Abstract
Activation of coagulation pathways may contribute to risk for non-AIDS related conditions among HIV positive patients. We measured tissue factor-dependent procoagulant activity on circulating microparticles (MP-TF) in the plasma of 163 HIV positive participants, both untreated and treated, with viral suppression. MP-TF activity was 39% lower among treated versus untreated participants (p<0.001), which persisted in adjusted models (−36%; p=0.03). Among treated participants, MP-TF activity correlated modestly with D-dimer (r=0.24; p=0.01), vWF (r=0.36; p<0.001), and IL-6 (r=0.20; p=0.04) levels. Future research should focus on mechanisms driving residual functional TF activity and whether these alterations have clinical consequences for non-AIDS defining complications.
INTRODUCTION
The spectrum of clinical disease among contemporary HIV positive patients receiving effective treatment with antiretroviral therapy (ART) now more commonly consists of non-AIDS defining chronic conditions such as cardiovascular disease.1,2 Plasma D-dimer, a marker of coagulation activity, strongly predicts risk for all-cause mortality (largely non-AIDS related) as well as specific conditions like CVD and thromboembolic events among HIV patients.3–7 In addition, D-dimer levels are correlated with HIV viral replication after starting or stopping ART, but remain elevated among treated patients when compared to uninfected controls.6,8,9 Currently, mechanisms underlying persistent HIV-related abnormalities in coagulation biology, and the potential consequences for non-AIDS related clinical risk, remain poorly understood.
Tissue factor (TF) is a transmembrane protein that when complexed with factor VII(a), is responsible for initiating coagulation through activation of the extrinsic pathway. Circulating TF exists on cell surfaces (e.g., activated monocytes), as soluble cell-free TF in plasma (where it is largely inactive in coagulation), and on cell-derived microparticles (MP). MPs are membrane-encapsulated vesicles released from activated or apoptotic cells that contain and express surface proteins derived from the parent cell.10 Though TF positive MPs (MP-TF) constitute only a small fraction of circulating TF, they likely represent a functionally active form of TF given their origin from activated cells (e.g., released form monocytes in response to endotoxemia).10,11 In this report we study the effect of ART treatment on MP-TF procoagulant activity, and explore associations between MP-TF activity with biomarkers of coagulation and systemic inflammation that in turn predict clinical risk among HIV positive patients.
METHODS
Study Population
Participants with HIV infection were recruited from 2007 to 2011 as part of ongoing research protocols at Hennepin County Medical Center (HCMC) HIV clinic in Minneapolis, MN. Plasma biomarkers of inflammation and coagulation had previously been measured among most of these participants. MP-TF activity was assessed for this study using stored specimens from participants who were either: a) naïve to treatment or off ART for ≥1 year (i.e., ‘untreated’), or b) receiving ART with HIV viral load <200 copies/mL for ≥6 months (i.e., ‘treated’). Patients were excluded for presence of a current bacterial infection, pregnancy, or known CVD. Study measures were obtained at a single study visit. Framingham Risk Score (FRS) for 10 year coronary heart disease risk was estimated using published algorithms.12 All study protocols, including use of stored specimens for future research, were approved by the HCMC human subject research committee, and participants underwent written informed consent prior to enrollment.
Laboratory Measures
Plasma specimens were collected using EDTA (ethylenediaminetetraacetic acid) tubes and processed within 30 minutes of collection and frozen at −70°C until analysis. Plasma was isolated after a 2500g spin for 15 minutes in a refrigerated centrifuged at 4°C. Measures obtained from fresh blood at HCMC clinical laboratory included: HIV RNA level, serologies for hepatitis B and C, total cholesterol, low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), and triglycerides. All samples were handled in a blinded fashion.
