Abstract
Diabetic cardiomyopathy (DCM) is defined as cardiac disease independent of vascular complications during diabetes. The number of new cases of DCM is rising at epidemic rates in proportion to newly diagnosed cases of diabetes mellitus (DM) throughout the world. DCM is a heart failure syndrome found in diabetic patients that is characterized by left ventricular hypertrophy and reduced diastolic function, with or without concurrent systolic dysfunction, occurring in the absence of hypertension and coronary artery disease. DCM and other diabetic complications are caused in part by elevations in blood glucose and lipids, characteristic of DM. Although there are pathological consequences to hyperglycemia and hyperlipidemia, the combination of the two metabolic abnormalities potentiates the severity of diabetic complications. A natural competition exists between glucose and fatty acid metabolism in the heart that is regulated by allosteric and feedback control and transcriptional modulation of key limiting enzymes. Inhibition of these glycolytic enzymes not only controls flux of substrate through the glycolytic pathway, but also leads to the diversion of glycolytic intermediate substrate through pathological pathways, which mediate the onset of diabetic complications. The present review describes the limiting steps involved in the development of these pathological pathways and the factors involved in the regulation of these limiting steps. Additionally, therapeutic options with demonstrated or postulated effects on DCM are described.
Diabetes mellitus
Diabetes mellitus (DM) is a global health epidemic whose rates have risen dramatically and are predicted to continue to rise during the next 20 years. It is estimated that 18.1 million people (8.0% of the adult population) in the United States have diagnosed DM, with another 7.1 million individuals having undiagnosed DM [1]. Similarly concerning is the 36.8% of the adult population who have abnormal fasting glucose levels, indicating clinical prediabetes. Type 2 DM (T2D) is particularly epidemic due to the rising rates of obesity throughout the world. Over one billion people worldwide are overweight (BMI >25 and <29.9) or obese (BMI >30) [2]. The projected obesity prevalence globally is 8.0% for men and 12.3% for women in 2010. DM is expected to rise worldwide from 175 million in 2000 to 353 million by 2030, creating a tremendous healthcare and financial burden [3]. The United States, with an overweight and obesity prevalence of 67.3% for adults older than twenty, is predicted to be the forerunner of the DM epidemic, increasing prevalence from 8.8% in 2000 to 11.2% by 2030 [1, 3].
Diabetes mellitus consists of several metabolic conditions in which there is a dysfunction in the cell’s ability to transport and utilize glucose. Type 1 DM (T1D), formerly called insulin dependent or juvenile diabetes, is caused by T lymphocyte-mediated autoimmune destruction of the pancreatic β-cells, resulting in insufficient insulin production and corresponding decrease in glucose utilization [4]. The etiology of type 2 DM (T2D), formerly called insulin independent or adult-onset diabetes, results from an insulin resistance that instigates hypertrophy of the β-cell to compensate, resulting in hyperinsulinemia leading to eventual insulin resistance [5, 6]. Progressive decompensatory failure of the β-cells in T2D decreases the amount of insulin produced. The end result is a decreased level of serum insulin, which is insufficient to overcome the developed insulin resistance. These pathophysiological changes lead to elevated blood glucose levels (hyperglycemia) and impaired cellular glycolysis and pyruvate oxidation [7]. Chronic hyperglycemia can result in numerous comorbidities, including kidney failure, nerve damage, retinopathy, peripheral vascular disease and cardiac dysfunction/failure [8]. The mechanisms causing these comorbidities, particularly cardiac dysfunction, include increased levels of advanced glycation end products, mitochondrial dysfunction, enhanced oxidative stress, altered cell metabolic function and altered calcium homeostasis [8-10].
Cardiovascular and cardiomyocyte dysfunction in DM
Cardiovascular disease (CVD) resulted in one out of every three deaths in the United States in 2008, making it the leading cause of death often resulting from other medical conditions, including hypertension, alcoholism, obesity, and diabetes [1]. Additionally, heart disease death rates among adult diabetics is 2-4 times more likely than adults without DM and 68% of adults with DM older than 65 years die of some form of heart disease [11]. The significance of DM has especially increasing significance in women, as females with diabetes have a five times greater incidence of heart diseases than their non-diabetic counterparts, compared to the two fold increase in heart disease observed in diabetic versus non-diabetic men [12]. This discordance may be attributable to the intrinsic difference in the myocardium and/or sex hormonal and neurohormonal differences, but more gender specific studies are needed to fully describe the differences in mechanisms [13]. One secondary CVD is diabetic cardiomyopathy (DCM). The early stages of DCM involve observable left ventricular hypertrophy (LVH), which along with myocardial remodeling, causes abnormal left ventricle (LV) filling and diastolic dysfunction [14]. The left ventricular diastolic dysfunction (LVDD) is detectable via echocardiography [15]. Progression of DCM can lead to systolic dysfunction, which may be unrecognized in its early stages due to compensatory mechanisms preserving a normal ejection fraction in these individuals [14]. Functional alterations in vivo include decreased fractional shortening, decreased ventricular filling, decreased ventricular ejection fraction, increased ventricular wall stiffness and increased pre-ejection time [8]. This leads to abnormal relaxation, including increased isovolumetric relaxation time and impaired diastole [16].
Gross differentiation of T1D and T2D cardiomypathy
Diabetic cardiomyopathy has been largely considered as a single condition equally affecting all diabetic individuals. However, in recent years there has been increasing work utilizing animal models to differentiate the mechanisms and effects of DCM caused by T1D verses T2D. In general, studies have found that although systolic dysfunction is present in both, it is more prevalent in type 1 DCM [17]. Type 2 DCM models have been associated with a larger amount of preclinical changes than type 1 DCM, including a greater impairment of ventricular filling, resulting in more prevalent diastolic dysfunction [15, 18]. On gross pathological analyses of the myocardium, a newer finding has suggested that T2D patients have a significantly higher level of myocardial steatosis preceding and possibly contributing to the early diastolic dysfunction [19, 20]. Although the exact mechanisms for differences in DCM presentation of T1D and T2D is unknown, one possible explanation involves insulin resistance that shows reduced protective effects to ischemic/reperfusion [21, 22]. Other factors with a greater association with T2D such as hypertriglyceridemia and reactive oxygen species (ROS) due to augmented fatty acid metabolism have also been implicated in functional cardiomyocyte alterations [19, 20, 23]. Additional studies are warranted to elucidate and categorize type 1 verses type 2 diabetic cardiomyopathy.
Animal models of type 1 and type 2 diabetic cardiomyopathy
Increasing efforts in recent years have been placed in determining the mechanisms of DCM at the cardiomyocyte level. Aiding in this process, several rodent models have been developed to mirror the pathogenesis of T1D and T2D and the effects on the myocardium. Models referred to in this review will be briefly summarized. T1D is induced in a murine model by injection of streptozotocin (STZ) into the animal. Streptozotocin is a glucosamine-nitrosurea antibiotic that selectively targets and destroys pancreatic β-cells, resulting in hypoinsulineaemia and hyperglycemia. There are two methods of inducing T1D with STZ: injected singly at high concentrations, STZ preferentially accumulates in and kills pancreatic β-cells or given in multiple low doses an inflammatory response occurs in the β-cells, leading to lymphocytic infiltrates and cell death [24]. Either mechanism has been shown to effectively model the autoimmune T cell-mediated destruction and hypoinsulinemia observed in human T1D [15, 25]. The T2D model widely utilized is the Zucker fatty rat (ZFR), which displays an obesity-related (fa) trait resulting from leptin receptor genetic mutation [26]. The ZFR male homozygous (fa/fa) rat develops obesity and insulin independent diabetes (T2D). Insulin resistance results from compensatory pancreatic β-cell hyperplasia contributing to hyperinsulinaemia that may induce resistance; this phase is followed by eventual β-cell loss [27]. In similar locations to the ZFR fa gene are the well characterized obese (ob) and diabetes (db) genes. The product of ob is the protein hormone leptin and db encodes the leptin receptor in mice (homologous to the fa genes in ZFR) [28, 29]. Leptin expressed in adipocytes is thought to serve as a feedback to the brain for fat storage levels and has a functional relation to proinflammatory cytokines [30]. Mutations of leptin (ob/ob) or disruption of leptin receptors/signaling pathways (db/db mice, fa/fa rats) results in obesity syndromes and corresponding T2D models in animals.
Regulation of glucose metabolism by insulin and fatty acids
Glucose utilization by the heart is stimulated by insulin and inhibited by fatty acids, effects attributed to the modulation of limiting steps in glucose metabolism. The distinct metabolic phenotype of the diabetic heart is characterized by elevations in fatty acid uptake and oxidation combined with a decrease in glucose uptake and oxidation, a pattern largely mediated by competition between glucose and fatty acid metabolism as defined by the Randle hypothesis and transcriptional regulation of limiting enzymes. Because the diversion of metabolites from glycolysis to diabetes-dependent pathological pathways contributes to the development of DCM, these limiting steps become potential sites of pathology [31].
One of the limiting steps in myocardial glucose metabolism is glucose uptake via the glucose transporters GLUT1 and GLUT4. It has been proposed that the basal rate of glucose transport is GLUT1 dependent while the facilitation of glucose transport by insulin involves both the translocation of GLUT4 to the cell membrane and the upregulation of the transporter, an effect that is impaired in diabetes [7]. Both hyperglycemia and hyperlipidemia attenuate insulin-mediated stimulation of glucose transport. However, elevations in plasma glucose enhance glucose uptake through mass action, partially overcoming impaired glucose uptake caused by severe insulin resistance and hyperlipidemia. Thus, despite the dramatic reduction in glucose uptake, the size of the intracellular glucose pool is elevated in the type 1 diabetic heart [32]. Through a similar mechanism, the intracellular glucose pool of the nondiabetic heart is increased by addition of palmitate to the medium [32]. Therefore, impaired GLUT4 activity in the diabetic heart does not limit glycolytic flux, as adequate amounts of glucose are available for the hexokinase reaction. These data imply that key bottlenecks in glycolysis develop downstream from the glucose transporters in the diabetic heart.
