Abstract
Hepatocellular carcinoma (HCC) is increasing in incidence and has a very high fatality rate. Cirrhosis due to chronic hepatitis B or hepatitis C is the leading risk factor for HCC. Global epidemiology of HCC is determined by prevalence of dominant viral hepatitis and the age it is acquired in the underlying population. Upcoming risk factors include obesity, diabetes and related non-alcoholic fatty liver disease. This review discusses the latest trends of HCC globally and in the United States. It also provides an evidence-based commentary on the risk factors and lists some preventive measures to reduce the incidence of HCC.
Keywords: Hepatocellular carcinoma, epidemiology, chronic hepatitis B, chronic hepatitis C, prevention
Hepatocellular carcinoma (HCC) is the most common primary malignancy of the liver. It is the fifth most common cancer in men, worldwide, and seventh among women, with over half a million new cases diagnosed annually worldwide. It is the second leading cause of cancer related mortality in the world.1, 2 Chronic liver disease due to hepatitis B virus (HBV) or hepatitis C virus (HCV) accounts for the majority of HCC cases and thus, highly amenable to preventive measures. 3
Global Epidemiology of HCC
The distribution of HCC varies according to geographic location (Figure 1). The disease burden is highest in areas with endemic HBV infection (where HBsAg prevalence is 8% or more), such as in sub-Saharan Africa and Eastern Asia, with incidence rates of over 20 per 100,000 individuals. Mediterranean countries such as Italy, Spain, and Greece have intermediate incidence rates of 10-20 per 100,000 individuals, while North and South America have a relatively low incidence (< 5 per 100,000 individuals). The global age distribution of HCC cases is related to dominant viral hepatitis in the underlying population and the age at which it was acquired. In regions of high incidence the most common cause is HBV transmitted at birth, the diagnosis of HCC is about one decade earlier as compared with North America and Europe where the most common etiology is HCV acquired later in life. HCC is more common in men than women as HBV, HCV, and alcohol consumption are more prevalent and possibly more carcinogenic in males. In 80 to 90% cases, HCC occurs in the setting of cirrhosis.4
Figure 1.
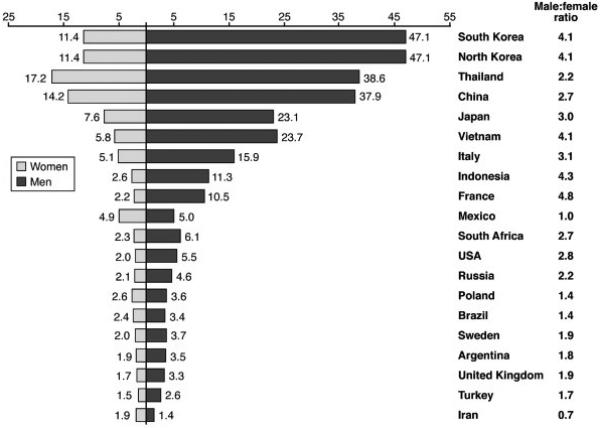
Age-standardized incidence rates of hepatocellular carcinoma per 100,000 populations at risk, in different regions of world (Source: GLOBOCAN 2002).
Trends of HCC in United States
Population-based data from SEER registries showed that overall annual age-adjusted incidence rates of HCC had doubled from 1.4 per 100,000 in 1975-77 to 4.8 per 100,000 in 2005-07.5 The greatest recent increase in incidence was seen in Hispanics and blacks among the ages of 45-65 years (Figure 2). 6 The overall 5-year survival is less than 12%, making HCC the fastest rising cause of cancer related death in United States. Both sexes showed a 3-fold increase in incidence of HCC from 1975-2007. Approximately half of the increase in HCC cases was attributed to the aging cohort with chronic hepatitis C infection. A study on veterans with chronic HCV showed that during 1996-2006 prevalence of cirrhosis and decompensated liver disease doubled, whereas the prevalence of HCC increased 10-fold (0.07% to 1.3%).7 HBV accounts for 10-15% of HCC cases in the US; less than 5% are infected with both viruses and 30-35% has neither HCV nor HBV. Increasing incidence of obesity and non-alcoholic fatty liver disease (NAFLD) is another factor responsible for the surge in HCC cases in United States.