D-dimer levels, interleukin-6 (IL-6), and high sensitivity CRP (hsCRP) were measured using methods previously described,13 and von Willebrand factor (vWF) levels were measured on Stago STA-R analyzer (Stago Diagnostics, Parsipanny NJ), at the Laboratory for Clinical Biochemistry Research at the University of Vermont. These markers were chosen to assess coagulation activity and inflammation, and because they have been associated with clinical risk for CVD and all-cause mortality in HIV infected and uninfected populations.6,7,14–16
MP-TF activity was measured as previously reported,17 on MPs isolated from stored EDTA plasma by performing another centrifugation at 20,000g for 30 minutes at 4oC. The MP pellet was re-suspended in HBSA (20mM HEPES, 120mM NaCl, 1mg/ml) via mild sonication. A previously described two stage chromogenic assay was employed with the following modifications: 1) MPs were incubated for 2 hours with 2.5mM CaCl2, 1nM FVIIa and 150nM FX in the presence and absence of a TF blocking antibody, 2) Absorbance measurements were made every 30 seconds for 30 minutes after the addition of EDTA and FXa chromogenic substrate (Pefachrome 8595, Centerchem, Norwalk, CT).18 MP-TF activity was calculated in relation to an Innovin™ tissue factor standard.
Statistical Methods
Descriptive statistics are reported as means with standard deviation (SD) and medians with inter-quartile range (IQR). Wilcoxon rank tests and the chi square test for categorical variables were used to compare characteristics of the untreated and ART-treated groups. For comparisons between groups, the relative percent difference (with 95% confidence interval) was obtained by exponentiating the mean difference on the natural log scale using generalized linear models. Fully adjusted models included the following covariates: age, gender, race/ethnicity, smoking status, co-infection with hepatitis B/C, prior AIDS, current CD4 count, total-to-HDL-C ratio and lipid lowering therapy. Hepatitis B/C co-infection was chosen over injection drug use (IDU) in covariate models as hepatitis B/C co-infection is more likely to influence the degree of current systemic inflammation, and findings were similar with and without inclusion of IDU in multivariate models. Total-to-HDL-C was included over other lipid parameters given the strong association with clinical event risk,19,20 and that these 2 lipid measures differed the most between groups. Determinants of residual MP-TF activity were then explored among treated participants. Correlations were assessed using non-parametric rank tests due non-normal distribution of data. The level of significance was defined as p<0.05, and all analyses were conducted with SAS (version 9.2) and R statistical software (Version 2.10.1; http://www.cran.r-project.org).
RESULTS
Study Sample
Among the 163 HIV positive participants, 54 were untreated and 109 were receiving ART with a suppressed viral load. Demographic and clinical characteristics are presented in Table 1. Compared with untreated participants, those receiving ART were older, had a higher CD4 count, a higher proportion with a prior AIDS event, and a lower proportion with a history of IDU or who currently smoked cigarettes. Overall, traditional cardiovascular disease risk factors were more abnormal for treated versus untreated participants as indicated by 10-year FRS, use of lipid lowering drugs and cholesterol levels. Among treated participants, 51% were taking tenofovir disoproxil fumarate (DF), 50% were taking abacavir, 27% were taking a non-nucleoside reverse transcriptase inhibitor (efavirenz for 23%), 12% were taking raltegravir, and 49% were taking a protease inhibitor (26% atazanavir, 8% lopinavir, and 6% darunavir).
TABLE 1.
