Abstract
Mesenchymal stem cells (MSCs), due to their paracrine, transdifferentiation, and immunosuppressive effects, hold great promise as a therapy for peripheral arterial disease. Diabetes is an important risk factor for peripheral arterial disease; however, little is known of how type II diabetes affects the therapeutic function of MSCs. This review summarizes the current status of preclinical and clinical studies that have been performed to determine the efficacy of MSCs in the treatment of peripheral arterial disease. We also present findings from our laboratory regarding the impact of type II diabetes on the therapeutic efficacy of MSCs neovascularization after the induction of hindlimb ischemia. In our studies, we documented that experimental type II diabetes in db/db mice impaired MSCs’ therapeutic function by favoring their differentiation towards adipocytes, while limiting their differentiation towards endothelial cells. Moreover, type II diabetes impaired the capacity of MSCs to promote neovascularization in the ischemic hindlimb. We further showed that these impairments of MSC function and multipotency were secondary to hyperinsulinemia-induced, Nox4-dependent oxidant stress in db/db MSCs. Should human MSCs display similar oxidant stress-induced impairment of function, these findings might permit greater leverage of the potential of MSC transplantation, particularly in the setting of diabetes or other cardiovascular risk factors, as well as provide a therapeutic approach by reversing the oxidant stress of MSCs prior to transplantation.
Keywords: mesenchymal stem cells, peripheral arterial disease, oxidative stress, limb ischemia
Introduction
Peripheral artery occlusive disease remains a leading cause of limb loss and mortality worldwide. Cell-based therapies were conceived initially as an innovative way to improve blood flow recovery to prevent amputation. Many potential candidate cells have been evaluated, including whole bone marrow cells, bone marrow mononuclear cells, hematopoietic stem cells, endothelial progenitor cells, and hemangiocytes [1–5]. Of the various types of stem and progenitor cells, mesenchymal stem cells (MSCs) are being pursued the most actively at both preclinical and clinical levels, owing to their greater ease of isolation and capacity for ex vivo expansion.
Mesenchymal stem cells are multipotent non-hematopoietic stem cells that have the capacity for self-renewal and terminal differentiation into a multitude of different cell types, the best characterized of which are osteocytes, chondrocytes and adipocytes [6, 7]. Mesenchymal stem cells can home to and survive in an ischemic environment. Through paracrine effects, they aid in the promotion of arteriogenesis and angiogenesis and by terminally differentiating into vascular cells and myocytes [8–10]. These features enable MSCs to promote post-ischemic neovascularization and blood flow recovery in ischemic diseases secondary to peripheral arterial occlusive disease; however, the specific mechanisms by which they do so have yet to be fully characterized.
Over the last two decades, extensive and breakthrough research into stem cell based therapies have shown great promise for the treatment of a variety of clinical disorders, including peripheral artery occlusive diseases (PAD) [11–16]. Here, we will focus on the current status of research into MSCs as a stem cell-based therapy for PAD and the unique challenges to their successful application towards a standard clinical therapy.
Origin and identification of MSCs
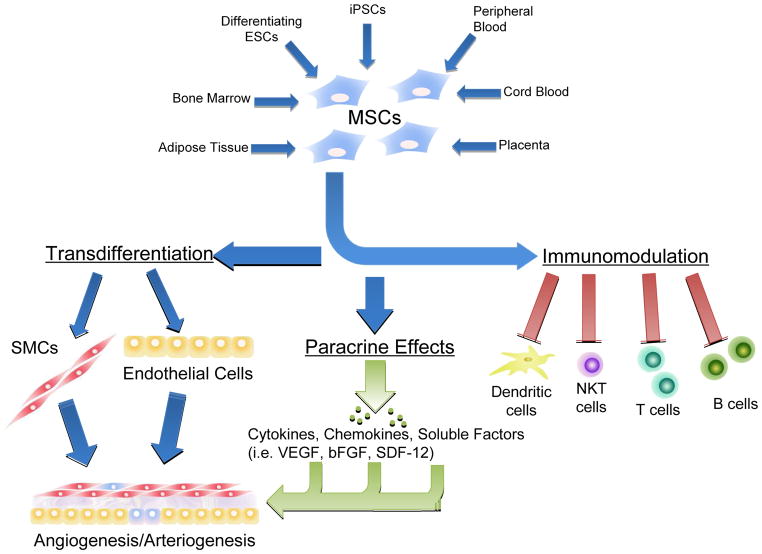
Mesenchymal stem cells were first isolated from the bone marrow and described in 1997 [6, 17]. Since then, MSCs have also been isolated from a variety of other sources: peripheral blood [18], cord blood [19–21], adipose tissue [22–24], placenta [25], lung, dental pulp, periodontal ligament tissue, along with fetal and amniotic membranes. (FIGURE 1) Mesenchymal stem cells derived from these diverse sources have all expressed a distinct pattern of cell surface markers, ex vivo differentiation capacity, and pro-angiogenic properties characteristic of these cells. Mesenchymal stem cells derived from each of these sources have also been shown in experimental models to be effective in the treatment of hindlimb ischemia. Despite the similarity of the cell surface marker expression, MSCs derived from different sources nonetheless do exhibit heterogeneity in colony formation rates and differentiation potential [26–28]. Bone marrow-derived MSCs showed the greatest therapeutic potential to reduce the area of myocardial infarction and improve myocardial performance and capillary density in preclinical mouse models of ischemia [29, 30]. In contrast, transplantation of human adipose tissue-derived MSCs showed better blood flow recovery in preclinical models of hindlimb ischemia [31].
Figure 1.
Biological sources and activity of Mesenchymal Stem Cells. MSCs can be isolated from multiple sources, and exert therapeutic effects on multiple systems to contribute to the therapy of peripheral arterial disease.
While studies of bone marrow-derived MSCs are the best established, due to clinical concerns surrounding the invasiveness and pain associated with bone marrow aspiration, alternative sources of MSCs have been explored. One recent breakthrough was the use of induced pluripotent stem cells (iPSC). With appropriate programming iPSCs can be induced towards differentiation into MSCs [32, 33]; these iPSC-derived MSCs have been shown to be effective in promoting neovascularization in preclinical models of limb ischemia [34, 35]. In one such study that compared the capacity of MSCs derived from different sources for engraftment and terminal differentiation, iPSC-MSCs were more effective than bone marrow-derived MSCs [34].
Mesenchymal stem cells have also been derived from human embryonic stem cells (ESCs) [36]. Human ESC-derived MSCs have the same typical cell surface markers and capacity to differentiate into characteristic cell types as do MSCs derived from either bone marrow or adipose tissue. Human ESC-MSCs have been shown to exert both immunosuppressive and anti-inflammatory effects [37]. In a rat hindlimb ischemic model, ESC-MSCs showed both pro-angiogenic and proliferative effects [38]. While multiple studies show undoubtedly that therapeutically active MSCs can be derived from a wide variety of tissues, considerable work needs to be performed to determine the optimal source of these cells before the initiation of human clinical trials.
Current impediments to the therapeutic utilization of MSCs include the lack of unique identifying markers for MSCs as well as sufficient methods to fully characterize their subpopulations. Furthermore, a discrepancy exists between the behavior of ex vivo expanded MSCs and fresh, non-manipulated MSCs. Currently described universal characteristics of MSCs cultured ex vivo include their adherence to uncoated plastic plates and fibroblast-like morphology [39, 40]. Mesenchymal stem cells isolated from different tissues also share several common cell surface markers including CD13, CD29, CD44, CD54, CD63 CD73, CD105, CD106 and CD90, and are negative for surface markers CD14, CD34, CD11b, CD79α, CD45 and HLA-DR. In addition, these cells have the capacity for osteogenic, adipogenic and chondrogenic differentiation. However, significant differences in the functional efficacy of MSCs derived from different sources has been suggested by studies showing differences in gene expression and multilineage differentiation dependent on the MSC source of origin [41, 42].
