Abstract
The adipocyte derived hormone leptin acts in the brain to regulate body weight, food intake and energy expenditure. Even though it is well accepted that leptin regulates energy expenditure at least in part by modulating thermogenesis, the exact mechanisms are not clear. Particularly, it is unclear which central circuits regulate thermogenic leptin actions and if and how these may interact with feeding circuits. Within the last decade our understanding of central thermoregulatory circuits has increased substantially and allowed the identification of leptin target neurons (those expressing the long form leptin receptor –LepRb) that are involved in the sympathetic control of the heat generating brown adipose tissue (BAT). Indeed, LepRb neurons in the preoptic area and dorsomedial hypothalamus are part of known thermoregulatory circuits controlling sympathetic premotor neurons that are located in the raphe pallidus. Thermoregulatory control and food intake are both regulated by leptin signaling pathways, even though distinct neuronal pathways have been described, respectively. Nevertheless, feeding status and control of body temperature and energy expenditure are tightly interconnected, but it is unknown how these aspects are connected within leptin signaling pathways to result in appropriate output signals (e.g. BAT thermogenesis). Indeed, cold-induced thermogenesis is potently blocked during fasting, which instead triggers an active decrease in energy expenditure and body temperature, a state known as torpor. In this article we will review recent data characterizing central thermoregulatory LepRb pathways and speculate on potential integration mechanisms that may relay anorexic and thermoregulatory leptin action to control energy homeostasis.
Keywords: leptin, thermoregulation, energy expenditure, dorsomedial hypothalamus, preoptic area, arcuate nucleus
1. Leptin, Thermogenesis, and Energy Expenditure
Leptin is a pleiotropic adipocyte derived hormone that controls body weight, food intake and energy expenditure [1]. This is most impressively demonstrated in rodents and humans that lack leptin or its receptor (LepRb), where dramatic hyperphagia and early onset obesity are most prominent features. In addition, it had been noticed early on, even before the identification of the leptin gene, that leptin deficient ob/ob mice display low body temperature (1–2 °C below control littermates) and do not survive acute cold-exposure [2–5]. Importantly, these thermogenic abnormalities associated with leptin deficiency can be corrected by exogenous leptin replacement [6], demonstrating that leptin also regulates thermoregulatory circuits.
Thermoregulation is one of the most important mechanisms in animal physiology. Not only is it crucial for preventing cellular damage from physiologically extreme temperature, but also for optimizing biological activity and consequently body function. For example poikilotherm animals such as lizards and snakes need to warm their bodies in the sun before they are able to move quickly and hunt effectively. Mammals – including humans – are homeotherms and are able to maintain body temperature despite fluctuations in environmental temperature. By employing behavioral and metabolic adaptations, homeotherms can promote heat dissipation (panting, wetting of fur) to prevent overheating or facilitate heat generation and preservation (huddling, piloerection, vasoconstriction, shivering, non-shivering thermogenesis) to prevent hypothermia (e.g. cold-induced thermogenesis). However, under thermoneutral conditions neither heat generating nor heat dissipating mechanisms are engaged. Interestingly, the hypothermia and hypometabolism observed in leptin deficient ob/ob mice is absent at thermoneutral conditions, further demonstrating an important role of leptin to enable proper cold-induced thermogenesis [2, 7]. Thermogenesis is also important in fighting infection. Following inflammatory responses to invading pathogens, body temperature is actively increased to produce fever (e.g. pyrogen-induced thermogenesis). Low leptin levels (during starvation) or leptin deficiency results in abnormal inflammatory responses and abnormal fever induction [8–10], although the latter remains controversial [11].
Heat production, to prevent hypothermia or to induce fever, requires energy demanding adaptations and will thus increase energy expenditure. The best studied effector organ that promotes heat production is the brown adipose tissue (BAT). BAT is specifically characterized by the expression of uncoupling protein 1 (UCP1), which is a mitochondrial proton channel that, when opened, enables proton influx to the mitochondrial lumen. UCP1 uncouples the proton motive force of the respiratory chain from ATP production and instead releases energy surplus as heat [12–14]. This uncoupling process is regulated by sympathetic activation via β3-adrenergic receptors (β3-AR), fatty acids and thyroid hormone [15–18]. β3-AR stimulation induces UCP1 gene expression, UCP1 activity and β-oxidation; the latter is particularly crucial to fuel the energy demanding thermogenic process. Induction of BAT thermogenesis has been also termed adaptive thermogenesis or non-shivering thermogenesis and distinguishes this tightly regulated heat producing process from other non-regulated processes (e.g. thermic effects of metabolic processes) or behavioral processes that also contribute to heat production(e.g. shivering, locomotor activity) [19].
Aside from its fundamental role in maintaining body temperature, BAT thermogenesis also has the capacity to prevent excessive body weight gain in response to a high fat diet (HFD), a phenomenon which has been termed diet-induced thermogenesis [20]. However, the importance of BAT thermogenesis in body weight control, particularly in humans, has been debated [21, 22]. Despite the controversy and supported by the recent demonstration of substantial amounts of functional BAT deposits in adult humans [23–26], BAT thermogenesis has reemerged as a potentially viable target to treat obesity.
While it is well established that β3-adrenergic signaling and thyroid hormone govern mitochondrial uncoupling in BAT, multiple lines of evidence have also implicated leptin as a regulator of BAT thermogenesis. In rodents, leptin deficiency is associated with BAT atrophy and low UCP1 expression, thus compromising BAT-derived heat production [27, 28] and exogenous leptin injections stimulate sympathetic BAT inputs [29]. Leptin also appears to be essential for weight loss mediated by BAT thermogenesis. The food intake suppressing effects of exogenously injected leptin in ob/ob mice is largely responsible for the observed rapid decrease in body weight (about 65%); as demonstrated in pairfeeding experiments where vehicle treated ob/ob mice are restricted to the low food intake of leptin treated ob/ob mice. However, this left a significant amount of weight loss (about 35%) that was attributed to leptin's food intake independent effects [30, 31]. Interestingly, this phenomenon is absent in UCP1-deficient mice, strongly suggesting that BAT thermogenesis is required for food intake independent leptin induced weight loss [32].
In humans, leptin similarly prevents the hypometabolism that is commonly associated with dieting [33, 34], but the mechanisms (central sites) and peripheral target tissues (e.g. BAT, muscle) involved in increasing energy expenditure are unclear. Thus, while leptin's thermoregulatory effects are well documented, the central mechanisms of thermoregulatory leptin action and the importance for body weight regulation are not well understood.
2. Central Control of BAT Thermogenesis
Most research on BAT thermogenesis and body weight regulation has focused on BAT function per se, disregarding central circuits that control BAT thermogenesis. However, over the last decade the pioneering work by Shaun F. Morrison and colleagues has accumulated substantial insight into the distinct neuronal circuits that are key players in the control of cold- and pyrogene-induced BAT thermogenesis. These data have been recently compiled in two excellent review articles [35, 36].
