Abstract
Long-term exposure to respirable dust containing silica leads to pneumoconiosis/silicosis. The disease is irreversible and incurable, and only preventive steps such as job rotation, use of personal protective equipment, etc., remain solutions to the problem. Under such a situation, early diagnosis or prediction may become very useful to control the disease. Biomarkers are biological parameters in blood serum that change their values with deposition of dust in the lung and onset of lung fibrosis. Since these biomarkers can help us to diagnose and in the prognosis of the disease before it is actually diagnosed by the conventional X-ray technique and lung function test used for diagnosis of silicosis. The present paper describes the various types of available biomarkers, their application and usefulness. The paper also suggests that further study of the behavior/level of these biomarkers on a specific subject with time may provide more useful information of a confirmatory nature for prevention of dust-linked diseases.
Keywords: Biomarker, coal workers pneumoconiosis, silicosis
INTRODUCTION
The Committee on Biological Markers of the National Research Council (NRC) in the United States of America has defined biomarkers as “Indicators of variation in cellular or biochemical components or processes, structure or function that are measurable in biologic systems or samples” (NRC, 1989). Therefore, biomarkers may represent signals in the intervening steps between exposure and resultant disease. There are a number of diseases, which do not have effective treatment and prevention is considered the best remedial step. Early diagnosis and prognosis for these diseases is therefore very critical to their treatment. Diagnosis of these diseases with the help of biomarkers enables one to take timely preventive steps and ensure a prolonged healthy life of the affected person. Silicosis and Coal Workers Pneumoconiosis (CWP) come under this category of diseases of workforces exposed to respirable mine dust containing free silica for a long time. This paper describes various available biomarkers as a potential remedial tool for air-borne respirable dust exposure-linked diseases such as silicosis and CWP.
SILICOSIS
Silicosis refers to the formation of small, typically round nodules in the lungs of people exposed to crystalline silica dust. Silicosis is a potentially fatal, irreversible, progressive and untreatable fibrotic lung disease caused by prolonged inhalation and deposition of respirable crystalline silica. Pulmonary tuberculosis is a common complication before silicosis. It may occur at any stage in the development of silicosis, but silicosis is more likely to occur in older workers having a prolonged history of respirable air-borne dust exposure. There are three kinds of silicosis that are recognized: Acute silicosis can develop after 2–5 years of frequent exposure to high levels of respirable dust containing silica. Acute silicosis occurs among underground miners working under uncontrolled conditions with no respiratory protection, in tunneling workers and sandblasters. Accelerated silicosis can occur in workers after 10 or more years of exposure to very high levels of silica dust. Chronic silicosis can occur in workers exposed to fairly high levels of silica dust for extended periods of time (more than 15 years). Coal mining workers contract a similar type of disease called CWP due to exposure to coal dust containing silica. Data from coal India Limited showed that up to 41 new cases have been diagnosed in the year 2004. Figure 1 shows the trends of diagnosing new cases of CWP in Coal India Limited over a period of 17 years from 1994 to 2010. Since the cases are mostly diagnosed at a later stage of service, total cases at a time may not be very high mainly due to their superannuation and there are only 52 proved cases of CWP as on 01 January 2010.
Figure 1.
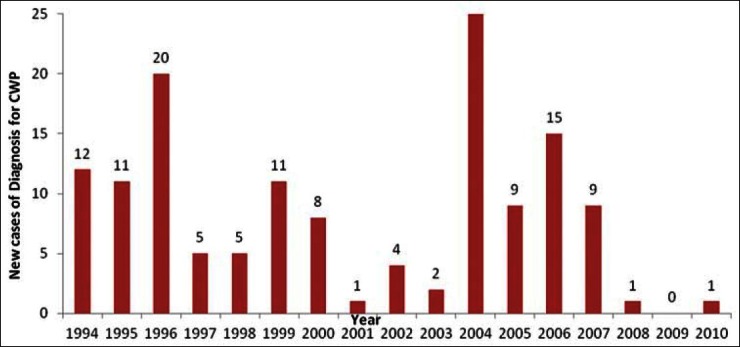
Pattern of new cases of CWP diagnosis in coal India limited
Diagnosis of silicosis is mainly based on clinical examination of subjects, with special emphasis on the respiratory system, measurement of pulmonary function, especially lung volume, and grading of profusion according to the International Labour Organization (ILO) classification of pneumoconiosis. Spirometry is a physiological test that measures an individual′s inhalation or exhalation air volume as a function of time.
