Abstract
Basal insulin have been developed over the years. In recent times newer analogues have been added to the armanentarium for diabetes therapy. This review specifically reviews the current status of different basal insulins
Keywords: Basal insulin, diabetes, degludec
INTRODUCTION
Since its first clinical use in 1920s, insulin remains the cornerstone in the management of both type 1 and type 2 diabetes mellitus. Further, in late 1970s, insulin therapy was complimented by the availability of recombinant human insulin,[1] which was a more acceptable form of insulin as it had fewer side effects. Subsequently, recombinant technology led to development of insulin analogs, in which structural modifications were introduced in the amino acid sequences to human insulin so as to get the desired pharmacokinetic and pharmacodynamic profile.
The strategy behind insulin therapy is to simulate the body's physiological insulin secretion. In this, there is a basal insulin secretion that constitutes approximately 40% of the total insulin secretion over 24-h period along with short bursts of insulin secretion that occurs with meals.[2] The main function of the basal insulin secretion is to inhibit hepatic glycogenolysis, ketogenesis, and gluconeogenesis and that of prandial insulin secretion is to combat the meal-related surge in glucose levels.[2] Our aim is to approximate this physiological insulin profile by providing a long-acting insulin that mimics the basal insulin secretion and to provide short-acting insulin with meals that approximates the insulin bursts occurring with meals [Figure 1].
Figure 1.
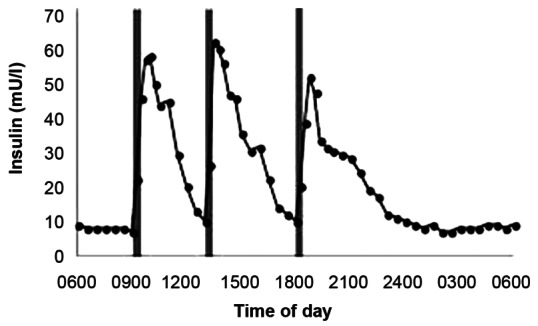
Physiological insulin profile that shows variations with respect to meals (source: http://www.biochemsoctrans.org/bst/default.htm)
EVOLUTION OF BASAL INSULINS
An ideal, basal insulin should have a peak-less pharmacodynamic profile, at least 24 h of duration of action, very low risk of hypoglycemia, should be tolerated well in both type 1 and type 2 diabetes mellitus, and should have predictable action without any intra and inter individual variability.[3] The quest for the search of such an ideal basal insulin continues.
Intermediate acting insulin neutal protamine hagedorn (NPH) and lente insulin has been used as basal insulin by giving two doses so as to give the basal insulin coverage for a period of 24 h. They have a gradual onset of action of 1-3 h with a peak of 4-8 h and a duration of action of 13-20 h.[4] NPH consists of protamine that ensures slow release of insulin and zinc, which allows protamine to form crystals with insulin. These insulin have a peak action that may overlap with the peaks of short-acting analogs and may result in frequent hypoglycemic episodes, especially at night.[3] They also have huge intra patient and inter patient variability in their pharmacokinetic and pharmacodynamic profile.[5] Ultralente insulin has longer duration of action than lente insulin and NPH, but it is associated with larger day to day variability, erratic peaks, and frequent hypoglycemic episodes.[4] Among all those mentioned above, only NPH is being used in clinical practice presently. These drawbacks necessitated more research that led to the discovery of long-acting analogs, namely glargine and detemir [Figure 2].
Figure 2.
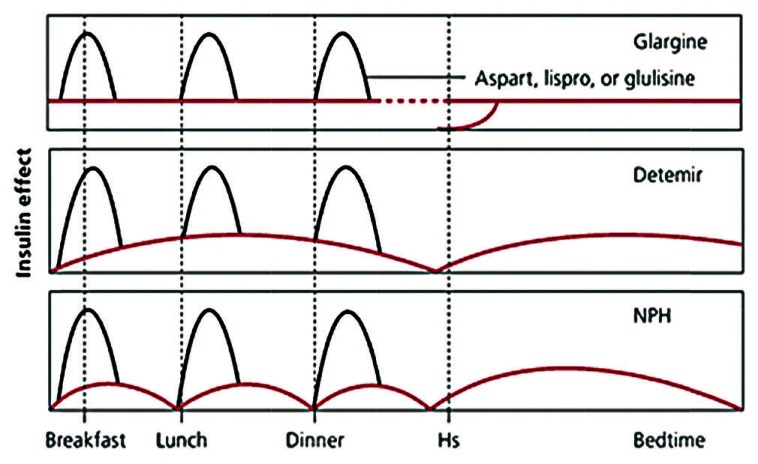
Comparison of effects of individual long-acting analogs when used in bolus basal regimen (source: http://www.aafp.org/afp/2011/0715/p183.html)
GLARGINE
The structure of glargine differs from that of human insulin such that, in A21 of A chain, there is substitution of aspargine with glycine, and, in position B30 of B chain, there is addition of two arginine residues.[6] It is stable in acidic solution, and, once it is injected into skin at neutral pH, it precipitates causing delays in its absorption and taking longer duration for action.[7] Its onset of action is 1-3 h up to 24 h, it has a peak-less profile, less variability in pharmacological profile,[8] and less hypoglycemia when compared with NPH.[9] The reduction of HbA1C is almost comparable with that of NPH.[10] The major drawback of glargine is the weight gain that occurs after prolonged use.[11] Also, there is a concern about cancer risk with this agent.