2.1. Raphe pallidus – Master Regulator of BAT Thermogenesis
The first central nervous system (CNS) sites identified as centers of BAT thermogenesis control were discovered using a modified pseudorabies virus (PRV) injected into the BAT. This viral retrograde and transsynaptic tracer is specifically taken up into sympathetic nerve endings that innervate the BAT. PRV replicates in nerves and is retrogradely and transsynaptically transported, resulting in effective labeling CNS neurons that are involved in the sympathetic regulation of BAT [37, 38]. Further comparison with cold-induced c-Fos (an immediate early gene used as a surrogate for neuronal activity) provided evidence for the paraventricular hypothalamus (PVN) and the rostral raphe pallidus (rRPa) as potential regulators of BAT thermogenesis [37]. Indeed, it was confirmed in anesthetized rats, that disinhibiting rRPa neurons with the gamma-aminobutyric acid (GABA)-receptor antagonist bicucullin (translating into activation of rRPa neurons, similar to cold-exposure) results in a robust elevation of sympathetic BAT activity and BAT temperature [39]. Conversely, inhibition of rRPa neurons resulted in suppression of cold- [40] as well as pyrogen-induced [39] sympathetic BAT activity and BAT temperature. Taken together, these findings indicated that both hypothermic and febrile responses mounted in BAT are mediated via rRPa neurons. These rRPa neurons are named BAT sympathetic premotor neurons, because they directly innervate BAT sympathetic motor neurons in the spinal cord [38]. Inhibition of serotonin receptors in the rRPa prevents leptin induced thermogenesis [41], even though LepRb is not directly expressed on rRPa neurons [42, 43]. Thus, thermogenic leptin action engages neuronal circuits that indirectly modulate rRPa neurons. Nevertheless, whether rRPa neurons play a role in the induction of diet-induced thermogenesis and thus body weight control has not been studied so far.
2.2. Hypothalamic Coordination of Diverse Sensory Inputs
As discussed above, premotor neurons in the rRPa receive both excitatory and inhibitory signals from synapsing neurons the release the neurotransmitters glutamate and GABA, respectively. Low ambient temperature or pyrogens are thought to strengthen excitatory over inhibitory inputs, to increase BAT thermogenesis, while strengthening inhibitory over excitatory inputs would result in decreased BAT thermogenesis.
Thermoregulatory control is initiated via sensory neurons in the skin, abdomen, spinal cord, but also via thermosensing neurons in the CNS, e.g. warm sensing neurons in the preoptic area (POA) [36]. Fever responses to lipopolysaccharide (LPS) can be initiated by activation of prostaglandin (PGE2) receptor expressing neurons in the POA [44], and inhibition of rRPa neurons originates at least from neurons within the POA [45], which sense and integrate temperature information from the CNS, periphery and deep-body via thermo-receptors [35]. POA neurons also provide inhibitory inputs to the dorsomedial hypothalamus/dorsal hypothalamic area (DMH/DHA) [46–50]. The DMH/DHA is another hypothalamic site that is labeled by BAT injected PRV [37]. DMH/DHA neurons are stimulated by cold and pyrogens [50, 51] and likely represent glutamatergic neurons [47]. DMH/DHA neurons innervate the rRPa and are critical to further stimulate rRPa neurons and sympathetic BAT activity [49, 52]. Thus, glutamatergic, excitatory inputs to the rRPa originate at least in part from DMH/DHA neurons [47]. The rRPa also receives direct inputs from orexin neurons in the lateral hypothalamus (LHA) [53]. And additional inputs from the nucleus of the solitary tract (NTS) had been hypothesized [54] and very recently confirmed [55].
Within this POA>DMH/DHA>rRPa>BAT neuronal pathway leptin receptor (LepRb) expressing neurons are found in the POA and DMH/DHA, but not the rRPa. Pointedly, LepRb neurons in the POA and DMH/DHA are labeled by PRV injections into the BAT, indicating that these thermoregulatory POA and DMH/DHA neurons are direct leptin targets [42]. Subsequent experiments further demonstrated that POA and DMH/DHA LepRb neurons are part of the known thermoregulatory circuit: POA LepRb neurons directly innervate the DMH/DHA, and POA and DMH/DHA LepRb neurons both innervate directly the rRPa [42] (Figure 1).
Figure 1.
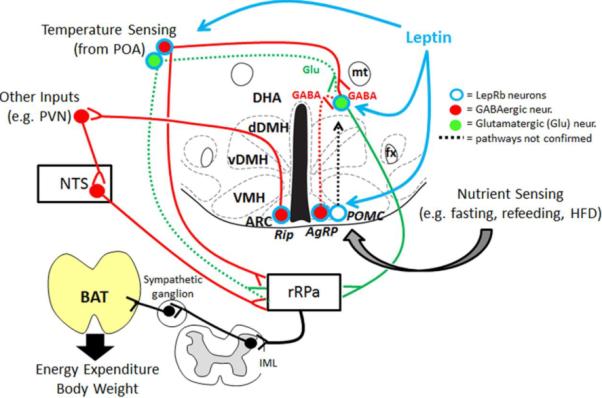
Schematic summary of known thermoregulatory LepRb circuits with emphasis on the DMH/DHA as possible relay site for the integration of diverse sensory inputs like nutrients and temperature. Low leptin levels, e.g. during fasting and in leptin signaling deficiency mice, increase neuronal firing of GABAergic AgRP neurons and may fail to stimulate glutamatergic DMH/DHA LepRb neurons, which prevents induction of BAT thermogenesis during cold exposure and would permit the induction of torpor. POA = preoptic area, DHA = dorsal hypothalamic area, dDMH = dorsal portion of the dorsomedial hypothalamus, vDMH = ventral portion of the dorsomedial hypothalamus, VMH = ventromedial e.g.hypothalamus, ARC = arcuate nucleus, mt = mammillothalamic tract, fx = fornix, rRPa = rostral raphe pallidus, IML = intermediolateral bundle, BAT = brown adipose tissue, Glu =glutamate, GABA = gamma-aminobutyrate acid, HFD = high fat diet, AgRP = agouti related peptide, POMC = pro-opiomelanocortin, PVN = paraventricular nucleus, NTS = nucleus of the solitary tract
Furthermore, cold exposure increased c-Fos expression in DMH/DHA LepRb neurons [42], strongly suggesting that the thermoregulatory defects observed in ob/ob mice involves defective neuronal activation of DMH/DHA LepRb neurons and possibly POA LepRb neurons. In line with this, leptin injections into the DMH have recently been shown to increase BAT temperature in wildtype mice [56], although this study targeted the DMH per se, not the DMH/DHA. Interestingly, thermogenenic leptin action in the DMH was not affected by leptin resistance because diet-induced obese mice responded equally well with a leptin-induced raise in BAT temperature [56]. Contrary, leptin-induced anorexia was severely reduced, which is thought to be mediated by leptin action in the arcuate nucleus [56, 57]. In fact, hyperleptinemic DIO mice even showed an increased BAT temperature in response to a high-fat-diet, suggesting that this diet-induced thermogenesis may indeed result from enhanced leptin signaling in hyperleptinemic DIO mice [56].
Further studies need to dissect in detail if the DMH/DHA is uniquely involved in thermogenic control, or if other DMH neurons either feed into the known thermoregulatory circuits or even describe additional thermoregulatory circuits. Indeed, neuropeptide Y expressing neurons in the compact portion of the DMH importantly regulate energy expenditure and BAT thermogenesis [58, 59], even though their projection into known thermoregulatory sites has not been investigated yet.