NEED FOR BIOMARKERS
Biological markers (biomarkers) are cellular, biochemical or molecular alterations that are measurable in biological media such as human tissues, cells or fluids. It is a biological characteristic that can be objectively measured and evaluated as an indicator of normal biological processes, pathogenic processes or pharmacological responses to a therapeutic intervention. Biomarkers include tools and technologies that can aid in understanding the prediction, cause, diagnosis, progression, regression or outcome of disease treatment. Alone or in combination, biomarkers can provide an early warning system for risk of future adverse health outcomes. Many of these biomarkers are relatively easy to measure and are often part of routine medical examinations. Blood pressure, heart rate and pulse are commonly measured indicators of cardiovascular functioning. Biomarkers may be better predictors of disease (illness) and death than self-reported health status. Even when individuals have already provided information on their physical, mental and cognitive health, biomarkers provide additional information that improves the ability to predict whether an individual is likely to live or die. Biomarkers collected in physical exams, such as markers of cardiovascular diseases and diabetes, and those not usually part of routine physical examinations, such as immune markers, are useful predictors of health. An individual biomarker, once it exceeds a certain threshold, is an indicator of risk of future illness or death due to problems in a particular biological system. By adding the risk indicators together, an index may be created that captures higher health risks signaled by biological processes that occur simultaneously in an individual. Although it is desirable to have a biomarker for any disease, its usefulness increases manifold when the disease is incurable and of a fatal nature like silicosis.
VARIOUS BIOMARKERS FOR SILOCOSIS
A number of researchers have experimented with a special type of parameter in blood serum and observed noticeable changes in that parameter with exposure of persons to silica dust. Various available and tried biomarkers are being presented as follows:
Serum Cu
The primary pathologic changes in silicosis are fibrosis in the lungs. Studies[1,2] conducted on Cu level in blood serum indicate that Cu plays a very important role in the progress of lung fibrosis, and it has a direct relationship with Cu level in serum. The studies conducted by Ren and Jiang[3] also confirmed that an increase in ceruloplasmin levels in a silicosis group is also one of the factors for increase in Cu level. Each ceruloplasmin molecule contains eight Cu atoms, and the Cu level in blood serum is maintained by ceruloplasmin. The Cu increase may therefore be explained by an increase in the ceruloplasmin level in blood serum. Thus fibrosis of lungs due to silicosis is accompanied by increases in ceruloplasmin as well as Cu levels in blood serum. An important study was conducted by Tiwari et al.,[4] on 134 workers of quartz stone crushing units to assess serum Cu activity. The study led to the following conclusions:
No significant difference was found among different categories of workers when mean serum Cu levels were compared according to duration of exposure
When workers were categorized according to presence or absence of respiratory diseases, those having respiratory diseases and exposed for ≥1 year were found to have significantly higher levels of serum Cu compared with those having a similar duration of exposure but free from disease
Smokers had significantly higher values of serum Cu compared with non-smokers
When pulmonary function tests were categorized according to respiratory morbidity, serum Cu levels were found to be higher among those having respiratory disorders compared with those free from diseases.
Neopterin
Neopterin is regarded as an early biomarker of the cellular immune response. Neopterin (D-erythro-6-[1′,2′,3-trihydroxypropyl]-pterin) is known to be in equilibrium with 7,8-dihydroneopterin, and the presence of high concentrations of both oxidized and reduced forms of pteridin may be associated with oxidation stress. Altindag et al.,[5] investigated serum neopterin levels in silica-exposed and healthy workers by ELISA, spectrophotometry and high-performance liquid chromatography (HPLC). They[5] found increased urinary and serum neopterin levels in silica-exposed workers compared with a control group. They[5] suggested that neopterin is an excellent marker for activation of the monocyte-macrophage axis in some clinical situations. Prakova et al.,[6] have done an investigation of silicosis patients. These patients were divided into three groups according to the characteristics of radio-morphological alternations (ILO, 2002). In the first group of 21 people, X-ray pictures showed a prevalence of unevenly spread striped shadows (s, t, u) and single oval shadows measuring up to 1.5 mm (p). The second group included 23 patients with X-ray alterations composed mainly of oval shadows with size up to 1.5 mm (p), from 1.5 to 3 mm (q) and above 3 mm (r). The third group comprised individuals with X-ray evidence for progressive massive fibrosis of types A and B. The control group consisted of 16 clinically healthy individuals not exposed to dust. Each subject was given a questionnaire, which included information on age, occupational history, health status and bad habits such as smoking. Prakova et al.,[6] used an ELISA kit (DRG Diagnostics, Germany; in ng/ml) to determine the serum neopterin level. According to Prakova et al.,[6] serum neopterin levels in silicosis patients was 2.37 ± 1.12 ng/ml and in controls 1.56 ± 0.39 ng/ml, which is significantly higher than those in the control. The increased serum neopterin level in the silicosis patients compared with the control group, noticed by Prakova et al.,[6] confirms the role of the cellular immune response and the persisting macrophage activation in the pathogenesis of the disease.