Figure 2.
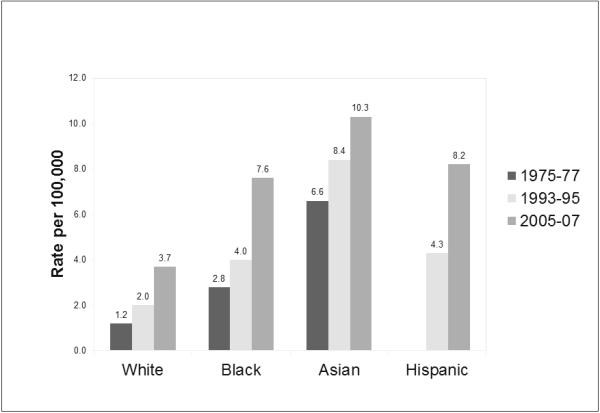
Age-adjusted incidence rate of hepatocellular carcinoma by race based on SEER registry data from 1975-2007.
Risk Factors for HCC
Hepatitis B Virus (HBV)
HBV is the leading risk factor for HCC globally and accounts for at least 50% cases of HCC.8 In endemic areas, HBV is mostly acquired by vertical and perinatal transmission with > 90% of these cases becoming chronic HBV carriers. In contrast, areas of low prevalence such as western countries, it is usually acquired in adulthood by horizontal transmission (through sexual and parenteral routes) with >90% of acute infections resolving spontaneously. HBV is a notorious HCC cause in the absence of cirrhosis; however, most (70%-90%) HBV-related HCC develops in cirrhotic livers.9 Several factors have been reported to increase HCC risk among HBV carriers, including demographic (male gender, older age, Asian or African ancestry, family history of HCC), viral (higher levels of HBV replication, HBV genotype, longer duration of infection, co-infection with HCV, HIV or HDV), clinical (cirrhosis) and environmental or life-style factors (exposure to aflatoxin, heavy alcohol drinking or tobacco smoking). In Asian studies, genotype C is associated with more severe liver disease, cirrhosis and the development of HCC, compared with genotype B; whereas in Western Europe and North America, genotype D is more associated with a higher incidence of HCC than genotype A, as well as the development of HCC in young carriers without cirrhosis.
Several meta-analysis have demonstrated that risk of HCC is 15-20 times greater among HBV infected individuals as compared to uninfected population.10, 11 A systematic review of the natural history of HBV in Asia summarized that incidence rate of HCC was 0.2 per 100 person-years in inactive carriers, 0.6 person-years with chronic HBV infection without cirrhosis, and 3.7 person-years in patients with compensated cirrhosis.12 The Risk Evaluation of Viral Load Elevation and Associated Liver Disease/ Cancer-Hepatitis B Virus (REVEAL-HBV) study from Taiwan showed that the risk of HCC in HBV infected participants increased in proportion to viral load (HBV DNA) independent of age, sex, smoking, alcohol consumption, and HbeAg status.13 There is a lack of high quality studies from Europe and North America, but reasonable estimates of HBV natural history in western population are incidence rate of 0.02 per 100 person-years in inactive carriers, 0.3 in chronic carriers without cirrhosis and 2.2 in subjects with compensated cirrhosis.14
Hepatitis C Virus (HCV)
HCV was detected in 44-66% of patients with HCC in Italy, and among 80% of HCC cases in Japan.15-17 In the United States, chronic HCV is the leading risk factor for HCC. Prospective studies have shown increased risk of HCC among HCV infected cohort.18 A meta-analysis of case control studies showed that individuals positive for anti-HCV had 17 times the risk of HCC as compared to anti-HCV negative cohort.19
HCV appears to increase the risk of HCC by inducing hepatic inflammation and importantly fibrosis, but also promoting malignant transformation of infected cells.20 The risk is highest among cirrhotics where HCC develops at rate of 1-4% per year, though rates up to 8% have been reported in Japan.21 HCC has also been reported in chronic HCV patients without cirrhosis but with intermediate to advanced hepatic fibrosis. The Hepatitis C Antiviral Long Term Treatment Against Cirrhosis trial (HALT-C) showed that 8% patients without cirrhosis, but with advanced fibrosis, developed HCC.22 Other risk factors that increase risk of HCC in infected patients include male sex, co-infection with HIV, HBV, HCV genotype 1b, old age, presence of diabetes and obesity, and a high level of chronic alcohol consumption. A meta-analysis of 21 studies presenting age-adjusted risk estimates for HCV genotype 1b vs. other HCV genotypes reported that patients infected with HCV genotype 1b have almost double the risk of developing HCC of those infected with other genotypes (pooled relative risk = 1.78, 95% CI: 1.36-2.32). 23 The pooled risk estimate was lower in an analysis limited to the 8 studies conducted in patients with liver cirrhosis (1.60; 1.07-2.39). There is no consistent evidence that HCV viral load or quasispecies are important in determining the risk of progression to HCC.