It is still unclear how thermogenic leptin actions are integrated at the level of DMH/DHA LepRb neuron, e.g. does leptin inhibit GABAergic POA inputs to the DMH/DHA and stimulate putative glutamatergic DMH/DHA LepRb neurons? Accordingly, future studies need to dissect the neurochemical nature of these thermoregulatory LepRb circuits and investigate what aspects of thermogenesis these neuronal populations control (cold-, pyrogen-, diet-induced thermogenesis).
2.3. Additional Modulation of Thermoregulatory Circuits
As mentioned earlier, the PVN has also been identified as potential regulator of BAT thermogenesis, based on BAT derived PRV labeling and cold-induced c-Fos expression in the PVN [37]. The PVN is a unique hypothalamic site where energy homeostasis is regulated via control of autonomic, neuroendocrine, and behavioral functions. In contrast to rRPa neurons, chemical activation of PVN neurons does not induce BAT sympathetic nerve activity or thermogenesis. However, PVN activation (or disinhibition) prevents cold- and pyrogen-induced thermogenesis [60]. This suggests that PVN neurons do not act as major BAT sympathetic premotor neurons, but instead control thermogenesis indirectly via sympathetic pre-motor neurons, e.g. in the rRPa. Furthermore, these thermoregulatory PVN signals are inhibitory and are under inhibitory control [60].
Such an inhibitory control signal to the PVN was recently identified and originates from GABAergic neurons in the arcuate nucleus (ARC). These GABAergic neurons express LepRb, but are distinct from typical LepRb expressing melanocortin neurons (agouti related peptide - AgRP neurons and pro-opiomelanocortin - POMC neurons). Instead, these GABAergic ARC neurons are characterized by driving expression from the rat insulin-2 promoter (Rip neurons) [55]. Indeed, stimulation of ARC Rip neurons induces energy expenditure, and deletion of GABA signaling from Rip neurons in the ARC suppresses energy expenditure and BAT thermogenesis. Interestingly, leptin induced BAT temperature is partially suppressed in animals that selectively lack GABA signaling in Rip neurons [55], further indicating that LepRb neurons in the ARC contribute the thermoregulatory control and thus integrate into thermoregulatory circuits.
Inhibitory PVN inputs lay downstream of the POA and DMH/DHA, but upstream of rRPa neurons, because disinhibition of the PVN blocks POA and DMH/DHA evoked thermogenesis, but not rRPa evoked thermogenesis [60]. Kong et. al. [55] suggested that GABAergic PVN neurons do not directly innervate the rRPa, but instead control inhibitory NTS neurons that directly inhibit rRPa neurons. However, inhibition of rRPa neurons has been repeatedly demonstrated to prevent BAT thermogenesis and thus is conflicting with known thermoregulatory circuits [36]. Therefore, future studies will have to address this discrepancy and dissect how PVN signals are integrated into the rRPa.
Also, other inhibitory inputs to the PVN are likely and have not been considered yet. For example, the vast majority of LepRb neurons in the ventral DMH is GABAergic (the ventral DMH does not include the DMH/DHA) [61] (and own unpublished observations), is stimulated by leptin and heavily innervates the PVN [42, 62]. Therefore, several sources of inhibitory PVN inputs may contribute to thermoregulatory modulation and parallel hypothalamic pathways exist that ultimately integrate into the control of rRPa neuronal activity and BAT thermogenesis.
The ventromedial hypothalamus (VMH) has been historically associated with thermoregulatory control due to early studies using pharmacological and deletion experiments [63–65], even though PRV tracing studies from BAT failed to reliably identify VMH neurons with thermoregulatory capacity. More recent work carefully revisited these original studies and suggested that the anatomically large size of deletions and injections may have confounded the original conclusions. Based on dose response studies it was rather hypothesized that the observed thermogenic effects could have resulted from leakage into the nearby DMH/DHA structure [52, 66, 67]. Nevertheless, given the strong interconnectivity of the DMH with virtually all other hypothalamic sites, including the VMH [68], it seems very possible that VMH neurons may contribute to thermoregulatory effects by feeding into the DMH/DHA or PVN network. Consistent with this, LepRb deletion selectively from SF-1 (steroidogenic factor-1) expressing VMH neurons, which are exclusively glutamatergic [61], fail to properly increase energy expenditure in response to a high fat diet and gain significantly more weight [69], suggesting that LepRb VMH neurons indeed could integrate nutritional information into thermoregulatory circuits.
Clearly, thermoregulation and thermoregulatory leptin action involves a tight interaction of hypothalamic and brainstem mechanisms. However, it is interesting that the brainstem, which is evolutionary an older part of the CNS, is able to mediate some thermoregulatory leptin functions independent of the hypothalamus, as observed in decerebrated rats [70] and other systems [54, 71, 72]. When leptin is injected into the 4th ventricle of decerebrated rats it only mildly raises brown fat and body temperature, but strikingly, if leptin injections are followed by thyroid releasing hormone (TRH) treatment, leptin robustly enhanced the thermogenic capacities of TRH alone [54, 72]. This sensitizing effect was dependent on phospholipase C and inositol-3-phosphate calcium release mechanisms and could be attributed to direct leptin effects on NTS neurons [73]. These NTS neurons were suggested to further stimulate rRPa neurons to control BAT thermogenesis, even though these connections remain to be validated. In fact, rRPa projecting NTS neurons have been particularly described as inhibitory GABAergic neurons [55], while LepRb expressing NTS neurons are exclusively glutamatergic [61].
Importantly, several other LepRb neurons co-localize with PRV labeling from the BAT including the retrochiasmatic area, ARC, NTS and Edinger Westphal nucleus (EW) [42], and the real challenge ahead will be to investigate how these pathways act together. Also, the parabrachial nucleus (PB) harbors a distinct population of LepRb expressing neurons, even though they rarely co-localized with BAT-related PRV labeled neurons [42] and their role in physiological leptin action is unknown. Interestingly, the PB plays a key role to mediate feeding behavior that is initiated by GABAergic projections from hypothalamic AgRP neurons [74]. Furthermore, the PB is an important relay for cutaneous thermosensory information [75, 76]. Thus, further studies are required to investigate if and how the PB integrates feeding and temperature information, and whether leptin plays a role to modulating these circuits either directly or indirectly.
It is possible that many or all of these different sites contribute equally to thermoregulatory control, however, given that many hypothalamic sites have been selectively attributed with specific physiological function it would be logical that hypothalamic and extra-hypothalamic inputs would be integrated at a certain level to guarantee that specific physiological situations (e.g. ambient and body temperature, nutritional state, humoral state, dehydration) are integrated and relayed into an appropriate output signal, such as an increase or decrease in BAT thermogenesis.
3. Integrating Sensory Inputs into BAT Thermogenesis
The discovery that leptin targets thermoregulatory circuits that control cold- and pyrogen-induced thermogenesis raises the question if LepRb neurons in this neuronal pathway further integrate other sensory inputs, e.g. the nutritional state of an animal that may initiate diet-induced thermogenesis or counteract cold-induced thermogenesis.
Understanding the integration of distinct neuronal circuits in body weight control is one of the most pressing tasks, because efforts to control excessive body weight gain with behavioral changes (e.g. dieting) have been largely unsuccessful partially due to weight loss induced hypometabolism, that promotes weight regain [33].
