PubMed] [Google Scholar]
- 4.Brandt, K. K., F. Vester, A. N. Jensen, and K. Ingvorsen. 2001. Sulfate reduction dynamics and enumeration of sulfate-reducing bacteria in hypersaline sediments of the Great Salt Lake. Microb. Ecol. 41:1-11. [DOI] [PubMed] [Google Scholar]
- 5.Canfield, D. E., and D. J. Desmarais. 1993. Biogeochemical cycles of carbon, sulfur, and free oxygen in a microbial mat. Geochim. Cosmochim. Acta 57:3971-3984. [DOI] [PubMed] [Google Scholar]
- 6.Caumette, P. 1993. Ecology and physiology of phototrophic bacteria and sulfate-reducing bacteria in marine salterns. Experientia 49:473-481. [Google Scholar]
- 7.Caumette, P., R. Baulaigue, and R. Matheron. 1988. Characterization of Chromatium salexigens sp. nov., a halophilic Chromatiaceae isolated from Mediterranean salinas. Syst. Appl. Microbiol. 10:284-292. [Google Scholar]
- 8.Caumette, P., R. Matheron, N. Raymond, and J. C. Relexans. 1994. Microbial mats in the hypersaline ponds of Mediterranean salterns (Salins-de-Giraud, France). FEMS Microbiol. Ecol. 13:273-286. [Google Scholar]
- 9.Cline, J. D. 1969. Spectrophotometric determination of hydrogen sulfide in natural waters. Limnol. Oceanogr. 14:454-458. [Google Scholar]
- 10.Conrad, R., P. Frenzel, and Y. Cohen. 1995. Methane emission from hypersaline microbial mats: lack of aerobic methane oxidation activity. FEMS Microbiol. Ecol. 16:297-306. [Google Scholar]
- 11.Dundas, I. 1998. Was the environment for primordial life hypersaline? Extremophiles 2:375-377. [DOI] [PubMed] [Google Scholar]
- 12.Fossing, H., and B. B. Jørgensen. 1989. Measurement of bacterial sulfate reduction in sediments. Evaluation of a single-step chromium reduction method. Biogeochemistry 8:205-222. [Google Scholar]
- 13.Garcia J.-L., B. K. C. Patel, and B. Ollivier. 2000. Taxonomic, phylogenetic, and ecological diversity of methanogenic Archaea. Anaerobe 6:205-226. [DOI] [PubMed] [Google Scholar]
- 14.Garcia-Pichel, F., U. Nübel, and G. Muyzer. 1998. The phylogeny of unicellular, extremely halotolerant cyanobacteria. Arch. Microbiol. 169:469-482. [DOI] [PubMed] [Google Scholar]
- 15.Golubic, S. 1980. Halophily and halotolerance in cyanophytes. Origins Life 10:169-183. [Google Scholar]
- 16.Imhoff, J. F. 2001. True marine and halophilic anoxygenic phototrophic bacteria. Arch. Microbiol. 176:243-254. [DOI] [PubMed] [Google Scholar]
- 17.Jørgensen, B. B. 1978. A comparison of methods for the quantification of bacterial sulfate reduction in coastal marine sediments. I. Measurement with radiotracer techniques. Geomicrobiol. J. 1:11-27. [Google Scholar]
- 18.Kamekura, M. 1998. Diversity of extremely halophilic bacteria. Extremophiles 2:289-295. [DOI] [PubMed] [Google Scholar]
- 19.Khmelenina, V. N., M. G. Kalyuzhnaya, V. G. Sakharovsky, N. E. Suzina, Y. A. Trotsenko, and G. Gottschalk. 1999. Osmoadaptation in halophilic and alkaliphilic methanotrophs. Arch. Microbiol. 172:321-329. [DOI] [PubMed] [Google Scholar]
- 20.Krekeler, D., P. Sigalevich, A. Teske, H. Cypionka, and Y. Cohen. 1997. A sulfate-reducing bacterium from the oxic layer of a microbial mat from Solar Lake (Sinai), Desulfovibrio oxyclinae sp. nov. Arch. Microbiol. 167:369-375. [Google Scholar]
- 21.Madigan, M. T., and A. Oren. 1999. Thermophilic and halophilic extremophiles. Curr. Opinion Microbiol. 2:265-269. [DOI] [PubMed] [Google Scholar]