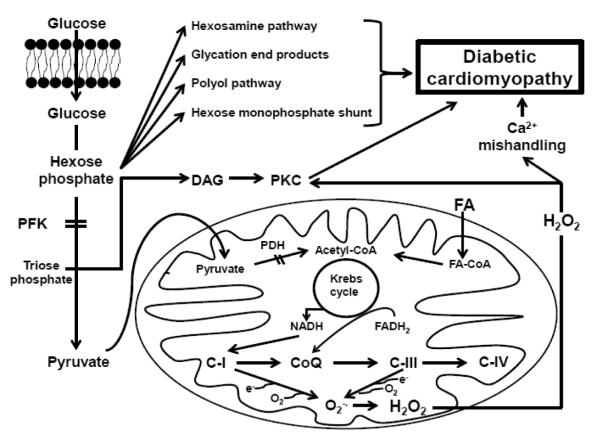
One of the most important limiting glycolytic enzymes in the diabetic heart is phosphofructokinase (PFK), which catalyzes the conversion of fructose-6-phosphate to fructose 1,6 bisphosphate. This enzyme is a key target of fatty acid-mediated regulation of glycolysis, as enhanced rates of fatty acid β-oxidation lead to elevations in cardiac levels of acetyl CoA and citrate, the latter a potent inhibitor of PFK [33]. The allosteric enzyme is also a major sensor of the high energy phosphate content of the heart, as both a low ATP/ADP ratio and elevations in AMP kinase activity stimulate the activity of the enzyme [33, 34]. Because diabetes is associated with enhanced rates of fatty acid β- oxidation and elevations in citrate levels, it has been proposed that PFK activity is diminished in the diabetic heart [33]. However, PFK is located in the cytosol, while citrate is largely produced in the mitochondria. Moreover, the observation that tricarboxylate carrier activity in heart mitochondria is low has raised further doubts about the assumption that citrate levels regulate PFK in the diabetic heart [35]. An alternate mechanism for the control of PFK is transcriptional modification. According to Finck et al [36], hearts overexpressing PPARα have reduced PFK expression, although the mechanism of regulation has not been clarified. Some of the most compelling data supporting a role for PFK in diabetes-mediated glycolytic impairment come from crossover analysis of metabolic intermediates of type 1 and lean type 2 diabetic hearts, suggesting elevations in glucose-6-phosphate levels and reductions in fructose 1,6 bisphosphate content [32, 37]. This analysis assumes that substrate levels increase and product levels decrease upon inhibition of the enzyme. Therefore, as a result of reduced PFK activity, a bottleneck develops in the glycolytic pathway of the diabetic heart [32, 37]. The resulting increases in myocardial levels of glucose-6-phosphate and fructose-6-phosphate have pathological consequences, as they serve as substrates for four pathological pathways involved in the development of DCM (Figure 1). Glucose-6- phosphate serves as a substrate for glucose-6-phosphate dehydrogenase, the initial enzyme in the hexose monophosphate shunt. In the obese T2D heart, the generation of NADPH by glucose-6-phosphate dehydrogenase provides substrate for NADPH oxidase, a key enzyme involved in the generation of ROS in the cytosol [38]. Glucose-6-phosphate can also contribute to the glycation of proteins, a reaction capable of generating ROS and damaging key proteins in the diabetic heart [39]. A receptor for advanced glycation end products initiates pathways that appear to contribute to the development of DCM [40]. In a similar manner, the diversion of fructose-6-phosphate to diabetes-linked pathological pathways contributes to the development of DCM. Fructose-6-phosphate initiates the hexosamine pathway, whose final reaction is a transferase-mediated modification of serine or threonine residues leading to the formation of an O-linked N-acetylglucosamine product. Stimulation of the hexosamine pathway has been linked to impaired calcium cycling and contractile dysfunction of the diabetic heart [41-43]. Glycolytic intermediates have also been implicated in the promotion of the polyol pathway [44]. Although the polyol pathway is reportedly involved in diabetes-mediated vascular injury, a role for the pathway in the development of DCM remains to be established.
Figure 1.
Metabolic Mechanisms of Diabetic Cardiomyopathy PFK- phosphofructokinase; DAG – diacylglycerol; PKC – protein kinase C; FA – fatty acid; PDH – pyruvate dehydrogenase; NADH – nicotinamide adenine dinucleotide (reduced); FADH – flavin adenine dinucleotide (reduced); CoQ – coenzyme Q; CI, III, IV – respiratory chain complexes
Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) is inhibited by high intracellular concentrations of NADH and ATP [33]. Since the levels of GAPDH in the heart are relatively high, the enzyme is not limiting in most pathological conditions. However, during myocardial ischemia, cellular levels of fructose 1,6 bisphosphate and triose-phosphate accumulate without a rise in metabolic intermediates located downstream of the enzyme, suggesting a rate controlling role for GAPDH in glycolysis [33]. Evidence that diabetes leads to respiratory chain dysfunction implies that diabetes must be associated with a rise in the NADH/NAD+ ratio that could inhibit GADPH. However, in the lean type 2 diabetic heart, crossover analysis fails to uncover a bottleneck in glycolysis at the GAPDH site [37] although an increase in glyceraldehyde-3-phosphate levels has been observed [45]. According to Ojaimi et al. [46], GAPDH is downregulated in the heart of type 1 diabetic dogs. Moreover, according to the unifying hypothesis of diabetes proposed by Brownlee and coworkers [31, 47, 48], GAPDH assumes a central role in the development of diabetic complications. In Brownlee’s hypothesis, the metabolism of glucose by endothelial cells leads to the formation of ROS in the mitochondria. The oxidants then cross the mitochondrial membrane and migrate to the nucleus where they produce oxidative DNA damage. The oxidized DNA products activate poly(ADP-ribose)polymerase, an enzyme that catalyzes ADP-ribosylation of proteins [49]. GAPDH is taken up by the nucleus, where it undergoes ADP ribosylation, inactivating the enzyme. Du et al. [50] maintain that ADP ribosylation of GAPDH diminishes the levels of active enzyme, forming a bottleneck in glycolysis that allows the diversion of trioses into pathological pathways. Although Brownlee’s theory introduced the novel idea that bottlenecks in glycolysis can cause the diversion of glycolytic intermediates into diabetes-linked pathological pathways, questions have been raised regarding the role of GAPDH in the development of one of these bottlenecks [51]. Clearly, further study of the role of GAPDH in the development of DCM is warranted.
Another limiting step of glucose metabolism in the heart is the pyruvate dehydrogenase complex (PDH), which consists of three enzymes. One enzyme catalyzes the irreversible conversion of pyruvate to acetyl CoA and two enzymes regulate the activity of the PDH reaction through phosphorylation/dephosphorylation. Pyruvate dehydrogenase kinase (PDH kinase) catalyzes the phosphorylation and inactivation of the PDH reaction, while pyruvate dehydrogenase phosphatase (PDH phosphatase) catalyzes the dephosphorylation and activation of the complex. The activity of PDH kinase is inhibited by pyruvate and ADP and activated by elevations in the acetyl CoA/CoA and the NADH/NAD+ ratios [33, 52, 53]. PDH phosphatase activity is enhanced by increases in Ca2+ and Mg2+ [33, 54]. Central to the ability of pyruvate to enter the mitochondria and undergo the irreversible conversion to acetyl CoA is the rate of fatty acid oxidation, which elevates acetyl CoA and inhibits PDH, and the generation of pyruvate by glycolysis, which increases the substrate and activator of PDH. Indeed, the acetyl CoA/CoA ratio and the PDH reaction are highly dependent on the fatty acid, glucose and insulin content of the perfusion medium [55]. The highest acetyl CoA/CoA ratios are observed in hearts perfused with medium containing 1.2 mM palmitate, 20 mM glucose and insulin, while the lowest ratios are detected in hearts perfused with 0.4 mM palmitate and 5 mM glucose in the absence of insulin. Diabetes elevates the acetyl CoA/CoA ratio in hearts perfused with buffer containing high palmitate and glucose content as well as the low substrate, insulin free condition. In accordance with expected PDH activity, palmitate addition and diabetes severely reduce glucose oxidation while enhancing palmitate oxidation, effects that are not appreciably altered by addition of insulin to the buffer. Severe reductions in glucose oxidation are observed in diabetic hearts perfused with buffer containing low insulin levels and the combination of either high concentrations of palmitate and glucose or low concentrations of the two substrates, indicating that the metabolic effects of diabetes cannot be reversed by acute changes in substrate content and insulin. However, PDH activity can be restored in the hearts of diabetic animals subjected to either chronic refeeding or insulin treatment [33, 52]. Thus, suppression of glucose oxidation in the diabetic heart depends not only upon the acute metabolic interactions that develop between fatty acid and glucose metabolism, but also the metabolic interactions mediated by chronic exposure of the diabetic heart to high circulating levels of fatty acids.
According to Finck et al [36], peroxisome proliferator activated receptor alpha (PPARα) levels are elevated in both T1D and obese T2D hearts, at least in part caused by the increase in fatty acid levels. One of the targets of PPARα is PDH kinase 4, whose expression is upregulated by PPARα [36]. In mice overexpressing PPARα, the upregulation of PDH kinase 4 is associated with reductions in glucose oxidation [55], while the downregulation of PPARα leads to elevated rates of glucose oxidation [56]. Chatham and Forder [57] found that dichloroacetate-mediated stimulation of PDH in the T1D heart significantly increases flux of pyruvate through the citric acid cycle and the overall rate of glucose oxidation, effects that appear responsible for the observed improvement in contractile function. The authors also found that T1D hearts perfused with buffer containing glucose as the sole substrate exhibit significant contractile dysfunction, an effect reversed by the addition of hexanoate to the perfusion buffer. The beneficial effect of hexanoate was attributed to acceleration in the rates of acetyl CoA generation, citric acid cycle flux and reducing equivalent generation.
Inhibition of PDH restricts the entry of pyruvate into the mitochondria while enhancing the accumulation of trioses upstream from pyruvate kinase. The diversion of these trioses into diacylglycerol biosynthesis contributes to the activation of several diacylglycerol-sensitive protein kinase C (PKC) isoforms. Connelly et al [58] have found that inhibition of PKC-β preserves cardiac function in Ren-2 diabetic rats. However, other PKC isoforms may also contribute to the complications of diabetes. Among other adverse actions, PKC activates NADPH oxidase, stimulating the generation of ROS [59].