Non-Alcoholic Fatty Liver Disease (NAFLD)
NAFLD is now the leading cause of chronic liver disease in the United States. Features of metabolic syndrome such as visceral obesity or insulin resistance are present in virtually all cases of NAFLD. A population-based study using SEER-Medicare database showed a statistically significant association between metabolic syndrome and HCC.24 Most likely, this increased risk is thought to be due to NASH.
There are several case-reports and series of well-documented patients with NAFLD/NASH who developed HCC. However, two population based cohort studies in patients with NAFLD/ NASH showed no increased incidence and 0.3% risk of HCC, respectively, over 6 years of follow up.25, 26 Studies of NAFLD or NASH cohorts, with few or no cirrhosis cases, demonstrated a minimal HCC risk (cumulative HCC mortality between 0% and 3% for study periods up to two decades). 27, 28 Similarly, a study published from Denmark that included biopsy proven NAFLD/NASH cases, but without significant fibrosis, found no increased risk of HCC when followed up for almost 21 years.29 Conversely, consistently increased risk was observed in NASH-cirrhosis cohorts. 30-35 They also showed that risk of HCC was lower in NAFLD-related cirrhosis as compared to its incidence in cirrhosis due to viral hepatitis. Natural history cohort studies that lacked control groups reported cumulative incidence of HCC mortality between 0.25% and 2.3%. 36-40 The determinants of elevated risk among NASH-C cohorts are unclear, as most studies were underpowered to perform multivariate analysis.
In summary, modest epidemiological evidence supports the association between NAFLD / NASH and increased risk of HCC that seems to be limited to patients with cirrhosis; and this risk is lower as compared to cirrhosis from other etiologies.
Aflatoxin
Aflatoxins are carcinogens produced by Aspergillus species (A flavus and A parasiticus) and infest grains, corn, cassava, peanuts, and fermented soybeans, particularly when stored in high moisture conditions. There is a high incidence of HCC in regions where aflatoxin contaminated food ingestion is common, such as in parts of sub-Saharan Africa and eastern Asia; however, these regions have high HBV prevalence as well. Majority of HCC in these regions were found to have mutation of tumor suppressor gene p53 that is most likely caused by aflatoxins.41 There is no evidence of aflatoxin related HCC cases in the US. A study from China demonstrated about 60 fold increased risk of HCC in patients with chronic HBV and aflatoxin exposure.42
Alcohol
Although heavy alcohol intake increases the risk of HCC through the development of cirrhosis, there is no definite evidence to show carcinogenic potential of alcohol. Alcohol acts in synergism with HCV and HBV infection, presumably accelerating the process of fibrosis and progression to cirrhosis. A meta-analysis showed that in chronic HCV patients, odds of developing cirrhosis were 2.3 higher in heavy alcohol drinkers as compared to none or low quantity drinkers.43 Another study showed that risk of HCC increased in a linear fashion with heavy alcohol intake (>60g/ day), and this risk doubled in those infected with HCV. Similarly, a study on patients with HBV related cirrhosis; HCC risk was increased three times by heavy alcohol intake.44 The effect of low or moderate amount of alcohol intake on risk of HCC is unclear.