Therapeutic Features of MSCs in Peripheral Arterial Disease
Transdifferentiation capacity
As a part of their multiple differentiation potential, MSCs can be induced to adopt endothelial properties in the presence of vascular endothelial growth factor (VEGF) and/or basic fibroblast growth factor (bFGF), and to express endothelial-specific markers, including CD31, vWF, VE-cadherin, and endothelial nitric oxide synthase (eNOS). In vitro transdifferentiation towards cardiomyocytes has been achieved with both physical and chemical stimuli [43–46]. Evidence for endothelial and cardiomyogenic differentiation in vivo has been reported in preclinical animal studies in which MSCs are transplanted into areas of ischemia or infraction. In these studies, de novo expression of endothelial and cardiomyocyte markers were confirmed with immunohistochemistry and fluorescence microscopy [45, 47]. In agreement with these findings, transplanted MSCs promoted angiogenesis, arteriogenesis, and thereby collateral artery enlargement that ultimately significantly increased limb blood flow recovery [43, 48]. (FIGURE 1)
Paracrine Effect
Mesenchymal stem cells can induce angiogenesis and arteriogenesis by releasing various angiogenic growth factors including VEGF, bFGF, and stromal cell derived factor-1α [49]. Genetic modification of MSCs has been shown to further increase the range of paracrine effects [50]. Administration of the conditioned medium of MSCs is able to recapitulate the benefits of MSCs for tissue repair in an in vitro system. A protein array analysis of MSC culture medium detected a significant fraction of total secreted molecules are growth factors, cytokines and chemokines which are well known for anti-apoptotic and regeneration stimulating effects [51]. Bone marrow-derived MSCs can also stimulate the proliferation and differentiation of endogenous cardiac stem cells, thereby restoring cardiac function after myocardial infarction in pigs. [52]. The specific mechanisms through which MSCs exert these effects to promote neovascularization has yet to be defined in a comprehensive manner. (FIGURE 1)
Immunosuppressive effect
Early studies showed that MSCs have unique immunological characteristics that allow their persistence in a xenogeneic environment [53]. Thereafter, evidence confirmed the immunomodulatory properties of MSCs. MSCs can induce immunosuppressive activity for a broad range of immune cells, including T lymphocytes, B lymphocytes, natural killer cells and dendritic cells [54–56]. This immunosuppressive effect may give MSCs unique advantages as a transplanted cell therapy.(FIGURE 1) However, the precise mechanism underlying the immunomodulatory effects is still unknown. Direct cell-to-cell contact or release of immunosuppressive factors may play major roles.
Methods of administration of MSCs
Mesenchymal stem cells can be derived from multiple potential sources and administered by a variety of techniques. Intramuscular (IM) injection has been the most commonly utilized technique both in preclinical and clinical studies [48]. The understanding from current studies is that MSCs injected into the muscle of the ischemic hindlimb remain localized to that area, and have not been detected in other tissues or organs in significant numbers [57]. MSCs have also been infused intravenously both in preclinical models as well as in patients [58]. A challenge of intravenous administration of MSCs is that they can become trapped in the lungs, thereby substantially reducing the number of cells that are able to migrate to the area of ischemic or injured tissue [59, 60]. Both direct intramuscular injection and intravenous administration also have several relative disadvantages, including a significant proportion of cell loss in the surrounding tissues, delayed loss of MSCs due to impaired homing, or in the case of IM injections, needle-mediated tissue damage. For these reasons, alternative methods have been investigated, such as manipulating the MSC cultures by utilizing magnetically engineered tissue technology or collagen scaffolds [61, 62]. Moreover, multiple clinical trials administering bone marrow derived-mononuclear cells are successfully utilizing intra-arterial injections [77–79].
Some investigators have shown that MSCs are therapeutically effective when injected immediately following the induction of hindlimb ischemia in mice [34, 63]. In a similar study, a delay of 24 hours after the induction of ischemia has also been shown to be more effective than immediate injection [49]. In fact, the delay between the onset of disease and therapeutically effective administration of MSCs seems to consist of a wide range. For example, bone marrow-derived MSCs transplanted at one hour, one week, or two weeks after myocardial infarction all proved to be effective, although injection at approximately one week after infarction showed the highest recovery of myocardial function [64]. As the acute inflammatory reaction is nearly complete at this time point, the success of delayed injections may be a consequence of increased survival of transplanted cells.
Further concern lays in elucidating the optimal dose for cell therapy as, in general, only a small proportion of MSCs survive and are capable of exerting a therapeutic effect either through paracrine mechanisms or terminal determination [47, 65, 66]. The tendency toward the use of large numbers of MSCs for transplantation has to be weighed against reports of complications from such efforts. In particular, there are reports of MSCs inducing tumor formation [67–69], though both short and long-term clinical studies have thus far shown no significant complications. Nevertheless, further investigation is still required to optimize the concentration, timing and delivery of MSCs that would be critical for effective neovascularization in PAD.
Clinical Role of MSCs in PAD
Following the potential of pre-clinical studies, promising results concerning the therapeutic efficacy of bone marrow-derived mononuclear cells (BM-MNCs) in humans with critical limb ischemia was first demonstrated almost a decade ago [80]. Multiple early stage clinical trials have since followed suit, though early results have been conflicting in regards to significance of blood flow recovery; larger trials are now underway to better elucidate its efficacy [Table 1, Table 2]. Interest is further growing regarding the benefits of specific cells types ranging from endothelial progenitor cells (EPCS) [81,82], ALDH-bright cells [83], mesenchymal stem cells (MSCs) [9,84,85], and combinations there-of.
Table 1.
Completed MSC Clinical Trials
| Author | Location | Patient Population | Cell Product | Injection Method | Product Control | Patient Enrollment | Time Frame | Outcomes | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Route | Dose (#cells) | Site # | Total | Therapy | Control | |||||||
| Powell, RJ; 2011 | USA | “no-option” CLI | (A)BM-MNC | IM | 35–295 × 106 | 20 | Electrolyte Solution | 86 | 58 | 28 | 12 mo | ↑TTF, ↑WH |
|
| ||||||||||||
| Lasala, GP; 2011 | USA | “no-option” CLI | (A)BM-MNC EPC + (A)BM-MSC | IM | (BM-MNC) 900 × 106 (BM-MSC) 9 × 106 |
40 | Infusion Media | 26 | 12 | CL | 04 mo | ↑ABI, ↑PS, ↑QoL, ↑WH, ↑WT, ↓Pain |
| (BM-MNC) 1800 × 106 (BM-MSC) 18 × 106 |
14 | |||||||||||
|
| ||||||||||||
| Lu, D; 2011 | China | Diabetic CLI | (A)BM-MNC | IM | 9.6 ± 1.1 ×108 | 20 | NS | 41 | 19 | CL | 8 mo | ↑ABI, ↑Angiogensis (MRA), ↑AFS, ↑TcPO2, ↑WH, ↑WT, ↓Pain |
| (A)BM-MSC | IM | 9.3 ± 1.1 ×108 | 20 | 18 | ||||||||
|
| ||||||||||||
| Dash, NR; 2009 | India | Diabetic Ulcer | (A)BM-MSC | IM | 45–60 × 106/cc | 5–6 cc | Standard Care | 24 | 3 | 3 | 3 mo | ↑Angiogesis (Bioposy), ↑WH, ↑WD, ↓Pain |
| TAO Ulcer | 9 | 9 | ||||||||||
Table 2.
On-going MSC Clinical Trials
| Identifier | Location | Study Status | Phase | Cell Product | Estimated Enrollment | Injection method | Main Study Design | Time Frame | Outcomes Measures |
|---|---|---|---|---|---|---|---|---|---|
| NCT01079403 | Spain | Unknown | I/II | (A)Adipose-MSC | 36 | IA | RT, PA, S/E | 12 mo | ABI, DSA, MRA |
| NCT01483898 | USA | On-going | III | ixmyelocel-T | 594 | IM | RT, PA, E, DB | 18 mo | AFS, WH |
| NCT01351610 | Germany | On-going | I/II | (A)BM-MSC | 30 | IV | RT, PA, S/E | 12 mo | ABI, QoL, RP, TcPO2, WH |
| NCT01257776 | Spain | On-going | I/II | (A)Adipose-MSC | 36 | IA | RT, PA, S/E | 12 mo | ABI, AFS, DSA |
| NCT01456819 | Malaysia | On-going | II | (A)BM-MNC+MSC | 50 | IM | RT, PA, E | 12 mo | ABI, DSA, ETT, VAS, TcPO2, WH |
| NCT01216865 | China | Not yet recruiting | I/II | Cord-MSC | 50 | IM | RT, PA, S/E | 6 mo | ABI, AFS, Pain, WT, WH |
| NCT01211028 | France | On-going | I/II | (A)Adipose-MSC | 15 | IM | NR, SGA, S | 6 mo | AE |
| NCT00883870 | India | On-going | I/II | (Al)MSC | 20 | IM | RT, PA, S/E, DB | 6 mo | ABI, TcPO2 |
| NCT01484574 | India | On-going | II | (Al)BM-MSC | 126 | IM | NR, SGA, S/E | 24 mo | ABI, AFS, MRA, QoL, TcPO2, WT |
| NCT01686139 | Israel | Not yet recruiting | I/II | (Al)BM-MSC | 20 | IM | NR, SGA, S | 6 mo | AE, Pain, VAS, WH |
Early results of phase I trials were promising, as patients injected with MSCs demonstrated improved walking time, ankle-brachial indices (ABI), and limb perfusion [84]. Follow-up Phase II studies evaluated the efficacy of intramuscular injections of a combination product of BM-MNCs and BM-MSCs in patients with severe limb ischemia, who were not candidates for surgical revascularization. The combination product included ex vivo-expanded autologous BM-MSCs, along with BM-MNC derived EPCs, as characterized by flow cytometry. The study further sought to evaluate the effect of cell number by separating patients into low- and high-dose groups. Regardless of dosage, injected patients showed significant improvements in ABI, walking time, pain, and limb perfusion, as well as significant wound healing [9]. The authors hypothesized that the therapeutic effect was due to the presence of specific cell types rather than absolute number of cells infused.
