SUMMARY
Systems of coupled oscillators abound in nature. How they establish stable phase relationships under diverse conditions is fundamentally important. The mammalian suprachiasmatic nucleus (SCN) is a self-sustained, synchronized network of circadian oscillators that coordinates daily rhythms in physiology and behavior. To elucidate the underlying topology and signaling mechanisms that modulate circadian synchrony, we discriminated the firing of hundreds of SCN neurons continuously over days. Using a novel analysis method to identify functional interactions between neurons based on changes in their firing, we characterized a GABAergic network comprised of fast, excitatory and inhibitory connections that is both stable over days and changes in strength with time of day. By monitoring PERIOD2 protein expression, we provide the first evidence that these millisecond-level interactions actively oppose circadian synchrony and inject jitter into daily rhythms. These results provide a new mechanism by which circadian oscillators can tune their phase relationships under different environmental conditions.
INTRODUCTION
The study of coupled oscillators is part of a broader movement towards understanding complex systems (Strogatz, 2000). In biology, intercellular communication can modulate the precision and synchronization of single-cell oscillations including glycolysis, somitogenesis, respiration and daily cycling (Jiang et al., 2000; Herzog, 2007). In many cases, coupled systems are inherently difficult to understand because the interactions are diverse and dynamic. The suprachiasmatic nucleus (SCN) of the mammalian brain provides an exceptional opportunity to reveal the topology, types, stability and function of diverse connections in a defined network of neural oscillators.
Neurons within the SCN express near 24-h (circadian) oscillations in electrical activity and gene expression, and entrain to regulate daily rhythms including metabolism, hormone release, and sleep-wake cycles. These cells depend on an intracellular transcription-translation feedback loop to generate daily rhythms and intercellular signaling for both synchronization and reliable rhythmicity (Yamaguchi et al., 2003; Webb et al., 2009). Importantly, it is unknown how intercellular signaling modulates the cycle-to-cycle precision of circadian rhythms.
Neural communication in the SCN includes gap junctions, neurotransmitters and neuropeptides. Of these, loss of vasoactive intestinal polypeptide (VIP) dramatically impairs circadian rhythms in the SCN and in behavior (Aton and Herzog, 2005). Recent links between VIP signaling and schizophrenia highlight the possibility that VIP determines the development of the circuits underlying circadian synchrony (Vacic et al., 2011). To test whether VIP is required to maintain network topology in the SCN, we established a novel method to reliably map the functional connections between SCN neurons.
Within the central nervous system, γ-amino-butyric acid (GABA) serves as the principal inhibitory neurotransmitter. Nearly every neuron within the SCN synthesizes GABA(Moore and Speh, 1993; Belenky et al., 2007) and exhibits inhibitory postsynaptic currents (IPSCs) that depend on GABA signaling and vary in frequency over the day (Itri et al., 2004). In spite of its predominance, however, the function of GABAergic signaling in the SCN remains unresolved. GABA has been reported to be inhibitory at all times (Aton et al., 2006; Liu and Reppert, 2000), mainly inhibitory during the day and excitatory during the night (Albus et al., 2005; Choi et al., 2008; De Jeu and Pennartz, 2002) and inhibitory during the night, excitatory during the day (Wagner et al., 1997). Furthermore, daily administration of exogenous GABA suffices to coordinate SCN neurons (Liu and Reppert, 2000) and GABA can transmit phase information between SCN populations (Albus et al., 2005); however, synchrony among SCN cells can persist during chronic blockade of intrinsic GABAergic signaling (Aton et al., 2006). To resolve these apparent contradictions, we discriminated the discharge patterns of large numbers of individual neurons over multiple days and identified the stability and polarity of GABA-dependent interactions in the SCN. Using real-time bioluminescence imaging, we discovered a novel role for these synapses in circadian timekeeping.
RESULTS
Spontaneous firing reveals fast connectivity
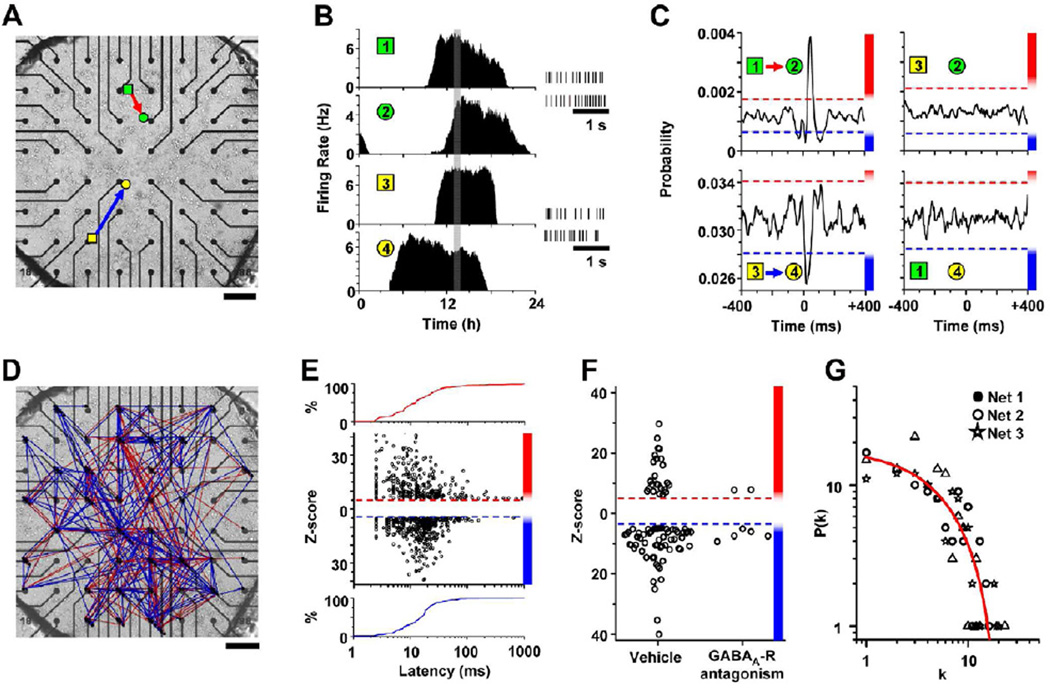
To assess functional communication between SCN neurons, we monitored gene expression and firing rates of individual SCN neurons in vitro. We found that in explants and dispersals, SCN neurons maintained synchronized circadian rhythms for as long as we recorded, demonstrating that the network mechanisms underlying coordinated circadian rhythmicity are intrinsic to these cultures (Figure S1). We took advantage of this self-sustained neural circuit to test the role and stability of specific connections in circadian rhythms. We recorded spontaneous action potentials from many SCN neurons simultaneously and continuously over days with 40-µs resolution on multi-electrode arrays (MEAs) (Figure 1A). Consistent with previous reports (Welsh et al., 1995), circadian neurons fired daily for 9.8 ± 0.3 h (mean ± SEM) with maximal firing frequencies of 4.7 ± 0.3 Hz (n = 101 neurons from 3 cultures). From the time-stamped spikes, we found spike trains that cross-correlated either positively or negatively between neurons (Figures 1B and 1C). These cross-correlations indicated that when one neuron fired, there was a low, but real, probability that its correlated partner increased or decreased its discharge rate with a short time delay.