Coffee
Several epidemiological studies have elucidated the link between heavy coffee consumption and low liver enzymes, reduced risk of cirrhosis and reduced risk of HCC. Chronic HCV patients who consumed high levels of coffee were found to have lower fibrosis score.45 A meta-analysis of studies on risk of HCC among coffee drinkers in European and Japanese studies showed a reduced risk of HCC. They concluded that relative risk of HCC among low or moderate coffee drinkers (defined as 1-2 cups / day) was 0.70 (95% CI 0.57-0.85), and that for high drinkers (defined as ≥ 3 cups / day) was 0.45 (95% CI 0.38-0.53) as compared to non-drinkers.46 Although the mechanism of this possible protective effect is unclear, coffee consumption lowers insulin levels and reduces the risk of diabetes, a known risk factors for HCC.47 Animal studies also suggest that coffee contains compounds with anti-carcinogenic properties.
Host Genetic Factors
HCC develops only in small percentage of those infected with HCV or HBV. Host genetic makeup may be an important factor that influences progression to HCC. Two meta-analysis indentified variants of tumor necrosis factor (TNF) associated with higher risk of HCC.48, 49 They showed that TNFα-308 AA and AG variants (vs. GG) were associated with a significantly increased risk of HCC. A recent meta-analysis concluded that null genotypes of glutathione S-transferase (GST) genes (GSTM1 or GSTT1) were associated with an increased risk of HCC.50
Prevention of HCC
HBV vaccination
Development of HBV vaccine has been a major success in reducing the incidence of HBV infection and subsequent development of HCC. The vaccine is safe and effective against all HBV genotypes and serotypes. HBV vaccine is recommended for all newborns, pregnant women at their first pre-natal visit, and high-risk individuals. Neonates born to HBV infected mothers should get a dose of hepatitis B immune globulin in addition to vaccination. Countries like Taiwan that have implemented universal hepatitis B vaccination program have demonstrated its success. Twenty years after adopting the program, HBV carrier rate among children in Taiwan has decreased to 1.2% and incidence of HCC among the vaccinated children has dropped by 70%. 51
Antiviral treatment
A metanalysis of non-randomized trials and observational studies demonstrated reduced risk of HCC after anti-viral treatment for HBV infection. A multicenter randomized controlled study on patients with chronic HBV and advanced hepatic fibrosis showed that 3.9% developed HCC on lamivudine therapy as compared to 7.4% in placebo arm when treated up to 5 years. 52
Randomized and non-randomized studies have shown that achieving sustained viral response in chronic HCV patients, both with and without cirrhosis, leads to a substantial reduction in risk of HCC. 53, 54 Although viremia of any level is a risk factor for HCC, viral load is not associated with HCC. HCV+ patients with advanced fibrosis who clear viremia with anti-viral treatment have a reduced, but not eliminated risk, of HCC and should undergo surveillance for HCC.
HCC Surveillance
Only one randomized trial from China showed that HCC related mortality was reduced by 37% in the surveillance arm that consisted of AFP and ultrasound every 6 months compared with a no surveillance control arm.55 Observational cohort and case control studies, with their inevitable limitations, have demonstrated similar benefits for surveillance.56, 57 This expected benefit and the fact that patients diagnosed early are eligible for liver transplantation, prompted some but not all professional societies such as American Association for the study of Liver diseases to recommend surveillance for patients at high risk of HCC.
Alpha-fetoprotein (AFP) surveillance with cutoff as 20 ng/ml has a sensitivity of only 60% and is inadequate as sole means of surveillance. Ultrasonography has sensitivity between 65-80% and specificity of over 95% as a surveillance modality. 58 Although AASLD guidelines recommend 6 monthly screening with ultrasound, we feel that using combination of alpha-fetoprotein and ultrasonography can increase the yield of screening. Adherence to guidelines is a problem as highlighted in a study conducted on Veterans Administration (VA) practice setting, where most patients who were eligible for surveillance did not receive regular imaged based surveillance.59 Computed tomography (CT) and magnetic resonance imaging (MRI) are better at imaging the liver as compared to ultrasonography, but they have not been examined as surveillance tools, and therefore should be used for diagnosis and staging HCC rather than surveillance.
