Abstract
We report a cell-phone based Escherichia coli (E. coli) detection platform for screening of liquid samples. In this compact and cost-effective design attached to a cell-phone, we utilize anti-E. coli O157:H7 antibody functionalized glass capillaries as solid substrates to perform a quantum dot based sandwich assay for specific detection of E. coli O157:H7 in liquid samples. Using battery-powered inexpensive light-emitting-diodes (LEDs) we excite/pump these labelled E. coli particles captured on the capillary surface, where the emission from the quantum dots is then imaged using the cell-phone camera unit through an additional lens that is inserted between the capillary and the cell-phone. By quantifying the fluorescent light emission from each capillary tube, the concentration of E. coli in the sample is determined. We experimentally confirmed the detection limit of this cell-phone based fluorescent imaging and sensing platform as ~5 to 10 cfu mL−1 in buffer solution. We also tested the specificity of this E. coli detection platform by spiking samples with different species (e.g., Salmonella) to confirm that non-specific binding/detection is negligible. We further demonstrated the proof-of-concept of our approach in a complex food matrix, e.g., fat-free milk, where a similar detection limit of ~5 to 10 cfu mL−1 was achieved despite challenges associated with the density of proteins that exist in milk. Our results reveal the promising potential of this cell-phone enabled field-portable and cost-effective E. coli detection platform for e.g., screening of water and food samples even in resource limited environments. The presented platform can also be applicable to other pathogens of interest through the use of different antibodies.
Water and food associated diseases still pose considerable public health threat even in highly industrialized parts of the world, causing significant amount of hospitalizations and deaths every year.1-5 For example, Escherichia coli (E. coli) can easily contaminate food or drinking water, posing a significant threat to public health safety.6,7 As few as 10–100 E. coli O157:H7 particles can kill the cells of the intestinal lining, destroy the kidneys, cause blood clots in the brain, as well as seizures, paralysis, and respiratory failures.8 In order to prevent such serious health problems, and economic losses due to outbreaks caused by water- and/or food-borne diseases, gold standard detection methods are established such as EPA Method 1604,9 which requires membrane filtration and colony culture in agar plates prior to fluorescent microscope inspection under ultraviolet light. Although these methods are very sensitive and accurate, field-portable as well as cost-effective tools which can provide rapid and quantitative results are still needed in field settings or even at home. Toward this important goal, various promising approaches, including flow cytometry, polymerase chain reaction (PCR), DNA microarrays, surface plasmon resonance (SPR), enzyme linked immunosorbent assay (ELISA), mass spectroscopy as well as optofluidics have been introduced over the past decade to identify and quantify pathogens in water and food samples.10-20 However, these existing approaches are relatively complex and bulky which makes them less effective in field conditions and resource limited settings.
To provide a compact, light-weight and cost-effective solution to this important need, here we demonstrate the use of a cell-phone based fluorescent imaging and sensing platform for specific detection of E. coli and quantification of its concentration in liquid samples. Since we already have >5 billion cell-phone subscribers world-wide (as of 2011), where >70% of these cell-phones are being used in developing parts of the world,21 cell-phones provide an extremely cost-effective and ubiquitous platform for conducting such screening tests, almost anywhere in the world. In addition to this massive scale and deployment of cell-phones and their connectivity, the imagers (i.e., the camera units) as well as the digital processors installed on cell-phones are rather advanced which provide us unique and timely opportunities to conduct smart micro-analysis on cell-phones.22-26
Following this vision, in this work we combined antibody functionalized glass capillaries with quantum dots as signal reporters to specifically detect E. coli particles in liquid samples using a light-weight (~28 grams) and compact (3.5 cm × 5.5 cm × 2.4 cm) attachment to the existing camera unit of the cell-phone (see Fig. 1). This cost-effective attachment to the cell-phone acts as a fluorescent microscope quantifying the emitted light from each capillary after specific capture of E. coli particles within the sample of interest. We experimentally confirmed the detection limit of this cell-phone based fluorescent imaging and sensing platform to be ~5 to 10 cfu mL−1 in buffer solution. As an example for a complex food matrix, we tested our approach on fat-free milk samples (Alta Dena), where our detection limit also remained at ~5 to 10 cfu mL−1 despite challenges associated with density of proteins that exist in milk.