Figure 1. Detection and mapping of GABA-dependent interactions in SCN networks.
(A) SCN neurons were recorded on a MEA. Colored shapes show the locations where four representative neurons were recorded. (B) The firing patterns of these neurons differed in their time of peak firing over the day (left) and in their spike patterns (right). (C) Spike trains from one recorded hour (gray box in 1B) crosscorrelated positively, negatively or not at all between each pair of neurons. These predictable changes in firing were illustrated as excitatory (red arrow) or inhibitory (blue arrow), directed connections on a micrograph of the SCN culture. Dashed lines represent significance thresholds as determined by BSAC and the magnitude of each Z-score is color coded (graded red and blue bars on the right). In these examples, the probability of neuron 2 firing increased by 233% after neuron 1 fired and the probability of neuron 4 firing decreased by 17% after neuron 3 fired. (D) A map of interactions detected from a representative 24-h recording. The 103 firing neurons (nodes) made 542 directed connections (arrows). (E) The Z-score of each interaction as a function of the latency to the peak of its cross-correlogram. Note from the cumulative distribution of these excitatory (red line) and inhibitory (blue line) interactions that most peaked between 2.5 and 15 ms, consistent with monosynaptic communication. (F) Treatment of SCN cultures with GABAA receptor antagonists (200 µM bicuculline or 100 µM gabazine) significantly decreased the number of excitatory and inhibitory connections. The remaining correlations were relatively weak and accounted for less than 7% of the connections found in vehicle-treated cultures. (G) Degree distributions of three representative SCN networks were not linear on log-log plots and therefore deviate from the power law predicted for scale-free networks. In these representative networks, the likelihood P(k) of finding a neuron with k outgoing connections decreased exponentially with increasing connections and the averaged degree distribution across networks was best fit by a first-order exponential decay function (r2 = 0.95, red line). Scale bars, 200 µm.
Between-network validation of connectivity
Correlated activity can reflect functional neural connectivity (Bialek et al., 1991; Gerstein and Perkel, 1969), but may also arise coincidentally. To determine the likelihood of detecting spurious versus functional connections, we developed a method (BSAC, see EXPERIMENTAL PROCEDURES) that generated an empirical distribution of Z-scores for false-positive connections in SCN circuits. Iterative pair-wise analysis of spike trains from 610 neurons recorded on 10 MEAs yielded cross-correlograms of the 185,745 possible pair-wise comparisons. Of these, 161,101 were impossible interactions because the neurons were in physically distinct MEAs (Figure S2). These false connections were more prevalent than would be expected based on the standard prediction intervals associated with their Z-scores. This indicates that studies that use Z-scores to determine the significance of correlated neural activity (e.g. functional neuro-imaging or neural circuit analyses) can overestimate the number of connections. By including nodes (neurons) from independent networks (SCN cultures), we were able to set an empirically derived false discovery rate (FDR) to 0.001 (1 in every 1000 correlations could be incorrect) and define functional connections as inter-neuronal firing correlations with either |Z|>5.6 (positive cross-correlations) or |Z|>4.68 (negative cross-correlations). Importantly, iterative comparisons across 3–10 cultures yielded similar Z-score thresholds (P>0.05, One-way ANOVAs for positive and negative correlations, respectively) indicating that connection detection was highly reproducible from culture to culture. For all SCN recordings, we calculated the frequency of detecting true neuronal interactions (hit rate) to be 96.0 ± 1.2% (mean ± SEM). Thus, BSAC recognizes functional connections with exceptionally high hit rates (96%) and low false-alarm rates (0.1%).
Sparse GABAergic connectivity in the SCN
We next sought to identify the signaling mechanism(s) underlying the identified communication between SCN neurons. Using the significantly cross-correlated firing patterns from 330 SCN neurons recorded in three cultures over 24 h (n= 103 – 121 neurons/culture), we generated spatial maps of connectivity with neurons represented as nodes and their interactions as directed edges (Figure 1D). We found interactions within cultures that were inhibitory (58 ± 4%, mean ± SEM of n = 3 cultures), excitatory (42 ± 4%) or switched polarity (10 ± 1%) over the day (where the proportions of the three types of interactions summed to 100% within each culture). The median lag times to maximal cross-correlation for inhibitory and excitatory connections were 15.4 ms and 12.5 ms, respectively, with 71% of correlations peaking within 20 ms and 93% within 50 ms (Figure 1E). Distributions of lag times did not differ significantly between inhibitory and excitatory connections (P=0.24, Student’s t-test). Taking into account delays due to action potential propagation (0.1 to 6.5 ms, Figure S3) and post-synaptic response (15.4 ms as measured by Itri et al., 2004), these results suggest that a majority of the deduced connections represent direct, fast synaptic interactions and a minority (i.e. those with longer lag times) may arise from polysynaptic interactions, common inputs, or post-inhibitory rebound.