Serum selenium
Selenoprotein-P (SeP) is a selenium (Se) supply protein that is an antioxidant micronutrient considered to be vital for human health. The Nutritional Prevention of Cancer Trial showed that a selenium supplement decreased the incidence of lung cancer.[7] Selenium is considered to be a chemopreventive agent for lung cancer in subjects with low selenium status, although it increases lung cancer risk in subjects with higher selenium status.[8] Muzemboa et al.,[9] conducted a control study where serum samples from 78 patients with silicosis and 20 healthy controls were assayed for Se and SeP. Medical and job history, lung function test and chest radiography examination were done. The level of serum Se was measured by electro-thermal atomic absorption spectrophotometry and SeP was assessed by sandwich enzyme immunoassay. These studies showed that serum Se levels were reduced in silicotic participants (74.0 μg/l and 4.2 mg/l, respectively) compared with control participants. Corresponding serum SeP (116 μg/l and 5.8 mg/l, respectively) levels decreased in a similar way in participants with silicosis. The studies by Muzemboa et al.,[9] confirmed that SeP is a good indicator of Se status in humans. Serum Se and SeP concentration were found to be inadequate in patients with silicosis, and decreased significantly with severity of disease.
Serum Zn
Pathologic changes in silicosis include collagen tissue in the lungs. Bai et al.,[1] indicated that zinc (Zn) takes part in the synthesis of collagen in the progression of lung fibrosis. Konishi et al.,[2] indicated that fibrosis is depressed by Zn. Wang et al.,[10] showed that Zn level is determined by using a flameless atomic absorption spectrophotometer (VIEO 22; Instrumentation Laboratory Inc., Lexington, MA, USA). Serum samples are diluted about five times with a mixture of 1% HNO3 and 2% Triton before atomic absorption. Each sample is analyzed in triplicate and the average value is adopted. Zinc level will be lower according the condition of a patient. If the patient′s condition is worsened and the compensating mechanism is destroyed, Zn level will decline.
Angiotensin-converting enzyme
Lieberman et al.,[11] reported an elevation of serum-angiotensin converting enzyme (SACE) levels in sarcoidosis. Subsequent studies[11,12] confirmed that SACE activity increases in a large proportion of patients having granulomatous diseases such as sarcoidosis and silicosis. Ryan et al.,[13] observed that angiotensin-1-converting enzyme (peptidyldipeptide hydrolase; EC 3.4.15.1) is a membrane-bound glycoprotein that converts angiotensin-1 to 2 and participates in bradykinin degradation. Tiwari et al.,[14] also confirmed elevation of SACE levels among silicotic patients. They observed the mean value of SACE level in silicotic patient to be 66.85 ± 14.38 IU/l compared with 64.28 ± 8.94 IU/l in controls. Studdy et al.,[12] explained that elevated SACE levels can be attributed to the fibrotic involvement of lung tissue, including capillaries as the endothelial cells in the capillary bed have high angiotensin-1-converting enzyme content.[12]
Heme oxygenase-1
Heme oxygenase-1 (HO-1), a rate-limiting enzyme in heme catabolism, has antioxidative, antiapoptotic and anti-inflammatory activities. Studies indicate that crystalline silica induces the production of reactive oxygen species (ROS), which play a key role in the development of silicosis. Generation of oxidants by crystalline silica particles and by silica-activated cells results in the activation of the following mechanisms:
Cell and lung injury
Activation of cell signaling pathways, which include mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase (ERK)
ERK phosphorylation
Increased expression of inflammatory cytokines
Activation of specific transcription factors
Mitochondrial dysfunction
Increase lipid peroxidation
Increase in gene expression of death receptors and ligands.