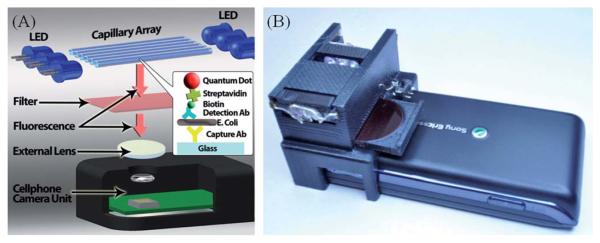
Fig. 1.
(A–B) Schematic diagram and picture of the optical attachment for E. coli detection on a cell-phone using the quantum dot based sandwich assay in glass capillary tubes. The entire attachment to the cell-phone weighs ~28 grams (~1 ounce) and has dimensions of ~3.5 × 5.5 × 2.4 cm. This compact and light-weight unit has an imaging field-of-view of 11 mm × 11 mm and can monitor ~10 capillary tubes all in parallel. It can also be repeatedly attached to and detached from the cell-phone body without the need for any fine alignment, making its interface quite easy to operate.
In our field-portable design, we employ glass capillaries (inner diameter: ~100 μm; outer diameter: ~170 μm; length: ~11 mm) as solid substrates to perform a quantum dot based sandwich assay to detect E. coli particles. The same capillary tube which functions as our microfluidic channel for liquid delivery into our imaging volume also serves as a waveguide (once filled with liquid) for guiding the excitation light.27-29 These glass capillaries were initially functionalized with anti-E. coli O157:H7 antibodies using standard surface chemistry protocols30,31 to specifically capture E. coli O157:H7 particles in liquid samples. This functionalization process involves various steps. First, the glass capillaries were cleaned and hydrophilized with a 1: 1 mixture of hydrochloric acid and methanol for ~30 minutes at room temperature and washed with DI water. Then the capillaries were filled with 1% (v/v) 3-(aminopropyl)triethoxysilane in 10% ethanol for 1 hour and thoroughly cleaned with DI water. The aminosilanized capillaries were activated with 5 mM homofunctionalized cross-linker bis(sulfosuccinimidyl) suberate (BS3) solution in PBS buffer for ~1 hour. After rinsing the capillaries with PBS buffer, the capillaries were filled with 100 μg mL−1 anti-E. coli O157:H7 antibody (KPL, MD, USA) solution to immobilize the antibodies onto the capillary interior surface covalently. The inner surfaces of these capillaries were further blocked with 2% gelatine in PBS to reduce the nonspecific binding. Following these steps, the capillaries were ready to specifically capture E. coli O157:H7 particles in liquid samples.
To experimentally test our approach, various concentrations of E. coli O157:H7 (KPL, MD, USA) were spiked in liquid samples (e.g., 2% gelatine–PBS buffer or fat-free milk) and the resulting contaminated sample was flushed through each capillary at a flow rate of ~50 μL min−1 for ~20 minutes using a syringe pump. Note that this step could also be done using a battery powered peristaltic pump or a mechanical spring based pump which might be more suitable for field use. After washing, the capillaries were then filled with 100 μg mL−1 biotinylated secondary anti-E. coli antibodies (KPL, MD, USA) and incubated for ~1 hour. Finally, streptavidin conjugated quantum dots (emission at 625 nm) (Life Technologies, NY, USA) at a concentration of 3 nM were introduced into the capillaries to bind to the biotinylated secondary antibodies incubating for ~30 minutes, serving as the fluorescent signal reporter.