Regulation of fatty acid metabolism by glucose and insulin
The healthy myocardium preferentially utilizes fatty acids as a source of energy, providing up to 70% of reducing equivalents for ATP synthesis. However, T2D causes a further stimulation in fatty acid uptake and oxidation through an elevation in circulating levels of fatty acid and an upregulation of both fatty acid translocase (FAT/CD36) and fatty acid binding protein (FATP1), proteins involved in fatty acid uptake by the heart [60-62]. Ironically, insulin also promotes both the upregulation of FAT/CD36 and its translocation to the sarcolemma [63, 64]. However, chronic elevations in circulating fatty acids enhance the expression of several enzymes of fatty acid metabolism. These effects are mediated in part by the activation of the PPAR family of nuclear receptors [65], with particular relevance being PPARα, whose activation results in the activation of both FAT/CD36 and FATP1 [62]. In obese T2D rats, the uptake of fatty acids into the heart is associated with enhanced rates of FAT/CD36 translocation to the sarcolemma [60, 62].
The myocardium contains triglyceride stores, which can be rapidly mobilized. Although the size of these stores is normally small, they increase in the diabetic heart. The mobilization of these stores limits the degree of glucose oxidation in glucose-perfused hearts by providing a source of fatty acids for oxidation. In the obese T2D rat, the accumulation of ceramides contribute to the development of glucose intolerance and is a mediator of apoptosis [66]. Fatty acyl CoA and carnitine accumulation have also been implicated in the development of DCM [67].
One of the most important regulatory steps of fatty acid metabolism is the transport of fatty acids into the mitochondria. Short and medium chain fatty acids are activated in the matrix by acyl CoA synthetase, therefore, their metabolism is independent of carnitine. However, the long chain fatty acids require carnitine to enter the mitochondria, a process catalyzed by carnitine palmitoyltransferase I (CPT-I) and CPT-II. CPT-I, which catalyzes the formation of fatty acyl carnitine from fatty acyl CoA, is allosterically inhibited by malonyl CoA [62]. In type 1 diabetic hearts perfused with medium containing 1.2 mM palmitate, 20 mM glucose and insulin, malonyl CoA levels decline, which mediates an increase in palmitate oxidation [68]. Malonyl CoA synthesis is catalyzed by acetyl CoA carboxylase while the degradation of malonyl CoA to acetyl CoA involves malonyl CoA decarboxylase. Acetyl CoA carboxylase activity is regulated by phosphorylation-dephosphorylation, with AMP kinase activation capable of completely inhibiting the enzyme through phosphorylation [69]. AMP kinase, which is an energy sensor, is stimulated by elevations in the AMP/ATP and creatine/creatine phosphate ratios while being inhibited by insulin, provided that the two ratios remain elevated [70]. The increase in fatty acid oxidation in the T1D and obese T2D heart has been attributed in part to an upregulation in malonyl CoA decarboxylase, an enzyme controlled by high fat feeding, diabetes and elevated PPARα levels [68, 71]. As expected, malonyl CoA decarboxylase inhibitors decrease fatty acid oxidation and enhance glucose oxidation through malonyl CoA-mediated regulation of CPT-1.
The diabetic phenotype does not develop in PPARα null mice [72]. On the other hand, cardiac hypertrophy and fractional shortening are worse in diabetic mice overexpressing PPARα, an effect associated with myocardial accumulation of triglycerides containing long chain fatty acids. Also defective in the diabetic-PPARα overexpressing heart is the upregulation of ACO, an enzyme involved in the generation of ROS in the peroxisomal fatty acid oxidation pathway. It is proposed that ACO may contribute to the observed elevation in ROS generation and decline in the glutathione redox ratio (GSH/GSSG). In a related study, Chen et al [73] showed that treatment of obese T2D mice and PPARα overexpressing mice with Astragalus, an active ingredient of traditional Chinese medicine, improves LV chamber dilatation, fractional shortening dysfunction and the expression of sarcoplasmic reticular Ca2+ ATPase 2a (SERCA2a). The contractile effects were correlated with increases in glucose oxidation and decreases in fatty acid metabolism. Recently, decreased PPARδ expression has been implicated in the development of DCM [74]. PPARδ is the predominant member of the PPAR family in the heart, where it regulates glucose and fatty acid metabolism [75]. However, mice overexpressing PPARβ/δ do not develop a cardiomyopathy or exhibit lipid accumulation [76]. Therefore, while the expression of PPARδ is diminished in the diabetic heart, the effects of PPARα appear to be the dominant regulator of glucose and fatty acid metabolism.
Mitochondrial dysfunction in the diabetic heart
The generation of acetyl CoA by fatty acid oxidation and pyruvate dehydrogenase is a key determinant of citric acid cycle flux, with fatty acid oxidation accounting for the increase in acetyl CoA generation by the diabetic heart [77]. Bowman [77] also observed elevations in the levels of citrate, isocitrate, malate and α-ketoglutarate in the T1D heart, changes which were consistent with the stimulation in acetyl CoA synthesis by fatty acid oxidation. Based on similar findings, Taegtmeyer and Passmore [78] suggested that diabetes mediates the unspanning of citric acid cycle flux, as excessive acetyl CoA generation leads to a deficiency in free CoA, which diminishes flux through α-ketoglutarate dehydrogenase but enhances flux through the transamination of α-ketoglutarate. However, slowing of respiratory chain flux likely contributes to the inhibition of α-ketoglutarate dehydrogenase, as respiratory chain inhibition elevates the NADH/NAD+ ratio, which diminishes citric acid cycle flux [79-82]. Among the respiratory chain complexes depressed in T1D and obese T2D hearts are I, III and V [79-82]. The activity of these complexes is regulated by the levels of mitochondrial encoded proteins, which join with nuclear encoded proteins to form the respiratory chain complexes. The biosynthesis of mitochondria encoded proteins occurs in the mitochondria and depends upon the levels of mitochondrial DNA, which are determined by the transcriptional activity of mitochondrial transcription factor A (tfam). Suarez et al [83] discovered that overexpression of tfam dramatically affects the function of neonatal rat cardiomyocytes incubated in medium containing 30 mM glucose. While control cells exposed to high glucose exhibit a delay in the relaxation phase of the Ca2+ transient, a reduction in cellular ATP content and diminished SERCA2a content, cells containing elevated levels of tfam show an improvement in Ca2+ delay and elevations in both cellular ATP and SERCA2a content. Hyperglycemia also increases the levels of O-GlcNAcylated tfam, which diminish the activity of tfam and reduce the activity of oxidative phosphorylation (complex V activity). Interestingly, chronic exposure of nondiabetic rats to the PPARα agonist Wy-14,643 leads to reductions in state 3 respiration, but elevations in state 4 respiration of the heart, indicating that the rate of ADP phosphorylation is significantly depressed in Wy-14,643 treated hearts [84]. The depression in respiration in Wy-14,643 treated rats was attributed to reductions in cyclooxygenase II (COXII), a mitochondria encoded protein subunit of cytochrome oxidase. Another consequence of impaired respiratory chain flux is the generation of ROS as electrons are diverted from the respiratory chain to the acceptor oxygen. Boudina et al [85] reported that heart mitochondria from obese T2D mice produce elevated rates of ROS. Besides causing mitochondrial damage, matrix ROS generation can affect cytosolic ROS formation. As shown in Figure 1, ROS from the mitochondria can leave the mitochondria as H2O2 and activate PKC in the cytosol. ROS-mediated PKC activation can significantly increase cytosolic ROS content by activating NADPH oxidase. Thus, diabetes-mediated mitochondrial dysfunction contributes to the development of DCM by altering ATP generation and Ca2+ movement.
Role of impaired energy metabolism in the development of DCM
Brownlee [31] introduced the novel hypothesis that the complications of diabetes arise from the development of a bottleneck in glycolysis that diverts glycolytic intermediates into diabetes-linked pathological pathways (Figure 1). The theory was based on the observation that glucose-treated bovine aortic endothelial cells generate superoxide that plays a central role in the development of diabetic complications [48]. Inhibition of mitochondrial superoxide generation prevents the activation of several pathological pathways of hyperglycemic injury (protein kinase C stimulation, hexosamine pathway, polyol pathway and glycation end product formation). In a follow-up study, Du et al. [50] localized the glycolytic bottleneck to glyceraldehyde-3-phosphate dehydrogenase (GAPDH), with more recent studies attributing the decline in GAPDH activity to ADP-ribosylation, a reaction mediated by poly (ADP-ribose)polymerase [49]. It was proposed that oxidants generated in the mitochondria enter the nucleus, causing oxidative DNA damage that stimulates poly (ADP-ribose) polymerase activity. Schaffer et al. [51] have questioned the role of ADP-ribosylation in the regulation of glycolysis in diabetes, as both GAPDH uptake by the nucleus and its ADP-ribosylation are commonly associated with cell death. Since diabetes leads to respiratory chain dysfunction, a more likely cause of GAPDH inhibition is the rise in the NADH/NAD+ ratio.
Treatment Strategies for DCM
Glycemic Control
Hyperglycemia has been shown to be instrumental in the development of DCM in T2D patients. Consequently, with improved glycemic control, diastolic dysfunction is also improved [86, 87]. Thiazolidinediones (TZDs) activate PPAR-γ allowing for increased peripheral insulin sensitivity and secondarily reduce hepatic glucose production, reducing hyperglycemia. Although effective glycemic controllers that decrease the effect of DCM, these drugs are contraindicated in advanced cases of heart disease (New York Heart Association classes III and IV). Thiazolidinediones also improve adipocyte function and can alter adipocyte storage and triglyceride levels [88, 89]. Metformin, a biguanide class antidiabetic drug, operates via decreasing hepatic glucose production and has demonstrated improvements in survival of T2D patients with heart failure and can also decrease the prevalence of heart failure development in T2D patients. Studies do not offer direct information on the effects of metformin on DCM, but the reduction in CVD rates justifies its usage to treat DCM [90, 91]. New agents utilizing incretin for glycemic control appear to have beneficial effects and reduce heart failure in DCM. Incretin mimetics include injectable glucagon like peptide-1 (GLP-1) analogs that activate the GLP-1 receptors, thereby increasing glucose dependent insulin secretion and decreasing glucagon secretion. Likewise, oral dipeptidyl peptidase inhibitors prolong the half-life of endogenous GLP-1, increasing its effect. Overall, these agents have a positive effect on myocardial function and left ventricular ejection fraction in patients with heart failure in both clinical studies and animal models [90, 92, 93]. Alpha-glucosidase inhibitors, specifically acarbose, prevent the absorption of glucose in the gut: increasing the effect of insulin, decreasing stress on β-cells and reducing CVD risk due to postprandial hyperglycemia [94, 95]. Additionally, for type I DM, it has been shown that DCM does not develop if the T1D is well controlled with insulin therapy. These data point to hyperglycemia not only as an exacerbating, but also a treatable factor of DCM [96].