3.1. Leptin, Thermogenesis and Torpor
Physiologically, the need for a proper integration of diverse sensory inputs becomes clear when conflicting sensory inputs are present. As outlined above, cold exposure requires the induction of heat generating mechanisms. In contrast, a fasting state requires the organism to save energy. Thus, a fasting animal in a cold environment requires the integration of these obvious conflicting sensory inputs into an appropriate thermoregulatory response. Indeed, it is known that the combination of fasting and cold exposure induces regular torpor events in mammals, which is characterized by an active down regulation of energy expenditure, heart rate and respiration over several hours and causes a substantial drop in body temperature [77, 78]. Leptin importantly controls torpor behavior: the low serum leptin levels during fasting are permissive to enter torpor, even though low leptin levels alone are not sufficient to induce torpor [79, 80]. Similarly, exogenously raising serum leptin levels effectively prevents torpor [81] and the absence of leptin in ob/ob mice signals extensive fasting and therefore initiates regular torpor in ob/ob mice despite food availability and severe hyperphagia [82]. Whether the thermoregulatory circuits via POA>DMH/DHA>rRPa, as described above, play a role in the expression of torpor is unknown, but it can be speculated that low leptin levels would result in low neuronal activity of glutamatergic DMH/DHA LepRb neurons and the resulting decrease in BAT thermogenesis would certainly support torpor.
3.2. The ARC as Sensory Hub for Nutrient State
Different nutritional states like single feeding bouts, high fat diet consumption, fasting or refeeding are also well known to cause changes in energy expenditure [83]. The hypothalamic ARC represents a known site where changes in nutritional status are sensed and result in robust modulation of neuronal activity, gene expression and even proportional changes of inhibitory versus stimulatory synaptic inputs [84–87]. More specifically, anorexigenic POMC neurons and orexigenic AgRP neurons are differentially regulated in response to feeding states, which is at least in part mediated by leptin [86]. Similarly, selective deletion of LepRb neurons in the ARC was able to attenuate leptin-induced stimulation of BAT sympathetic activity [88].
POMC derived neuropeptides bind to and stimulate melanocortin-4-receptors (MC4R) to inhibit food intake and increase energy expenditure, while AgRP acts as a reverse agonist on MC4R to increase food intake and decrease energy expenditure [86]. POMC and AgRP neurons both project broadly within the hypothalamus including the POA and DMH [89] and MC4Rs are associated with the regulation of sympathetic BAT inputs [90]. In fact, MC4R function in cholinergic motor neurons in the spinal cord (likely including BAT sympathetic motor neurons) is sufficient to recover the low energy expenditure of MC4R-deficient mice [91]. However, this does not rule out that MC4Rs further upstream e.g. in LepRb DMH/DHA neurons importantly contribute to regulate thermogenesis. Indeed, MC4Rs are strongly expressed in the dorsal portion of the DMH consistent with the distribution of DMH/DHA LepRb neurons [92] as well as in rRPa neurons [93]. Thus, AgRP and POMC neurons in the ARC could provide the nutrient dependent sensory input, which is integrated into the rRPa either directly or indirectly via the DMH/DHA.
Intriguingly, MC4R deficient mice are very sensitive to HFD induced body weight gain and fail to raise UCP1 in response to a HFD [90, 94], strongly suggesting MC4R signaling as an important component for diet-induced thermogenesis. Furthermore, intra-DMH injections of the MC4R agonist MTII was able to increase body temperature, which could be prevented by intra-DMH AgRP. However, in the same study it was also concluded that leptin dependent induction of BAT temperature was independent of functional MC4R, because leptin induced BAT temperature was still inducible in MC4R deficient mice [56]. Thus, both pathways may act on parallel neuronal populations within the same thermoregulatory pathway.
3.3. Inhibitory action of AgRP neurons and Thermoregulation
Recent data suggest that the orexigenic function of AgRP neurons is independent of MC4R signaling [95–97] and instead the inhibitory acting neurotransmitter GABA is responsible for the orexigenic function of AgRP neurons [74, 98]. Neuronal activation of AgRP neurons does not only regulate food intake, but also importantly modulates energy expenditure. Activation of AgRP neurons induces food intake and decreases energy expenditure, while silencing of AgRP neurons inhibits food intake and increases energy expenditure [98]. Even though it is unknown if AgRP neurons synaptically couple with DMH/DHA LepRb neurons, the strong stimulation of the inhibitory acting AgRP neurons during fasting or in leptin deficient mice [85] would indeed add a potent inhibitory signal onto DMH/DHA LepRb neurons, consistent with a decrease in BAT thermogenesis and energy expenditure. This putative inhibition of DMH/DHA LepRb neurons by AgRP neurons combined with the lack of leptin induced activation of DMH/DHA LepRb neurons [42] could be potent enough to prevent cold-induced thermogenesis and instead would enable torpor induction. Contrarily, it could be speculated that the selective lack of GABAergic AgRP signals would enhance thermogenic function, and thus prevent torpor in fasted, cold exposed animals (Figure 1). Indeed, the selective deletion of the neurotransmitter GABA from AgRP neurons results in enhanced energy expenditure which was even more pronounced on a high fat diet [99], consistent with an enhanced diet-induced thermogenesis. Certainly, AgRP projections to the DMH/DHA require further confirmation and the interplay and relay of nutrient state with other thermogenic parameters requires further testing based on this neuronal circuits. However, given the unique position of the DMH, which has been highlighted early on as a hypothalamic nucleus that shows exceptional interconnections with every hypothalamic site [68], it would be perfectly located to integrate several hypothalamic sensory inputs (e.g. temperature sensing from the POA and nutrient sensing from the ARC) into a coordinated output signal.
4. Concluding remarks
The continuing rise in obesity among adults and children, and our inability to counteract excessive body weight gain by behavioral modifications or the develop safe and effective drugs has shown that the very essentials of body weight control are still unknown. While body weight gain is generally a simple interplay of energy intake (food) and energy expenditure (exercise and thermogenesis), we still miss a clear understanding how the fine balancing of energy homeostasis is controlled in the central nervous system. Particularly, the recent renaissance of BAT thermogenesis as a potential drug target sparked new ideas on how changes in energy expenditure are initiated. Many neuronal circuits have been discovered to control energy expenditure, but less work has been done to study known thermoregulatory circuits on the basis of body weight regulation and to identify the central sites that may integrate and relay distinct hoemostatic states (e.g. nutrients, temperature, inflammation etc.). The leptin signaling system is a perfect example to illustrate how one effector can modulate a multitude of homeostatic systems. The wealth of molecular genetic tools to study leptin signaling pathways and existing data on leptin action show a promising direction to understand the interplay of distinct homeostatic systems.