The fluorescence emission from the quantum dots that are attached to E. coli particles was imaged and quantified using our cost-effective and light-weight (~28 grams) microscope attachment to the cell-phone (Sony-Ericsson U10i Aino™ with an 8 megapixel camera unit). As illustrated in Fig. 1, 11 mm long capillaries are inserted into the sample holder and for each acquired cell-phone camera image, ~6 to 10 capillaries can be microscopically imaged all in parallel over a wide field-of-view of ~11 mm × 11 mm. For exciting the quantum dots within each capillary tube, battery powered ultra-violet (UV) LEDs are directly butt coupled to the capillaries without the use of any lenses. Since each capillary tube that is filled with the liquid sample acts as a waveguide for this UV light, it enables uniform excitation of quantum dot labelled E. coli particles captured on the capillary interior surface. The emitted fluorescence light is then imaged by the cell-phone camera unit through an additional lens (focal length: 15 mm) that is inserted between the capillary and the cell-phone lens (Fig. 1). Since the guided excitation light propagates perpendicular to the detection path, this opto-fluidic pumping scheme permits the use of an inexpensive long pass glass filter (cut-off wavelength at ~610 nm) to remove the scattered UV light and create the dark-field background that is required for fluorescent imaging. These cell-phone captured capillary fluorescent images (each acquired with an integration time of ~1 s) were then analyzed using ImageJ software and the E. coli concentration in each capillary was quantified by measuring the fluorescent intensity integrated along the width of our imaging field of view, i.e., over a capillary length of ~11 mm. Note that since our numerical aperture is rather low, small variations in the height of our sample capillaries would not cause focusing related issues.
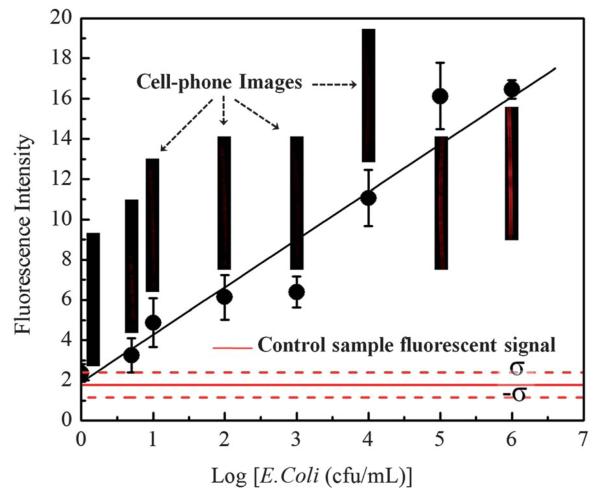
To demonstrate the sensitivity of this quantum dot based sandwich assay for detection of E. coli using a cell-phone we flushed through each functionalized capillary tube spiked samples in 2% gelatine–PBS buffer at various concentrations ranging from 0 cfu mL−1 (control) up to 106 cfu mL−1. Representative capillary images captured by our cell-phone fluorescent microscope are shown in Fig. 2 as insets. Based on these experiments, the titration curve can be plotted against the spiked E. coli concentration as shown in Fig. 2, which shows a strong linear relationship between the fluorescence signal (F) and the logarithm of the E. coli concentration (i.e., F = 1.89 × log[E. coli] + 2.36 with R = 0.985). Based on Fig. 2, the fluorescent signal from the ~5 cfu mL−1 sample remains consistently higher than the background signal arising from the control buffer. Thus the detection limit32 of our system can be estimated to be ~5 cfu mL−1 in PBS buffer. Using a more conservative metric, which defines the detection limit of a system as the control signal plus 3 times the standard deviation of the control signal level,33 we can alternatively calculate our detection limit to be ~10 cfu mL−1 (see Fig. 2). We should also mention that our E. coli O157:H7 samples (purchased from KPL, MD, USA) contain lysed cell fragments in addition to whole bacterial cells. This, however, is not an issue for the presented approach since our cell-phone based fluorescent microscope has a very large field-of-view (e.g., 11 mm × 11 mm) and quantifies the concentration of E. coli particles based on integration of the entire fluorescent intensity across the 11 mm length of each capillary, rather than counting individual bacteria pieces per image.
Fig. 2.
Dose–response curve for E. coli O157:H7 in 2% gelatine–PBS buffer using the quantum dot based sandwich assay implemented on a cell-phone (Fig. 1B). This response curve provides a linear fit (F = 1.89 × log[E. coli] + 2.36) with R = 0.985. The detection limit is ~5 to 10 cfu mL−1. Inset images show the raw capillary fluorescent images that are captured by our cell-phone fluorescent microscope at different E. coli concentrations. For each measurement point, 3 different samples are used (i.e., n = 3). Standard deviation (σ) for the control sample fluorescent signal level is 0.62.