Gossart et al.,[15] have done experiments on rats and observed that intra-tracheal instillation of silica in rat lungs led to fibrosis and triggered the production of ROS.[15] These ROS caused direct tissue injury and activation of alveolar macrophages to undergo apoptosis, enhance the synthesis of proinflammatory cytokines and augment the induction of inflammatory responses via MAPK-dependent pathways.[16] Shi et al.,[17] suggested that silica-induced oxidative DNA damage, reflected by an increase in 8-hydroxydeoxyguanosine, is associated with increased risk of carcinogenesis. Another study[18] explained that HO-1, an enzyme that degrades heme into bilirubin, Fe2+ and carbon monoxide (CO) protect against ROS-induced airway inflammation. The study by Otterbein et al.,[19] reported similar findings.
Sato et al.,[20] conducted experiments on the characteristics of 46 patients with silicosis, 27 patients with chronic obstructive pulmonary diseases (COPDs) and 42 normal control subjects. Patients with silicosis and COPDs had longer smoking histories than control subjects, and the majority of patients were ex-smokers. Spirometry test for these patients show that the vital capacity (VC) and forced expiratory volume in 1 s (FEV1) were less than 80% of the predicted values for 21/46 patients with silicosis. Patients with COPDs had reduced FEV1 but not VC when compared with the control subjects but not patients with silicosis. The silicosis group included patients with disease of varying radiologic severity. Of the 46 patients, 43 had an ILO profusion >1/0 (with or without large opacities) and three had large opacities only. Some of the patients with silicosis or COPDs were being treated with theophylline (32.6% and 40.7%, respectively), β-agonists (28.3% and 25.9%, respectively), anti-cholinergics (26.1% and 37.0%, respectively) and/or inhaled corticosteroids (8.7% and 22.2%, respectively). Serum HO-1 levels were also correlated inversely with serum 8-hydroxydeoxyguanosine levels and positively with vital capacity and forced expiratory volume in 1 s in patients with silicosis.
HO-1 was present in the lungs of humans and mice with silicosis, especially at sites of silica particle deposition. In mice, silica exposure was associated with acute leukocyte infiltration, leading to development of silicotic lung lesions. Inflammation was suppressed by treatment with hemin, an inducer of HO-1, and enhanced by zinc protoporphyrin, an inhibitor of HO-1. Pulmonary HO-1 expression is increased in silicosis. HO-1 suppresses ROS activity, and subsequent pathologic changes, thereby attenuating disease progression. Another study[20] shows that HO-1 is persistently expressed in lung lesions of patients with silicosis. This appears to reflect the induction of ROS by silica, leading to elevation in serum HO-1 levels. The increased HO-1 can protect the host by suppressing silica-induced ROS activity. Thus regulation of HO-1 may represent a novel strategy for treatment of silicosis.