Because most, if not all, SCN neurons are GABAergic and express GABAA receptors, we tested whether the mapped interactions depend on GABA-mediated signaling using gabazine (Gbz, 100 µM) or bicuculline (200 µM). These GABAA receptor antagonists decreased the number of significant connections by 90 ± 2% (mean ± SEM) compared to vehicle (P=0.03, Figure 1F; comparing connections from 120 randomly selected neurons in 3 cultures during vehicle and drug application). This loss in functional connections was not due to a decrease in firing since discharge rates actually increased slightly under GABA blockade (vehicle = 4.06 ± 0.31 Hz; mean± SEM; GABAA-R antagonists = 5.41 ± 0.38 Hz; P<0.05). Those few cross-correlations that remained had low Z-scores, suggesting that they could have been weakened by the blockers or could depend on an alternate, weak intercellular signal. Given the 96% hit rate of BSAC, we conclude that at least 93% of all detected interactions in these SCN cultures, both inhibitory and excitatory, depend on GABAA receptor signaling.
Specific network topologies have been postulated to underlie coordinated activity in a variety of biological systems including neural networks (Grinstein and Linsker, 2005; Harris et al., 2003; Bonifazi et al., 2009). These topologies are considered scale-free if their degree distributions follow a power law. To assess the architecture of the GABA-dependent network in the SCN, we measured the number of connections from and to each neuron (out and in node degrees, respectively). As indicated by spatial network maps (e.g. Figure 1D and Figure S2), levels of single-cell connectivity were heterogeneous. Neurons received connections from a median of 4.4% of the network and sent connections to 4.5% of the network; strikingly, some cells (15 of 330 cells) were directly connected to greater than 25% of the whole population and only 1.8% of nodes remained unconnected. Out and in degree distributions did not differ significantly across cultures (P>0.05 respectively, One-way ANOVAs) and were fit better by first-order exponential decay functions (r2 = 0.95 and 0.87, respectively) than power functions (out: r2=0.82, F(1,20) = 51.83, P<0.0001; in: r2= 0.81, F(1,15) = 8.13, P=0.012, extra-sum-of-squares F-test). That is, most cells sent and received approximately 1–4 connections with exponentially fewer sending or receiving progressively more connections (Figure 1G). In these cultures (n=3), the proportion of possible interactions identified as functional (i.e. the network density) ranged from 0.047 to 0.061. Together, these data suggest that SCN neurons reliably form networks of fast neurotransmission comprising 5 to 6% of the possible connections with patterns that are not purely scale-free.
Because we were concerned that the density of recording electrodes might affect the deduced topology of neural networks, we sub-sampled known networks to model the effects of undersampling and hidden nodes. We found that network density, clustering coefficient and path length were unaffected by including as little as 70% of the recorded neurons (Figure S4). These results suggest that BSAC accurately revealed network properties from recordings of 50–100 SCN neurons.
To determine if physiologically identifiable subgroups of SCN cells were more or less connected, we linearly correlated node degree (sending, receiving and total interactions) with measures of each neuron’s firing pattern at its daily peak of firing. Interestingly, no metric of the inter-spike interval distribution (i.e. the coefficient of variation, mode, median or mean) predicted the degree of connectivity of single neurons. We conclude that fast neurotransmission between SCN neurons has no apparent preference for neurons with specific firing patterns.
VIP signaling does not alter fast network architecture
Because VIP has been implicated in both synchronization of circadian neurons in the SCN(Aton and Herzog, 2005) and neural development (Muller et al., 1995), we tested whether VIP is required for normal GABA-dependent communication. We mapped connections within high-density, VIP-null SCN cultures and found they did not differ from wild-type cultures in network density (0.057 ± 0.015 vs. 0.045 ± 0.009, respectively; P=0.50, n=7 cultures per genotype), average path length (2.84 ± 0.32 nodes vs. 3.30 ± 0.23; P=0.27), mean node degree (0.11 ± 0.03 vs. 0.09 ± 0.01; P=0.49) or mean clustering coefficient (0.18 ± 0.03 vs. 0.23 ± 0.03, respectively; P=0.41). Together, these data indicate that VIP signaling is not required to determine the topology of the fast connections in the SCN. We conclude that VIP provides a synchronizing, not a trophic, signal to coordinate circadian cells within the SCN.
Connectivity fluctuates in circadian time
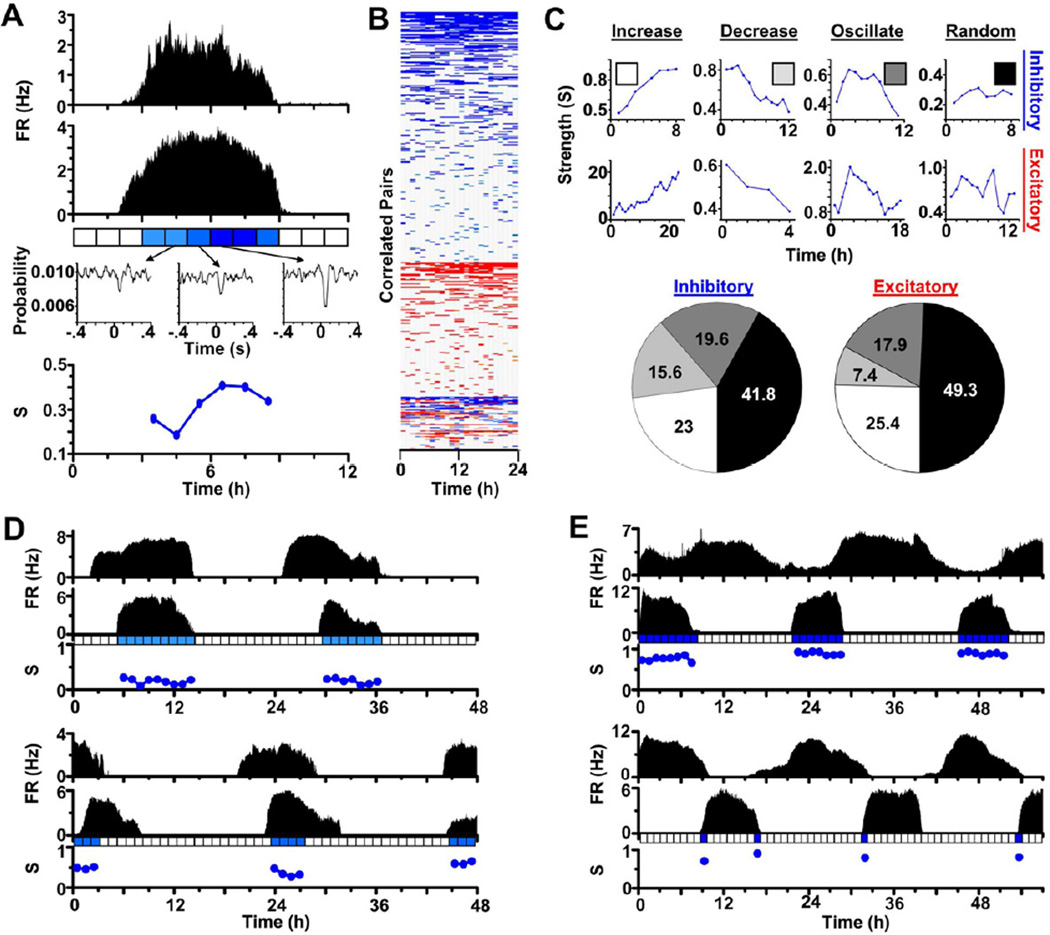