Currently, the largest scale combination cell study reported is the RESTORE-CLI trial, which has just completed phase II studies in patients with critical limb ischemia [86,87]. Their cellular product takes autologous BM-MNCs, and through ex-vivo treatment, yields cells that primarily express markers for MSCs and alternatively activated macrophages. This double-blind randomized, placebo-controlled trial specifically targeted the safety and efficacy of the product, while also showing significantly improved time to occurrence of first treatment failure (as defined by major amputation, mortality, doubling of wound surface area from baseline, and de novo gangrene), and wound healing. However, although there appeared to be improved amputation-free survival, this improvement was not statistically significant, and no data was reported on other potential markers of clinical blood flow recovery. A large phase III study utilizing this product is currently underway with a target goal of 594 patients powered for significance.
Thus far, there has only been one study to directly evaluate the effect of intramuscular injections of ex vivo-expanded autologous BM-MSCs in comparison to BM-MNCs [85]. In a double-blind, randomized, placebo-controlled trial in 41 type II diabetic patients with bilateral CLI, significant improvements were noted in ABI, pain, walking time, wound healing, and collateral artery enlargement following administration of either BM-MNCs or BM-MSCs. Moreover, these significant improvements were more demonstrable in the BM-MSCs group than in the BM-MNCs group.
Since treatment with MSCs has demonstrated an impressive potential for improved blood flow recovery in patients[8, 85], a number of new early phase clinical trials utilizing MSCs, rather than BM-MNCs, are underway [Table 2] However, as with clinical trials that utilize BM-MNCs, a number of questions remain: patient selection, cellular processing, dosing, methods of implantation, and even the exact markers of clinical efficacy are all still widely debated. Moreover, the exact mechanisms by which administration of MSCs benefits blood flow recovery in PAD have still yet to be fully elucidated.
Experimental Type II Diabetes Impairs the Regenerative Capacity of MSCs in Post-ischemic Neovascularization
Peripheral artery disease is frequently associated with diabetes, hypertension, atherosclerosis, and aging, all of which could impair the regenerative function of stem cells and progenitor cells [70–76]. We have recently shown that MSCs derived from mice with experimental type II diabetes causes hyperinsulinemia-induced oxidant stress in MSCs that restricts their multipotency and impairs their capacity to promote neovascularization [43].
We first compared post-ischemic neovascularization in preclinical models of type I (streptazotocin) and type II (db/db mouse) diabetes and found that the type II diabetic mice exhibited greater impairment in neovascularization than did the type I diabetic mice [70]. The duration of diabetes was identical in both models. A substantial adipocyte infiltration into ischemic muscles was unique to the type II diabetic db/db mice; no such infiltration occurred in the type I diabetic mouse model. In fact, we have seen no such adipocytic infiltration in any other models of limb ischemia, including mouse models of hypercholesterolemia and hindlimb ischemia induction in wild type mice. As a consequence of this finding, we tested the hypothesis that the adipocytes in the post-ischemic muscle of type II diabetic db/db mice are derived from MSCs [43].
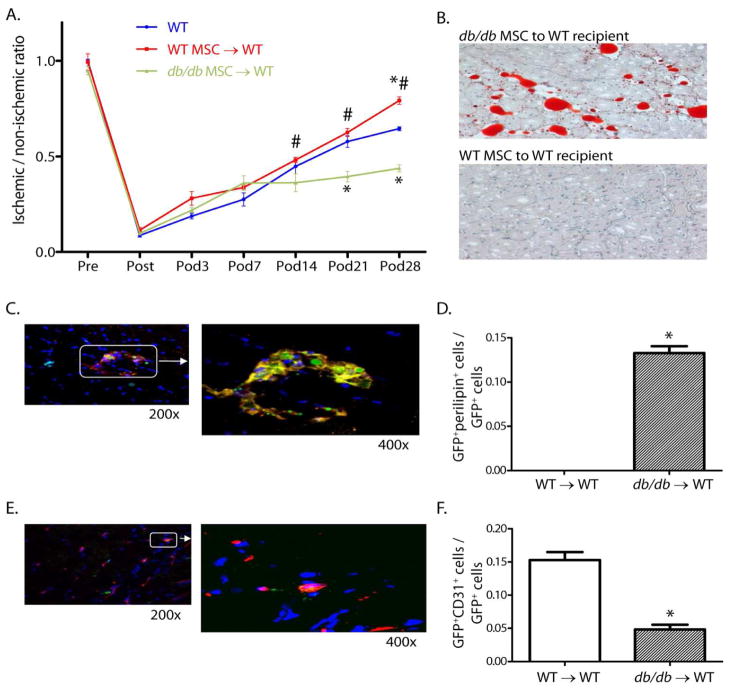
MSCs were harvested from both db/db mice and wild type mice and were transduced with an adenovirus that expressed the green fluorescent protein (GFP). Both types of MSCs were then injected into the bone marrow of different groups of recipient wild type mice 24 hours after the induction of hindlimb ischemia. We found that the wild type recipient mice that received MSCs from db/db mice exhibited less blood flow in the ischemic leg than did wild type mice receiving wild type MSCs, and more importantly showed extensive adipocytic infiltration within the ischemic muscle. Confocal microscopy revealed co-localization of the adipocyte surface marker, perilipin, and GFP confirmed that the infiltrated adipocytes originated from the transplanted db/db MSCs. In the wild type mice that had received db/db MSCs, approximately 14% of the GFP-positive cells were adipocytes, whereas those that received wild type MSCs had no GFP-positive cells that were adipocyte–positive The inverse was true of endothelial cells. In the wild type recipient mice that received db/db MSCs, approximately 5% of the GFP-positive cells had differentiated into endothelial cells, whereas 17% of the GFP-positive cells differentiated into endothelial cells in the recipient mice that received wild type MSCs. Finally, transplantation of db/db MSCs into wild type recipient mice resulted in impaired blood flow recovery in the ischemic leg compared to mice receiving wild type MSCs (FIGURE 2). Interestingly, these effects occurred despite the existence of the recipients’ endogenous MSC population and the absence of type II diabetes. Thus, the db/db MSCs generated a more dominant phenotype during the neovascularization process.
Figure 2.
Foot blood flow recovery, muscle histology, and co-localization studies in WT mice transplanted with db/db or WT MSC after induction of hindlimb ischemia. (A) Foot blood flow recovery by LDPI (mean±SEM; n=6; *P<0.05 vs. WT; #P<0.05 vs. db/db MSC → WT transplant group). (B) Histology of gastrocnemius muscle from the ischemic hindlimb (Oil Red O for identification of adipocytes, hematoxylin counter stain, 200x). (C) Representative confocal images (GFP for identification of MSC [green] and perilipin for identification of adipocytes [red]. (D) Ratio of GFP+periplipin+ cells to GFP+ cells in the ischemic hindlimb muscle (mean±SEM; n=5; *P<0.05). (E) Representative confocal images (GFP for the identification of MSC [green] and CD31 for the identification of endothelial cells [red]). (F) Ratio of GFP+CD31+ cells to GFP+ cells in the ischemic hindlimb muscle (mean±SEM; n=5; *P<0.05). Reprinted with permission from JAHA with the specific citation[43].