Clara cell protein
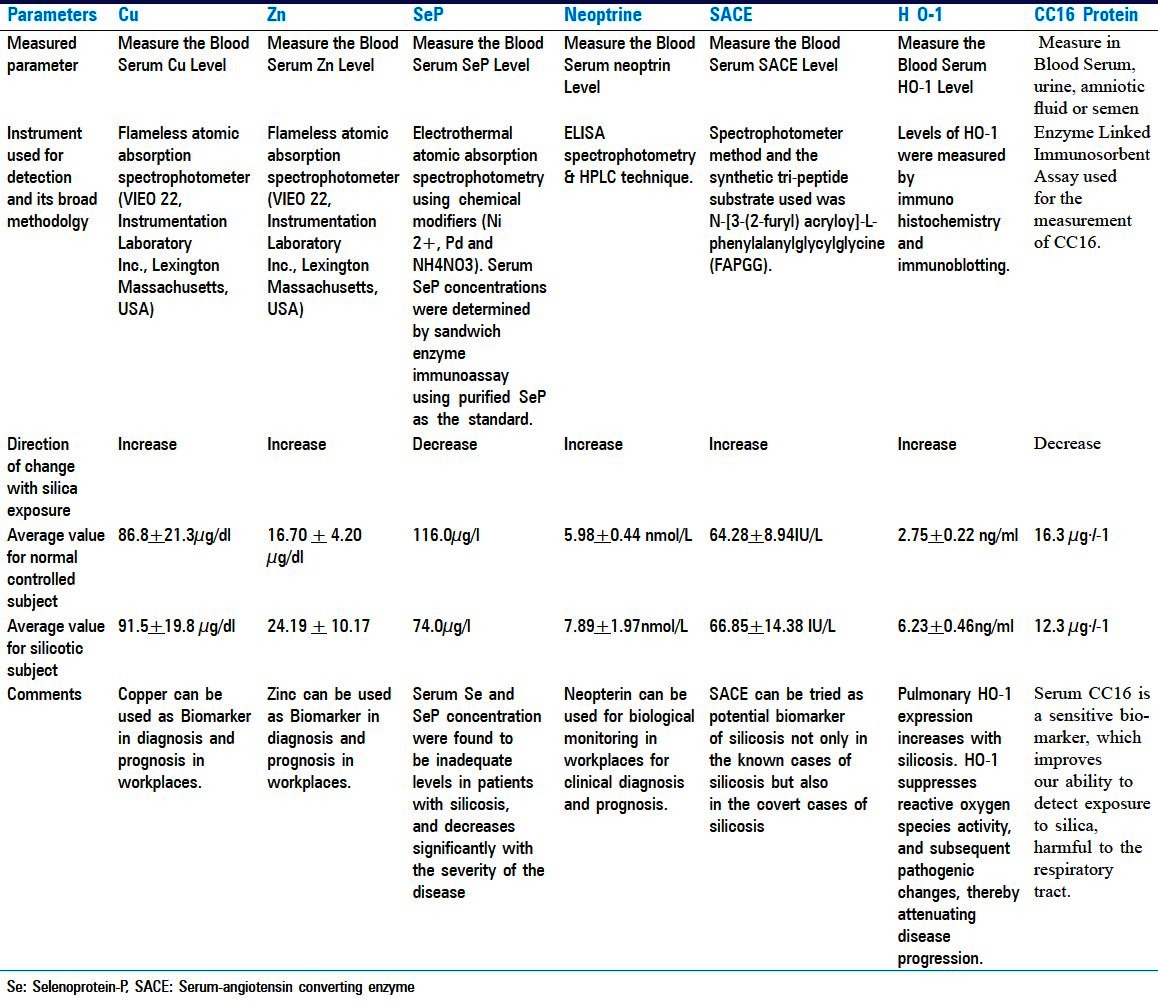
Clara cell protein (CC16) is a 16-kDa protein secreted by non-silicated cells of the tracheobronchial tree. It is proposed as a peripheral marker of respiratory epithelial injury. Bernard et al.,[21] observed that the highest concentrations of CC16 are observed in sputum and bronchoalveolar lavage fluid, reflecting intense secretion of the protein in the airways.[21] The protein also occurs, but in smaller concentrations, in other fluids such as urine, amniotic fluid or semen. Broeckaert and Bernard[22] found that Clara cells are one of the most multifunctional and heterogeneous cell types in the mammalian lung, with their main function being protection of the respiratory tract. Bernard et al.,[23] measured the concentration of CC16 in the serum of 86 miners exposed to silica and 86 control subjects matched for age, body mass index and smoking status (26 lifelong non-smokers and 60 current smokers in both groups). Workers were exposed to silica-rich dust in a quarry for 15.2 months on average. No appreciable difference could be detected between exposed and control workers with regard to respiratory symptoms, chest radiographs or lung function tests. However, the concentration of CC16 in serum decreased in silica-exposed workers (geometric mean 12.3 μg/l) compared with controls (16.3 μg/l). Decrease was found both in lifelong non-smokers (14.7 vs 21.9) and current smokers (11.3 vs 14.5). Tobacco smoking caused a decrease in serum CC16 that was further lowered after silica exposure. The serum concentrations of CC16 probably reflect the very early toxic effects of silica particles on the respiratory epithelium. This reinforces the view that serum CC16 is a sensitive marker, which might improve our ability to detect exposure to chemicals potentially harmful to the respiratory tract. Various information/parameters for all these seven biomarkers have been summarized and presented in Table 1 for a comparative view and easy comprehension.
Table 1.
Comparative view of all seven biomarkers for silicosis
DISCUSSION
It is important to know that silicosis is a non-curable yet preventable occupational disease and can be avoided with proper preventive strategies. These strategies include proper ventilation, dust collectors, wetting techniques, use of approved personal protective equipment, training workers and regular medical examinations. Trained management and workers fully realizing the debilitating effects of silica quartz exposure are key to the success of any silicosis preventive strategy. Biomarkers, which are biological measurable indicators or products of physiological processes that represent a critical step in the development of toxicity, show promise as a diagnostic tool for silicosis. A sensitive biomarker detects early changes at the cellular or molecular level, which may be indicative of lung damage.