β-Adrenergic Blockade
Significant evidence has accumulated showing the benefits of β-blocker therapy in treating heart failure and left ventricular systolic dysfunction. Meta-analysis of multiple trials with focus on DM patients confirms the benefits of β-blockade in heart failure patients with diabetes. Also shown is that β-blockers with an additional treatment such as angiotensin converting enzyme inhibitors (ACEIs) show increased relative risk reduction in mortality rates of diabetic congestive heart failure (CHF) patients [97, 98].
Cholesterol Reduction
3-Hydroxy-3-methylglutaryl coenzyme A reductase inhibitors (statins, HMG-CoA reductase inhibitors) reduce the synthesis of cholesterol and have a multitude of effects known to reduce the incidence of DCM. Statins have also been shown to reduce mortality in patients with ischemic and non-ischemic heart failure, however some disagreement exists with the results [99]. In the realm of DCM, not enough studies have been performed to allow definitive indications for the effective use of statins.
Exercise Training
Exercise training has been shown to prevent and improve DCM. Reviews of system studies show that exercise training reverses DM induced free fatty acid metabolism, increases sensitivity to insulin, allows for increased glucose metabolism, increases cardiac output and can even reverse contractile dysfunction observed in DCM [18]. A study in T2D mice found that interval training reverses myocyte Ca2+ handling dysfunction. Mice that underwent the training protocol exhibited a return to normal sarcoplasmic reticulum Ca2+ load and a decrease in spontaneous Ca2+ waves. The training did not lead to normal blood glucose or insulin levels, suggesting that it may have had a more direct effect on the heart [100]. In recent studies of vigorous long lasting exercise in a T1D model (STZ-induced), results demonstrated altered dynamics of cardiomyocyte contraction, however they did not show a change in amplitude of contraction or calcium transients, implicating a possible effect of exercise on other states of Ca2+ homeostasis [101]. These results illustrate positive overall effects of exercise training in combating DCM.
Fibrates
Fibrates are a class of drugs acting as PPARα agonists with the demonstrated ability to lower triglycerides/low density lipoproteins (LDL) levels, raise high density lipoproteins (HDL) levels and increase insulin sensitivity in humans and animals [102, 103]. These actions make fibrates an attractive treatment option for the hyperlipidemia and hyperinsulinemia that accompany T2D. Additionally, clinical trials have shown that fibrate therapy may reduce CVD risk factors associated with dyslipidemia and decrease occurrence of coronary events in the diabetic populations [104]. More specifically for diabetic cardiomyopathy, fibrate treatment (bezafibrate) has been shown to prevent not only metabolic dysfunction in sucrose fed animals, but also prevent cardiomyocyte dysfunction seen in these T2D models [105]. The clinical effects of fibrates and other classes of PPARα agonists should continue to be pursued to determine possible protective effects against type 2 DCM.
Antioxidants
Antioxidants may also be a promising preventative treatment strategy for DCM. A study in pre-diabetic insulin resistant mice showed a significant increase in ROS production, along with abnormal Ca2+ signaling and decreased Na+/Ca2+ exchanger protein expression. Transgenic mice with an overexpression of catalase, an enzyme that catalyzes the breakdown of hydrogen peroxide, restored normal Ca2+ signaling and contractile properties despite having no effect on insulin sensitivity and post-insulin receptor signaling [106]. As mentioned above, metallothionien overexpression relieved almost all of the cellular DCM dysfunction except for decreased SERCA2a function, however contractile parameters were restored [107]. This suggests that antioxidants may be an important treatment for DCM.
RAAS Modification
Hyperglycemia has been shown to trigger the renin angiotensin aldosterone system (RAAS), which is known to directly affect the heart. Angiotensin II depresses Ca2+ sparks and inhibits SERCA2a in heart failure. Candesartan, an angiotensin II type 1 receptor blocker (ARB), has been shown to restore normal sparks in atrial tissue that are impaired by Angiotensin II. Based on this, candesartan was administed to myocytes isolated from STZ-induced diabetic rats, which reduced PKC levels and restored Ca2+ transients and sparks and SR Ca2+ loading [108]. These results indicate that further research on candesartan and angiotensin II receptor blockers at the in vivo level may show improvements in Ca2+ signaling parameters in DCM. Angiotensin convering enzyme inhibitors (ACEIs) block the conversion of angiotensin I to angiotensin II, resulting in increased levels of bradykinin. This increased vasoactivity can facilitate blood flow to muscle and adipose tissue including cardiac tissue, leading to reduced effects on cardiovascular fibrosis. ACEIs have been shown to decrease CVD rates and all-cause mortality in patients with diabetes, in particular diabetic hypertensive patients. Also, treatment with ACEIs has been shown to increase insulin sensitivity of cells [109-111]. Angiotensin II receptor blockers (ARBs) and ACEI can prevent coronary perivascular fibrosis and collagen deposition [112]. Along with the aforementioned medications, aldosterone antagonists have been shown to cause positive remodeling in patients with LVDD, an effect that may be applicable to T2D [113]. These drugs modifying the RAAS system have significant positive effects for the treatment of DCM.
Calcium Upregulation
Calcium channel blockers (CCBs) can increase intracellular calcium to prevent DM-induced cardiomyocyte changes. Diltiazem showed possible suppression of muscle hypertrophy, degeneration and fibrosis caused by hyperglycemia in animal T1D models [114]. Early studies with verapamil demonstrated marked improvement of myosin/myofibrillary ATPase and SERCA activity in ventricular tissue of STZ induced T1D animals. Additionally, decreases in diastolic ventricular pressure and increases in rate of contraction and relaxation were all shown in STZ-treated mice [115, 116]. These data suggest the use of verapamil in treating type 1 DCM. STZ treated animals similarly saw a beneficial effect in insulin sensitivity and cardiac function, as well as reductions in hypertriglyceridemia from treatments with nifedipine [117, 118]. Clearly, there is a potential for use for CCBs in DCM, however additional studies are required to determine the mechanisms of action of CCBs in DCM.
Future Treatment Strategies
Recent years have yielded new therapies for DCM focusing on advanced glycation endproducts (AGEs) including AGE inhibitors and AGE cross-link breakers, which may provide future directions for DCM intervention, treatment and prevention [119].
The calcium signaling pathway lends itself to several potential DCM treatment targets. The most obvious therapeutic strategy for impaired calcium signaling is improving SERCA2a function. Overexpression of SERCA2a in transgenic rats prevented drug-induced DM-induced contractile dysfunction by inhibiting changes in contractile function and calcium handling. A follow up study showed that doxycycline-induced SERCA2a overexpression following the induction of DCM improved contractile and calcium signaling parameters [120]. This both confirms the importance of SERCA2a downregulation in DCM and suggests a potential therapeutic target for patients. Overall, PKC inhibition in preventing DCM via the calcium signaling pathway seems to be worth pursuing as PKC appears to prevent changes in expression of calcium handling proteins [121].
There are numerous options for the treatment of DCM (Table 1), but the best treatment option will depend on, among other things, the type of DM, the individual’s response to prescribed therapies and complicating comorbidities.
Table 1.
Therapeutic Strategies for Diabetic Cardiomyopathy
| Diabetic Cardiomyopathy Treatment | Therapeutic | strategy DCM Type |
|---|---|---|
| Glycemic Control: | Insulin therapy | T1D |
| Thiazolidinediones | T2D | |
| Metformin | T2D | |
| Glucagon-like peptide-1 Dipeptidyl peptidase inhibitors |
T2D | |
| Alpha-glucosidase inhibitors | T2D | |
| Adrenergic Blockade: | β-blockers | T1D & T2D |
| Cholesterol Reduction: | Statins | Efficacy Questionable for DCM |
| Exercise Training: | – | T1D & T2D |
| Antioxidants: | – | T1D & T2D |
| RAAS: | ACEIs | T1D & T2D |
| ARBs | T1D & T2D | |
| Aldosterone Antagonist | T1D & T2D | |
| Calcium Upregulation: | Calcium Channel Blockers | Efficacy Questionable for T1D |
ACEI – Angiotensin converting enzyme inhibitor, ARB- Angiotensin receptor blockers, T1D – type 1 diabetes mellitus, T2D – type 2 diabetes mellitus.
Conclusions
The epidemic rise in DM worldwide has made DCM an increasing health concern. In the present review, we introduce a modified version of the Brownlee hypothesis. We suggest that inhibition of phosphofructokinase (PFK) leads to the formation of a bottleneck that diverts hexose intermediates into the hexosamine pathway, glycosylated end products, the polyol pathway and the hexose monophosphate shunt (Figure 1). The role of these pathways in the generation of ROS, protein damage and calcium mishandling are widely recognized. PFK is inhibited by citrate, which increases as a result of enhanced fatty acid metabolism. Also regulating PFK is fatty acid-mediated elevation in PPARα. The other major bottleneck in the glucose oxidation pathway is pyruvate dehydrogenase (PDH), whose inhibition by phosphorylation is mediated by the fatty acid-mediated accumulation of acetyl CoA and PPARα. Inhibition of PDH leads to the accumulation of trioses that are diverted into diacylglycerol, an activator of protein kinase C. Several PKC isoforms (α, β, δ ,ε and ξ) are activated in the diabetic heart and glucose-treated cardiomyocytes [122- 126]. Overexpression of PKC-β2, whose levels are upregulated in diabetes, leads to the development of a cardiomyopathy [127]. Moreover, PKCδ plays a role in apoptosis, which is enhanced in diabetes [125]. The activation of PKCε is associated with the phosphorylation of troponin I and troponin T, which inhibits myofibrillar ATPase [124]. PKC has also been implicated in the generation of ROS by NADPH oxidase. Thus, the regulation of PDH represents an important bottleneck site for the modulation of contractile function. Additionally, we offer a variety of treatment options shown to be effective in treating DCM (Table 1) including glycemic control, adrenergic blockade, calcium up-regulation, RAAS modification, antioxidant treatment and exercise training.