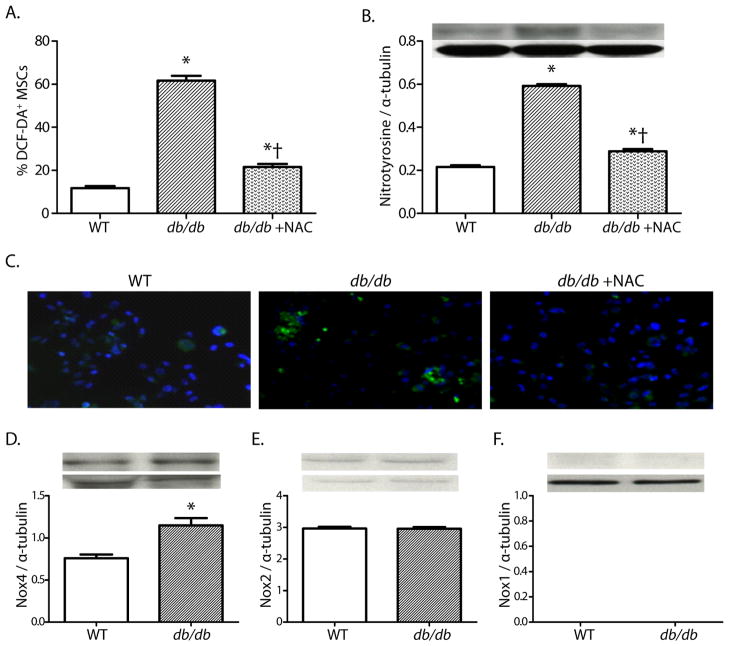
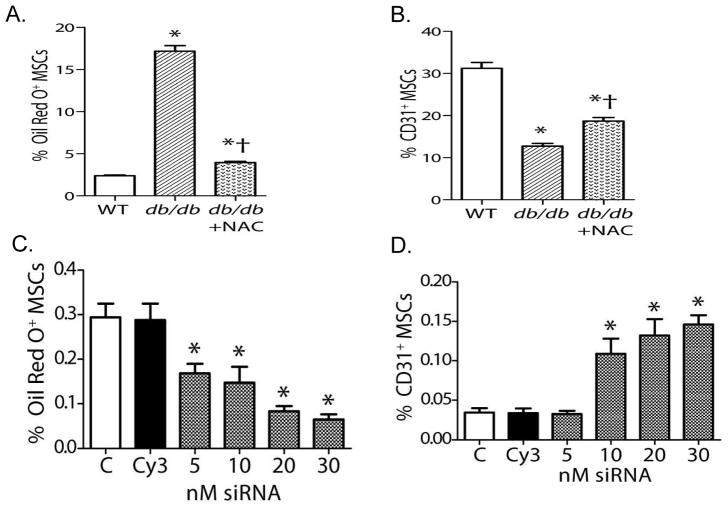
In order to explain these results, we hypothesized that the type II diabetic phenotype induces oxidant stress in MSCs that restricts their multipotency, specifically enhancing their potential to differentiate into an adipocyte phenotype and reducing their potential to differentiate into an endothelial cell phenotype. A series of in vitro studies confirmed greater oxidant stress in MSCs derived from db/db mice than in those from wild type mice. This increase in oxidant stress could be reversed with pretreatment with the antioxidant N-acetylcysteine (NAC) (FIGURE 3). NADPH oxidases are important sources of reactive oxygen species in type II diabetes. We found a two-fold higher expression of NOX4 in db/db MSCs than in WT MSCs, while levels of NOX2 and NOX1 were low to undetectable in either group. When tested in vitro, db/db MSCs had a ten-fold greater propensity to differentiate into adipocytes than did MSCs from wild type mice, and this increased propensity for adipocytic differentiation could be reversed by pretreatment with NAC or by knockdown of NOX4 expression by siRNA techniques. Db/db MCSs also showed an impaired capacity to differentiate into endothelial cells in vitro that also could be reversed by pretreatment with NAC or knockdown of NOX4 expression by siRNA. (FIGURE 4).
Figure 3.
Oxidant stress, and NADPH oxidase expression in db/db and WT MSCs. (A, B) Oxidant levels, as determined by DCF staining and nitrotyrosine accumulation in MSCs (mean±SEM; n=6; *P<0.05 vs. WT; †P<0.05 vs. db/db). (C) Representative photomicrographs of DCF staining in MSCs (200x). (D–F) Quantitative expressions of Nox4, Nox2, and Nox1 proteins in MSC cell lysates (D–E: mean±SEM; n=6; *P<0.05; F: n=6). Reprinted with permission from JAHA with the specific citation[43].
Figure 4.
In vitro responses of MSCs to selective differentiation media. (A, B) Quantification of MSC differentiation towards adipocytes(A)or endothelial cells(B) (For panels A,B: mean±SEM; n=6; *P<0.05 vs. WT; †P<0.05 vs. db/db.) (C) Nox4 siRNA reduced differentiation of db/db MSCs into an adipocyte phenotype. (D) Nox4 siRNA increased differentiation of db/db MSCs into an endothelial phenotype. (For panels C and D: mean±SEM, n=6; *P<0.05 vs. control [C]; Cy3 is the siRNA control). Reprinted with permission from JAHA with the specific citation[43].
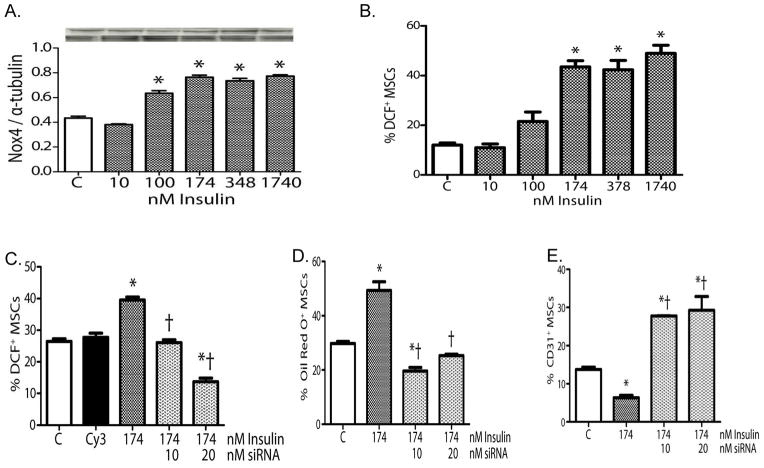
Because it is known that insulin can induce NOX4 expression in pre-adipocytes [74] and that this action is a requisite for terminal differentiation into adipocytes, we explored whether the increase of NOX4 expression in db/db MSCs was due to hyperinsulinemia in experimental type II diabetic mice. These studies showed that in db/db MSCs, hyperinsulinemia induces NOX4-derived oxidant stress that restricts MSCs multipotency in vitro (FIGURE 5). Knockdown of NOX4 expression with a specific siRNA decreased the insulin-induced oxidant stress and restored MSC multipotency in db/db mesenchymal cells in vitro (FIGURE 5).
Figure 5.
Effects of insulin on WT MSCs. (A) Insulin increased Nox4 expression in WT MSCs. (B) Insulin increased oxidant levels in WT MSCs. (C) Pretreatment of WT MSCs with Nox4 siRNA blocked the pro-oxidant effects of insulin in WT MSCs. (D) Pretreatment of WT MSCs with Nox4 siRNA blocked the effects of insulin on adipocyte differentiation. (E) Pretreatment of WT MSCs with Nox4 siRNA blocked the effects of insulin on endothelial differentiation. (For all panels: mean±SEM, n=6; *P<0.05 vs. control [C]; †P<0.05 vs. insulin alone; Cy3 was used as the siRNA control.). Reprinted with permission from JAHA with the specific citation[43].
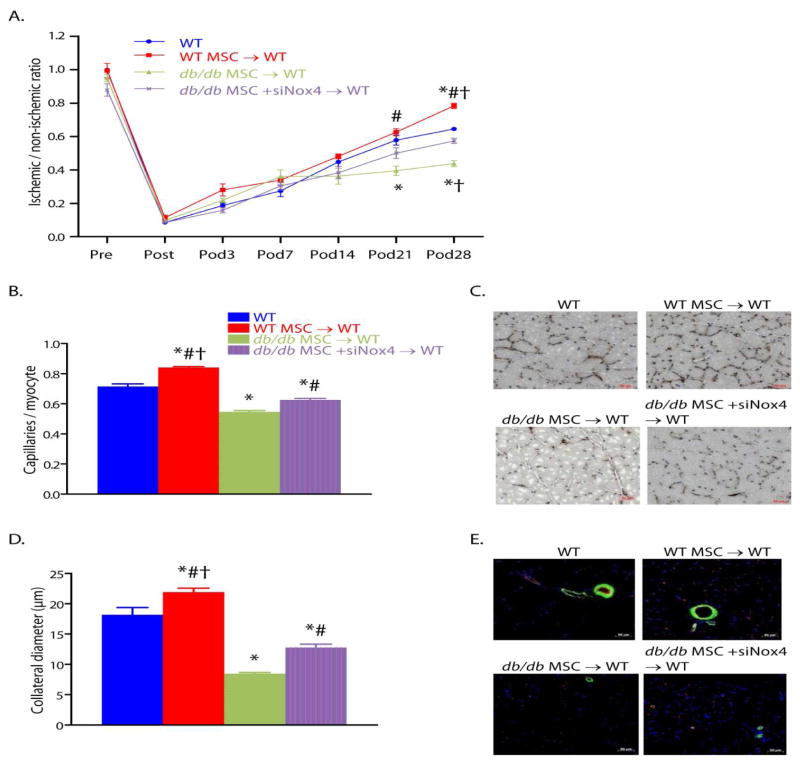
In order to determine whether these in vitro findings accounted for our in vivo findings of adipocytic infiltration and impaired blood flow recovery after hindlimb ischemia in mice that received MSCs from db/db mice, we tested the hypothesis that pretreatment of db/db MSCs with NOX4 siRNA prior to transplantation reverses their propensity for differentiation into adipocytes in ischemic calf muscles as well as their impaired capacity to promote ischemic neovascularization. Transplantation of db/db MSCs after siRNA-induced NOX4 knockdown increased the blood flow recovery to normal levels for WT mice. Knockdown of NOX4 expression in transplanted db/db MSCs also increased collateral artery diameters and capillary density in recipient mice. These results indicate that the impaired capacity of db/db MSCs to augment post ischemic neovascularization in vivo is due in large part to NOX4-induced oxidant stress (FIGURE 6).