Silica is one of the most documented workplace contaminants for miners. Long-term occupational exposure to silica is associated with increased risk for respiratory diseases such as silicosis, tuberculosis, chronic bronchitis, chronic obstructive pulmonary diseases and lung cancer. A variety of these dysfunctions are related to different biomarkers. Increase in ACE, copper (Cu) and ceruloplasmin levels can help in the diagnosis of silicosis. These particles activate macrophages to produce cytokines and enhance the production of tumor necrosis factor-α. These studies are insufficient to identify the disease in its early stages. More studies are required for target identification, validation and elucidation of its mechanism of action. Neopterin has been determined as an innovative tool for monitoring diseases associated with activation of cell-mediated immunity. Enhanced levels of neopterin reflect stimulation of the cell-mediated immune response with alterations in lymphocyte subsets and serum immunoglobulin levels.
Silicosis always accompanies lung fibrosis, which increases ceruloplasmin as well as Cu levels. Increase in ceruloplasmin levels in a silicosis group is one of the factors for increase in Cu levels. Selenium can be used as a chemo-preventive agent, and it also reduces the toxicities and side effects associated with cisplatin and radiation therapy. Neopterin levels may be used for assessment of occupational exposure to silica. Neopterin biological monitoring should be performed in workplaces for clinical diagnosis and prognosis. HO-1 is persistently expressed in lung lesions of patients with silicosis. Increase in HO-1 can protect the host by suppressing silica-induced ROS activity. SACE may be used to assist in distinguishing between silicosis and silico-tuberculosis. The significant reduction of serum CC16 in workers inhaling crystalline silica-rich dust could be used as a biomarker for early toxicity from exposure to crystalline silica and to subsequently improve detection of risk.
Understanding the probable action mechanism of silica after its inhalation by human body will be useful not only in developing a biomarker but also in adopting effective silicosis prevention strategies. Generation of free radicals by surface activity of crystalline silica induces oxidative stress. Once free silica reaches an alveolar macrophage, it is engulfed or phagocytosed by the macrophage, which may lead to damage of the lysosomal membrane. This is mainly due to the interaction between crystalline silica and hydrogen ions in membranes of cells. Peroxidation of phospholipids and unsaturated fatty acids in the membranes of cellular and intracellular structures results in damage of the membranes and generation of oxygen-free radicals, which causes damage to the intracellular structures. As a result, the membrane structure of the cells becomes more permeable and ions move more freely across the cellular membranes. This renders cells more vulnerable to chemical and physical stresses. Due to the damage to the intracellular structures, proteolytic enzymes are released into the cytoplasm, which results in the death of the macrophage. Destruction of the alveolar macrophage leads to increased proteolytic enzyme release in the lung tissue. The outcome of this is initiation of fibrotic reactions in the lung. Clinical detection of silicosis is dependent on the detection of radiological abnormalities, which is a late process. Crystalline silica-induced fibrosis has contributed greatly to the identification of a number of biological responses that are involved in the pathogenesis of silicosis. The presence of crystalline silica in biopsy and autopsy samples of lung tissues could be determined by electron microscopy using an electronic probe microanalyzer, electron sound analysis and scanning electron microscopy together with energy-dispersive X-ray microanalysis.
There are several reasons why such emphasis is currently placed on biomarkers. Biomarkers may enhance the diagnostic accuracy of occupational and environmental illness, and ultimately result in prevention of diseases in other individuals. In addition, biomarkers are likely to enhance the understanding of the dose-response relationship between exposure to a hazard and an illness. Ultimately, their use may help to evaluate the effectiveness of various control measures.
These biomarkers play a very crucial role in early diagnosis of the disease by observing characteristic changes in any of these biomarkers. Periodic monitoring of these biomarker parameters may be prognostic. Most of the earlier studies focused on one or the other parameter in blood serum and noticed marked changes (increase/decrease) in these parameters with respect to those of control subjects (not exposed to respirable dust). In most of the cases, the changes in the characteristics′ value were not more than the variation within the group itself. Despite, these changes suggest a definite change in lung fibrosis and as such have a diagnostic value. A major limitation of all these studies is that these studies had been conducted in a lateral dimension over a small time period and within a group population, which gives only statistical and probabilistic results with respect to an individual subject. Therefore, a more conclusive/definite finding may be obtained when the observations from these biomarkers are monitored in a cohort or for a specific subject over a long time and the data are interpreted with a time scale. Although such studies may take longer time (in years) to provide any meaningful changes in the characteristics′ values, they will be conclusive and have a definite prognostic value with respect to the health status of the subjects under monitoring. Mining industry of large public undertakings provide a vulnerable cohort such as drill operators, which remains in the similar profession/dust exposure profile for a significantly long time and therefore provides an opportunity to establish a conclusive prognostic value of the aforementioned biomarkers. Such a study will also help adopt preventive strategies and reducing cases of CWP in the industry.