Highlights
Basics of lLeptin, thermogenesis, and energy expenditure
Central control of brown adipose tissue (BAT) thermogenesis, with emphasis on the raphe pallidus, hypothalamic coordination of diverse sensory inputs
Integrating Sensory Inputs into BAT Thermogenesis with emphasis on temperature and nutrient integration into the DMH/DHA
Acknowledgements
This review is based on a presentation made during the 2012 Annual Meeting of the Society for the Study of Ingestive Behavior (SSIB), July 10–14, 2012. The SSIB meeting was made possible in part by generous unrestricted donations from NovoNordisk, Research Diets Inc., Sanofi-Aventis Group, and TSE Systems. This work was supported by NIH P20 RR02195, P/F NORC #2P30-DK072476-06, R01-DK092587 (HM), F32-DK097896 (KRZ). This work utilized the facilities of the Cell Biology and Bioimaging Core and Phenotyping Core, supported in part by COBRE (NIH P20-RR021945), CNRU (NIH 1P30-DK072476) center grants from the National Institutes of Health. We thank Dr. Hans-Rudolf Berthoud for the helpful discussion of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Myers MG, Cowley MA, Munzberg H. Mechanisms of leptin action and leptin resistance. Annu. Rev. Physiol. 2008;70:537–556. doi: 10.1146/annurev.physiol.70.113006.100707. [DOI] [PubMed] [Google Scholar]
- 2.DAVIS TR, MAYER J. Imperfect homeothermia in the hereditary obese-hyperglycemic syndrome of mice. Am. J. Physiol. 1954;177:222–226. doi: 10.1152/ajplegacy.1954.177.2.222. [DOI] [PubMed] [Google Scholar]
- 3.Trayhurn P, Thurlby PL, James WP. Thermogenic defect in pre-obese ob/ob mice. Nature. 1977;266:60–62. doi: 10.1038/266060a0. [DOI] [PubMed] [Google Scholar]
- 4.Trayhurn P, Thurlby PL, James WP. A defective response to cold in the obese (obob) mouse and the obese Zucker (fafa) rat [proceedings] Proc. Nutr. Soc. 1976;35:133A. [PubMed] [Google Scholar]
- 5.Joosten HF, van der Kroon PH. Role of the thyroid in the development of the obese-hyperglycemic syndrome in mice (ob ob) Metabolism. 1974;23:425–436. doi: 10.1016/0026-0495(74)90090-0. [DOI] [PubMed] [Google Scholar]
- 6.Harris RB, Zhou J, Redmann SM, Jr., Smagin GN, Smith SR, Rodgers E, Zachwieja JJ. A leptin dose-response study in obese (ob/ob) and lean (+/?) mice. Endocrinology. 1998;139:8–19. doi: 10.1210/endo.139.1.5675. [DOI] [PubMed] [Google Scholar]
- 7.Trayhurn P, James WP. Thermoregulation and non-shivering thermogenesis in the genetically obese (ob/ob) mouse. Pflugers Arch. 1978;373:189–193. doi: 10.1007/BF00584859. [DOI] [PubMed] [Google Scholar]
- 8.Inoue W, Luheshi GN. Acute starvation alters lipopolysaccharide-induced fever in leptin-dependent and -independent mechanisms in rats. Am. J. Physiol Regul. Integr. Comp Physiol. 2010;299:R1709–R1719. doi: 10.1152/ajpregu.00567.2010. [DOI] [PubMed] [Google Scholar]
- 9.Faggioni R, Moser A, Feingold KR, Grunfeld C. Reduced leptin levels in starvation increase susceptibility to endotoxic shock. Am. J. Pathol. 2000;156:1781–1787. doi: 10.1016/S0002-9440(10)65049-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Faggioni R, Fantuzzi G, Gabay C, Moser A, Dinarello CA, Feingold KR, Grunfeld C. Leptin deficiency enhances sensitivity to endotoxin-induced lethality. Am. J. Physiol. 1999;276:R136–R142. doi: 10.1152/ajpregu.1999.276.1.R136. [DOI] [PubMed] [Google Scholar]
- 11.Steiner AA, Krall CM, Liu E. A reappraisal on the ability of leptin to induce fever. Physiol Behav. 2009;97:430–436. doi: 10.1016/j.physbeh.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 12.Aquila H, Link TA, Klingenberg M. The uncoupling protein from brown fat mitochondria is related to the mitochondrial ADP/ATP carrier. Analysis of sequence homologies and of folding of the protein in the membrane. EMBO J. 1985;4:2369–2376. doi: 10.1002/j.1460-2075.1985.tb03941.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heaton GM, Wagenvoord RJ, Kemp A, Jr., Nicholls DG. Brown-adipose-tissue mitochondria: photoaffinity labelling of the regulatory site of energy dissipation. Eur. J. Biochem. 1978;82:515–521. doi: 10.1111/j.1432-1033.1978.tb12045.x. [DOI] [PubMed] [Google Scholar]
- 14.Nicholls DG, Rial E. A history of the first uncoupling protein, UCP1. J. Bioenerg. Biomembr. 1999;31:399–406. doi: 10.1023/a:1005436121005. [DOI] [PubMed] [Google Scholar]
- 15.Himms-Hagen J, Cui J, Danforth E, Jr, Taatjes DJ, Lang SS, Waters BL, Claus TH. Effect of CL-316,243, a thermogenic beta 3-agonist, on energy balance and brown and white adipose tissues in rats. Am. J. Physiol. 1994;266:R1371–R1382. doi: 10.1152/ajpregu.1994.266.4.R1371. [DOI] [PubMed] [Google Scholar]
- 16.Bartness TJ, Vaughan CH, Song CK. Sympathetic and sensory innervation of brown adipose tissue. Int. J. Obes. (Lond) 2010;34(Suppl 1):S36–S42. doi: 10.1038/ijo.2010.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Silva JE. Thermogenic mechanisms and their hormonal regulation. Physiol Rev. 2006;86:435–464. doi: 10.1152/physrev.00009.2005. [DOI] [PubMed] [Google Scholar]
- 18.Prusiner SB, Cannon B, Lindberg O. Oxidative metabolism in cells isolated from brown adipose tissue. 1. Catecholamine and fatty acid stimulation of respiration. Eur. J. Biochem. 1968;6:15–22. doi: 10.1111/j.1432-1033.1968.tb00413.x. [DOI] [PubMed] [Google Scholar]
- 19.Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- 20.Rothwell NJ, Stock MJ. A role for brown adipose tissue in diet-induced thermogenesis. Nature. 1979;281:31–35. doi: 10.1038/281031a0. [DOI] [PubMed] [Google Scholar]
- 21.Kozak LP. Brown fat and the myth of diet-induced thermogenesis. Cell Metab. 2010;11:263–267. doi: 10.1016/j.cmet.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cannon B, Nedergaard J. Nonshivering thermogenesis and its adequate measurement in metabolic studies. J. Exp. Biol. 2011;214:242–253. doi: 10.1242/jeb.050989. [DOI] [PubMed] [Google Scholar]
- 23.van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND, Schrauwen P, Teule GJ. Cold-activated brown adipose tissue in healthy men. N. Engl. J. Med. 2009;360:1500–1508. doi: 10.1056/NEJMoa0808718. [DOI] [PubMed] [Google Scholar]
- 24.Ravussin E, Kozak LP. Have we entered the brown adipose tissue renaissance? Obes. Rev. 2009;10:265–268. doi: 10.1111/j.1467-789X.2008.00559.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fruhbeck G, Becerril S, Sainz N, Garrastachu P, Garcia-Velloso MJ. BAT: a new target for human obesity? Trends Pharmacol. Sci. 2009;30:387–396. doi: 10.1016/j.tips.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 26.Nedergaard J, Bengtsson T, Cannon B. Unexpected evidence for active brown adipose tissue in adult humans. Am. J. Physiol Endocrinol. Metab. 2007;293:E444–E452. doi: 10.1152/ajpendo.00691.2006. [DOI] [PubMed] [Google Scholar]