Figure 6.
Reversal of Nox4 induced oxidant stress in type 2 diabetic MSCs restores their capacity to augment post-ischemic neovascularization in db/db mice. (A) Foot blood flow recovery measurement by LDPI. (B, C) Quantification (B) of capillary density by CD31 immunostaining and representative images (C). (D, E) Quantification (D) of collateral diameter by CD31 and alpha-SMA double staining and representative images (E). (For all panels: mean±SEM; n=6; *P<0.05 vs. WT; †P<0.05 vs. db/db MSC +siNox4 → WT transplant group; #P<0.05 vs. db/db MSC→ WT transplant group; scale bar, 50 μm.). Reprinted with permission from JAHA with the specific citation[43].
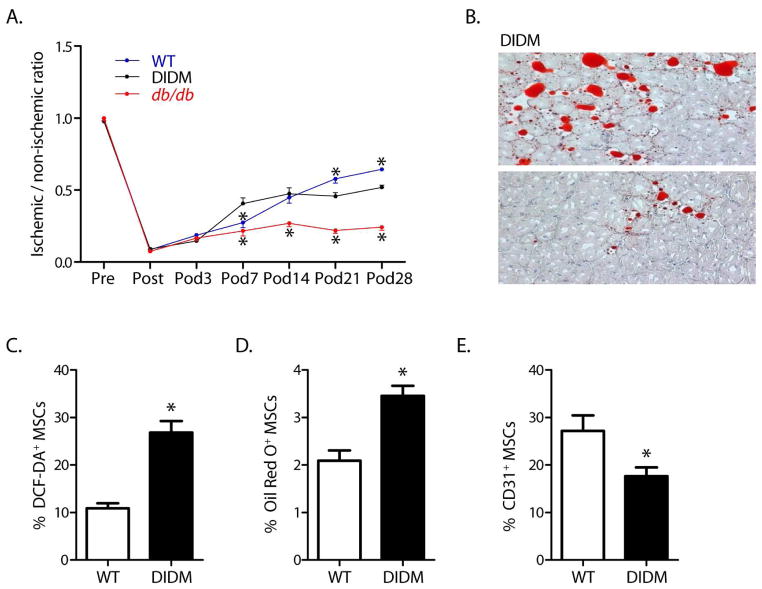
Db/db mice are an experimental model of type II diabetes because they exhibit hypercholesterolemia, hyperinsulinemia, obesity and dyslipidemia. However, they are also leptin receptor-deficient mice, and this could affect their capacity for post-ischemic neovascularization. To account for this potentially confounding variable, we repeated these experiments in wild type mice fed a high fat diet to induce type II diabetes. In these diet-induced type II diabetic mice, blood flow recovery following hindlimb ischemia was significantly less than that in wild type mice. In addition, the diet-induced type II diabetic mice also showed an adipocytic infiltration within the ischemic muscle similar to that in db/db mice, although to a much lesser extent. The latter is likely due to the much lower levels of hyperinsulinemia in the diet-induced diabetic mice than in the db/db mice. Finally, the MSCs from diet-induced diabetic mice showed the same restriction in multipotency determined in vitro as observed in MSCs from db/db mice. (FIGURE 7)
Figure 7.
Diet-induced diabetes (DIDM) impairs recovery from hindlimb ischemia, induces adipocyte differentiation in ischemic muscle, and restricts MSC multipotency. (A) Foot blood flow recovery in DIDM mice (mean±SEM, n=6; *P<0.05 vs. DIDM; note that the WT data shown here were generated specifically for comparison to DIDM mice and are different from those displayed in Figure 3). (B) Intramuscular adipocyte infiltration within the ischemic hindlimb muscle from DIDM mice. (C) Oxidant levels, as evidenced by DCF staining (n=7). (D) MSCs from DIDM demonstrated increased differentiation to an adipocyte phenotype (n=8). (E) MSCs from DIDM mice demonstrated reduced differentiation to an endothelial phenotype (n=7–8). (For panels C–E: mean±SEM; *P<0.05.). Reprinted with permission from JAHA with the specific citation[43].
Conclusions
MSCs are being implemented in a wide variety of preclinical and clinical studies as a therapeutic option for the treatment of complications resulting from peripheral arterial occlusive disease. Our work has shown that MSCs harvested from type II diabetic mice show several oxidant stress-dependent dysfunctions. Rather than increasing post-ischemic neovascularization and limb blood flow, injection of MSCs from type II diabetic mice impaired blood flow recovery. Should human MSCs display similar oxidant stress-induced impairment of function, these findings recommend a therapeutic approach towards maximizing the potential of MSC transplantation, particularly in the increasingly common setting of diabetes or other cardiovascular risk factors. We propose that either in vivo systemic treatment with an antioxidant and/or ex vivo treatment of MSCs with antioxidants will significantly increase the intended clinical benefit.
Acknowledgments
The authors acknowledge financial support from grant HL75353 from the National Institute for Heart, Lung, and Blood (to L.M.M.).
Abbreviation Key
- A
autologous
- Al
allogenic
- ABI
ankle-brachial index
- AE
adverse events
- AFS
amputation-free survival
- CLI
critical limb ischemia
- CL
contralateral leg
- DSA
digital subtraction angiography
- MRA
magnetic resonance angiography
- PS
perfusion scintigraphy
- QoL
quality of life survey
- RP
rest pain
- TAO
thromboangiitis obliterans
- TcPO2
transcutaneous oxygen pressure
- TTF
time to treatment failure
- WH
wound healing
- WT/D
pain free walking time/distance
Footnotes
Conflict of interest
The authors declare no potential conflicts of interest.
References
- 1.Takahashi TKC, Masuda H, Chen D, Silver M, Kearney M, Magner M, Isner JM, Asahara T. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med. 1999;5(4):434–438. doi: 10.1038/7434. [DOI] [PubMed] [Google Scholar]
- 2.Kinnaird TSE, Burnett MS, Epstein SE. Bone-marrow-derived cells for enhancing collateral development: mechanisms, animal data, and initial clinical experiences. Circ Res. 2004;95(4):354–363. doi: 10.1161/01.RES.0000137878.26174.66. [DOI] [PubMed] [Google Scholar]
- 3.Asahara TMT, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275(5302):964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 4.Crosby KW, JR, Schatteman G, Martin PJ, Raines EW, Seifert RA, Bowen-Pope DF. Endothelial cells of hematopoietic origin make a significant contribution to adult blood vessel formation. Circ Res. 2000;87(9):728–30. doi: 10.1161/01.res.87.9.728. [DOI] [PubMed] [Google Scholar]
- 5.Sneider EB, NP, Messina LM. Regenerative medicine in the treatment of peripheral arterial disease. J Cell Biochem. 2009;108(4):753–61. doi: 10.1002/jcb.22315. [DOI] [PubMed] [Google Scholar]
- 6.Pittenger MF, MA, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284(5411):143–7. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 7.Bianco P, GRP Marrow stromal stem cells. J Clin Invest. 2000;105(12):1663–8. doi: 10.1172/JCI10413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iwase T, NN, Fujii T, Itoh T, Murakami S, Matsumoto T, Kangawa K, Kitamura S. Comparison of angiogenic potency between mesenchymal stem cells and mononuclear cells in a rat model of hindlimb ischemia. Cardiovasc Res. 2005;66(3):543–51. doi: 10.1016/j.cardiores.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 9.Lasala GP, SJ, Minguell JJ. Therapeutic angiogenesis in patients with severe limb ischemia by transplantation of a combination stem cell product. J Thorac Cardiovasc Surg. 2012;144(2):377–82. doi: 10.1016/j.jtcvs.2011.08.053. [DOI] [PubMed] [Google Scholar]
- 10.Lawall H, BP, Amann B. Stem cell and progenitor cell therapy in peripheral artery disease. A critical appraisal. Thromb Haemost. 2010;103(4):696–709. doi: 10.1160/TH09-10-0688. [DOI] [PubMed] [Google Scholar]
- 11.Webber MJ, HX, Murthy SN, Rajangam K, Stupp SI, Lomasney JW. Capturing the stem cell paracrine effect using heparin-presenting nanofibres to treat cardiovascular diseases. J Tissue Eng Regen Med. 2010;4(8):600–10. doi: 10.1002/term.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lasala GP, MJ Vascular disease and stem cell therapies. Br Med Bull. 2011;98:187–97. doi: 10.1093/bmb/ldr017. [DOI] [PubMed] [Google Scholar]
- 13.de Nigris F, BM, Williams-Ignarro S, D’Armiento FP, Lerman LO, Byrns R, Crimi E, Palagiano A, Fatigati G, Ignarro LJ, Napoli C. Therapeutic effects of autologous bone marrow cells and metabolic intervention in the ischemic hindlimb of spontaneously hypertensive rats involve reduced cell senescence and CXCR4/Akt/eNOS pathways. J Cardiovasc Pharmacol. 2007;50(4):424–33. doi: 10.1097/FJC.0b013e31812564e4. [DOI] [PubMed] [Google Scholar]