ABBREVIATIONS
- ACEI
angiotensin converting enzyme inhibitor
- ADP
adenosine diphosphate
- AGES
advanced glycation end products
- AMP
adenosine monophosphate
- ARB
angiotensin receptor blockers
- ATP
adenosine triphosphate
- BMI
body mass index
- CASQ2
calsequestrin
- CCB
calcium channel blockers
- CHF
congestive heart failure
- COX
cytochrome oxidase
- CPT-1
carnitine palmitoyltransferase-1
- CVD
cardiovascular disease
- DCM
diabetic cardiomyopathy
- DM
diabetes mellitus
- FAT
fatty acid translocase
- FKBP
FK 506 Binding Protein
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- GLP-1
glucagon-like peptide-1
- GLUT
glucose transporter
- GSH/GSSG
glutathione redox ratio
- HMG-COA
3-hydroxy-3-methylglutaryl coenzyme A
- LV
left ventricle
- LVDD
left ventricular diastolic dysfunction
- LVH
left ventricular hypertrophy
- NADPH
nicotinamide adenine dinucleotide phosphate
- NCX
Na+/Ca2+ exchanger
- O-GlcNAC
O-linked N-acetylglucosamine
- PDH
pyruvate dehydrogenase complex
- PFK
phosphofructokinase
- PKC
protein kinase C
- PPAR
peroxisome proliferation activated receptor
- RAAS
renin angiotensin aldosterone system
- ROS
reactive oxygen species
- SERCA2A
sarco(endo)plasmic reticulum calcium ATPase
- SR
sarcoplasmic reticulum
- STZ
streptozotocin
- TFAM
mitochondrial transcription factor A
- TZD
Thiazolidinediones
- T1D
type 1 diabetes mellitus
- T2D
type 2 diabetes mellitus
- ZFR
Zucker fatty rats
Footnotes
Disclosure of Interests Mr. Isfort and Drs. Stevens, Schaffer, Jong and Wold declare that they have no conflicts of interest or financial ties to disclose.
Disclosure of Interests Mr. Isfort and Drs. Stevens, Schaffer, Jong and Wold declare that they have no conflicts of interest or financial ties to disclose.
References
- 1.Roger VL, Go AS, Lloyd-Jones DM, et al. Heart disease and stroke statistics--2012 update: a report from the American Heart Association. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anand SS, Yusuf S. Stemming the global tsunami of cardiovascular disease. Lancet. 2011;377:529–32. doi: 10.1016/S0140-6736(10)62346-X. [DOI] [PubMed] [Google Scholar]
- 3.Yach D, Stuckler D, Brownell KD. Epidemiologic and economic consequences of the global epidemics of obesity and diabetes. Nat Med. 2006;12:62–6. doi: 10.1038/nm0106-62. [DOI] [PubMed] [Google Scholar]
- 4.Shao CH, Rozanski GJ, Patel KP, et al. Dyssynchronous (non-uniform) Ca2+ release in myocytes from streptozotocin-induced diabetic rats. J Mol Cell Cardiol. 2007;42:234–46. doi: 10.1016/j.yjmcc.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 5.Pereira L, Matthes J, Schuster I, et al. Mechanisms of [Ca2+]i transient decrease in cardiomyopathy of db/db type 2 diabetic mice. Diabetes. 2006;55:608–15. doi: 10.2337/diabetes.55.03.06.db05-1284. [DOI] [PubMed] [Google Scholar]
- 6.Schafer SA, Machicao F, Fritsche A, et al. New type 2 diabetes risk genes provide new insights in insulin secretion mechanisms. Diabetes Res Clin Pract. 2011;93(Suppl 1):S9–24. doi: 10.1016/S0168-8227(11)70008-0. [DOI] [PubMed] [Google Scholar]
- 7.An D, Rodrigues B. Role of changes in cardiac metabolism in development of diabetic cardiomyopathy. Am J Physiol Heart Circ Physiol. 2006;291:H1489–506. doi: 10.1152/ajpheart.00278.2006. [DOI] [PubMed] [Google Scholar]
- 8.Boudina S, Abel ED. Diabetic cardiomyopathy revisited. Circulation. 2007;115:3213–23. doi: 10.1161/CIRCULATIONAHA.106.679597. [DOI] [PubMed] [Google Scholar]
- 9.Barlovic DP, Soro-Paavonen A, Jandeleit-Dahm KA. RAGE biology, atherosclerosis and diabetes. Clin Sci (Lond) 2011;121:43–55. doi: 10.1042/CS20100501. [DOI] [PubMed] [Google Scholar]
- 10.Duncan JG. Mitochondrial dysfunction in diabetic cardiomyopathy. Biochim Biophys Acta. 2011;1813:1351–9. doi: 10.1016/j.bbamcr.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Department of Health and Human Services CfDCaP, Atlanta, GA National Diabetes Fact Sheet: National Estimates and General Information on Diabetes and Prediabetes in the United States. 2011.
- 12.Garcia MJ, McNamara PM, Gordon T, et al. Morbidity and mortality in diabetics in the Framingham population. Sixteen year follow-up study. Diabetes. 1974;23:105–11. doi: 10.2337/diab.23.2.105. [DOI] [PubMed] [Google Scholar]
- 13.Ren J, Ceylan-Isik AF. Diabetic Cardiomyopathy: Do Women Differ from Men? Endocrine. 2004;25:73–83. doi: 10.1385/ENDO:25:2:073. [DOI] [PubMed] [Google Scholar]
- 14.Schilling JD, Mann DL. Diabetic cardiomyopathy: bench to bedside. Heart Fail Clin. 2012;8:619–31. doi: 10.1016/j.hfc.2012.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lacombe VA, Viatchenko-Karpinski S, Terentyev D, et al. Mechanisms of impaired calcium handling underlying subclinical diastolic dysfunction in diabetes. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1787–97. doi: 10.1152/ajpregu.00059.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Howarth FC, Qureshi MA, Hassan Z, et al. Changing pattern of gene expression is associated with ventricular myocyte dysfunction and altered mechanisms of Ca2+ signalling in young type 2 Zucker diabetic fatty rat heart. Exp Physiol. 2011;96:325–37. doi: 10.1113/expphysiol.2010.055574. [DOI] [PubMed] [Google Scholar]
- 17.Van Buren P, LeWinter MM. Chapter 26: Heart Failure as a Consequence of Diabetic Cardiomyopathy. Heart Failure: A Companion to Braunwald’s Heart Disease (Second Edition) 2011:408–18. [Google Scholar]
- 18.Fang ZY, Prins JB, Marwick TH. Diabetic cardiomyopathy: evidence, mechanisms, and therapeutic implications. Endocr Rev. 2004;25:543–67. doi: 10.1210/er.2003-0012. [DOI] [PubMed] [Google Scholar]
- 19.McGavock JM, Lingvay I, Zib I, et al. Cardiac steatosis in diabetes mellitus: a 1H-magnetic resonance spectroscopy study. Circulation. 2007;116:1170–5. doi: 10.1161/CIRCULATIONAHA.106.645614. [DOI] [PubMed] [Google Scholar]
- 20.Ng AC, Delgado V, Bertini M, et al. Myocardial steatosis and biventricular strain and strain rate imaging in patients with type 2 diabetes mellitus. Circulation. 2010;122:2538–44. doi: 10.1161/CIRCULATIONAHA.110.955542. [DOI] [PubMed] [Google Scholar]
- 21.Greer JJ, Ware DP, Lefer DJ. Myocardial infarction and heart failure in the db/db diabetic mouse. Am J Physiol Heart Circ Physiol. 2006;290:H146–53. doi: 10.1152/ajpheart.00583.2005. [DOI] [PubMed] [Google Scholar]
- 22.Hoshida S, Yamashita N, Otsu K, et al. Cholesterol feeding exacerbates myocardial injury in Zucker diabetic fatty rats. Am J Physiol Heart Circ Physiol. 2000;278:H256–62. doi: 10.1152/ajpheart.2000.278.1.H256. [DOI] [PubMed] [Google Scholar]
- 23.Fauconnier J, Andersson DC, Zhang SJ, et al. Effects of palmitate on Ca(2+) handling in adult control and ob/ob cardiomyocytes: impact of mitochondrial reactive oxygen species. Diabetes. 2007;56:1136–42. doi: 10.2337/db06-0739. [DOI] [PubMed] [Google Scholar]
- 24.Graham ML, Janecek JL, Kittredge JA, et al. The streptozotocin-induced diabetic nude mouse model: differences between animals from different sources. Comp Med. 2011;61:356–60. [PMC free article] [PubMed] [Google Scholar]
- 25.Rodrigues B, Poucheret P, Battell M, et al. STZ-induced diabetes: induction, mechanism(s) and dose-dependency. Experimental Models of Diabetes. 1999 [Google Scholar]
- 26.Corsetti JP, Sparks JD, Peterson RG, et al. Effect of dietary fat on the development of non-insulin dependent diabetes mellitus in obese Zucker diabetic fatty male and female rats. Atherosclerosis. 2000;148:231–41. doi: 10.1016/s0021-9150(99)00265-8. [DOI] [PubMed] [Google Scholar]
- 27.Tokuyama Y, Sturis J, DePaoli AM, et al. Evolution of beta-cell dysfunction in the male Zucker diabetic fatty rat. Diabetes. 1995;44:1447–57. doi: 10.2337/diab.44.12.1447. [DOI] [PubMed] [Google Scholar]
- 28.Iida M, Murakami T, Ishida K, et al. Phenotype-linked amino acid alteration in leptin receptor cDNA from Zucker fatty (fa/fa) rat. Biochem Biophys Res Commun. 1996;222:19–26. doi: 10.1006/bbrc.1996.0691. [DOI] [PubMed] [Google Scholar]
- 29.Martin SS, Qasim A, Reilly MP. Leptin resistance: a possible interface of inflammation and metabolism in obesity-related cardiovascular disease. J Am Coll Cardiol. 2008;52:1201–10. doi: 10.1016/j.jacc.2008.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tilg H, Moschen AR. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol. 2006;6:772–83. doi: 10.1038/nri1937. [DOI] [PubMed] [Google Scholar]