CONCLUSIONS
Based on the aforementioned discussion on the silica-containing respirable dust exposure of miners and their early diagnostic cum prognostic approach using monitoring of a few characteristic biomarkers, the following conclusions can be drawn:
Since respirable dust exposure-linked lung fibrosis disease is non-curable, their early diagnosis and prognosis using indirect biological parameters called biomarkers provides a better way to deal with the deadly disease.
Research on biomarkers for silicosis is still at its nascent stage and it should be encouraged as part of a silicosis removal program.
Considering the nature of the disease, there is a need for change of the study approach for establishing a biomarker for silicosis. The existing approach of lateral study on a population group has only statistical prediction potential. It should be changed to subject-specific or a stable control population (cohort) of a vulnerable group with respect to these biomarkers over a long period of time. This will enable us to have a prognostic/predictive approach for the deadly disease and an effective silicosis preventive strategy leading to a silicosis-free world in the near future. Specialized industry such as mining may provide such a cohort for long-term study for establishing biomarkers for silicosis.
The problem of respirable dust exposure-linked lung fibrosis should be addressed by a three-pronged approach, a medico-socio-engineering approach, rather than any one in isolation as a combination of a medical, social and engineering approach may provide an optimum solution to this deadly disease.
ACKNOWLEDGMENTS
The authors are thankful to the Director, CSIR - Central Institute of Mining and Fuel Research, Dhanbad for giving his kind permission to publish the paper.
Footnotes
Source of Support: CSIR - Central Institute of Mining & Fuel Research, Dhanbad, India.
Conflict of Interest: No.
REFERENCES
- 1.Bai Y, Wang JJ, Wei SC, Hao DQ. Changes of trace element copper and zinc in sera of cases with silicosis. Chin J Indian Hyg Occup Dis. 1993;11:280. [Google Scholar]
- 2.Konishi A, Iguchi H, Ochi J, Kinoshita R, Miura K, Uchino H. Changes of trace element copper and zinc in sera of cases with silicosis. Gastroenterol. 1985;89:709–15. doi: 10.1016/0016-5085(85)90563-3. [DOI] [PubMed] [Google Scholar]
- 3.Ren ML, Jiang XL. Detection of coal silicosis by radioimmunoassay used for ceruloplasmin. Chin J Ind Hyg Occup Dis. 1993;11:145–6. [Google Scholar]
- 4.Tiwari RR, Sathwara NG, Saiyed HN. Serum copper levels among quartz stone crushing workers: A cross sectional study. Indian J Physiol Pharmacol. 2004;48:337–42. [PubMed] [Google Scholar]
- 5.Altindag ZZ, Baydar T, Isimer A, Sahin G. Neopterin as a new biomarker for the evaluation of occupational exposure to silica. Int Arch Occup Environ Health. 2003;76:318–22. doi: 10.1007/s00420-003-0434-9. [DOI] [PubMed] [Google Scholar]
- 6.Prakova G, Gidikova P, Slavov E, Sandeva G, Stanilova S. The potential role of neopterin as a biomarker for silicosis. Trakia J Sci. 2005;3:37–41. [Google Scholar]
- 7.Clark LC, Combs GF, Jr, Turnbull BW, Slate EH, Chalker DK, Chow J, et al. Nutritional Prevention of Cancer Study Group. Effects of selenium supplementation for cancer prevention in patients with carcinoma of the skin. A randomized controlled trial. JAMA. 1996;276:1957–63. [PubMed] [Google Scholar]