- 14.Kang Y, PC, Kim D, Seong CM, Kwon K, Choi C. Unsorted human adipose tissue-derived stem cells promote angiogenesis and myogenesis in murine ischemic hindlimb model. Microvasc Res. 2010;80(3):310–6. doi: 10.1016/j.mvr.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 15.Chen J, LY, Wang L, Lu M, Zhang X, Chopp M. Therapeutic benefit of intracerebral transplantation of bone marrow stromal cells after cerebral ischemia in rats. J Neurol Sci. 2001;189(1–2):49–57. doi: 10.1016/s0022-510x(01)00557-3. [DOI] [PubMed] [Google Scholar]
- 16.Jin HK, CJ, Huntley GW, Schuchman EH. Intracerebral transplantation of mesenchymal stem cells into acid sphingomyelinase-deficient mice delays the onset of neurological abnormalities and extends their life span. J Clin Invest. 2002;109(9):1183–91. doi: 10.1172/JCI14862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DJ P. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276(5309):71–4. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- 18.Ukai R, HO, Harada K, Houkin K, Hamada H, Kocsis JD. Mesenchymal stem cells derived from peripheral blood protects against ischemia. J Neurotrauma. 2007;24(3):508–20. doi: 10.1089/neu.2006.0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim SW, HH, Chae GT, Lee SH, Bo S, Yoon JH, Lee YS, Lee KS, Park HK, Kang KS. Successful stem cell therapy using umbilical cord blood-derived multipotent stem cells for Buerger’s disease and ischemic limb disease animal model. Stem Cells. 2006;24(6):1620–6. doi: 10.1634/stemcells.2005-0365. [DOI] [PubMed] [Google Scholar]
- 20.Romanov YA, SV, Smirnov VN. Searching for alternative sources of postnatal human mesenchymal stem cells: candidate MSC-like cells from umbilical cord. Stem Cells. 2003;21(1):105–10. doi: 10.1634/stemcells.21-1-105. [DOI] [PubMed] [Google Scholar]
- 21.Xu Y, MH, Li C, Hao M, Wang Y, Yu Z, Li Q, Han J, Zhai Q, Qiu L. Umbilical cord-derived mesenchymal stem cells isolated by a novel explantation technique can differentiate into functional endothelial cells and promote revascularization. Stem Cells Dev. 2010;19(10):1511–22. doi: 10.1089/scd.2009.0321. [DOI] [PubMed] [Google Scholar]
- 22.Nakagami H, MK, Morishita R, Iguchi S, Nishikawa T, Takami Y, Kikuchi Y, Saito Y, Tamai K, Ogihara T, Kaneda Y. Novel autologous cell therapy in ischemic limb disease through growth factor secretion by cultured adipose tissue-derived stromal cells. Arterioscler Thromb Vasc Biol. 2005;25(12):2542–7. doi: 10.1161/01.ATV.0000190701.92007.6d. [DOI] [PubMed] [Google Scholar]
- 23.Cho HH, KY, Kim JT, Song JS, Shin KK, Bae YC, Jung JS. The role of chemokines in proangiogenic action induced by human adipose tissue-derived mesenchymal stem cells in the murine model of hindlimb ischemia. Cell Physiol Biochem. 2009;24(5–6):511–8. doi: 10.1159/000257495. [DOI] [PubMed] [Google Scholar]
- 24.Iwashima S, OT, Maruyama S, Saka Y, Kobori M, Omae K, Yamaguchi H, Niimi T, Toriyama K, Kamei Y, Torii S, Murohara T, Yuzawa Y, Kitagawa Y, Matsuo S. Novel culture system of mesenchymal stromal cells from human subcutaneous adipose tissue. Stem Cells Dev. 2009;18(4):533–43. doi: 10.1089/scd.2008.0358. [DOI] [PubMed] [Google Scholar]
- 25.Fukuchi Y, NH, Sugiyama D, Hirose I, Kitamura T, Tsuji K. Human placenta-derived cells have mesenchymal stem/progenitor cell potential. Stem Cells. 2004;22(5):649–58. doi: 10.1634/stemcells.22-5-649. [DOI] [PubMed] [Google Scholar]
- 26.Kern S, EH, Stoeve J, Klüter H, Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2011;24(5):1294–301. doi: 10.1634/stemcells.2005-0342. [DOI] [PubMed] [Google Scholar]
- 27.Bieback K, KS, Kocaömer A, Ferlik K, Bugert P. Comparing mesenchymal stromal cells from different human tissues: bone marrow, adipose tissue and umbilical cord blood. Biomed Mater Eng. 2008;18(1 Suppl):S71–6. [PubMed] [Google Scholar]
- 28.Rebelatto CK, AA, Moretão MP, Senegaglia AC, Hansen P, Barchiki F, Oliveira J, Martins J, Kuligovski C, Mansur F, Christofis A, Amaral VF, Brofman PS, Goldenberg S, Nakao LS, Correa A. Dissimilar differentiation of mesenchymal stem cells from bone marrow, umbilical cord blood, and adipose tissue. Exp Biol Med (Maywood) 2008;233(7):901–13. doi: 10.3181/0712-RM-356. [DOI] [PubMed] [Google Scholar]
- 29.Gaebel R, FD, Sorg H, Polchow B, Frank J, Bieback K, Wang W, Klopsch C, Ong LL, Li W, Ma N, Steinhoff G. Cell origin of human mesenchymal stem cells determines a different healing performance in cardiac regeneration. PLoS One. 2011;6(2):e15652. doi: 10.1371/journal.pone.0015652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hoffmann J, GA, Doyle TC, Robbins RC, Schrepfer S, Pelletier MP. Angiogenic effects despite limited cell survival of bone marrow-derived mesenchymal stem cells under ischemia. Thorac Cardiovasc Surg. 2010;58(3):136–42. doi: 10.1055/s-0029-1240758. [DOI] [PubMed] [Google Scholar]
- 31.Kim Y, KH, Cho H, Bae Y, Suh K, Jung J. Direct comparison of human mesenchymal stem cells derived from adipose tissues and bone marrow in mediating neovascularization in response to vascular ischemia. Cell Physiol Biochem. 2007;20(6):867–76. doi: 10.1159/000110447. [DOI] [PubMed] [Google Scholar]
- 32.Takahashi K, TK, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–72. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 33.Yu J, VM, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin II, Thomson JA. Induced pluripotent stem cell lines derived from human somatic cells. science. 2007;318(5858):1917–20. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 34.Lian Q, ZY, Zhang J, Zhang HK, Wu X, Zhang Y, Lam FF, Kang S, Xia JC, Lai WH, Au KW, Chow YY, Siu CW, Lee CN, Tse HF. Functional mesenchymal stem cells derived from human induced pluripotent stem cells attenuate limb ischemia in mice. Circulation. 2010;121(9):1113–23. doi: 10.1161/CIRCULATIONAHA.109.898312. [DOI] [PubMed] [Google Scholar]
- 35.Jung Y, BG, Nolta JA. Concise review: Induced pluripotent stem cell-derived mesenchymal stem cells: progress toward safe clinical products. Stem Cells. 2012;30(1):42–7. doi: 10.1002/stem.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gruenloh W, KA, Sondergaard C, McGee J, Nacey C, Kalomoiris S, Pepper K, Olson S, Fierro F, Nolta JA. Characterization and in vivo testing of mesenchymal stem cells derived from human embryonic stem cells. Tissue Eng Part A. 2011;17(11–12):1517–25. doi: 10.1089/ten.tea.2010.0460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sánchez L, G-AI, Ligero G, Rubio R, Muñoz-López M, García-Pérez JL, Ramos V, Real PJ, Bueno C, Rodríguez R, Delgado M, Menendez P. Enrichment of human ESC-derived multipotent mesenchymal stem cells with immunosuppressive and anti-inflammatory properties capable to protect against experimental inflammatory bowel disease. Stem Cells. 2011;29(2):251–62. doi: 10.1002/stem.569. [DOI] [PubMed] [Google Scholar]
- 38.Laurila JP, LL, Castellone MD, Trivedi P, Heikkila J, Hinkkanen A, Hematti P, Laukkanen MO. Human embryonic stem cell-derived mesenchymal stromal cell transplantation in a rat hind limb injury model. Cytotherapy. 2009;11(6):726–737. doi: 10.3109/14653240903067299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Horwitz EM, LBK, Dominici M, Mueller I, Slaper-Cortenbach I, Marini FC, Deans RJ, Krause DS, Keating A. Clarification of the nomenclature for MSC: The International Society for Cellular Therapy position statement. Cytotherapy. 2005;7(5):393–395. doi: 10.1080/14653240500319234. [DOI] [PubMed] [Google Scholar]