This decent detection limit of ~5 to 10 cfu mL−1 achieved with our cell-phone based field-portable system is largely due to the employment of quantum dots, which exhibit better brightness and photostability, increasing the detection sensitivity in immunoassay compared to traditional organic dyes.34-36 In addition to this, the use of glass capillaries with a high surface-to-volume ratio also increases the capture efficiency of bacteria, further improving our detection limit.
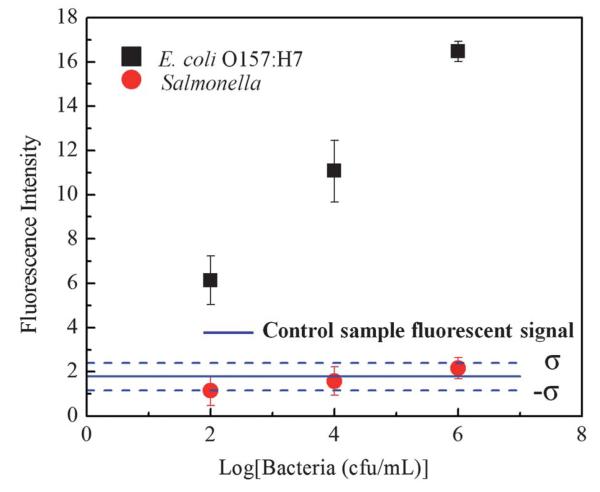
Next, to evaluate the specificity of our detection system we tested our platform with Salmonella, which is another common food borne pathogen. The capillary surface was prepared in the same way as described above. Instead of E. coli particles, Salmonella contaminated samples at high concentrations of 102 cfu mL−1, 104 cfu mL−1, and 106 cfu mL−1 were flushed through capillaries functionalized with anti-E. coli O157:H7 antibodies. As shown in Fig. 3, the fluorescent signal arising from Salmonella spiked samples at high concentrations was comparable to the signal level of control buffer solution and remained much lower than the fluorescent signal arising from the E. coli contaminated samples at the same concentration levels. These cross-reactivity control experiments demonstrated the specificity of our platform toward detection of E. coli O157:H7 in liquid samples.
Fig. 3.
Experimental results of our specificity test are summarized. The fluorescent signals from Salmonella contaminated buffer samples at different concentrations are compared against the fluorescent signals from E. coli O157:H7 contaminated buffer samples. As desired, Salmonella contaminated samples did not cause cross-reactivity. For each measurement point, 3 different samples are used (i.e., n = 3). The standard deviation (σ) for the control sample fluorescence signal level is 0.62.
To further explore the performance of our system for screening of a complex food matrix, next we imaged fat-free milk samples that were spiked with known concentrations of E. coli O157:H7. The detection protocol was the same as described above. The concentrations of the spiked bacteria ranged between 1 cfu mL−1 and 106 cfu mL−1 (see Fig. 4). The raw cellphone images corresponding to the low E. coli concentrations in fat-free milk (i.e., between 1 and 10 cfu mL−1) are also shown in Fig. S1†. The overall fluorescent signal generated from these spiked milk samples was similar to the fluorescent signal of the same concentration of E. coli O157: H7 in PBS buffer. The titration curve in these fat-free milk experiments (Fig. 4) provided a linear fit with an equation of F = 1.58 × log[E. coli] + 2.2 with R = 0.986. The detection limit in these milk experiments was estimated to be ~5 to 10 cfu mL−1, similar to the earlier experiments reported in Fig. 2.
Fig. 4.
Dose–response curve for E. coli O157:H7 detection in fat-free milk using the quantum dot based sandwich assay implemented on a cell-phone (Fig. 1B). The curve provides a linear fit (F = 1.58 × log[E. coli] + 2.2) with R = 0.986. The detection limit can be estimated to be ~5 to 10 cfu mL−1 in fat-free milk. Inset images show the raw capillary fluorescent images that are captured by our cell-phone fluorescent microscope at different E. coli concentrations in fat-free milk. For each measurement point, 3 different samples are used (i.e., n = 3). The standard deviation (s) for the control sample fluorescence signal level is 0.4.