- 31.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–20. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 32.Randle PJ, Garland PB, Hales CN, et al. Interactions of metabolism and the physiological role of insulin. Recent Prog Horm Res. 1966;22:1–48. doi: 10.1016/b978-1-4831-9825-5.50004-x. [DOI] [PubMed] [Google Scholar]
- 33.Neely JR, Morgan HE. Relationship between carbohydrate and lipid metabolism and the energy balance of heart muscle. Annu Rev Physiol. 1974;36:413–59. doi: 10.1146/annurev.ph.36.030174.002213. [DOI] [PubMed] [Google Scholar]
- 34.Sambandam N, Lopaschuk GD. AMP-activated protein kinase (AMPK) control of fatty acid and glucose metabolism in the ischemic heart. Prog Lipid Res. 2003;42:238–56. doi: 10.1016/s0163-7827(02)00065-6. [DOI] [PubMed] [Google Scholar]
- 35.Chappell JB, Robinson BH. Penetration of the mitochondrial membrane by tricarboxylic acidanions. Biochem Soc Symp. 1968;27:123–33. [PubMed] [Google Scholar]
- 36.Finck BN, Lehman JJ, Leone TC, et al. The cardiac phenotype induced by PPARalpha overexpression mimics that caused by diabetes mellitus. J Clin Invest. 2002;109:12–30. doi: 10.1172/JCI14080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schaffer SW, Seyed-Mozaffari M, Cutcliff CR, et al. Postreceptor myocardial metabolic defect in a rat model of non-insulin-dependent diabetes mellitus. Diabetes. 1986;35:593–7. doi: 10.2337/diab.35.5.593. [DOI] [PubMed] [Google Scholar]
- 38.Serpillon S, Floyd BC, Gupte RS, et al. Superoxide production by NAD(P)H oxidase and mitochondria is increased in genetically obese and hyperglycemic rat heart and aorta before the development of cardiac dysfunction. The role of glucose-6-phosphate dehydrogenase-derived NADPH. Am J Physiol Heart Circ Physiol. 2009;297:H153–62. doi: 10.1152/ajpheart.01142.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li SY, Sigmon VK, Babcock SA, et al. Advanced glycation endproduct induces ROS accumulation, apoptosis, MAP kinase activation and nuclear O-GlcNAcylation in human cardiac myocytes. Life Sci. 2007;80:1051–6. doi: 10.1016/j.lfs.2006.11.035. [DOI] [PubMed] [Google Scholar]
- 40.Yan SF, Ramasamy R, Bucciarelli LG, et al. RAGE and its ligands: a lasting memory in diabetic complications? Diab Vasc Dis Res. 2004;1:10–20. doi: 10.3132/dvdr.2004.001. [DOI] [PubMed] [Google Scholar]
- 41.Fulop N, Mason MM, Dutta K, et al. Impact of Type 2 diabetes and aging on cardiomyocyte function and O-linked N-acetylglucosamine levels in the heart. Am J Physiol Cell Physiol. 2007;292:C1370–8. doi: 10.1152/ajpcell.00422.2006. [DOI] [PubMed] [Google Scholar]
- 42.Hu Y, Belke D, Suarez J, et al. Adenovirus-mediated overexpression of O-GlcNAcase improves contractile function in the diabetic heart. Circ Res. 2005;96:1006–13. doi: 10.1161/01.RES.0000165478.06813.58. [DOI] [PubMed] [Google Scholar]
- 43.Clark RJ, McDonough PM, Swanson E, et al. Diabetes and the accompanying hyperglycemia impairs cardiomyocyte calcium cycling through increased nuclear O-GlcNAcylation. J Biol Chem. 2003;278:44230–7. doi: 10.1074/jbc.M303810200. [DOI] [PubMed] [Google Scholar]
- 44.Cotter MA, Cameron NE, Robertson S. Polyol pathway-mediated changes in cardiac muscle contractile properties: studies in streptozotocin-diabetic and galactose-fed rats. Exp Physiol. 1992;77:829–38. doi: 10.1113/expphysiol.1992.sp003649. [DOI] [PubMed] [Google Scholar]
- 45.Trueblood N, Ramasamy R. Aldose reductase inhibition improves altered glucose metabolism of isolated diabetic rat hearts. Am J Physiol. 1998;275:H75–83. doi: 10.1152/ajpheart.1998.275.1.H75. [DOI] [PubMed] [Google Scholar]
- 46.Ojaimi C, Kinugawa S, Recchia FA, et al. Oxidant-NO dependent gene regulation in dogs with type I diabetes: impact on cardiac function and metabolism. Cardiovasc Diabetol. 2010;9:43. doi: 10.1186/1475-2840-9-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Du XL, Edelstein D, Rossetti L, et al. Hyperglycemia-induced mitochondrial superoxide overproduction activates the hexosamine pathway and induces plasminogen activator inhibitor-1 expression by increasing Sp1 glycosylation. Proc Natl Acad Sci U S A. 2000;97:12222–6. doi: 10.1073/pnas.97.22.12222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nishikawa T, Edelstein D, Du XL, et al. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature. 2000;404:787–90. doi: 10.1038/35008121. [DOI] [PubMed] [Google Scholar]
- 49.Pacher P, Szabo C. Role of poly(ADP-ribose) polymerase 1 (PARP-1) in cardiovascular diseases: the therapeutic potential of PARP inhibitors. Cardiovasc Drug Rev. 2007;25:235–60. doi: 10.1111/j.1527-3466.2007.00018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Du X, Matsumura T, Edelstein D, et al. Inhibition of GAPDH activity by poly(ADP-ribose) polymerase activates three major pathways of hyperglycemic damage in endothelial cells. J Clin Invest. 2003;112:1049–57. doi: 10.1172/JCI18127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schaffer SW, Jong CJ, Mozaffari M. Role of oxidative stress in diabetes-mediated vascular dysfunction: unifying hypothesis of diabetes revisited. Vascul Pharmacol. 2012;57:39–49. doi: 10.1016/j.vph.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 52.Wieland O, Siess E, Schulze-Wethmar FH, et al. Active and inactive forms of pyruvate dehydrogenase in rat heart and kidney: effect of diabetes, fasting, and refeeding on pyruvate dehydrogenase interconversion. Arch Biochem Biophys. 1971;143:593–601. doi: 10.1016/0003-9861(71)90244-x. [DOI] [PubMed] [Google Scholar]
- 53.Hansford RG, Cohen L. Relative importance of pyruvate dehydrogenase interconversion and feedback inhibition in the effect of fatty acids on pyruvate oxidation by rat heart mitochondria. Arch Biochem Biophys. 1978;191:65–81. doi: 10.1016/0003-9861(78)90068-1. [DOI] [PubMed] [Google Scholar]
- 54.McCormack JG, Halestrap AP, Denton RM. Role of calcium ions in regulation of mammalian intramitochondrial metabolism. Physiol Rev. 1990;70:391–425. doi: 10.1152/physrev.1990.70.2.391. [DOI] [PubMed] [Google Scholar]
- 55.Hopkins TA, Sugden MC, Holness MJ, et al. Control of cardiac pyruvate dehydrogenase activity in peroxisome proliferator-activated receptor-alpha transgenic mice. Am J Physiol Heart Circ Physiol. 2003;285:H270–6. doi: 10.1152/ajpheart.00852.2002. [DOI] [PubMed] [Google Scholar]
- 56.Campbell FM, Kozak R, Wagner A, et al. A role for peroxisome proliferator-activated receptor alpha (PPARalpha ) in the control of cardiac malonyl-CoA levels: reduced fatty acid oxidation rates and increased glucose oxidation rates in the hearts of mice lacking PPARalpha are associated with higher concentrations of malonyl-CoA and reduced expression of malonyl-CoA decarboxylase. J Biol Chem. 2002;277:4098–103. doi: 10.1074/jbc.M106054200. [DOI] [PubMed] [Google Scholar]
- 57.Chatham JC, Forder JR. Relationship between cardiac function and substrate oxidation in hearts of diabetic rats. Am J Physiol. 1997;273:H52–8. doi: 10.1152/ajpheart.1997.273.1.H52. [DOI] [PubMed] [Google Scholar]
- 58.Connelly KA, Kelly DJ, Zhang Y, et al. Inhibition of protein kinase C-beta by ruboxistaurin preserves cardiac function and reduces extracellular matrix production in diabetic cardiomyopathy. Circ Heart Fail. 2009;2:129–37. doi: 10.1161/CIRCHEARTFAILURE.108.765750. [DOI] [PubMed] [Google Scholar]
- 59.Ricci C, Pastukh V, Leonard J, et al. Mitochondrial DNA damage triggers mitochondrial-superoxide generation and apoptosis. Am J Physiol Cell Physiol. 2008;294:C413–22. doi: 10.1152/ajpcell.00362.2007. [DOI] [PubMed] [Google Scholar]
- 60.Luiken JJ, Arumugam Y, Dyck DJ, et al. Increased rates of fatty acid uptake and plasmalemmal fatty acid transporters in obese Zucker rats. J Biol Chem. 2001;276:40567–73. doi: 10.1074/jbc.M100052200. [DOI] [PubMed] [Google Scholar]
- 61.Coort SL, Willems J, Coumans WA, et al. Sulfo-N-succinimidyl esters of long chain fatty acids specifically inhibit fatty acid translocase (FAT/CD36)-mediated cellular fatty acid uptake. Mol Cell Biochem. 2002;239:213–9. [PubMed] [Google Scholar]
- 62.Lopaschuk GD, Ussher JR, Folmes CD, et al. Myocardial fatty acid metabolism in health and disease. Physiol Rev. 2010;90:207–58. doi: 10.1152/physrev.00015.2009. [DOI] [PubMed] [Google Scholar]
- 63.Chabowski A, Coort SL, Calles-Escandon J, et al. Insulin stimulates fatty acid transport by regulating expression of FAT/CD36 but not FABPpm. Am J Physiol Endocrinol Metab. 2004;287:E781–9. doi: 10.1152/ajpendo.00573.2003. [DOI] [PubMed] [Google Scholar]