- 40.Reiser J, ZX, Hemenway CS, Mondal D, Pradhan L, La Russa VF. Potential of mesenchymal stem cells in gene therapy approaches for inherited and acquired diseases. Expert Opin Biol Ther. 2005;5(12):1571–1584. doi: 10.1517/14712598.5.12.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wieczorek G, SC, Schulz R, Scheller M, Vingron M, Ropers HH, Nuber UA. Gene expression profile of mouse bone marrow stromal cells determined by cDNA microarray analysis. Cell Tissue Res. 2003;311(2):227–37. doi: 10.1007/s00441-002-0671-3. [DOI] [PubMed] [Google Scholar]
- 42.Camarillo C, SM, Hart RP. Comparison of microarray and quantitative real-time PCR methods for measuring MicroRNA levels in MSC cultures. Methods Mol Biol. 2011;698:419–29. doi: 10.1007/978-1-60761-999-4_30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yan J, TG, Wang S, Messina KE, DiDato S, Guo S, Messina LM. Type 2 diabetes restricts mesenchymal stem cell multipotency and Impairs their capacity to augment post-ischemic neovascularization in db/db Mice. Journal of the American Heart Association. 2012;1:e002238. doi: 10.1161/JAHA.112.002238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hamou C, CM, Thangarajah H, Chang E, Chang EI, Grogan RH, Paterno J, Vial IN, Jazayeri L, Gurtner GC. Mesenchymal stem cells can participate in ischemic neovascularization. Plast Reconstr Surg. 2009;123(2 suppl):45S–55S. doi: 10.1097/PRS.0b013e318191be4a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li M, YJ, Li Y, Li D, Yan D, Ruan Q. CXCR4+ progenitors derived from bone mesenchymal stem cells differentiate into endothelial cells capable of vascular repair after arterial injury. Cell Reprogram. 2010;12(4):405–15. doi: 10.1089/cell.2009.0088. [DOI] [PubMed] [Google Scholar]
- 46.Gong Z, NL Use of human mesenchymal stem cells as alternative source of smooth muscle cells in vessel engineering. Methods Mol Biol. 2011;698:279–94. doi: 10.1007/978-1-60761-999-4_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Toma C, PM, Cahill KS, Byrne BJ, Kessler PD. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation. 2002;105(1):93–8. doi: 10.1161/hc0102.101442. [DOI] [PubMed] [Google Scholar]
- 48.Zhu CJ, DJ, Li J, Zhang MJ, Wang LP, Luo L. Preliminary study on the mechanism of acupoint injection of bone marrow mesenchymal stem cells in improving blood flow in the rat of hind limb ischemia. J Tradit Chin Med. 2011;31(3):241–5. doi: 10.1016/s0254-6272(11)60050-2. [DOI] [PubMed] [Google Scholar]
- 49.Kinnaird T, SE, Burnett MS, Shou M, Lee CW, Barr S, Fuchs S, Epstein SE. Local delivery of marrow-derived stromal cells augments collateral perfusion through paracrine mechanisms. Circulation. 2004;109(12):1543–9. doi: 10.1161/01.CIR.0000124062.31102.57. [DOI] [PubMed] [Google Scholar]
- 50.Li H, ZS, He Z, Yang Y, Pasha Z, Wang Y, Xu M. Paracrine factors released by GATA-4 overexpressed mesenchymal stem cells increase angiogenesis and cell survival. Am J Physiol Heart Circ Physiol. 2010;299(6):H1772–81. doi: 10.1152/ajpheart.00557.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Parekkadan B, vPD, Suganuma K, Carter EA, Berthiaume F, Tilles AW, Yarmush ML. Mesenchymal stem cell-derived molecules reverse fulminant hepatic failure. PLoS One. 2007;2(9):e941. doi: 10.1371/journal.pone.0000941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hatzistergos KE, QH, Oskouei BN, Hu Q, Feigenbaum GS, Margitich IS, Mazhari R, Boyle AJ, Zambrano JP, Rodriguez JE, Dulce R, Pattany PM, Valdes D, Revilla C, Heldman AW, McNiece I, Hare JM. Bone marrow mesenchymal stem cells stimulate cardiac stem cell proliferation and differentiation. Circ Res. 2010;107(7):913–22. doi: 10.1161/CIRCRESAHA.110.222703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liechty KW, MT, Shaaban AF, Radu A, Moseley AM, Deans R, Marshak DR, Flake AW. Human mesenchymal stem cells engraft and demonstrate site-specific differentiation after in utero transplantation in sheep. Nat Med. 2000;6(11):1282–6. doi: 10.1038/81395. [DOI] [PubMed] [Google Scholar]
- 54.JS Immune regulation by mesenchymal stem cells: two sides to the coin. Tissue Antigens. 2007;69(1):1–9. doi: 10.1111/j.1399-0039.2006.00739.x. [DOI] [PubMed] [Google Scholar]
- 55.Uccelli A, ML, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008;8(9):726–36. doi: 10.1038/nri2395. [DOI] [PubMed] [Google Scholar]
- 56.Parekkadan B, vPD, Suganuma K, Carter EA, Berthiaume F, Tilles AW, Yarmush ML. Mesenchymal stem cell-derived molecules reverse fulminant hepatic failure. PLoS One. 2007;2(9):e941. doi: 10.1371/journal.pone.0000941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shabbir A, ZD, Suzuki G, Lee T. Heart failure therapy mediated by the trophic activities of bone marrow mesenchymal stem cells: a noninvasive therapeutic regimen. Am J Physiol Heart Circ Physiol. 2009;296(6):H1888–97. doi: 10.1152/ajpheart.00186.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Guiducci S, PF, Saccardi R, Guidi S, Ibba-Manneschi L, Manetti M, Mazzanti B, Dal Pozzo S, Milia AF, Bellando-Randone S, Miniati I, Fiori G, Fontana R, Amanzi L, Braschi F, Bosi A, Matucci-Cerinic M. Autologous mesenchymal stem cells foster revascularization of ischemic limbs in systemic sclerosis: a case report. Ann Intern Med. 2010;153(10):650–4. doi: 10.7326/0003-4819-153-10-201011160-00007. [DOI] [PubMed] [Google Scholar]
- 59.Schrepfer S, DT, Reichenspurner H, Fischbein MP, Robbins RC, Pelletier MP. Stem cell transplantation: the lung barrier. Transplant Proc. 2007;39(2):573–6. doi: 10.1016/j.transproceed.2006.12.019. [DOI] [PubMed] [Google Scholar]
- 60.Morikawa S, MY, Kubota Y, Nagai Y, Niibe K, Hiratsu E, Suzuki S, Miyauchi-Hara C, Nagoshi N, Sunabori T, Shimmura S, Miyawaki A, Nakagawa T, Suda T, Okano H, Matsuzaki Y. Prospective identification, isolation, and systemic transplantation of multipotent mesenchymal stem cells in murine bone marrow. J Exp Med. 2009;206(11):2483–96. doi: 10.1084/jem.20091046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ishii M, SR, Numaguchi Y, Kito T, Suzuki H, Shimizu K, Ito A, Honda H, Murohara T. Enhanced angiogenesis by transplantation of mesenchymal stem cell sheet created by a novel magnetic tissue engineering method. Arterioscler Thromb Vasc Biol. 2011;31(10):2210–5. doi: 10.1161/ATVBAHA.111.231100. [DOI] [PubMed] [Google Scholar]
- 62.Wang J, CW, Ye J, Ji S, Zhao X, Zhan L, Feng J, Zhang Z, Zhao Y. A cellular delivery system fabricated with autologous BMSCs and collagen scaffold enhances angiogenesis and perfusion in ischemic hind limb. J Biomed Mater Res A. 2012;100(6):1438–47. doi: 10.1002/jbm.a.34081. [DOI] [PubMed] [Google Scholar]
- 63.Zhang Y, ZR, Li Y, He G, Zhang D, Zhang F. Simvastatin augments the efficacy of therapeutic angiogenesis induced by bone marrow-derived mesenchymal stem cells in a murine model of hindlimb ischemia. Mol Biol Rep. 2012;39(1):285–93. doi: 10.1007/s11033-011-0737-y. [DOI] [PubMed] [Google Scholar]
- 64.Jiang CY, GC, He AN, Hu XY, Chen J, Jiang Y, Wang JA. Optimal time for mesenchymal stem cell transplantation in rats with myocardial infarction. J Zhejiang Univ Sci B. 2008;9(8):630–7. doi: 10.1631/jzus.B0820004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jackson KA, MS, Wang H, Pocius J, Hartley CJ, Majesky MW, Entman ML, Michael LH, Hirschi KK, Goodell MA. Regeneration of ischemic cardiac muscle and vascular endothelium by adult stem cells. J Clin Invest. 2001;107(11):1395–402. doi: 10.1172/JCI12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang G, HQ, Braunlin EA, Suggs LJ, Zhang J. Enhancing efficacy of stem cell transplantation to the heart with a PEGylated fibrin biomatrix. Tissue Eng Part A. 2008;14(6):1025–36. doi: 10.1089/ten.tea.2007.0289. [DOI] [PubMed] [Google Scholar]