Clinical Characteristics and Laboratory Measures
| Untreated | ART-Treated | p-value* | |
|---|---|---|---|
| Number | 54 | 109 | |
| Characteristic | |||
| Age, median years (IQR) | 41 (31, 45) | 47 (43, 54) | <0.001 |
| Male Gender, # (%) | 45 (83) | 98 (90) | 0.23 |
| Race/Ethnicity White, # (%) African American, # (%) Other, # (%) |
23 (43) 24 (44) 7 (13) |
63 (58) 36 (33) 10 (9) |
-- |
| Current Smoker, # (%) | 35 (65) | 48 (44) | 0.01 |
| Prior IDU, # (%) | 17 (35) | 8 (7) | <0.001 |
| Hepatitis B or C, # (%) | 14 (26) | 15 (14) | 0.12 |
| Prior AIDS, # (%) | 6 (12) | 48 (47) | <0.001 |
| BMI, median kg/m2 (IQR) | 26 (23, 29) | 27 (24, 29) | 0.65 |
| Diabetes, # (%) | 3(6) | 9(8) | 0.81 |
| Blood pressure medication, # (%) | 11 (20) | 17 (16) | 0.44 |
| Lipid lowering medication, # (%) | 1 (3) | 30 (28) | 0.001 |
| 10-year FRS, median % (IQR) | 5.14 (1.23, 9.15) | 7.13 (4.51, 10.44) | 0.008 |
| ART regimen Tenofovir, # (%) NNRTI, # (%) PI, # (%) |
-- -- -- |
56 (51) 29 (27) 53 (49) |
-- -- -- |
| Laboratory Measure | |||
| CD4+ count, median cells/mm3 (IQR) | 342 (259, 446) | 522 (377, 745) | <0.001 |
| HIV RNA, median log10 copies/mL (IQR) | 4.2 (3.9, 4.7) | -- | -- |
| Total Chol./HDL-C, median (IQR) | 4.0 (3.3, 5.5) | 4.0 (3.4, 5.2) | 0.25 |
| Total Cholesterol, median mg/dL (IQR) | 162 (129, 185) | 185 (167, 212) | <0.001 |
| LDL-C, median mg/dL (IQR) | 88 (71, 112) | 103 (85, 123) | 0.02 |
| HDL-C, median mg/dL (IQR) | 37 (30, 48) | 46 (36, 54) | <0.001 |
| Triglycerides, median mg/dL (IQR) | 118 (78, 155) | 142 (99, 226) | 0.002 |
| MP-TF activity, median pg/mL (IQR) | 0.35 (0.24, 0.54) | 0.22 (0.14, 0.34) | <0.001 |
| D-dimer, median μg/mL (IQR) | 0.35 (0.19, 0.57) | 0.15 (0.11, 0.25) | <0.001 |
| vWF, median (IQR) | 224.0 (178.5, 303.0) | 131.5 (107.0, 181.0) | <0.001 |
| IL-6, median pg/mL (IQR) | 2.35 (1.53, 4.64) | 1.67 (1.17, 2.35) | <0.001 |
| hsCRP, median μg/mL (IQR) | 1.99 (0.80, 5.18) | 1.66 (0.83, 3.74) | 0.02 |
p-value for comparison by ART use; IDU=injection drug use; BMI=body mass index; FRS=Framingham risk score; NNRTI=non-nucleoside reverse transcriptase inhibitor; PI=protease inhibitor; LDL-C=low-density lipoprotein cholesterol; HDL-C=high-density lipoprotein cholesterol; MP-TF= microparticles expressing active tissue factor; IL=6=interleukin-6; hsCRP=high sensitivity C-reactive protein; sICAM-1=soluble intercellular adhesion molecule; vWF=von Willebrand Factor
Comparison Between Untreated and ART-Treated Participants
Median values for MP-TF activity and other plasma biomarkers, including differences by ART use from unadjusted comparisons, are presented in Table 1. When compared to untreated participants, treated participants have 39% lower MP-TF activity, 52% lower D-dimer, 41% lower vWF, and 36% lower IL-6, but hsCRP levels were not significantly different. In fully adjusted models, differences were similar for MP-TF activity (36% lower; p=0.03), D-dimer (61% lower; p<0.001), wWF (37% lower; p<0.001), IL-6 (49% lower; p<0.001), and hsCRP (47% lower; p=0.02).
MP-TF Activity, Co-morbid Disease, Inflammation, and Coagulation Activity
Among untreated participants, MP-TF activity was higher for those with hepatitis B or C co-infection (74%; p=0.03), and was correlated with age (r=0.30; p=0.03), 10-year FRS (r=0.34; p=0.01), and current CD4 count (r=0.43; p=0.001). In contrast, among treated participants, MP-TF activity was not correlated with age, 10-year FRS or current CD4 count, but was 28% lower (p=0.09) among those taking lipid-lowering medication versus not.