HBV and HCV will continue to be the leading cause of HCC. Antiviral treatment for chronic hepatitis B and C while very efficacious, their effectiveness in community practice is low due to the barriers in access, diagnosis, cost of medications, and presence of co-morbidities and contraindications. Large proportions of infected population live undiagnosed with HBV or HCV for decades and only get detected when they seek medical care for advanced liver disease or HCC. In a new initiative, U.S. Centers for Disease Control and Prevention now recommends routine screening for HCV in all baby boomers born between the years 1945-1965. Measures to increase awareness among general population, primary care providers and improving access to specialist care are some other steps that can help in reducing the incidence of HCC.
Acknowledgments
Funding: Authors report no funding for this article.
Footnotes
Conflict of Interest: The authors disclose no conflicts.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.GLOBOCAN International Agency for Research on Cancer (IARC) 2002 Available at: http://www-dep.iarc.fr. [Google Scholar]
- 2.World Health Organization Mortality Database. WHO Statistical Information System. 2008 Available at: http://www.who.int/whosis. [Google Scholar]
- 3.Bosch FX, Ribes J, Cleries R, Diaz M. Epidemiology of hepatocellular carcinoma. Clin Liver Dis. 2005;9:191–211. doi: 10.1016/j.cld.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 4.Colombo M, de Franchis R, Del Ninno E, et al. Hepatocellular carcinoma in Italian patients with cirrhosis. N Engl J Med. 1991;325:675–680. doi: 10.1056/NEJM199109053251002. [DOI] [PubMed] [Google Scholar]
- 5.Davila JA, El-Serag HB. The Rising Incidence of Hepatocellular Carcinoma in the United States: an Update. Gastroenterology. 2012;142(suppl 1):S914–S914. [Google Scholar]
- 6.El-Serag HB, Lau M, Eschbach K, Davila J, Goodwin J. Epidemiology of hepatocellular carcinoma in Hispanics in the United States. Arch Intern Med. 2007;167:1983–1989. doi: 10.1001/archinte.167.18.1983. [DOI] [PubMed] [Google Scholar]
- 7.Kanwal F, Hoang T, Kramer JR, et al. Increasing prevalence of HCC and cirrhosis in patients with chronic hepatitis C virus infection. Gastroenterology. 2011;140:1182–1188. doi: 10.1053/j.gastro.2010.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parkin DM. The global health burden of infection-associated cancers in the year 2002. Int J Cancer. 2006;118:3030–3044. doi: 10.1002/ijc.21731. [DOI] [PubMed] [Google Scholar]
- 9.Yang JD, Kim WR, Coelho R, et al. Cirrhosis is present in most patients with hepatitis B and hepatocellular carcinoma. Clin Gastroenterol Hepatol. 2011;9:64–70. doi: 10.1016/j.cgh.2010.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shi J, Zhu L, Liu S, Xie WF. A meta-analysis of case-control studies on the combined effect of hepatitis B and C virus infections in causing hepatocellular carcinoma in China. Br J Cancer. 2005;92:607–612. doi: 10.1038/sj.bjc.6602333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Donato F, Boffetta P, Puoti M. A meta-analysis of epidemiological studies on the combined effect of hepatitis B and C virus infections in causing hepatocellular carcinoma. Int J Cancer. 1998;75:347–354. doi: 10.1002/(sici)1097-0215(19980130)75:3<347::aid-ijc4>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 12.Fattovich G, Bortolotti F, Donato F. Natural history of chronic hepatitis B: special emphasis on disease progression and prognostic factors. J Hepatol. 2008;48:335–352. doi: 10.1016/j.jhep.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 13.Chen CJ, Yang HI, Su J, et al. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. Jama. 2006;295:65–73. doi: 10.1001/jama.295.1.65. [DOI] [PubMed] [Google Scholar]