- 67.Jeong JO, HJ, Kim JM, Cho HJ, Park C, Lee N, Kim DW, Yoon YS. Malignant tumor formation after transplantation of short-term cultured bone marrow mesenchymal stem cells in experimental myocardial infarction and diabetic neuropathy. Circ Res. 2011;108(11):1340–7. doi: 10.1161/CIRCRESAHA.110.239848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Miura M, MY, Padilla-Nash HM, Molinolo AA, Fu B, Patel V, Seo BM, Sonoyama W, Zheng JJ, Baker CC, Chen W, Ried T, Shi S. Accumulated chromosomal instability in murine bone marrow mesenchymal stem cells leads to malignant transformation. Stem Cells. 2006;24(4):1095–103. doi: 10.1634/stemcells.2005-0403. [DOI] [PubMed] [Google Scholar]
- 69.Hatzistergos KE, BA, Ince T, Grichnik JM, Hare JM. What is the oncologic risk of stem cell treatment for heart disease? Circ Res. 2011;108(11):1300–3. doi: 10.1161/CIRCRESAHA.111.246611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yan J, TG, Park B, Yan Y, Nowicki PT, Messina LM. Recovery from hind limb ischemia is less effective in type 2 than in type 1 diabetic mice: roles of endothelial nitric oxide synthase and endothelial progenitor cells. J Vasc Surg. 2009;50(6):1412–22. doi: 10.1016/j.jvs.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tie G, YJ, Yang Y, Park B, Messina LM. Oxidized low density lipoprotein inactivates PI3 kinase/Akt pathway in murine endothelial progenitor cell. Journal of Vascular Research. 2010;47(6):519–30. doi: 10.1159/000313879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Heeschen C, LR, Honold J, Assmus B, Aicher A, Walter DH, Martin H, Zeiher AM, Dimmeler S. Profoundly reduced neovascularization capacity of bone marrow mononuclear cells derived from patients with chronic ischemic heart disease. Circulation. 2004;109(13):1615–22. doi: 10.1161/01.CIR.0000124476.32871.E3. [DOI] [PubMed] [Google Scholar]
- 73.Dimmeler S, LA Aging and disease as modifiers of efficacy of cell therapy. Circ Res. 2008;102(11):1319–30. doi: 10.1161/CIRCRESAHA.108.175943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.MacKenzie TC, FA Human mesenchymal stem cells: insights from a surrogate in vivo assay system. Cells Tissues Organs. 2002;171(1):90–5. doi: 10.1159/000057694. [DOI] [PubMed] [Google Scholar]
- 75.Urbich C, DS Risk factors for coronary artery disease, circulating endothelial progenitor cells, and the role of HMG-CoA reductase inhibitors. Kidney Int. 2005;67(5):1672–6. doi: 10.1111/j.1523-1755.2005.00261.x. [DOI] [PubMed] [Google Scholar]
- 76.Amin AH, AEZ, Nair D, Partyka MI, Kadowitz PJ, Belmadani S, Matrougui K. Modified multipotent stromal cells with epidermal growth factor restore vasculogenesis and blood flow in ischemic hind-limb of type II diabetic mice. Lab Invest. 2010;90(7):985–96. doi: 10.1038/labinvest.2010.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cobellis G, Silvestroni A, Lillo S, Sica G, Botti C, Maione C, Schiavone V, Rocco S, Brando G, Sica V. Long-term effects of repeated autologous transplantation of bone marrow cells in patients affected by peripheral arterial disease. Bone Marrow Transplant. 2008 Nov;42(10):667–72. doi: 10.1038/bmt.2008.228. [DOI] [PubMed] [Google Scholar]
- 78.Walter DH, Krankenberg H, Balzer JO, Kalka C, Baumgartner I, Schlüter M, Tonn T, Seeger F, Dimmeler S, Lindhoff-Last E, Zeiher AM PROVASA Investigators. Intraarterial administration of bone marrow mononuclear cells in patients with critical limb ischemia: a randomized-start, placebo-controlled pilot trial (PROVASA) Circ Cardiovasc Interv. 2011 Feb 1;4(1):26–37. doi: 10.1161/CIRCINTERVENTIONS.110.958348. [DOI] [PubMed] [Google Scholar]
- 79.Ruiz-Salmeron R, de la Cuesta-Diaz A, Constantino-Bermejo M, Pérez-Camacho I, Marcos-Sánchez F, Hmadcha A, Soria B. Angiographic demonstration of neoangiogenesis after intra-arterial infusion of autologous bone marrow mononuclear cells in diabetic patients with critical limb ischemia. Cell Transplant. 2011;20(10):1629–39. doi: 10.3727/096368910X0177. [DOI] [PubMed] [Google Scholar]
- 80.Tateishi-Yuyama E, Matsubara H, Murohara T, Ikeda U, Shintani S, Masaki H, Amano K, Kishimoto Y, Yoshimoto K, Akashi H, Shimada K, Iwasaka T, Imaizumi T Therapeutic Angiogenesis using Cell Transplantation (TACT) Study Investigators. Therapeutic angiogenesis for patients with limb ischaemia by autologous transplantation of bone-marrow cells: a pilot study and a randomised controlled trial. Lancet. 2002 Aug 10;360(9331):427–35. doi: 10.1016/S0140-6736(02)09670-8. [DOI] [PubMed] [Google Scholar]
- 81.Miyamoto M, Yasutake M, Takano H, Takagi H, Takagi G, Mizuno H, Kumita S, Takano T. Therapeutic angiogenesis by autologous bone marrow cell implantation for refractory chronic peripheral arterial disease using assessment of neovascularization by 99mTc-tetrofosmin (TF) perfusion scintigraphy. Cell Transplant. 2004;13(4):429–37. doi: 10.3727/000000004783983837. [DOI] [PubMed] [Google Scholar]
- 82.Mizuno H, Miyamoto M, Shimamoto M, Koike S, Hyakusoku H, Kuroyanagi Y. Therapeutic angiogenesis by autologous bone marrow cell implantation together with allogeneic cultured dermal substitute for intractable ulcers in critical limb ischaemia. J Plast Reconstr Aesthet Surg. 2010 Nov;63(11):1875–82. doi: 10.1016/j.bjps.2009.11.037. Epub 2010 Jan. [DOI] [PubMed] [Google Scholar]
- 83.Perin EC, Silva G, Gahremanpour A, Canales J, Zheng Y, Cabreira-Hansen MG, Mendelsohn F, Chronos N, Haley R, Willerson JT, Annex BH. A randomized, controlled study of autologous therapy with bone marrow-derived aldehyde dehydrogenase bright cells in patients with critical limb ischemia. Catheter Cardiovasc Interv. 2011 Dec 1;78(7):1060–7. doi: 10.1002/ccd.23066. [DOI] [PubMed] [Google Scholar]
- 84.Lasala GP, Silva JA, Gardner PA, Minguell JJ. Combination stem cell therapy for the treatment of severe limb ischemia: safety and efficacy analysis. Angiology. 2010 Aug;61(6):551–6. doi: 10.1177/0003319710364213. [DOI] [PubMed] [Google Scholar]
- 85.Lu D, Chen B, Liang Z, Deng W, Jiang Y, Li S, Xu J, Wu Q, Zhang Z, Xie B, Chen S. Comparison of bone marrow mesenchymal stem cells with bone marrow-derived mononuclear cells for treatment of diabetic critical limb ischemia and foot ulcer: a double-blind, randomized, controlled trial. Diabetes Res Clin Pract. 2011 Apr;92(1):26–36. doi: 10.1016/j.diabres.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 86.Powell RJ, Comerota AJ, Berceli SA, Guzman R, Henry TD, Tzeng E, Velazquez O, Marston WA, Bartel RL, Longcore A, Stern T, Watling S. Interim analysis results from the RESTORE-CLI, a randomized, double-blind multicenter phase II trial comparing expanded autologous bone marrow-derived tissue repair cells and placebo in patients with critical limb ischemia. J Vasc Surg. 2011 Oct;54(4):1032–41. doi: 10.1016/j.jvs.2011.04.006. [DOI] [PubMed] [Google Scholar]
- 87.Powell RJ, Marston WA, Berceli SA, Guzman R, Henry TD, Longcore AT, Stern TP, Watling S, Bartel RL. Cellular therapy with Ixmyelocel-T to treat critical limb ischemia: the randomized, double-blind, placebo-controlled RESTORE-CLI trial. Mol Ther. 2012 Jun;20(6):1280–6. doi: 10.1038/mt.2012.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dash NR, Dash SN, Routray P, Mohapatra S, Mohapatra PC. Targeting nonhealing ulcers of lower extremity in human through autologous bone marrow-derived mesenchymal stem cells. Rejuvenation Res. 2009 Oct;12(5):359–66. doi: 10.1089/rej.2009.0872. [DOI] [PubMed] [Google Scholar]