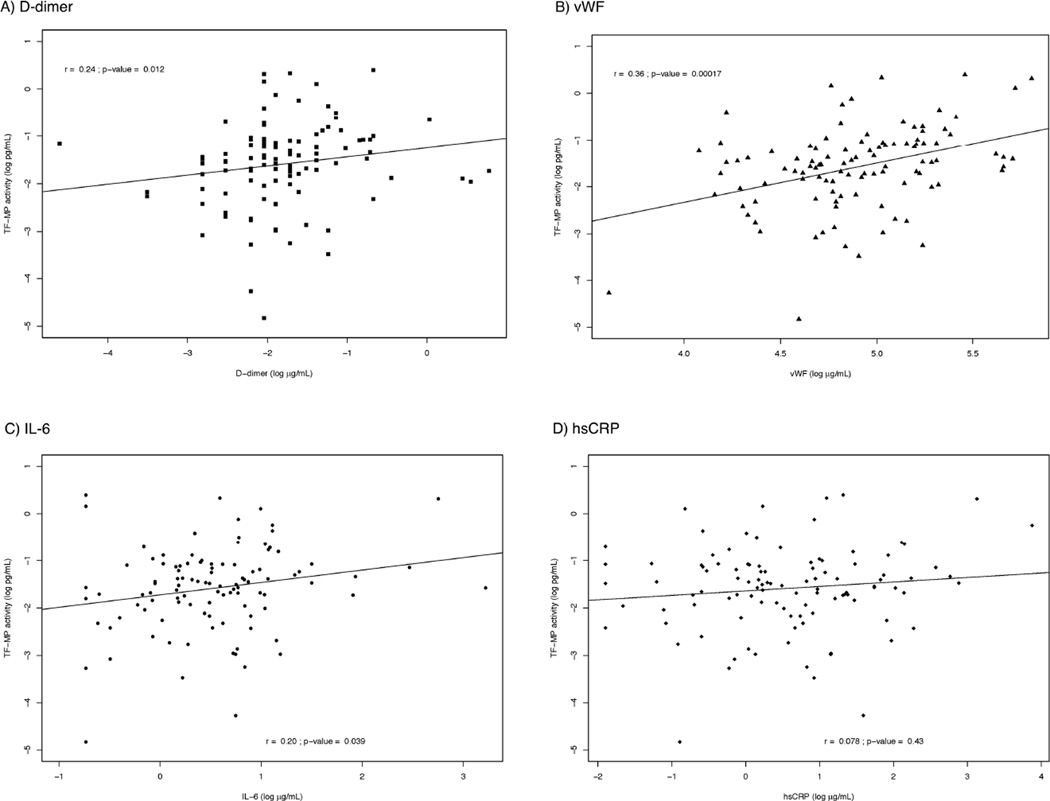
Finally, we then explored associations between MP-TF activity among treated patients with plasma biomarker levels to see if TF activity corresponded to residual abnormalities in biomarkers reflecting ongoing inflammation and coagulation. Scatter plots with correlations between MP-TF activity and inflammation and coagulation biomarkers are presented in Figure 1. In linear regression models, the change in log-e MP-TF activity for each 1 log-e increase in biomarker level was 0.19 for D-dimer (95% CI −0.02, 0.41; p=0.08), 0.84 for vWF (95% CI 0.44, 1.23; p<0.001), 0.26 for IL-6 (95% CI 0.02, 0.50; p=0.04), and 0.09 for hsCRP (95% CI −0.06, 0.24; p=0.22). Corresponding associations tended to be stronger in fully adjusted models at 0.31 for D-dimer (p=0.01), 0.86 for vWF (p<0.001), 0.32 for IL-6 (p=0.02), and 0.21 for hsCRP (p=0.01).
Figure 1. Correlations Between MP-TF Activity and Plasma Biomarker Levels.
Legend: Scatter plot with trend line describes the correlation between MP-TF activity and D-dimer (a; square), vWF (b; triangle), IL-6 (c; circle) and hsCRP (d; diamond) levels. Spearman’s rank correlation coefficient and p-value are reported.