In conclusion, we demonstrated a cost-effective and field-portable cell-phone based fluorescent imaging and sensing platform for sensitive detection of E. coli O157:H7 particles using the quantum dot enabled sandwich assay implemented within glass capillary tubes. This cell-phone based platform demonstrated a detection limit of ~5 to 10 cfu mL−1 in both PBS buffer and fat-free milk. The specificity of the developed system was also verified through negative cross-reactivity tests using Salmonella spiked samples. Our results illustrate the promising potential of this cell-phone enabled field-portable and cost-effective E. coli detection platform for e.g., screening of water and food samples even in resource limited environments. Finally, the presented platform can also be applicable to other pathogens of interest through the use of different specific antibodies.
Supplementary Material
Acknowledgements
The authors acknowledge the support of The Office of Naval Research (ONR).
Footnotes
Electronic supplementary information (ESI) available. See DOI: 10.1039/c2an35071h
Notes and references
- 1.Dziuban EJ, Liang JL, Graun GF, Hill V, Painter J, Moore MR, Calderon RL, Roy SL, Beach MJ. Surveillance Summaries. 2006;55:1–24. [PubMed] [Google Scholar]
- 2.Shannon MA, Bohn PW, Elimelech M, Georgiadis JG, Marinas BJ, Mayes AM. Nature. 2008;452:301–310. doi: 10.1038/nature06599. [DOI] [PubMed] [Google Scholar]
- 3.Fenwick A. Science. 2006;313:1077. doi: 10.1126/science.1127184. [DOI] [PubMed] [Google Scholar]
- 4.Waterborne disease could cost over $ 500 million annually in U.S., http://www.cdc.gov/media/pressrel/2010/r100714.htm.
- 5.Marris E. Nature. 2008;452:288. doi: 10.1038/452273a. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization 2011 http://www.euro.who.int/en/what-we-do/health-topics/emergencies/international-health-regulations/news/news/2011/06/ehec-outbreak-update-16.
- 7.Hunter PR. J. Water Health. 2003;1:65–72. [PubMed] [Google Scholar]
- 8.Foodborne Pathogenic Microorganisms and Natural Toxins Handbook. U.S. Food & Drug Administration, Center for Food Safety & Applied Nutrition; Feb, 2002. Escherichia coli O157:H7. 12 Nov 2002. [Google Scholar]
- 9.Method 1604: Total Coliforms and Escherichia coli in Water by Membrane Filtration Using a Simultaneous Detection Technique (MI Medium) Environmental Protection Agency; United States: 2002. [Google Scholar]
- 10.Raybourne RB. Current Protocols in Cytometry. John Wiley and Sons, Inc.; 2003. [Google Scholar]
- 11.Vesey G, Hutton P, Champion A, Ashbolt N, Williams KL, Warton A, Veal D. Cytometry. 1994;16:1–6. doi: 10.1002/cyto.990160102. [DOI] [PubMed] [Google Scholar]
- 12.Fratamico PM, Sackitey SK, Wiedmann M, Deng MY. J. Clin. Microbiol. 1995;33:2188–2191. doi: 10.1128/jcm.33.8.2188-2191.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aldeen WE, hale D, Robison AJ, Carroll K. Parasitology. 1995;21:77–79. doi: 10.1016/0732-8893(94)00142-j. [DOI] [PubMed] [Google Scholar]
- 14.Song J, Vo-Dinh T. Anal. Chim. Acta. 2004;507:115–121. [Google Scholar]
- 15.Zourob M, Mohr S, Brown BJT, Fielden PR, McDonnell MB, Goddard NJ. Anal. Chem. 2005;77:232–242. doi: 10.1021/ac049627g. [DOI] [PubMed] [Google Scholar]
- 16.Ivnitski D, Abdel-Hamid I, Atanasov P, Wilkins E. Biosens. Bioelectron. 1999;14:599–624. doi: 10.1016/s0956-5663(99)00004-4. [DOI] [PubMed] [Google Scholar]
- 17.Perkins EA, Squirrell DJ. Biosens. Bioelectron. 2000;14:853–859. doi: 10.1016/s0956-5663(99)00069-x. [DOI] [PubMed] [Google Scholar]
- 18.Zhou J. Curr. Opin. Microbiol. 2003;6:288–294. doi: 10.1016/s1369-5274(03)00052-3. [DOI] [PubMed] [Google Scholar]
- 19.Kiesel P, Martini J, Huck M, Bern M, Johnson N. CLEO: 2011-Laser Applications to Photonic Applications, OSA Technical Digest (CD) Optical Society of America; 2011. paper CWL1. [Google Scholar]
- 20.Sauer S, Freiwald A, Maier T, Kube M, Reinhardt R, Kostrzewa M, Geider K. PLoS One. 2008;3:e2843. doi: 10.1371/journal.pone.0002843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.International Telecommunication Union Market information and statistics. 2010 http://www.itu.int/ITU-D/ict/statistics/index.html.