- 27.Commins SP, Watson PM, Padgett MA, Dudley A, Argyropoulos G, Gettys TW. Induction of uncoupling protein expression in brown and white adipose tissue by leptin. Endocrinology. 1999;140:292–300. doi: 10.1210/endo.140.1.6399. [DOI] [PubMed] [Google Scholar]
- 28.Himms-Hagen J. Defective brown adipose tissue thermogenesis in obese mice. Int. J. Obes. 1985;9(Suppl 2):17–24. [PubMed] [Google Scholar]
- 29.Haynes WG, Morgan DA, Walsh SA, Mark AL, Sivitz WI. Receptor-mediated regional sympathetic nerve activation by leptin. J. Clin. Invest. 1997;100:270–278. doi: 10.1172/JCI119532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rafael J, Herling AW. Leptin effect in ob/ob mice under thermoneutral conditions depends not necessarily on central satiation. Am. J. Physiol Regul. Integr. Comp Physiol. 2000;278:R790–R795. doi: 10.1152/ajpregu.2000.278.3.R790. [DOI] [PubMed] [Google Scholar]
- 31.Thurlby PL, Trayhurn P. The role of thermoregulatory thermogenesis in the development of obesity in genetically-obese (ob/ob) mice pair-fed with lean siblings. Br. J. Nutr. 1979;42:377–385. doi: 10.1079/bjn19790127. [DOI] [PubMed] [Google Scholar]
- 32.Commins SP, Watson PM, Frampton IC, Gettys TW. Leptin selectively reduces white adipose tissue in mice via a UCP1-dependent mechanism in brown adipose tissue. Am. J. Physiol Endocrinol. Metab. 2001;280:E372–E377. doi: 10.1152/ajpendo.2001.280.2.E372. [DOI] [PubMed] [Google Scholar]
- 33.Rosenbaum M, Goldsmith R, Bloomfield D, Magnano A, Weimer L, Heymsfield S, Gallagher D, Mayer L, Murphy E, Leibel RL. Low-dose leptin reverses skeletal muscle, autonomic, and neuroendocrine adaptations to maintenance of reduced weight. J. Clin. Invest. 2005;115:3579–3586. doi: 10.1172/JCI25977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Galgani JE, Greenway FL, Caglayan S, Wong ML, Licinio J, Ravussin E. Leptin replacement prevents weight loss-induced metabolic adaptation in congenital leptin-deficient patients. J. Clin. Endocrinol. Metab. 2010;95:851–855. doi: 10.1210/jc.2009-1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morrison SF, Nakamura K, Madden CJ. Central control of thermogenesis in mammals. Exp. Physiol. 2008;93:773–797. doi: 10.1113/expphysiol.2007.041848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morrison SF, Madden CJ, Tupone D. Central control of brown adipose tissue thermogenesis. Front Endocrinol. (Lausanne) 2012;3 doi: 10.3389/fendo.2012.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cano G, Passerin AM, Schiltz JC, Card JP, Morrison SF, Sved AF. Anatomical substrates for the central control of sympathetic outflow to interscapular adipose tissue during cold exposure. J. Comp Neurol. 2003;460:303–326. doi: 10.1002/cne.10643. [DOI] [PubMed] [Google Scholar]
- 38.Bamshad M, Song CK, Bartness TJ. CNS origins of the sympathetic nervous system outflow to brown adipose tissue. Am. J. Physiol. 1999;276:R1569–R1578. doi: 10.1152/ajpregu.1999.276.6.R1569. [DOI] [PubMed] [Google Scholar]
- 39.Madden CJ, Morrison SF. Excitatory amino acid receptor activation in the raphe pallidus area mediates prostaglandin-evoked thermogenesis. Neuroscience. 2003;122:5–15. doi: 10.1016/s0306-4522(03)00527-x. [DOI] [PubMed] [Google Scholar]
- 40.Nakamura K, Morrison SF. Central efferent pathways mediating skin cooling-evoked sympathetic thermogenesis in brown adipose tissue. Am. J. Physiol Regul. Integr. Comp Physiol. 2007;292:R127–R136. doi: 10.1152/ajpregu.00427.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morrison SF. Activation of 5-HT1A receptors in raphe pallidus inhibits leptin-evoked increases in brown adipose tissue thermogenesis. Am. J. Physiol Regul. Integr. Comp Physiol. 2004;286:R832–R837. doi: 10.1152/ajpregu.00678.2003. [DOI] [PubMed] [Google Scholar]
- 42.Zhang Y, Kerman IA, Laque A, Nguyen P, Faouzi M, Louis GW, Jones JC, Rhodes C, Munzberg H. Leptin-receptor-expressing neurons in the dorsomedial hypothalamus and median preoptic area regulate sympathetic brown adipose tissue circuits. J. Neurosci. 2011;31:1873–1884. doi: 10.1523/JNEUROSCI.3223-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lam DD, Leinninger GM, Louis GW, Garfield AS, Marston OJ, Leshan RL, Scheller EL, Christensen L, Donato J, Jr., Xia J, Evans ML, Elias C, Dalley JW, Burdakov DI, Myers MG, Jr., Heisler LK. Leptin does not directly affect CNS serotonin neurons to influence appetite. Cell Metab. 2011;13:584–591. doi: 10.1016/j.cmet.2011.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lazarus M, Yoshida K, Coppari R, Bass CE, Mochizuki T, Lowell BB, Saper CB. EP3 prostaglandin receptors in the median preoptic nucleus are critical for fever responses. Nat. Neurosci. 2007;10:1131–1133. doi: 10.1038/nn1949. [DOI] [PubMed] [Google Scholar]
- 45.Hermann DM, Luppi PH, Peyron C, Hinckel P, Jouvet M. Afferent projections to the rat nuclei raphe magnus, raphe pallidus and reticularis gigantocellularis pars alpha demonstrated by iontophoretic application of choleratoxin (subunit b) J. Chem. Neuroanat. 1997;13:1–21. doi: 10.1016/s0891-0618(97)00019-7. [DOI] [PubMed] [Google Scholar]
- 46.Nakamura K, Matsumura K, Kaneko T, Kobayashi S, Katoh H, Negishi M. The rostral raphe pallidus nucleus mediates pyrogenic transmission from the preoptic area. J. Neurosci. 2002;22:4600–4610. doi: 10.1523/JNEUROSCI.22-11-04600.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cao WH, Morrison SF. Glutamate receptors in the raphe pallidus mediate brown adipose tissue thermogenesis evoked by activation of dorsomedial hypothalamic neurons. Neuropharmacology. 2006;51:426–437. doi: 10.1016/j.neuropharm.2006.03.031. [DOI] [PubMed] [Google Scholar]