Changes in functional connectivity over milliseconds to hours can be critical for experience-dependent plasticity, synchronization or metastability in the nervous system (Harris et al., 2003). To date, it is not known if reliable changes in functional connectivity are inherent to specific synapses. To examine the dynamics of specific connections, we monitored the strength of correlated electrical activity from identified pairs of SCN cells over a circadian cycle (Figure 2A). Iterative pair-wise analysis yielded heat-maps that convey the temporal dynamics of all connections found within representative synchronized networks (Figure 2B). Importantly, when both neurons fired during the day, changes in connection strength occurred independently of circadian changes in firing frequencies (r2 = 0.006, P=0.27). We found that the strength of SCN connections fluctuated (coefficient of variation = 0.24 ± 0.01, mean ± SEM, n=189 pairwise interactions from 3 cultures), with a majority of identified connections increasing, decreasing or oscillating in strength over the day as opposed to varying randomly (Figure 2C).
Figure 2. Functional connectivity is dynamic over circadian time.
(A) Circadian firing patterns with firing rate (FR) measured in Hertz (Hz) of two representative SCN neurons and the strength (S, blue circles) of their pair-wise spike-for-spike interaction every hour over one day. Note that the strength of this inhibitory communication increased over the day. (B) A temporal heat map demonstrates how pair-wise interactions between neurons in one SCN network changed in number and strength over 24 h. Each row represents the strength of the connection between a different pair of neurons every hour. Note that most interactions were inhibitory (blue) or excitatory (red) throughout the day while some switched polarity (red and blue). (C) We categorized these representative neuron-neuron interactions as inhibitory (top) or excitatory (middle) and, using least squares fitting, determined whether their strength increased, decreased, oscillated or varied randomly over the day. Pie charts (bottom) show the percentages of each type of interaction within SCN networks (n=189 pairwise interactions that had significant interactions for at least 4 consecutive hours from 3 cultures). Note that the majority of inhibitory and excitatory connections changed with time of day with approximately 25% increasing in strength after the neurons started firing. Smaller fractions of neurons either consistently decreased or increased and then decreased their interaction strength. (D,E) Connectivity persists over days between identified neurons. Here, four representative pairs of neurons with in-phase (D) or anti-phase (E) circadian firing patterns had significant communication over multiple days. Note that an absence of strength measurements does not signify a lack of anatomic connectivity, but rather insufficient spikes to assess activity-dependent functional connectivity.
To assess the relative stability of SCN connections, we tracked individual, fast connections over multiple days from 3 cultures. We found millisecond-level connectivity between identified pairs of neurons that persisted over multiple days regardless of whether their circadian patterns were in phase or anti-phase (Figures 2D and 2E, respectively). Together, these data strongly suggest that sparse GABAergic connections can change strength over hours, persist over multiple days within synchronized SCN networks, and do not define a unique phase relationship between circadian SCN neurons.
GABA makes daily rhythms less precise
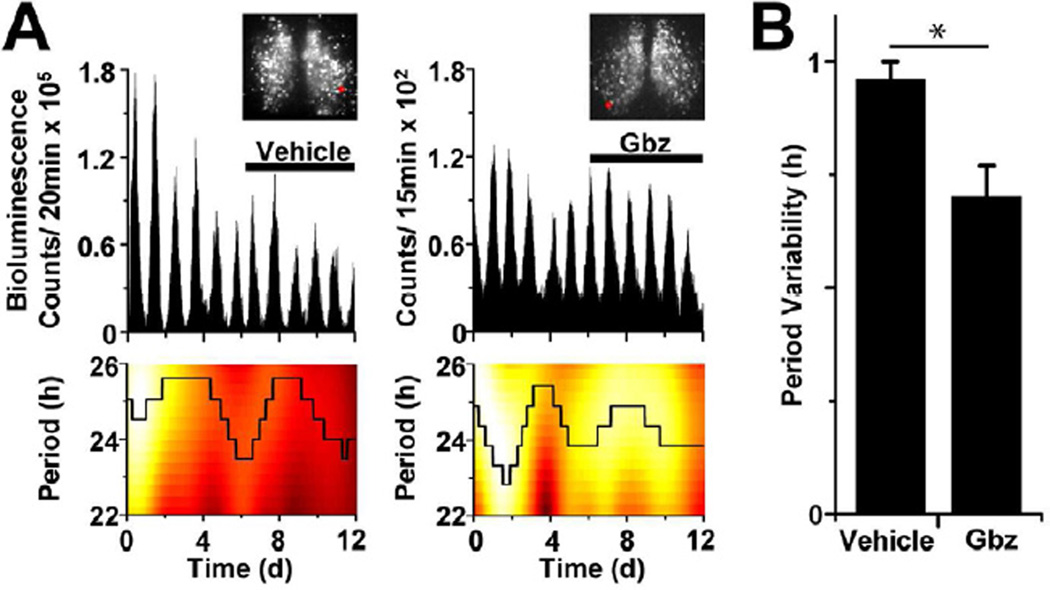
Given that we found significant GABAA receptor-mediated interactions within SCN networks that can change in strength over time, we tested the role of these connections in modulating circadian rhythmicity. Using a CCD camera, we monitored single-cell rhythms in Period2::Luciferase (PER2::LUC) expression from SCN explants over 12 days with 1 to 15 min resolution (Figure 3A). Period measurements for single cells were derived by continuous wavelet transform analysis (CWT) and period precision was calculated based upon the variance of the continuous period time-series. Consistent with a prior report (Aton et al., 2006), we found that GABAA receptor antagonism with 100 µM gabazine did not alter the level (Figure S5) or average period (23.63 ± 0.10 h; mean ± SEM, n=122 neurons in three SCN explants) of cellular PER2 rhythms compared to baseline (23.42 ± 0.12 h; P>0.05). Importantly, GABA blockade significantly decreased period variability of individual cells (0.70 ± 0.04 h, mean ± SEM) compared to vehicle (0.96 ± 0.07 h; P=0.002; n=3 SCN explants per treatment, 218 total cells; Figure 3B). The variability of the interpeak intervals was also decreased during GABA blockade (0.76 ± 0.05 h) compared to vehicle (0.98 ± 0.08 h, P=0.02). Together these data show that endogenous GABAA signaling decreases precision of circadian oscillations in networked SCN neurons.