- 22.Smith ZJ, Chu K, Espenson AR, Rahimzadeh M, Gryshuk A, Molinaro M, Dwyre DM, Lane S, Matthews D, Wachsmann-Hogiu S. PLoS One. 2011;6:e17150. doi: 10.1371/journal.pone.0017150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Breslauer DN, Maamari RN, Switz NA, Lam WA, Fletcher DA. PLos One. 2009;4:e6320. doi: 10.1371/journal.pone.0006320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tseng D, Mudanvali O, Oztoprak C, Iskiman SO, Sencan I, Yaglidere O, Ozcan A. Lab Chip. 2010;10:1787–1792. doi: 10.1039/c003477k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhu H, Yaglidere O, Su TW, Tseng D, Ozcan A. Lab Chip. 2011;11:315–322. doi: 10.1039/c0lc00358a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu H, Mavandadi S, Coskun AF, Yaglidere O, Ozcan A. Anal. Chem. 2011;83:6641–6647. doi: 10.1021/ac201587a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu P, Shelton DR, Karns JS, Sundaram A, Li S, Amstutz P, Tang C. Biosens. Bioelectron. 2005;21:678–683. doi: 10.1016/j.bios.2005.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ligler FS, Breimer M, Golden M, Nivens JP, Dodson DA, Green JP, Haders TM, Sadik OA. Anal. Chem. 2002;74:713–719. doi: 10.1021/ac015607s. [DOI] [PubMed] [Google Scholar]
- 29.Babu S, Mohapatra S, Zuokov L, Murthy S, Papazoglon E. Biosens. Bioelectron. 2009;24:3467–3474. doi: 10.1016/j.bios.2009.04.043. [DOI] [PubMed] [Google Scholar]
- 30.Jung Y, Jeong JY, Chung BH. Analyst. 2008;133:697–701. doi: 10.1039/b800014j. [DOI] [PubMed] [Google Scholar]
- 31.Rusmini F, Zhong Z, Feijen J. Biomacromolecules. 2007;8:1775–1789. doi: 10.1021/bm061197b. [DOI] [PubMed] [Google Scholar]
- 32.Xue X, Pan J, Xie H, Wang J, Zhang S. Talanta. 2009;77:1808–1813. doi: 10.1016/j.talanta.2008.10.025. [DOI] [PubMed] [Google Scholar]
- 33.Long GL, Winefordner JD. Anal. Chem. 1983;55:245–258. [Google Scholar]
- 34.Sanvicens N, Pascual N, Fernandez-Arguelles MT, Adrian J, Costa-Fernandez JM, Sanchez-Baeza F, Sanz-Medel A, Macro MP. Anal. Bioanal. Chem. 2011;399:2755–2762. doi: 10.1007/s00216-010-4624-5. [DOI] [PubMed] [Google Scholar]
- 35.Yang L, Li Y. Analyst. 2006;131:394–401. doi: 10.1039/b510888h. [DOI] [PubMed] [Google Scholar]
- 36.Hahn MA, Tabb JS, Krauss TD. Anal. Chem. 2006;77:4861–4869. doi: 10.1021/ac050641i. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.