DISCUSSION
Activation of coagulation pathways has been identified as a potential mechanism contributing to risk for non-AIDS-defining complications among HIV positive patients.4–6 We assessed the functional procoagulant activity of MP-TF, an active form of circulating cell-free TF, and determined that ART treatment with viral suppression was associated with lower TF pro-coagulant activity. Coagulation activity, as assessed by D-dimer levels, is persistently elevated among HIV-infected persons, despite viral suppression, though questions remain about the mechanisms up-regulating coagulation pathways.9 Our findings that residual MP-TF activity among treated patients is associated with biomarkers of inflammation and coagulation is consistent with the hypothesis that residual inflammation may be contributing to HIV-related coagulation abnormalities through increased circulating TF activity.
In a sentinel study by Funderburg et. al., monocyte expression of TF was increased between HIV infected versus uninfected persons and also correlated directly with HIV RNA and D-dimer levels.21 The authors subsequently reported that HIV increases the frequency of ‘activated’ monocyte phenotypes (CD14+/-CD16+), which express TF at high levels.21,22 Monocyte and tissue macrophage activation is an important mediator of coagulation and disease risk (e.g., premature atherosclerosis),23,24 and sCD14, a plasma marker of monocyte activation, is an independent predictor of mortality (largely non-AIDS related) among HIV positive patients.25 Circulating MP-TF typically originate from monocytes that express TF, and our findings describing associations between MP-TF procoagulant activity with D-dimer and vWF levels are consistent with the notion that activation of TF-pathways is an important component of HIV-related coagulation abnormalities.
The clinical significance of MP-TF activity among HIV positive patients is unknown, but is potentially important given IL-6 and D-dimer associations with clinical risk. Epidemiologic data show that these plasma biomarkers are elevated among HIV infected persons, even after viral suppression, and elevated levels predict increased risk for myocardial infarction and all-cause mortality.4–7,9 Specifically, in the SMART (Strategic Timing of AntiRetroviral Therapy) study, mortality risk associations with IL-6 and D-dimer were robust, present for those on and off ART, present for events in short and longer (>2 years) term, and were more extreme than what has been reported in uninfected populations.6,26,27 The hypothesis that persistent up-regulation of TF pathways in HIV patients explains, at least in part, D-dimer clinical risk prediction requires validation with additional measures of TF activity in larger studies.
The vast majority of TF in circulating blood is contained within mononuclear cells,10,11 and the inability to assess this fraction of peripheral blood was a limitation of our study. Newer methods that assess TF activity in whole blood (i.e., reflecting both cell-free and cell-associated activity) may now be applied to study epidemiologic associations with clinical event risk.10 Similarly, we were not able to determine whether monocyte activation is driving TF activity, or accounts for the associations with inflammatory biomarkers. Additional limitations of our study include the cross-sectional design and lack of an HIV uninfected control group. Although median MP-TF activity for both treated and untreated participants are higher that what we’ve found in healthy controls while developing the assay, comparisons of TF activity between treated HIV positive patients and uninfected persons need to account for additional confounding factors and this will be important to clarify in future studies.
In summary, ART treatment is associated with reduced MP-TF pro-coagulant activity, though residual activity among virally suppressed patients is related to ongoing inflammation and may be an important contributor to coagulation abnormalities. Future research should focus on the underlying mechanisms driving TF activity and whether these alterations contribute to clinical event risk.
ACKNOWLEDGEMENTS
We would like to thank all of our study participants, Edie Gunderson, Bette Bordenave, Rachel Givot, Jack Hall, and Miki Olson.
FUNDING: NIH 1UL1RR033183, NIH 1KL2RR033182-01, NIH 5 K12 RR023247, AHA 09CRP2230357, Gilead Pharmaceuticals.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: None reported.