- 14.El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142:1264–1273. doi: 10.1053/j.gastro.2011.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fasani P, Sangiovanni A, De Fazio C, et al. High prevalence of multinodular hepatocellular carcinoma in patients with cirrhosis attributable to multiple risk factors. Hepatology. 1999;29:1704–1707. doi: 10.1002/hep.510290604. [DOI] [PubMed] [Google Scholar]
- 16.Stroffolini T, Andreone P, Andriulli A, et al. Gross pathologic types of hepatocellular carcinoma in Italy. Oncology. 1999;56:189–192. doi: 10.1159/000011963. [DOI] [PubMed] [Google Scholar]
- 17.Yoshizawa H. Hepatocellular carcinoma associated with hepatitis C virus infection in Japan: projection to other countries in the foreseeable future. Oncology. 2002;62(Suppl 1):8–17. doi: 10.1159/000048270. [DOI] [PubMed] [Google Scholar]
- 18.Goodgame B, Shaheen NJ, Galanko J, El-Serag HB. The risk of end stage liver disease and hepatocellular carcinoma among persons infected with hepatitis C virus: publication bias? Am J Gastroenterol. 2003;98:2535–2542. doi: 10.1111/j.1572-0241.2003.07678.x. [DOI] [PubMed] [Google Scholar]
- 19.Donato F, Tagger A, Gelatti U, et al. Alcohol and hepatocellular carcinoma: the effect of lifetime intake and hepatitis virus infections in men and women. Am J Epidemiol. 2002;155:323–331. doi: 10.1093/aje/155.4.323. [DOI] [PubMed] [Google Scholar]
- 20.Lemon SM, McGivern DR. Is hepatitis C virus carcinogenic? Gastroenterology. 2012;142:1274–1278. doi: 10.1053/j.gastro.2012.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fattovich G, Giustina G, Degos F, et al. Morbidity and mortality in compensated cirrhosis type C: a retrospective follow-up study of 384 patients. Gastroenterology. 1997;112:463–472. doi: 10.1053/gast.1997.v112.pm9024300. [DOI] [PubMed] [Google Scholar]
- 22.Lok AS, Everhart JE, Wright EC, et al. Maintenance peginterferon therapy and other factors associated with hepatocellular carcinoma in patients with advanced hepatitis C. Gastroenterology. 2011;140:840–849. doi: 10.1053/j.gastro.2010.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raimondi S, Bruno S, Mondelli MU, Maisonneuve P. Hepatitis C virus genotype 1b as a risk factor for hepatocellular carcinoma development: a meta-analysis. J Hepatol. 2009;50:1142–1154. doi: 10.1016/j.jhep.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 24.Welzel TM, Graubard BI, Zeuzem S, et al. Metabolic syndrome increases the risk of primary liver cancer in the United States: a study in the SEER-Medicare database. Hepatology. 2011;54:463–471. doi: 10.1002/hep.24397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sanyal A, Poklepovic A, Moyneur E, Barghout V. Population-based risk factors and resource utilization for HCC: US perspective. Curr Med Res Opin. 2010;26:2183–2191. doi: 10.1185/03007995.2010.506375. [DOI] [PubMed] [Google Scholar]
- 26.Ong JP, Pitts A, Younossi ZM. Increased overall mortality and liver-related mortality in non-alcoholic fatty liver disease. J Hepatol. 2008;49:608–612. doi: 10.1016/j.jhep.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 27.Soderberg C, Stal P, Askling J, et al. Decreased survival of subjects with elevated liver function tests during a 28-year follow-up. Hepatology. 2010;51:595–602. doi: 10.1002/hep.23314. [DOI] [PubMed] [Google Scholar]
- 28.Arase Y, Kobayashi M, Suzuki F, et al. Difference in malignancies of chronic liver disease due to non-alcoholic fatty liver disease or hepatitis C in Japanese elderly patients. Hepatol Res. 2012;42:264–272. doi: 10.1111/j.1872-034X.2011.00915.x. [DOI] [PubMed] [Google Scholar]