- 64.Luiken JJ, Coort SL, Koonen DP, et al. Regulation of cardiac long-chain fatty acid and glucose uptake by translocation of substrate transporters. Pflugers Arch. 2004;448:1–15. doi: 10.1007/s00424-003-1199-4. [DOI] [PubMed] [Google Scholar]
- 65.Carley AN, Severson DL. Fatty acid metabolism is enhanced in type 2 diabetic hearts. Biochim Biophys Acta. 2005;1734:112–26. doi: 10.1016/j.bbalip.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 66.Holland WL, Brozinick JT, Wang LP, et al. Inhibition of ceramide synthesis ameliorates glucocorticoid-, saturated-fat-, and obesity-induced insulin resistance. Cell Metab. 2007;5:167–79. doi: 10.1016/j.cmet.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 67.Rodrigues B, Xiang H, McNeill JH. Effect of L-carnitine treatment on lipid metabolism and cardiac performance in chronically diabetic rats. Diabetes. 1988;37:1358–64. doi: 10.2337/diab.37.10.1358. [DOI] [PubMed] [Google Scholar]
- 68.Sakamoto J, Barr RL, Kavanagh KM, et al. Contribution of malonyl-CoA decarboxylase to the high fatty acid oxidation rates seen in the diabetic heart. Am J Physiol Heart Circ Physiol. 2000;278:H1196–204. doi: 10.1152/ajpheart.2000.278.4.H1196. [DOI] [PubMed] [Google Scholar]
- 69.Kudo N, Barr AJ, Barr RL, et al. High rates of fatty acid oxidation during reperfusion of ischemic hearts are associated with a decrease in malonyl-CoA levels due to an increase in 5′-AMP-activated protein kinase inhibition of acetyl-CoA carboxylase. J Biol Chem. 1995;270:17513–20. doi: 10.1074/jbc.270.29.17513. [DOI] [PubMed] [Google Scholar]
- 70.Gamble J, Lopaschuk GD. Insulin inhibition of 5′ adenosine monophosphate-activated protein kinase in the heart results in activation of acetyl coenzyme A carboxylase and inhibition of fatty acid oxidation. Metabolism. 1997;46:1270–4. doi: 10.1016/s0026-0495(97)90229-8. [DOI] [PubMed] [Google Scholar]
- 71.Young ME, Goodwin GW, Ying J, et al. Regulation of cardiac and skeletal muscle malonyl-CoA decarboxylase by fatty acids. Am J Physiol Endocrinol Metab. 2001;280:E471–9. doi: 10.1152/ajpendo.2001.280.3.E471. [DOI] [PubMed] [Google Scholar]
- 72.Finck BN, Han X, Courtois M, et al. A critical role for PPARalpha-mediated lipotoxicity in the pathogenesis of diabetic cardiomyopathy: modulation by dietary fat content. Proc Natl Acad Sci U S A. 2003;100:1226–31. doi: 10.1073/pnas.0336724100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chen W, Xia Y, Zhao X, et al. The Critical Role of Astragalus Polysaccharides for the Improvement of PPRAalpha-Mediated Lipotoxicity in Diabetic Cardiomyopathy. PLoS One. 2012;7:e45541. doi: 10.1371/journal.pone.0045541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yu BC, Chang CK, Ou HY, et al. Decrease of peroxisome proliferator-activated receptor delta expression in cardiomyopathy of streptozotocin-induced diabetic rats. Cardiovasc Res. 2008;80:78–87. doi: 10.1093/cvr/cvn172. [DOI] [PubMed] [Google Scholar]
- 75.Cheng L, Ding G, Qin Q, et al. Cardiomyocyte-restricted peroxisome proliferator-activated receptor-delta deletion perturbs myocardial fatty acid oxidation and leads to cardiomyopathy. Nat Med. 2004;10:1245–50. doi: 10.1038/nm1116. [DOI] [PubMed] [Google Scholar]
- 76.Burkart EM, Sambandam N, Han X, et al. Nuclear receptors PPARbeta/delta and PPARalpha direct distinct metabolic regulatory programs in the mouse heart. J Clin Invest. 2007;117:3930–9. doi: 10.1172/JCI32578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bowman RH. Effects of diabetes, fatty acids, and ketone bodies on tricarboxylic acid cycle metabolism in the perfused rat heart. J Biol Chem. 1966;241:3041–8. [PubMed] [Google Scholar]
- 78.Taegtmeyer H, Passmore JM. Defective energy metabolism of the heart in diabetes. Lancet. 1985;1:139–41. doi: 10.1016/s0140-6736(85)91907-5. [DOI] [PubMed] [Google Scholar]
- 79.Kuo TH, Moore KH, Giacomelli F, et al. Defective oxidative metabolism of heart mitochondria from genetically diabetic mice. Diabetes. 1983;32:781–7. doi: 10.2337/diab.32.9.781. [DOI] [PubMed] [Google Scholar]
- 80.Pierce GN, Dhalla NS. Heart mitochondrial function in chronic experimental diabetes in rats. Can J Cardiol. 1985;1:48–54. [PubMed] [Google Scholar]
- 81.Tomita M, Mukae S, Geshi E, et al. Mitochondrial respiratory impairment in streptozotocin- induced diabetic rat heart. Jpn Circ J. 1996;60:673–82. doi: 10.1253/jcj.60.673. [DOI] [PubMed] [Google Scholar]
- 82.Boudina S, Sena S, O’Neill BT, et al. Reduced mitochondrial oxidative capacity and increased mitochondrial uncoupling impair myocardial energetics in obesity. Circulation. 2005;112:2686–95. doi: 10.1161/CIRCULATIONAHA.105.554360. [DOI] [PubMed] [Google Scholar]
- 83.Suarez J, Hu Y, Makino A, et al. Alterations in mitochondrial function and cytosolic calcium induced by hyperglycemia are restored by mitochondrial transcription factor A in cardiomyocytes. Am J Physiol Cell Physiol. 2008;295:C1561–8. doi: 10.1152/ajpcell.00076.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zungu M, Young ME, Stanley WC, et al. Chronic treatment with the peroxisome proliferator- activated receptor alpha agonist Wy-14,643 attenuates myocardial respiratory capacity and contractile function. Mol Cell Biochem. 2009;330:55–62. doi: 10.1007/s11010-009-0100-y. [DOI] [PubMed] [Google Scholar]
- 85.Boudina S, Sena S, Theobald H, et al. Mitochondrial energetics in the heart in obesity-related diabetes: direct evidence for increased uncoupled respiration and activation of uncoupling proteins. Diabetes. 2007;56:2457–66. doi: 10.2337/db07-0481. [DOI] [PubMed] [Google Scholar]
- 86.von Bibra H, Hansen A, Dounis V, et al. Augmented metabolic control improves myocardial diastolic function and perfusion in patients with non-insulin dependent diabetes. Heart. 2004;90:1483–4. doi: 10.1136/hrt.2003.020842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.von Bibra H, Siegmund T, Hansen A, et al. [Augmentation of myocardial function by improved glycemic control in patients with type 2 diabetes mellitus] Dtsch Med Wochenschr. 2007;132:729–34. doi: 10.1055/s-2007-973608. [DOI] [PubMed] [Google Scholar]
- 88.McGuire DK, Inzucchi SE. New drugs for the treatment of diabetes mellitus: part I: Thiazolidinediones and their evolving cardiovascular implications. Circulation. 2008;117:440–9. doi: 10.1161/CIRCULATIONAHA.107.704080. [DOI] [PubMed] [Google Scholar]
- 89.Sharma AM, Staels B. Review: Peroxisome proliferator-activated receptor gamma and adipose tissue--understanding obesity-related changes in regulation of lipid and glucose metabolism. J Clin Endocrinol Metab. 2007;92:386–95. doi: 10.1210/jc.2006-1268. [DOI] [PubMed] [Google Scholar]
- 90.Masoudi FA, Inzucchi SE. Diabetes mellitus and heart failure: epidemiology, mechanisms, and pharmacotherapy. Am J Cardiol. 2007;99:113B–32B. doi: 10.1016/j.amjcard.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 91.Masoudi FA, Inzucchi SE, Wang Y, et al. Thiazolidinediones, metformin, and outcomes in older patients with diabetes and heart failure: an observational study. Circulation. 2005;111:583–90. doi: 10.1161/01.CIR.0000154542.13412.B1. [DOI] [PubMed] [Google Scholar]
- 92.Nikolaidis LA, Elahi D, Hentosz T, et al. Recombinant glucagon-like peptide-1 increases myocardial glucose uptake and improves left ventricular performance in conscious dogs with pacing-induced dilated cardiomyopathy. Circulation. 2004;110:955–61. doi: 10.1161/01.CIR.0000139339.85840.DD. [DOI] [PubMed] [Google Scholar]
- 93.Sokos GG, Nikolaidis LA, Mankad S, et al. Glucagon-like peptide-1 infusion improves left ventricular ejection fraction and functional status in patients with chronic heart failure. J Card Fail. 2006;12:694–9. doi: 10.1016/j.cardfail.2006.08.211. [DOI] [PubMed] [Google Scholar]
- 94.Chiasson JL, Josse RG, Gomis R, et al. Acarbose for prevention of type 2 diabetes mellitus: the STOP-NIDDM randomised trial. Lancet. 2002;359:2072–7. doi: 10.1016/S0140-6736(02)08905-5. [DOI] [PubMed] [Google Scholar]
- 95.Hanefeld M, Josse RG, Chiasson JL. Alpha-glucosidase inhibitors for patients with type 2 diabetes: response to van de Laar et al. Diabetes Care. 2005;28:1840. doi: 10.2337/diacare.28.7.1840. author reply1. [DOI] [PubMed] [Google Scholar]