Figure 3. GABAA-receptor signaling decreases precision of single-cell circadian rhythmicity.
(A) Blockade of GABAA signaling with gabazine (Gbz) reduced the variability of circadian periodicity in SCN cells. Traces (top) show PER2::LUC bioluminescence from two representative cells in SCN explants (insets, red circle shows cell location) treated with either vehicle (left) or 100 µM Gbz (right). Heat maps (bottom) indicate the instantaneous amplitude of the CWT and show the stability of the dominant period (black line) for each cell over 12 days. (B) Blockade of GABAA-dependent signaling significantly decreased period variability compared to vehicle (n=120 and 98 cells, respectively; * indicates P<0.01).
Desynchronization of rhythmicity by GABA
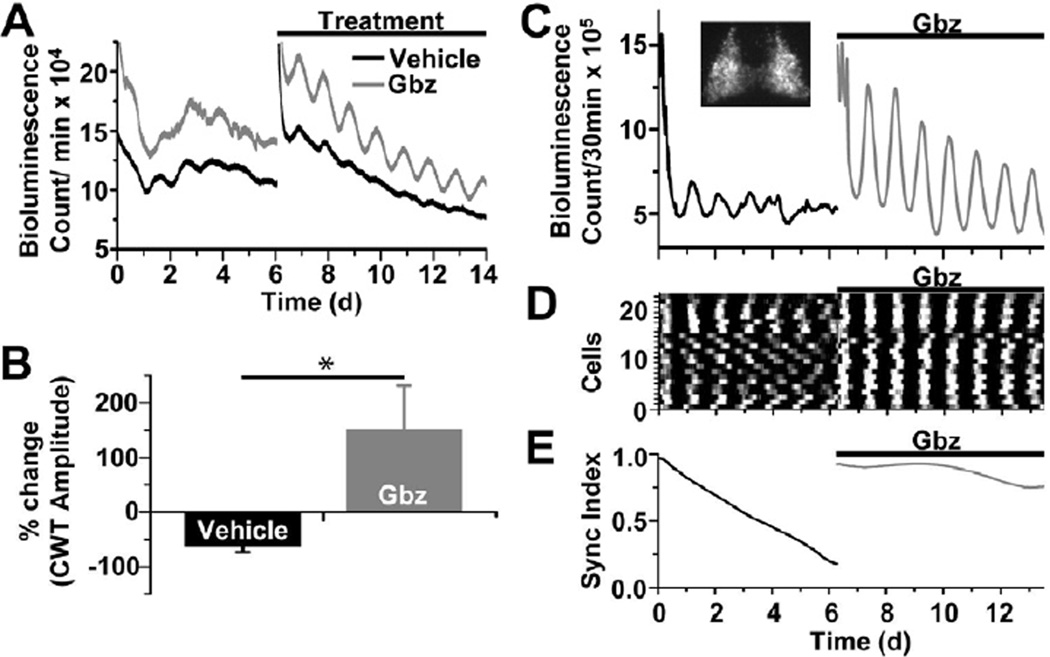
Because GABAA receptor signaling decreased precision of circadian gene expression in the presence of VIP, we postulated that GABAA receptor activation opposes the synchronizing effects of VIP in the SCN. We monitored PER2::LUC expression from VIP-null SCN explants (Vip−/−;PER2::LUC). Consistent with previous reports of Per1 transcription and PER2 protein in VIP-deficient SCN (Maywood et al., 2011), circadian bioluminescence damped over 6 days of baseline recording (relative amplitude error (RAE) = 0.044 ± 0.0003). SCN were then placed in fresh media with either 100 uM gabazine or vehicle and monitored for an additional 6 days (Figure 4A). Remarkably, blockade of GABAA signaling prevented significant damping in VIP-deficient SCN slices (for vehicle vs. gabazine, RAEtreated/RAEbaseline= 1.13 ± 0.26 vs. 0.51 ± 0.10, respectively; n=10 SCN explants per treatment; P<0.05). CWT analysis of the same data provided an independent quantification of the increased amplitude of circadian rhythmicity during GABA blockade (Figure 4B). We conclude that GABA is critical for the loss of circadian rhythmicity in VIP-deficient SCN.
Figure 4. Endogenous GABAdesychronizes circadian cells in the SCN.
(A) Representative traces of raw bioluminescence from two Vip−/−;PER2::LUC SCN explants show that gabazine (Gbz, 100 µM) rescued circadian rhythmicity compared to vehicle. (B) In the absence of VIP-signaling, gabazine increased the amplitude of the dominant period compared to baseline while vehicle treatment did not (n=10 explants in each condition; * indicates P<0.05). Total explant bioluminescence (C) and synchrony among cells (D,E) imaged from a Vip−/−;PER2::LUC explant (inset in C) quickly damped over 6 days of baseline recording while gabazine prevented damping of the bioluminescence rhythm and maintained circadian synchrony of the population.