- 29.Dam-Larsen S, Becker U, Franzmann MB, et al. Final results of a long-term, clinical follow-up in fatty liver patients. Scand J Gastroenterol. 2009;44:1236–1243. doi: 10.1080/00365520903171284. [DOI] [PubMed] [Google Scholar]
- 30.Ratziu V, Bonyhay L, Di Martino V, et al. Survival, liver failure, and hepatocellular carcinoma in obesity-related cryptogenic cirrhosis. Hepatology. 2002;35:1485–1493. doi: 10.1053/jhep.2002.33324. [DOI] [PubMed] [Google Scholar]
- 31.Sanyal AJ, Banas C, Sargeant C, et al. Similarities and differences in outcomes of cirrhosis due to nonalcoholic steatohepatitis and hepatitis C. Hepatology. 2006;43:682–689. doi: 10.1002/hep.21103. [DOI] [PubMed] [Google Scholar]
- 32.Kojima H, Sakurai S, Matsumura M, et al. Cryptogenic cirrhosis in the region where obesity is not prevalent. World J Gastroenterol. 2006;12:2080–2085. doi: 10.3748/wjg.v12.i13.2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yatsuji S, Hashimoto E, Tobari M, et al. Clinical features and outcomes of cirrhosis due to non-alcoholic steatohepatitis compared with cirrhosis caused by chronic hepatitis C. J Gastroenterol Hepatol. 2009;24:248–254. doi: 10.1111/j.1440-1746.2008.05640.x. [DOI] [PubMed] [Google Scholar]
- 34.Ascha MS, Hanouneh IA, Lopez R, et al. The incidence and risk factors of hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. Hepatology. 2010;51:1972–1978. doi: 10.1002/hep.23527. [DOI] [PubMed] [Google Scholar]
- 35.Bhala N, Angulo P, van der Poorten D, et al. The natural history of nonalcoholic fatty liver disease with advanced fibrosis or cirrhosis: an international collaborative study. Hepatology. 2011;54:1208–1216. doi: 10.1002/hep.24491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matteoni CA, Younossi ZM, Gramlich T, et al. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116:1413–1419. doi: 10.1016/s0016-5085(99)70506-8. [DOI] [PubMed] [Google Scholar]
- 37.Adams LA, Lymp JF, St Sauver J, et al. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 2005;129:113–121. doi: 10.1053/j.gastro.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 38.Ekstedt M, Franzen LE, Mathiesen UL, et al. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006;44:865–873. doi: 10.1002/hep.21327. [DOI] [PubMed] [Google Scholar]
- 39.Hashimoto E, Yatsuji S, Tobari M, et al. Hepatocellular carcinoma in patients with nonalcoholic steatohepatitis. J Gastroenterol. 2009;44(Suppl 19):89–95. doi: 10.1007/s00535-008-2262-x. [DOI] [PubMed] [Google Scholar]
- 40.Kawamura Y, Arase Y, Ikeda K, et al. Large-scale long-term follow-up study of Japanese patients with non-alcoholic Fatty liver disease for the onset of hepatocellular carcinoma. Am J Gastroenterol. 2012;107:253–261. doi: 10.1038/ajg.2011.327. [DOI] [PubMed] [Google Scholar]
- 41.Bressac B, Kew M, Wands J, Ozturk M. Selective G to T mutations of p53 gene in hepatocellular carcinoma from southern Africa. Nature. 1991;350:429–431. doi: 10.1038/350429a0. [DOI] [PubMed] [Google Scholar]
- 42.Qian GS, Ross RK, Yu MC, et al. A follow-up study of urinary markers of aflatoxin exposure and liver cancer risk in Shanghai, People’s Republic of China. Cancer Epidemiol Biomarkers Prev. 1994;3:3–10. [PubMed] [Google Scholar]
- 43.Hutchinson SJ, Bird SM, Goldberg DJ. Influence of alcohol on the progression of hepatitis C virus infection: a meta-analysis. Clin Gastroenterol Hepatol. 2005;3:1150–1159. doi: 10.1016/s1542-3565(05)00407-6. [DOI] [PubMed] [Google Scholar]
- 44.Ikeda K, Saitoh S, Suzuki Y, et al. Disease progression and hepatocellular carcinogenesis in patients with chronic viral hepatitis: a prospective observation of 2215 patients. J Hepatol. 1998;28:930–938. doi: 10.1016/s0168-8278(98)80339-5. [DOI] [PubMed] [Google Scholar]