REFERENCES
- 1.Neuhaus J, Angus B, Kowalska JD, La Rosa A, Sampson J, Wentworth D, et al. Risk of all-cause mortality associated with nonfatal AIDS and serious non-AIDS events among adults infected with HIV. AIDS. 2010;24(5):697–706. doi: 10.1097/QAD.0b013e3283365356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mocroft A, Reiss P, Gasiorowski J, Ledergerber B, Kowalska J, Chiesi A, et al. Serious fatal and nonfatal non-AIDS-defining illnesses in Europe. Journal of acquired immune deficiency syndromes. 2010;55(2):262–270. doi: 10.1097/QAI.0b013e3181e9be6b. [DOI] [PubMed] [Google Scholar]
- 3.Hunt P, Rodriguez B, Shive C, Clagett B, Funderburg N, Natta MV, et al. Gut Epithelial Barrier Dysfunction, Inflammation, and Coagulation Predict Higher Mortality during Treated HIV/AIDS. 19th Conference on Retroviruses and Opportunistic Infections (CROI); Seattle, WA USA. 2012. [Google Scholar]
- 4.Musselwhite LW, Sheikh V, Norton TD, Rupert A, Porter BO, Penzak SR, et al. Markers of endothelial dysfunction, coagulation and tissue fibrosis independently predict venous thromboembolism in HIV. AIDS. 2011;25(6):787–795. doi: 10.1097/QAD.0b013e3283453fcb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ford ES, Greenwald JH, Richterman AG, Rupert A, Dutcher L, Badralmaa Y, et al. Traditional risk factors and D-dimer predict incident cardiovascular disease events in chronic HIV infection. AIDS. 2010;24(10):1509–1517. doi: 10.1097/QAD.0b013e32833ad914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuller LH, Tracy R, Belloso W, De Wit S, Drummond F, Lane HC, et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 2008;5(10):e203. doi: 10.1371/journal.pmed.0050203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duprez DA, Neuhaus J, Kuller LH, Tracy R, Belloso W, De Wit S, et al. Inflammation, Coagulation and Cardiovascular Disease in HIV-Infected Individuals. PLoS One. 2012;7(9):e44454. doi: 10.1371/journal.pone.0044454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baker JV, Neuhaus J, Duprez D, Kuller LH, Tracy R, Belloso W, et al. Changes in inflammatory and coagulation biomarkers: a randomized comparison of immediate versus deferred antiretroviral therapy in patients with HIV infection. JAIDS. 2011;56(1):36–43. doi: 10.1097/QAI.0b013e3181f7f61a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Neuhaus J, Jacobs DR, Jr, Baker JV, Calmy A, Duprez D, La Rosa A, et al. Markers of inflammation, coagulation, and renal function are elevated in adults with HIV infection. J Infect Dis. 2010;201(12):1788–1795. doi: 10.1086/652749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Key NS, Mackman N. Tissue factor and its measurement in whole blood, plasma, and microparticles. Seminars in Thrombosis and Hemostasis. 2010;36(8):865–875. doi: 10.1055/s-0030-1267040. [DOI] [PubMed] [Google Scholar]
- 11.Satta N, Toti F, Feugeas O, Bohbot A, Dachary-Prigent J, Eschwege V, et al. Monocyte vesiculation is a possible mechanism for dissemination of membrane-associated procoagulant activities and adhesion molecules after stimulation by lipopolysaccharide. Journal of immunology. 1994;153(7):3245–3255. [PubMed] [Google Scholar]
- 12.Anderson KM, Wilson PW, Odell PM, Kannel WB. An updated coronary risk profile. A statement for health professionals. Circulation. 1991;83(1):356–362. doi: 10.1161/01.cir.83.1.356. [DOI] [PubMed] [Google Scholar]
- 13.Baker J, Ayenew W, Quick H, Hullsiek KH, Tracy R, Henry K, et al. High-density lipoprotein particles and markers of inflammation and thrombotic activity in patients with untreated HIV infection. J Infect Dis. 2010;201(2):285–292. doi: 10.1086/649560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harris TB, Ferrucci L, Tracy RP, Corti MC, Wacholder S, Ettinger WHJ, et al. Associations of elevated interleukin-6, C-reative protein levels with mortality in the elderly. American Journal of Medicine. 1999;106(5):506–5012. doi: 10.1016/s0002-9343(99)00066-2. [DOI] [PubMed] [Google Scholar]