- 48.Cao WH, Fan W, Morrison SF. Medullary pathways mediating specific sympathetic responses to activation of dorsomedial hypothalamus. Neuroscience. 2004;126:229–240. doi: 10.1016/j.neuroscience.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 49.Nakamura Y, Nakamura K, Matsumura K, Kobayashi S, Kaneko T, Morrison SF. Direct pyrogenic input from prostaglandin EP3 receptor-expressing preoptic neurons to the dorsomedial hypothalamus. Eur. J. Neurosci. 2005;22:3137–3146. doi: 10.1111/j.1460-9568.2005.04515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yoshida K, Li X, Cano G, Lazarus M, Saper CB. Parallel preoptic pathways for thermoregulation. J. Neurosci. 2009;29:11954–11964. doi: 10.1523/JNEUROSCI.2643-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sarkar S, Zaretskaia MV, Zaretsky DV, Moreno M, Dimicco JA. Stress- and lipopolysaccharide-induced c-fos expression and nNOS in hypothalamic neurons projecting to medullary raphe in rats: a triple immunofluorescent labeling study. Eur. J. Neurosci. 2007;26:2228–2238. doi: 10.1111/j.1460-9568.2007.05843.x. [DOI] [PubMed] [Google Scholar]
- 52.Dimicco JA, Zaretsky DV. The dorsomedial hypothalamus: a new player in thermoregulation. Am. J. Physiol Regul. Integr. Comp Physiol. 2007;292:R47–R63. doi: 10.1152/ajpregu.00498.2006. [DOI] [PubMed] [Google Scholar]
- 53.Tupone D, Madden CJ, Cano G, Morrison SF. An orexinergic projection from perifornical hypothalamus to raphe pallidus increases rat brown adipose tissue thermogenesis. J. Neurosci. 2011;31:15944–15955. doi: 10.1523/JNEUROSCI.3909-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rogers RC, Barnes MJ, Hermann GE. Leptin “gates” thermogenic action of thyrotropin-releasing hormone in the hindbrain. Brain Res. 2009;1295:135–141. doi: 10.1016/j.brainres.2009.07.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kong D, Tong Q, Ye C, Koda S, Fuller PM, Krashes MJ, Vong L, Ray RS, Olson DP, Lowell BB. GABAergic RIP-Cre Neurons in the Arcuate Nucleus Selectively Regulate Energy Expenditure. Cell. 2012;151:645–657. doi: 10.1016/j.cell.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Enriori PJ, Sinnayah P, Simonds SE, Garcia RC, Cowley MA. Leptin action in the dorsomedial hypothalamus increases sympathetic tone to brown adipose tissue in spite of systemic leptin resistance. J. Neurosci. 2011;31:12189–12197. doi: 10.1523/JNEUROSCI.2336-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Munzberg H, Flier JS, Bjorbaek C. Region-specific leptin resistance within the hypothalamus of diet-induced obese mice. Endocrinology. 2004;145:4880–4889. doi: 10.1210/en.2004-0726. [DOI] [PubMed] [Google Scholar]
- 58.Chao PT, Yang L, Aja S, Moran TH, Bi S. Knockdown of NPY expression in the dorsomedial hypothalamus promotes development of brown adipocytes and prevents diet-induced obesity. Cell Metab. 2011;13:573–583. doi: 10.1016/j.cmet.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang L, Scott KA, Hyun J, Tamashiro KL, Tray N, Moran TH, Bi S. Role of dorsomedial hypothalamic neuropeptide Y in modulating food intake and energy balance. J. Neurosci. 2009;29:179–190. doi: 10.1523/JNEUROSCI.4379-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Madden CJ, Morrison SF. Neurons in the paraventricular nucleus of the hypothalamus inhibit sympathetic outflow to brown adipose tissue. Am. J. Physiol Regul. Integr. Comp Physiol. 2009;296:R831–R843. doi: 10.1152/ajpregu.91007.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vong L, Ye C, Yang Z, Choi B, Chua S, Jr, Lowell BB. Leptin action on GABAergic neurons prevents obesity and reduces inhibitory tone to POMC neurons. Neuron. 2011;71:142–154. doi: 10.1016/j.neuron.2011.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Elmquist JK, Ahima RS, Elias CF, Flier JS, Saper CB. Leptin activates distinct projections from the dorsomedial and ventromedial hypothalamic nuclei. Proc. Natl. Acad. Sci. U. S. A. 1998;95:741–746. doi: 10.1073/pnas.95.2.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yoshida T, Bray GA. Catecholamine turnover in rats with ventromedial hypothalamic lesions. Am. J. Physiol. 1984;246:R558–R565. doi: 10.1152/ajpregu.1984.246.4.R558. [DOI] [PubMed] [Google Scholar]
- 64.Perkins MN, Rothwell NJ, Stock MJ, Stone TW. Activation of brown adipose tissue thermogenesis by the ventromedial hypothalamus. Nature. 1981;289:401–402. doi: 10.1038/289401a0. [DOI] [PubMed] [Google Scholar]
- 65.Kelly L, Bielajew C. Ventromedial hypothalamic regulation of brown adipose tissue. Neuroreport. 1991;2:41–44. doi: 10.1097/00001756-199101000-00010. [DOI] [PubMed] [Google Scholar]
- 66.Samuels BC, Zaretsky DV, Dimicco JA. Dorsomedial hypothalamic sites where disinhibition evokes tachycardia correlate with location of raphe-projecting neurons. Am. J. Physiol Regul. Integr. Comp Physiol. 2004;287:R472–R478. doi: 10.1152/ajpregu.00667.2003. [DOI] [PubMed] [Google Scholar]
- 67.Dimicco JA, Samuels BC, Zaretskaia MV, Zaretsky DV. The dorsomedial hypothalamus and the response to stress: part renaissance, part revolution. Pharmacol. Biochem. Behav. 2002;71:469–480. doi: 10.1016/s0091-3057(01)00689-x. [DOI] [PubMed] [Google Scholar]
- 68.Ter Horst GJ, Luiten PG. The projections of the dorsomedial hypothalamic nucleus in the rat. Brain Res. Bull. 1986;16:231–248. doi: 10.1016/0361-9230(86)90038-9. [DOI] [PubMed] [Google Scholar]
- 69.Dhillon H, Zigman JM, Ye C, Lee CE, McGovern RA, Tang V, Kenny CD, Christiansen LM, White RD, Edelstein EA, Coppari R, Balthasar N, Cowley MA, Chua S, Jr, Elmquist JK, Lowell BB. Leptin directly activates SF1 neurons in the VMH, and this action by leptin is required for normal body-weight homeostasis. Neuron. 2006;49:191–203. doi: 10.1016/j.neuron.2005.12.021. [DOI] [PubMed] [Google Scholar]
- 70.Skibicka KP, Grill HJ. Hindbrain leptin stimulation induces anorexia and hyperthermia mediated by hindbrain melanocortin receptors. Endocrinology. 2009;150:1705–1711. doi: 10.1210/en.2008-1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cao WH, Madden CJ, Morrison SF. Inhibition of brown adipose tissue thermogenesis by neurons in the ventrolateral medulla and in the nucleus tractus solitarius. Am. J. Physiol Regul. Integr. Comp Physiol. 2010;299:R277–R290. doi: 10.1152/ajpregu.00039.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hermann GE, Barnes MJ, Rogers RC. Leptin and thyrotropin-releasing hormone: cooperative action in the hindbrain to activate brown adipose thermogenesis. Brain Res. 2006;1117:118–124. doi: 10.1016/j.brainres.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 73.Rogers RC, McDougal DH, Hermann GE. Leptin amplifies the action of thyrotropin-releasing hormone in the solitary nucleus: an in vitro calcium imaging study. Brain Res. 2011;1385:47–55. doi: 10.1016/j.brainres.2011.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wu Q, Boyle MP, Palmiter RD. Loss of GABAergic signaling by AgRP neurons to the parabrachial nucleus leads to starvation. Cell. 2009;137:1225–1234. doi: 10.1016/j.cell.2009.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kobayashi A, Osaka T. Involvement of the parabrachial nucleus in thermogenesis induced by environmental cooling in the rat. Pflugers Arch. 2003;446:760–765. doi: 10.1007/s00424-003-1119-7. [DOI] [PubMed] [Google Scholar]