- 22.Moune, S., P. Caumette, R. Matheron, and J. C. Willison. 2003. Molecular sequence analysis of prokaryotic diversity in the anoxic sediments underlying cyanobacterial mats of two hypersaline ponds in Mediterranean salterns. FEMS Microbiol. Ecol. 44:117-130. [DOI] [PubMed] [Google Scholar]
- 23.Nübel, U., F. Garcia-Pichel, E. Clavero, and G. Muyzer. 2000. Matching molecular diversity and ecophysiology of benthic cyanobacteria and diatoms in communities along a salinity gradient. Environ. Microbiol. 2:217-226. [DOI] [PubMed] [Google Scholar]
- 24.Nübel, U., F. Garcia-Pichel, and G. Muyzer. 2000. The halotolerance and phylogeny of cyanobacteria with tightly coiled trichomes (Spirulina turpin) and the description of Halospirulina tapeticola gen. nov., sp. nov. Int. J. Syst. Evol. Microbiol. 50:1265-1277. [DOI] [PubMed] [Google Scholar]
- 25.Ollivier, B., P. Caumette, J. L. Garcia, and R. A. Mah. 1994. Anaerobic bacteria from hypersaline environments. Microbiol. Rev. 58:27-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oremland, R. S., and D. G. Capone. 1988. Use of “specific” inhibitors in biogeochemistry and microbial ecology. Adv. Microb. Ecol. 10:285-383. [Google Scholar]
- 27.Oren, A. 1999. Bioenergetic aspects of halophilism. Microbiol. Mol. Biol. Rev. 63:334-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oren, A. 2002. Diversity of halophilic microorganisms: environments, phylogeny, physiology, and applications. J. Ind. Microbiol. Biotechnol. 28:55-63. [DOI] [PubMed] [Google Scholar]
- 29.Oren, A., M. Kühl, and U. Karsten. 1995. An endoevaporitic microbial mat within a gypsum crust: zonation of phototrophs, photopigments, and light penetration. Mar. Ecol. Prog. Ser. 128:151-159. [Google Scholar]
- 30.Revsbech, N. P., B. B. Jørgensen, and O. Brix. 1981. Primary production of microalgae in sediments measured by oxygen microprofile, H14CO3− fixation, and oxygen exchange methods. Limnol. Oceanogr. 26:717-730. [Google Scholar]
- 31.Revsbech, N. P., and B. B. Jørgensen. 1983. Photosynthesis of benthic microflora measured with high spatial resolution by the oxygen microprofile method: capabilities and limitations of the method. Limnol. Oceanogr. 28:749-756. [Google Scholar]
- 32.Revsbech, N. P., B. B. Jørgensen, and T. H. Blackburn. 1983. Microelectrode studies of the photosynthesis and O2, H2S, and pH profiles of a microbial mat. Limnol. Oceanogr. 28:1062-1074. [Google Scholar]
- 33.Rothschild, L., L. J. Giver, M. R. White, and R. L. Mancinelli. 1994. Metabolic activity of microorganisms in evaporites. J. Phycol. 30:431-438. [DOI] [PubMed] [Google Scholar]
- 34.Shen, Y., R. Buick, and D. E. Canfield. 2000. Isotopic evidence for microbial sulfate reduction in the early Archaean era. Nature 410:77-81. [DOI] [PubMed] [Google Scholar]
- 35.Sokolov, A. P., and Y. A. Trotsenko. 1995. Methane consumption in (hyper)saline habitats of Crimea (Ukraine). FEMS Microbiol. Ecol. 18:299-304. [Google Scholar]
- 36.Spear, J. R., R. E. Ley, A. B. Berger, and N. E. Pace. 2003. Complexity in natural microbial ecosystems: the Guerrero Negro experience. Biol. Bull. 204:168-173. [DOI] [PubMed] [Google Scholar]
- 37.Zhilina, T. N., and G. A. Zavarzin. 1987. Methanosarcina vacuolata sp. nov., a vacuolated Methanosarcina. Int. J. Syst. Bacteriol. 37:281-283. [Google Scholar]