- 15.Triant VA, Meigs JB, Grinspoon SK. Association of C-reactive protein and HIV infection with acute myocardial infarction. J Acquir Immune Defic Syndr. 2009;51(3):268–273. doi: 10.1097/QAI.0b013e3181a9992c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tzoulaki I, Murray GD, Lee AJ, Rumley A, Lowe GD, Fowkes FG. Relative value of inflammatory, hemostatic, and rheological factors for incident myocardial infarction and stroke: the Edinburgh Artery Study. Circulation. 2007;115(16):2119–2127. doi: 10.1161/CIRCULATIONAHA.106.635029. [DOI] [PubMed] [Google Scholar]
- 17.Stravitz RT, Bowling R, Bradford RL, Key NS, Glover S, Thacker LR, et al. Role of procoagulant microparticles in mediating complications and outcome of acute liver injury/Acute liver failure. Hepatology. 2013 doi: 10.1002/hep.26307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee RD, Barcel DA, Williams JC, Wang JG, Boles JC, Manly DA, et al. Pre-analytical and analytical variables affecting the measurement of plasma-derived microparticle tissue factor activity. Thrombosis research. 2012;129(1):80–85. doi: 10.1016/j.thromres.2011.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lewington S, Whitlock G, Clarke R, Sherliker P, Emberson J, Halsey J, et al. Blood cholesterol and vascular mortality by age, sex, and blood pressure: a meta-analysis of individual data from 61 prospective studies with 55,000 vascular deaths. Lancet. 2007;9602;370:1829–1839. doi: 10.1016/S0140-6736(07)61778-4. [DOI] [PubMed] [Google Scholar]
- 20.McQueen MJ, Hawken S, Wang X, Ounpuu S, Sniderman A, Probstfield J, et al. Lipids, lipoproteins, and apolipoproteins as risk markers of myocardial infarction in 52 countries (the INTERHEART study): a case-control study. Lancet. 2008;372(9634):224–233. doi: 10.1016/S0140-6736(08)61076-4. [DOI] [PubMed] [Google Scholar]
- 21.Funderburg NT, Mayne E, Sieg SF, Asaad R, Jiang W, Kalinowska M, et al. Increased tissue factor expression on circulating monocytes in chronic HIV infection: relationship to in vivo coagulation and immune activation. Blood. 2010;115(2):161–167. doi: 10.1182/blood-2009-03-210179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Funderburg NT, Zidar DA, Shive C, Lioi A, Mudd J, Musselwhite LW, et al. Shared monocyte subset phenotypes in HIV-1 infection and in uninfected subjects with acute coronary syndromes. Blood. 2012 doi: 10.1182/blood-2012-05-433946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. The New England journal of medicine. 2005;352(16):1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 24.Woollard KJ, Geissmann F. Monocytes in atherosclerosis: subsets and functions. Nat Rev Cardiol. 2010;7(2):77–86. doi: 10.1038/nrcardio.2009.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sandler NG, Wand H, Roque A, Law M, Nason MC, Nixon DE, et al. Plasma levels of soluble CD14 independently predict mortality in HIV infection. J Infect Dis. 2011;203(6):780–790. doi: 10.1093/infdis/jiq118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paton NI for the INSIGHT SMART Study Group. Association between activation of inflammatory and coagulation pathways and mortality during long-term follow-up in SMART. 5th IAS Conference on HIV Pathogenesis Treatment and Prevention; 2009. MOPEA034. [Google Scholar]
- 27.Folsom AR, Delaney JA, Lutsey PL, Zakai NA, Jenny NS, Polak JF, et al. Associations of factor VIIIc, D-dimer, and plasmin-antiplasmin with incident cardiovascular disease and all-cause mortality. Am J Hematol. 2009;84(6):349–353. doi: 10.1002/ajh.21429. [DOI] [PMC free article] [PubMed] [Google Scholar]