To further determine how GABAA receptor signaling impacts circadian rhythms in single cells, we recorded bioluminescence using a cooled-CCD camera from SCN explants. We found that over 7 days of baseline treatment, Vip−/− cells exhibited low amplitude PER2::LUC oscillations (RAE = 0.116 ± 0.001; Figures 4C and 4D) and progressively desynchronized (Figure 4E). Upon medium change and addition of gabazine (100 µM), single cell PER2 expression (Figure S6) and rhythmicity dramatically increased (RAE = 0.004 ± 0.000, P<0.000001 vs. baseline; n=23 cells) and failed to damp or desynchronize for the duration of the recording. Together these results indicate that GABAA receptor-mediated signaling induces cycle-to-cycle jitter, weakly opposing the stabilizing and synchronizing effects of VIP on circadian rhythms in the SCN.
DISCUSSION
Fast connectivity of coupled circadian oscillators
Intercellular communication is necessary for proper SCN timekeeping and regulation of daily behaviors (Yamaguchi et al., 2003). By iteratively analyzing spike trains, we have produced the first maps of the functional, fast connections in the SCN network. Based on their probability to change firing within 50 ms, sensitivity to antagonists, and reproducibility across cultures and days, we posit that at least 93% of these connections represent direct, GABAA receptor-mediated interactions between SCN neurons. This is consistent with numerous studies that found most, if not all, SCN neurons receive GABAergic inputs (Moore and Speh, 1993; Belenky et al., 2007). The remaining 7% of connections we detected were relatively weak and could reflect GABAergic communication incompletely blocked by the concentrations of the antagonists used or weak signaling via other pathways (e.g. glycine neurotransmission (Mordel et al., 2011) or gap junctions (Long et al., 2005)). We conclude that GABA mediates nearly all interactions capable of influencing the millisecond firing patterns among SCN neurons.
Electron microscopic studies have found that individual SCN neurons receive between 300–1200 synaptic contacts (Guldner, 1976), a relatively low number compared to many brain areas. Whole-cell recordings in acute slices report typical SCN neurons fire during the day at 5 Hz with spontaneous IPSC frequencies at 3 – 12 Hz (Itri et al., 2004). Consistent with this evidence for inputs from small numbers of neurons, we found that functional GABAergic connectivity is sparse within SCN networks with approximately 55% of neurons responding to inputs from 1–4 neurons. We also found that nearly every SCN neuron sends at least one GABA-dependent output, such that most functionally connect to approximately 5% of the network and some connect to over 25% of the network. Taken together, these results suggest GABA provides sparse, weak and fast connectivity among SCN neurons.
Many biological networks have been hypothesized to be scale free and follow a power-law distribution. Within the SCN, recent computational models have predicted that a small-world scale-free network would be the most efficient topology for circadian synchrony (Vasalou et al., 2009; Hafner et al., 2012); however, here we provide clear evidence that signaling through GABAA receptors is not strictly patterned as a small-world network and, rather than enhance synchrony, decreases the precision and synchrony of neuronal rhythms. This is distinct from the well-described role of GABAergic signaling in coordinating higher-frequency (e.g., gamma [40–80 Hz]) neocortical oscillations (Ermentrout et al., 2008), but is consistent with the previous report that GABA is not required for SCN neurons to maintain circadian synchrony (Aton et al., 2006). In contrast to an extensive literature predicting GABA-induced synchrony (Liu and Reppert, 2000; Diekman and Forger, 2009), only a few theoretical studies have predicted that GABA could lead to reduced precision and synchrony in neural networks (Kopell and Ermentrout, 2004). We posit that GABAA signaling may accelerate reentrainment of the SCN to phase-shifted timing cues by increasing cycle-to-cycle jitter so that the cells are more easily phase-shifted by another signal (e.g. VIP). Our results lead us to predict that drugs that enhance GABAA signaling (e.g. benzodiazepines) in the SCN could reduce the precision of circadian rhythms, but enhance their adjustment to new schedules. Indeed, benzodiazepine administration speeds entrainment of human behavioral and hormonal circadian rhythms after simulated travel across time zones (Buxton et al., 2000).
Although it is highly unusual for GABA to be excitatory in the adult mammalian nervous system, previous studies have provided apparently contradictory evidence that within the SCN, GABA can be excitatory, inhibitory or both. We found that approximately 60% of all GABA-dependent connections were inhibitory and 40% were excitatory throughout the circadian day. A small fraction of these connections switched polarity for at least one hour during the day. The differential role of inhibitory and excitatory currents in the SCN remains unresolved.
Desynchronizers opposing synchronizers within the circadian clock
A central focus of biological timing research has been to understand the processes that promote synchronization. It is currently appreciated that activation of Gαs and Gαi/o protein-coupled receptors is vital to maintain synchrony among SCN cells (Aton and Herzog, 2005; Aton et al., 2006; Doi et al., 2011). VIP and, to a lesser extent, vasopressin and gastrin releasing peptide have been shown to mediate rhythm stability in and synchrony among circadian cells (Maywood et al., 2011). In contrast, we have found GABA to destabilize and desynchronize circadian cells. Synchronization and desynchronization are thus both active processes that can be differentially modulated. It is interesting to speculate that developmental and seasonal changes may alter the balance between fast neurotransmission and slower neuropeptide signaling to adjust the timing among SCN neurons. It is possible that GABA plays an important role within this setting to actively drive networks of oscillators to new phase relationships.
Additionally, recent work suggests that a hierarchy of neuropeptidergic signals may differentially promote or sustain rhythmicity and synchrony among SCN cells (Maywood et al., 2011). In light of our results, we must place GABAA signaling within this hierarchy and classify potential synchronizing agents by their ability to overcome the destabilizing effects of GABA. Our data suggest that VIP may be the only agent capable of overcoming this destabilizing effect since it is only after VIP signaling is eliminated that the desynchronizing effects of GABA are unmasked. Given that VIP signaling diminishes during aging (Cayetanot et al., 2005), increasingly unopposed GABAergic signaling may weaken SCN neuronal synchrony and contribute to sleep/wake cycle fragmentation in the elderly.