- 45.Modi AA, Feld JJ, Park Y, et al. Increased caffeine consumption is associated with reduced hepatic fibrosis. Hepatology. 2010;51:201–209. doi: 10.1002/hep.23279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bravi F, Bosetti C, Tavani A, et al. Coffee drinking and hepatocellular carcinoma risk: a meta-analysis. Hepatology. 2007;46:430–435. doi: 10.1002/hep.21708. [DOI] [PubMed] [Google Scholar]
- 47.Huxley R, Lee CM, Barzi F, et al. Coffee, decaffeinated coffee, and tea consumption in relation to incident type 2 diabetes mellitus: a systematic review with meta-analysis. Arch Intern Med. 2009;169:2053–2063. doi: 10.1001/archinternmed.2009.439. [DOI] [PubMed] [Google Scholar]
- 48.Guo YM, Wei WY, Shen XZ. Tumour necrosis factor 308 polymorphisms and hepatocellular carcinoma risk: a meta-analysis. Hepatogastroenterology. 2010;57:926–931. [PubMed] [Google Scholar]
- 49.Qin H, Liu B, Shi T, Liu Y, Sun Y, Ma Y. Tumour necrosis factor-alpha polymorphisms and hepatocellular carcinoma: a meta-analysis. J Int Med Res. 2010;38:760–768. doi: 10.1177/147323001003800304. [DOI] [PubMed] [Google Scholar]
- 50.White DL, Li D, Nurgalieva Z, El-Serag HB. Genetic variants of glutathione S-transferase as possible risk factors for hepatocellular carcinoma: a HuGE systematic review and meta-analysis. Am J Epidemiol. 2008;167:377–389. doi: 10.1093/aje/kwm315. [DOI] [PubMed] [Google Scholar]
- 51.Zanetti AR, Van Damme P, Shouval D. The global impact of vaccination against hepatitis B: a historical overview. Vaccine. 2008;26:6266–6273. doi: 10.1016/j.vaccine.2008.09.056. [DOI] [PubMed] [Google Scholar]
- 52.Liaw YF, Sung JJ, Chow WC, et al. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med. 2004;351:1521–1531. doi: 10.1056/NEJMoa033364. [DOI] [PubMed] [Google Scholar]
- 53.Singal AG, Volk ML, Jensen D, Di Bisceglie AM, Schoenfeld PS. A sustained viral response is associated with reduced liver-related morbidity and mortality in patients with hepatitis C virus. Clin Gastroenterol Hepatol. 2010;8:280–288. doi: 10.1016/j.cgh.2009.11.018. 288. [DOI] [PubMed] [Google Scholar]
- 54.Singal AK, Singh A, Jaganmohan S, et al. Antiviral therapy reduces risk of hepatocellular carcinoma in patients with hepatitis C virus-related cirrhosis. Clin Gastroenterol Hepatol. 2010;8:192–199. doi: 10.1016/j.cgh.2009.10.026. [DOI] [PubMed] [Google Scholar]
- 55.Zhang BH, Yang BH, Tang ZY. Randomized controlled trial of screening for hepatocellular carcinoma. J Cancer Res Clin Oncol. 2004;130:417–422. doi: 10.1007/s00432-004-0552-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Trevisani F, De NS, Rapaccini G, et al. Semiannual and annual surveillance of cirrhotic patients for hepatocellular carcinoma: effects on cancer stage and patient survival (Italian experience) Am J Gastroenterol. 2002;97:734–744. doi: 10.1111/j.1572-0241.2002.05557.x. [DOI] [PubMed] [Google Scholar]
- 57.Sherman M. Surveillance for hepatocellular carcinoma and early diagnosis. Clin Liver Dis. 2007;11:817–837. doi: 10.1016/j.cld.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 58.Singal A, Volk ML, Waljee A, et al. Meta-analysis: surveillance with ultrasound for early-stage hepatocellular carcinoma in patients with cirrhosis. Aliment Pharmacol Ther. 2009;30:37–47. doi: 10.1111/j.1365-2036.2009.04014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.El-Serag HB, Kramer JR, Chen GJ, et al. Effectiveness of AFP and ultrasound tests on hepatocellular carcinoma mortality in HCV-infected patients in the USA. Gut. 2011;60:992–997. doi: 10.1136/gut.2010.230508. [DOI] [PubMed] [Google Scholar]