- 76.Nakamura K, Morrison SF. A thermosensory pathway that controls body temperature. Nat. Neurosci. 2008;11:62–71. doi: 10.1038/nn2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Melvin RG, Andrews MT. Torpor induction in mammals: recent discoveries fueling new ideas. Trends Endocrinol. Metab. 2009;20:490–498. doi: 10.1016/j.tem.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Heldmaier G, Klingenspor M, Werneyer M, Lampi BJ, Brooks SP, Storey KB. Metabolic adjustments during daily torpor in the Djungarian hamster. Am. J. Physiol. 1999;276:E896–E906. doi: 10.1152/ajpendo.1999.276.5.E896. [DOI] [PubMed] [Google Scholar]
- 79.Swoap SJ, Weinshenker D. Norepinephrine controls both torpor initiation and emergence via distinct mechanisms in the mouse. PLoS. One. 2008;3:e4038. doi: 10.1371/journal.pone.0004038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Swoap SJ, Gutilla MJ, Liles LC, Smith RO, Weinshenker D. The full expression of fasting-induced torpor requires beta 3-adrenergic receptor signaling. J. Neurosci. 2006;26:241–245. doi: 10.1523/JNEUROSCI.3721-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Doring H, Schwarzer K, Nuesslein-Hildesheim B, Schmidt I. Leptin selectively increases energy expenditure of food-restricted lean mice. Int. J. Obes. Relat Metab Disord. 1998;22:83–88. doi: 10.1038/sj.ijo.0800547. [DOI] [PubMed] [Google Scholar]
- 82.Himms-Hagen J. Food restriction increases torpor and improves brown adipose tissue thermogenesis in ob/ob mice. Am. J. Physiol. 1985;248:E531–E539. doi: 10.1152/ajpendo.1985.248.5.E531. [DOI] [PubMed] [Google Scholar]
- 83.Westerterp KR. Diet induced thermogenesis. Nutr. Metab (Lond) 2004;1:5. doi: 10.1186/1743-7075-1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Schwartz MW, Seeley RJ, Woods SC, Weigle DS, Campfield LA, Burn P, Baskin DG. Leptin increases hypothalamic pro-opiomelanocortin mRNA expression in the rostral arcuate nucleus. Diabetes. 1997;46:2119–2123. doi: 10.2337/diab.46.12.2119. [DOI] [PubMed] [Google Scholar]
- 85.Munzberg H, Jobst EE, Bates SH, Jones J, Villanueva E, Leshan R, Bjornholm M, Elmquist J, Sleeman M, Cowley MA, Myers MG., Jr. Appropriate inhibition of orexigenic hypothalamic arcuate nucleus neurons independently of leptin receptor/STAT3 signaling. J. Neurosci. 2007;27:69–74. doi: 10.1523/JNEUROSCI.3168-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schwartz MW, Woods SC, Porte D, Jr., Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404:661–671. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- 87.Pinto S, Roseberry AG, Liu H, Diano S, Shanabrough M, Cai X, Friedman JM, Horvath TL. Rapid rewiring of arcuate nucleus feeding circuits by leptin. Science. 2004;304:110–115. doi: 10.1126/science.1089459. [DOI] [PubMed] [Google Scholar]
- 88.Harlan SM, Morgan DA, Agassandian K, Guo DF, Cassell MD, Sigmund CD, Mark AL, Rahmouni K. Ablation of the leptin receptor in the hypothalamic arcuate nucleus abrogates leptin-induced sympathetic activation. Circ. Res. 2011;108:808–812. doi: 10.1161/CIRCRESAHA.111.240226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bagnol D, Lu XY, Kaelin CB, Day HE, Ollmann M, Gantz I, Akil H, Barsh GS, Watson SJ. Anatomy of an endogenous antagonist: relationship between Agouti-related protein and proopiomelanocortin in brain. J. Neurosci. 1999;19:RC26. doi: 10.1523/JNEUROSCI.19-18-j0004.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Voss-Andreae A, Murphy JG, Ellacott KL, Stuart RC, Nillni EA, Cone RD, Fan W. Role of the central melanocortin circuitry in adaptive thermogenesis of brown adipose tissue. Endocrinology. 2007;148:1550–1560. doi: 10.1210/en.2006-1389. [DOI] [PubMed] [Google Scholar]
- 91.Rossi J, Balthasar N, Olson D, Scott M, Berglund E, Lee CE, Choi MJ, Lauzon D, Lowell BB, Elmquist JK. Melanocortin-4 receptors expressed by cholinergic neurons regulate energy balance and glucose homeostasis. Cell Metab. 2011;13:195–204. doi: 10.1016/j.cmet.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Liu H, Kishi T, Roseberry AG, Cai X, Lee CE, Montez JM, Friedman JM, Elmquist JK. Transgenic mice expressing green fluorescent protein under the control of the melanocortin-4 receptor promoter. J. Neurosci. 2003;23:7143–7154. doi: 10.1523/JNEUROSCI.23-18-07143.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fan W, Voss-Andreae A, Cao WH, Morrison SF. Regulation of thermogenesis by the central melanocortin system. Peptides. 2005;26:1800–1813. doi: 10.1016/j.peptides.2004.11.033. [DOI] [PubMed] [Google Scholar]
- 94.Butler AA, Marks DL, Fan W, Kuhn CM, Bartolome M, Cone RD. Melanocortin-4 receptor is required for acute homeostatic responses to increased dietary fat. Nat. Neurosci. 2001;4:605–611. doi: 10.1038/88423. [DOI] [PubMed] [Google Scholar]
- 95.Aponte Y, Atasoy D, Sternson SM. AGRP neurons are sufficient to orchestrate feeding behavior rapidly and without training. Nat. Neurosci. 2011;14:351–355. doi: 10.1038/nn.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wu Q, Howell MP, Cowley MA, Palmiter RD. Starvation after AgRP neuron ablation is independent of melanocortin signaling. Proc. Natl. Acad. Sci. U. S. A. 2008;105:2687–2692. doi: 10.1073/pnas.0712062105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wu Q, Palmiter RD. GABAergic signaling by AgRP neurons prevents anorexia via a melanocortin-independent mechanism. Eur. J. Pharmacol. 2011;660:21–27. doi: 10.1016/j.ejphar.2010.10.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Krashes MJ, Koda S, Ye C, Rogan SC, Adams AC, Cusher DS, Maratos-Flier E, Roth BL, Lowell BB. Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. J. Clin. Invest. 2011;121:1424–1428. doi: 10.1172/JCI46229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tong Q, Ye CP, Jones JE, Elmquist JK, Lowell BB. Synaptic release of GABA by AgRP neurons is required for normal regulation of energy balance. Nat. Neurosci. 2008;11:998–1000. doi: 10.1038/nn.2167. [DOI] [PMC free article] [PubMed] [Google Scholar]