EXPERIMENTAL PROCEDURES
See a detailed description in the Supplemental Experimental Procedures
Connectivity and Network Analysis
Spike trains from SCN neurons were recorded using MEAs and the Z-score and strength of each interaction was measured. We graphed functional connections with GUESS software and analyzed network architecture with NodeXL software.
BSAC (Between-Sample Analysis of Connectivity)
We developed an empirical method to discriminate correlated activity that derived from real versus coincidental neuronal interactions. We reasoned that correlations between spike trains recorded from neurons in physically distinct culture dishes do not signify connectivity. Using this logic, we iteratively cross-correlated spike trains from all neurons across 10 cultures (samples) over 1 h to determine the distribution of Z-scores associated with inherently false across-sample correlations. Using the full distribution of false across-sample correlations, we determined Z-score magnitude thresholds that corresponded with likelihoods of discovering false-positive across-array correlations.
Temporal changes in connection strength
To determine if connection strength systematically changed with time of day, we fit the strength vs. time data with linear and cosine functions and estimated the resultant p-value using the F test.
Bioluminescence recording and analysis
PER2::LUC expression from SCN explants was measured using photomultiplier tubes or a CCD camera. Circadian period, amplitude and period variability were assessed using FFT-NLLS and CWT. Additional statistical tests were done with Origin 7 software. Mean periods were compared by two-tailed t-tests. Distributions of periods were compared using both Levene's and Brown-Forsythe's tests for equal variance.
Drug Treatments
Bicuculline methiodide (Sigma) and SR95531 (gabazine; Tocris) were diluted in water or DMSO. Drugs or vehicle controls were added at less than 0.5% of total volume).
Supplementary Material
ACKNOWLEDGEMENTS
We thank B. Carlson, T. Holy, J. Huettner, P. Taghert, C. Zorumski, H. Herzel and members of the Herzog lab for helpful discussion. This work was supported by NIH grants MH63104 (to EDH) and F30NS070376 (to GMF)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
SUPPLEMENTAL INFORMATION
Supplemental Information includes seven figures and Supplemental Experimental Procedures and can be found with this article online.
REFERENCES
- Albus H, Vansteensel MJ, Michel S, Block GD, Meijer JH. A GABAergic mechanism is necessary for coupling dissociable ventral and dorsal regional oscillators within the circadian clock. Current Biology. 2005;15:886–893. doi: 10.1016/j.cub.2005.03.051. [DOI] [PubMed] [Google Scholar]
- Aton SJ, Colwell CS, Harmar AJ, Waschek J, Herzog ED. Vasoactive intestinal polypeptide mediates circadian rhythmicity and synchrony in mammalian clock neurons. Nat. Neurosci. 2005;8:476–483. doi: 10.1038/nn1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aton SJ, Huettner JE, Straume M, Herzog ED. GABA and Gi/o differentially control circadian rhythms and synchrony in clock neurons. Proc.Natl.Acad.Sci.U.S.A. 2006;103:19188–19193. doi: 10.1073/pnas.0607466103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belenky MA, Yarom Y, Pickard GE. Heterogeneous expression of gamma-aminobutyric acid and gamma-aminobutyric acid-associated receptors and transporters in the rat suprachiasmatic nucleus. J.Comp Neurol. 2007;506:708–732. doi: 10.1002/cne.21553. [DOI] [PubMed] [Google Scholar]
- Bialek W, Rieke F, De Ruyter Van Steveninck RR, Warland D. Reading a Neural Code. Science. 1991;252:1854–1857. doi: 10.1126/science.2063199. [DOI] [PubMed] [Google Scholar]
- Bonifazi P, Goldin M, Picardo MA, Jorquera I, Cattani A, Bianconi G, Represa A, Ben-Ari Y, Cossart R. GABAergic hub neurons orchestrate synchrony in developing hippocampal networks. Science. 2009;326:1419–1424. doi: 10.1126/science.1175509. [DOI] [PubMed] [Google Scholar]
- Buxton OM, Copinschi G, Van Onderbergen A, Karrison TG, Van Cauter E. A benzodiazepine hypnotic facilitates adaptation of circadian rhythms and sleep-wake homeostasis to an eight hour delay shift simulating westward jet lag. Sleep. 2000;23:915–927. [PubMed] [Google Scholar]
- Cayetanot F, Bentivoglio M, Aujard F. Arginine-vasopressin and vasointestinal polypeptide rhythms in the suprachiasmatic nucleus of the mouse lemur reveal aging-related alterations of circadian pacemaker neurons in a non-human primate. EurJNeurosci. 2005;22:902–910. doi: 10.1111/j.1460-9568.2005.04268.x. [DOI] [PubMed] [Google Scholar]
- Choi HJ, Lee CJ, Schroeder A, Kim YS, Jung SH, Kim JS, Kim DY, Sohn EJ, Han HC, Hong SK, Colwell CS, Kim YI. Excitatory actions of GABA in the suprachiasmatic nucleus. J Neurosci. 2008;28:5450–5459. doi: 10.1523/JNEUROSCI.5750-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jeu M, Pennartz C. Circadian modulation of GABA function in the rat suprachiasmatic nucleus: excitatory effects during the night phase. J. Neurophysiology. 2002;87:834–844. doi: 10.1152/jn.00241.2001. [DOI] [PubMed] [Google Scholar]
- Diekman CO, Forger DB. Clustering predicted by an electrophysiological model of the suprachiasmatic nucleusJBiol. Rhythms. 2009;24:322–333. doi: 10.1177/0748730409337601. [DOI] [PubMed] [Google Scholar]