- 8.Fritz H, Kennedy D, Fergusson D, Fernandes R, Cooley K, Seely A, et al. Selenium and lung cancer: A systematic review and meta analysis. PLoS One. 2011;6:26259. doi: 10.1371/journal.pone.0026259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muzemboa BA, Dumavibhata N, Ngatua NR, Eitokua M, Hirota R, Kondo S, et al. Serum selenium and selenoprotein P in patients with silicosis. J Trace Elem Med Biol. 2013;27:40–4. doi: 10.1016/j.jtemb.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 10.Wang W, Wang L, Lu Y. Serum concentration of copper and zinc in patients with silicosis. J Occup Health. 1998;40:230–1. [Google Scholar]
- 11.Lieberman J. Elevation of serum angiotensin-converting-enzyme (ACE) level in sarcoidosis. Am J Med. 1975;59:365–72. doi: 10.1016/0002-9343(75)90395-2. [DOI] [PubMed] [Google Scholar]
- 12.Studdy P, Bird R, James DG. Serum angiotensin-converting enzyme (SACE) in sarcoidosis and other granulomatous disorders. Lancet. 1978;2:1331–4. doi: 10.1016/s0140-6736(78)91972-4. [DOI] [PubMed] [Google Scholar]
- 13.Ryan JW, Ryan US, Schultz DR, Whitaker C, Chung A. Subcellular localization of pulmonary angiotensin-converting enzyme (kininase II) Biochem J. 1975;146:497–9. doi: 10.1042/bj1460497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tiwari RR, Sharma YK, Karnik AB, Sathwara NG, Saiyed HN. Serum angiotensin converting enzyme activity and serum copper levels in convert silicosis. Indian J Occup Environ Med. 2005;9:124–6. [Google Scholar]
- 15.Gossart S, Cambon C, Orfila C, Seguelas MH, Lepert JC, Rami J, et al. Reactive oxygen intermediates as regulators of TNF-alpha production in rat lung inflammation induced by silica. J Immunol. 1996;156:1540–8. [PubMed] [Google Scholar]
- 16.Janssen-Heininger YM, Macara I, Mossman BT. Cooperativity between oxidants and tumor necrosis factor in the activation of nuclear factor (NF)-kappaB: Requirement of Ras/mitogen-activated protein kinases in the activation of NF-kappaB by oxidants. Am J Respir Cell Mol Biol. 1999;20:942–52. doi: 10.1165/ajrcmb.20.5.3452. [DOI] [PubMed] [Google Scholar]
- 17.Shi X, Castranova V, Halliwell B, Vallyathan V. Reactive oxygen species and silica-induced carcinogenesis. J Toxicol Environ Health B Crit Rev. 1998;1:181–97. doi: 10.1080/10937409809524551. [DOI] [PubMed] [Google Scholar]
- 18.Choi AM, Alam J. Heme oxygenase-1: Function, regulation, and implication of a novel stress-inducible protein in oxidant-induced lung injury. Am J Respir Cell Mol Biol. 1996;15:9–19. doi: 10.1165/ajrcmb.15.1.8679227. [DOI] [PubMed] [Google Scholar]
- 19.Otterbein LE, Mantell LL, Choi AM. Carbon monoxide provides protection against hyperoxic lung injury. Am J Physiol. 1999;276:688–94. doi: 10.1152/ajplung.1999.276.4.L688. [DOI] [PubMed] [Google Scholar]
- 20.Sato T, Takeno M, Honma K, Yamauchi H, Saito Y, Sasaki T, et al. Heme oxygenase-1, a potential biomarker of chronic silicosis, attenuates silica-induced lung injury. Am J Respir Crit Care Med. 2006;174:906–14. doi: 10.1164/rccm.200508-1237OC. [DOI] [PubMed] [Google Scholar]
- 21.Bernard A, Dumont X, Roels H, Lauwerys R, Dierynck I, De Ley M, et al. The molecular mass and concentrations of protein 1 or Clara cell protein in biological fluids: A reappraisal. Clin Chim Acta. 1993;223:189–91. doi: 10.1016/0009-8981(93)90077-h. [DOI] [PubMed] [Google Scholar]
- 22.Broeckaert F, Bernard A. Clara cell secretory protein (CC16): Characteristics and perspectives as lung peripheral biomarker. Clin Exp Allergy. 2000;30:469–75. doi: 10.1046/j.1365-2222.2000.00760.x. [DOI] [PubMed] [Google Scholar]
- 23.Bernard AM, Gonzalez-Lorenzo JM, Siles E, Trujillano G, Lauwerys R. Early decrease of serum Clara cell protein in silica-exposed workers. Eur Respir J. 1994;7:1932–7. [PubMed] [Google Scholar]