- 96.Konduracka E, Gackowski A, Rostoff P, et al. Diabetes-specific cardiomyopathy in type 1 diabetes mellitus: no evidence for its occurrence in the era of intensive insulin therapy. Eur Heart J. 2007;28:2465–71. doi: 10.1093/eurheartj/ehm361. [DOI] [PubMed] [Google Scholar]
- 97.Haas SJ, Vos T, Gilbert RE, et al. Are beta-blockers as efficacious in patients with diabetes mellitus as in patients without diabetes mellitus who have chronic heart failure? A meta-analysis of large-scale clinical trials. Am Heart J. 2003;146:848–53. doi: 10.1016/S0002-8703(03)00403-4. [DOI] [PubMed] [Google Scholar]
- 98.Fonseca V, Bakris GL, Bell DS, et al. Differential effect of beta-blocker therapy on insulin resistance as a function of insulin sensitizer use: results from GEMINI. Diabet Med. 2007;24:759–63. doi: 10.1111/j.1464-5491.2007.02151.x. [DOI] [PubMed] [Google Scholar]
- 99.Ramasubbu K, Estep J, White DL, et al. Experimental and clinical basis for the use of statins in patients with ischemic and nonischemic cardiomyopathy. J Am Coll Cardiol. 2008;51:415–26. doi: 10.1016/j.jacc.2007.10.009. [DOI] [PubMed] [Google Scholar]
- 100.Stolen TO, Hoydal MA, Kemi OJ, et al. Interval training normalizes cardiomyocyte function, diastolic Ca2+ control, and SR Ca2+ release synchronicity in a mouse model of diabetic cardiomyopathy. Circ Res. 2009;105:527–36. doi: 10.1161/CIRCRESAHA.109.199810. [DOI] [PubMed] [Google Scholar]
- 101.Howarth FC, Almugaddum FA, Qureshi MA, et al. The effects of heavy long-term exercise on ventricular myocyte shortening and intracellular Ca2+ in streptozotocin-induced diabetic rat. J Diabetes Complications. 2010;24:278–85. doi: 10.1016/j.jdiacomp.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 102.Rubenstrunk A, Hanf R, Hum DW, et al. Safety issues and prospects for future generat ons of PPAR modulators. Biochim Biophys Acta. 2007;1771:1065–81. doi: 10.1016/j.bbalip.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 103.Goa KL, Barradell LB, Plosker GL. Bezafibrate. An update of its pharmacology and use in the management of dyslipidaemia. Drugs. 1996;52:725–53. doi: 10.2165/00003495-199652050-00008. [DOI] [PubMed] [Google Scholar]
- 104.Gross B, Staels B. PPAR agonists: multimodal drugs for the treatment of type-2 diabetes. Best Pract Res Clin Endocrinol Metab. 2007;21:687–710. doi: 10.1016/j.beem.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 105.Davidoff AJ, Mason MM, Davidson MB, et al. Sucrose-induced cardiomyocyte dysfunction is both preventable and reversible with clinically relevant treatments. Am J Physiol Endocrinol Metab. 2004;286:E718–24. doi: 10.1152/ajpendo.00358.2003. [DOI] [PubMed] [Google Scholar]
- 106.Dong F, Fang CX, Yang X, et al. Cardiac overexpression of catalase rescues cardiac contractile dysfunction induced by insulin resistance: Role of oxidative stress, protein carbonyl formation and insulin sensitivity. Diabetologia. 2006;49:1421–33. doi: 10.1007/s00125-006-0230-7. [DOI] [PubMed] [Google Scholar]
- 107.Wold LE, Ceylan-Isik AF, Fang CX, et al. Metallothionein alleviates cardiac dysfunction in streptozotocin-induced diabetes: role of Ca2+ cycling proteins, NADPH oxidase, poly(ADP- Ribose) polymerase and myosin heavy chain isozyme. Free Radic Biol Med. 2006;40:1419–29. doi: 10.1016/j.freeradbiomed.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 108.Yaras N, Bilginoglu A, Vassort G, et al. Restoration of diabetes-induced abnormal local Ca2+ release in cardiomyocytes by angiotensin II receptor blockade. Am J Physiol Heart Circ Physiol. 2007;292:H912–20. doi: 10.1152/ajpheart.00824.2006. [DOI] [PubMed] [Google Scholar]
- 109.Shekelle PG, Rich MW, Morton SC, et al. Efficacy of angiotensin-converting enzyme inhibitors and beta-blockers in the management of left ventricular systolic dysfunction according to race, gender, and diabetic status: a meta-analysis of major clinical trials. J Am Coll Cardiol. 2003;41:1529–38. doi: 10.1016/s0735-1097(03)00262-6. [DOI] [PubMed] [Google Scholar]
- 110.Sowers JR, Epstein M, Frohlich ED. Diabetes, hypertension, and cardiovascular disease: an update. Hypertension. 2001;37:1053–9. doi: 10.1161/01.hyp.37.4.1053. [DOI] [PubMed] [Google Scholar]
- 111.Murarka S, Movahed MR. Diabetic cardiomyopathy. J Card Fail. 2010;16:971–9. doi: 10.1016/j.cardfail.2010.07.249. [DOI] [PubMed] [Google Scholar]
- 112.Zaman AK, Fujii S, Goto D, et al. Salutary effects of attenuation of angiotensin II on coronary perivascular fibrosis associated with insulin resistance and obesity. J Mol Cell Cardiol. 2004;37:525–35. doi: 10.1016/j.yjmcc.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 113.Orea-Tejeda A, Colin-Ramirez E, Castillo-Martinez L, et al. Aldosterone receptor antagonists induce favorable cardiac remodeling in diastolic heart failure patients. Rev Invest Clin. 2007;59:103–7. [PubMed] [Google Scholar]
- 114.Shimada T. Correlation between metabolic and histopathological changes in the myocardium of the KK mouse. Effect of diltiazem on the diabetic heart. Jpn Heart J. 1993;34:617–6. doi: 10.1536/ihj.34.617. [DOI] [PubMed] [Google Scholar]
- 115.Afzal N, Ganguly PK, Dhalla KS, et al. Beneficial effects of verapamil in diabetic cardiomyopathy. Diabetes. 1988;37:936–42. doi: 10.2337/diab.37.7.936. [DOI] [PubMed] [Google Scholar]
- 116.Afzal N, Pierce GN, Elimban V, et al. Influence of verapamil on some subcellular defects in diabetic cardiomyopathy. Am J Physiol. 1989;256:E453–8. doi: 10.1152/ajpendo.1989.256.4.E453. [DOI] [PubMed] [Google Scholar]
- 117.Shah TS, Satia MC, Gandhi TP, et al. Effects of chronic nifedipine treatment on streptozotocin- induced diabetic rats. J Cardiovasc Pharmacol. 1995;26:6–12. doi: 10.1097/00005344-199507000-00002. [DOI] [PubMed] [Google Scholar]
- 118.Higa S, Shimabukuro M, Shinzato T, et al. Long-term nifedipine treatment reduces calcium overload in isolated reperfused hearts of diabetic rats. Gen Pharmacol. 1995;26:1679–86. doi: 10.1016/0306-3623(95)00036-4. [DOI] [PubMed] [Google Scholar]
- 119.Aneja A, Tang WH, Bansilal S, et al. Diabetic cardiomyopathy: insights into pathogenesis, diagnostic challenges, and therapeutic options. Am J Med. 2008;121:748–57. doi: 10.1016/j.amjmed.2008.03.046. [DOI] [PubMed] [Google Scholar]
- 120.Suarez J, Scott B, Dillmann WH. Conditional increase in SERCA2a protein is able to reverse contractile dysfunction and abnormal calcium flux in established diabetic cardiomyopathy. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1439–45. doi: 10.1152/ajpregu.00736.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wang M, Zhang WB, Zhu JH, et al. Breviscapine ameliorates cardiac dysfunction and regulates the myocardial Ca(2+)-cycling proteins in streptozotocin-induced diabetic rats. Acta Diabetol. 2010;47:209–18. doi: 10.1007/s00592-009-0164-x. [DOI] [PubMed] [Google Scholar]
- 122.Giles TD, Ouyang J, Kerut EK, et al. Changes in protein kinase C in early cardiomyopathy and in gracilis muscle in the BB/Wor diabetic rat. Am J Physiol. 1998;274:H295–307. doi: 10.1152/ajpheart.1998.274.1.H295. [DOI] [PubMed] [Google Scholar]
- 123.Liu X, Wang J, Takeda N, et al. Changes in cardiac protein kinase C activities and isozymes in streptozotocin-induced diabetes. Am J Physiol. 1999;277:E798–804. doi: 10.1152/ajpendo.1999.277.5.E798. [DOI] [PubMed] [Google Scholar]
- 124.Malhotra A, Kang BP, Cheung S, et al. Angiotensin II promotes glucose-induced activation of cardiac protein kinase C isozymes and phosphorylation of troponin I. Diabetes. 2001;50:1918–26. doi: 10.2337/diabetes.50.8.1918. [DOI] [PubMed] [Google Scholar]
- 125.Shizukuda Y, Buttrick PM. Protein kinase C(epsilon) modulates apoptosis induced by beta - adrenergic stimulation in adult rat ventricular myocytes via extracellular signal-regulated kinase (ERK) activity. J Mol Cell Cardiol. 2001;33:1791–803. doi: 10.1006/jmcc.2001.1442. [DOI] [PubMed] [Google Scholar]
- 126.Pastukh V, Wu S, Ricci C, et al. Reversal of hyperglycemic preconditioning by angiotensin II: role of calcium transport. Am J Physiol Heart Circ Physiol. 2005;288:H1965–75. doi: 10.1152/ajpheart.00855.2004. [DOI] [PubMed] [Google Scholar]
- 127.Wakasaki H, Koya D, Schoen FJ, et al. Targeted overexpression of protein kinase C beta2 isoform in myocardium causes cardiomyopathy. Proc Natl Acad Sci U S A. 1997;94:9320–5. doi: 10.1073/pnas.94.17.9320. [DOI] [PMC free article] [PubMed] [Google Scholar]