- Doi M, Ishida A, Miyake A, Sato M, Komatsu R, Yamazaki F, Kimura I, Tsuchiya S, Kori H, Seo K, et al. Circadian regulation of intracellular G-protein signaling mediates intercellular synchrony and rhythmicity in the suprachiasmatic nucleus. Nature Comm. 2011;2:327. doi: 10.1038/ncomms1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ermentrout GB, Galán RF, Urban NN. Reliability, synchrony and noise. Trends Neurosci. 2008;31:428–434. doi: 10.1016/j.tins.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerstein GL, Perkel DH. Simultaneously recorded trains of action potentials: analysis and functional interpretation. Science. 1969;164:828–830. doi: 10.1126/science.164.3881.828. [DOI] [PubMed] [Google Scholar]
- Grinstein G, Linsker R. Synchronous neural activity in scale-free network models versus random network models. Proc.Natl.Acad.Sci.U.S.A. 2005;102:9948–9953. doi: 10.1073/pnas.0504127102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guldner FH. Synaptology of the rat suprachiasmatic nucleus. Cell Tissue Res. 1976;165:509–544. doi: 10.1007/BF00224478. [DOI] [PubMed] [Google Scholar]
- Hafner M, Koeppl H, Gonze D. Effect of Network Architecture on Synchronization and Entrainment Properties of the Circadian Oscillations in the Suprachiasmatic Nucleus. PLoS Comp. Biol. 2012;8:e1002419. doi: 10.1371/journal.pcbi.1002419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris KD, Csicsvari J, Hirase H, Dragoi G, Buzsáki G. Organization of cell assemblies in the hippocampus. Nature. 2003;424:552–556. doi: 10.1038/nature01834. [DOI] [PubMed] [Google Scholar]
- Herzog ED. Neurons and networks in daily rhythms. Nat.Rev.Neurosci. 2007;8:790–802. doi: 10.1038/nrn2215. [DOI] [PubMed] [Google Scholar]
- Itri J, Michel S, Waschek JA, Colwell CS. Circadian rhythm in inhibitory synaptic transmission in the mouse suprachiasmatic nucleus. J. Neurophysiology. 2004;92:311–319. doi: 10.1152/jn.01078.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang YJ, Aerne BL, Smithers L, Haddon C, Ish-Horowicz D, Lewis J. Notch signalling and the synchronization of the somite segmentation clock. Nature. 2000;408:475–479. doi: 10.1038/35044091. [DOI] [PubMed] [Google Scholar]
- Kopell N, Ermentrout B. Chemical and electrical synapses perform complementary roles in the synchronization of interneuronal networks. Proc.Natl.Acad.Sci.U.S.A. 2004;101:15482–15487. doi: 10.1073/pnas.0406343101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Reppert SM. GABA synchronizes clock cells within the suprachiasmatic circadian clock. Neuron. 2000;25:123–128. doi: 10.1016/s0896-6273(00)80876-4. [DOI] [PubMed] [Google Scholar]
- Long MA, Jutras MJ, Connors BW, Burwell RD. Electrical synapses coordinate activity in the suprachiasmatic nucleus. Nat. Neurosci. 2005;8:61–66. doi: 10.1038/nn1361. [DOI] [PubMed] [Google Scholar]
- Maywood ES, Chesham JE, O’Brien JA, Hastings MH. A diversity of paracrine signals sustains molecular circadian cycling in suprachiasmatic nucleus circuits. Proc.Natl.Acad.Sci.U.S.A. 2011;108:14306–14311. doi: 10.1073/pnas.1101767108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore RY, Speh JC. GABA is the principal neurotransmitter of the circadian system. Neurosci. Letters. 1993;150:112–116. doi: 10.1016/0304-3940(93)90120-a. [DOI] [PubMed] [Google Scholar]
- Mordel J, Karnas D, Inyushkin A, Challet E, Pévet P, Meissl H. Activation of glycine receptor phase-shifts the circadian rhythm in neuronal activity in the mouse suprachiasmatic nucleus. J.Physiol. 2011;589:2287–2300. doi: 10.1113/jphysiol.2010.204693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller JM, Lelievre V, Becq-Giraudon L, Meunier AC. VIP as a cellgrowth and differentiation neuromodulator role in neurodevelopment. Mol. Neurobiology. 1995;10:115–134. doi: 10.1007/BF02740671. [DOI] [PubMed] [Google Scholar]
- Rao VR, Finkbeiner S. NMDA and AMPA receptors: old channels, new tricks. Trends Neurosci. 2007;30:284–291. doi: 10.1016/j.tins.2007.03.012. [DOI] [PubMed] [Google Scholar]
- Strogatz SH. From Kuramoto to Crawford: exploring the onset of synchronization in populations of coupled oscillators. Physica D. 2000;143:1–20. [Google Scholar]
- Vacic V, McCarthy S, Malhotra D, Murray F, Chou HH, Peoples A, Makarov V, Yoon S, Bhandari A, Corominas R, et al. Duplications of the neuropeptide receptor gene VIPR2 confer significant risk for schizophrenia. Nature. 2011;471:499–503. doi: 10.1038/nature09884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasalou C, Herzog ED, Henson MA. Small-World Network Models of Intercellular Coupling Predict Enhanced Synchronization in the Suprachiasmatic Nucleus. J.Biol.Rhythms. 2009;24:243–254. doi: 10.1177/0748730409333220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner S, Castel M, Gainer H, Yarom Y. GABA in the mammalian suprachiasmatic nucleus and its role in diurnal rhythmicity. Nature. 1997;387:598–603. doi: 10.1038/42468. [DOI] [PubMed] [Google Scholar]
- Webb AB, Angelo N, Huettner JE, Herzog ED. Intrinsic, nondeterministic circadian rhythm generation in identified mammalian neurons. Proc.Natl.Acad.Sci.U.S.A. 2009;106:16493–16498. doi: 10.1073/pnas.0902768106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh DK, Logothetis DE, Meister M, Reppert SM. Individual neurons dissociated from rat suprachiasmatic nucleus express independently phased circadian firing rhythms. Neuron. 1995;14:697–706. doi: 10.1016/0896-6273(95)90214-7. [DOI] [PubMed] [Google Scholar]
- Yamaguchi S, Isejima H, Matsuo T, Okura R, Yagita K, Kobayashi M, Okamura H. Synchronization of cellular clocks in the suprachiasmatic nucleus. Science. 2003;302:1408–1412. doi: 10.1126/science.1089287. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.