DETEMIR
The structure of detemir differs from that of human insulin in two perspectives: Deletion of amino acid threonine at position 30 of beta chain and addition of 14 carbon aliphatic fatty acid to epsilon amino group of lysine in position B29.[12] These changes enable detemir to bind with albumin reversibly and to form multimeric complexes within subcutaneous tissue, which prolongs its duration of action.[13] Its pharmacological profile is very similar to that of glargine, except that it may require 2 doses in some patients to provide optimal basal coverage, which occurs rarely with glargine.[14] Furthermore, it is weight neutral, which makes it more preferable in obese population,[15] seems to have lesser cancer risks, and has less inter individual variability when compared with glargine.[16]
DEGLUDEC-IDEAL BASAL INSULIN?
In recent times, degludec has attracted the attention of many people with its extended duration of action and thrice weekly injection to give full coverage of basal insulin. The structure of degludec differs from that of human insulin with the deletion of threonine of B30 and addition of 16 chain carbon fatty di-acid to lysine of B29 with a glutamic acid as a spacer. This formulation allows degludec to form multiple hexamers, which makes them dissociate slowly after subcutaneous injection and thus take longer duration for action. In addition, binding to albumin also compliments its longevity.[17] It results in long, flat, extended insulin profile, released smoothly without any peaks and has no inter subject variability. Its levels are detectable even after 96 h of injection.[18] The advantage over glargine and detemir is that it has ultra-long acting profile that reduces the number of doses for basal coverage. They showed that degludec provided comparable results between once daily and thrice weekly doses without any significant difference in adverse effects or glycemic control.[19] Also, it has less occurrence of hypoglycemia in type 1 diabetes[20] and better control of postprandial blood sugars in type 2 diabetes when compared to glargine.[16] The drug has completed phase III trial and is awaiting FDA approval.
Degludec can be formulated with aspart, a short-acting insulin. Two formulations are available: 70-30% and 55-45%.[21] It can be used in the treatment of both type 1 and type 2 diabetes as basal bolus regimen with aspart, and, in type 2, it can be used alone with other oral hypoglycemic agents.[22] The common adverse drug reactions are nausea, vomiting, rash, headache, hypoglycemia, weight gain, and upper respiratory tract infections.[22]
With the amount of evidence that is evolving, it is very likely that glucedec may replace the existing long-acting analogs in the near future [Figures 3 and 4].
Figure 3.
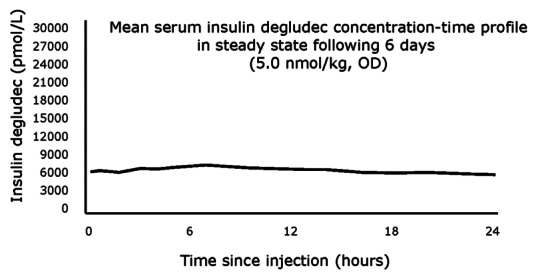
The steady state concentration of degludec (source: http://www.diabetologica.com/2011/03/ultra-long-acting-insulin-degludec-three-imes-per-week/)
Figure 4.
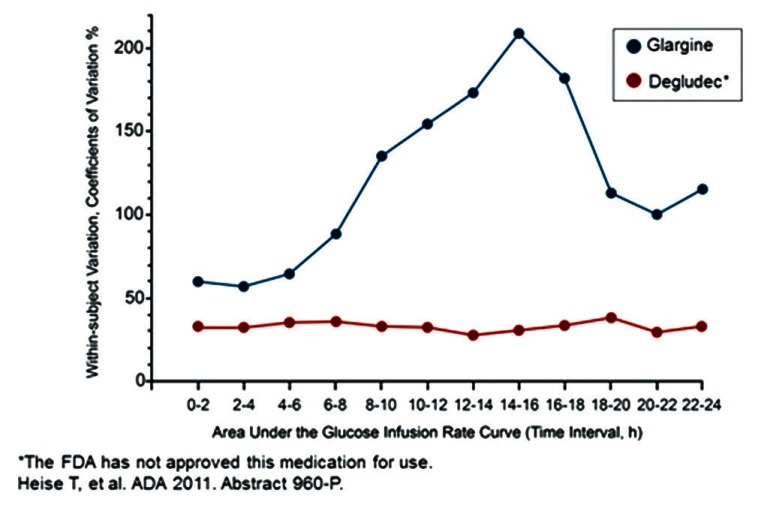
Comparison of pharmacological variability between degludec and glargine. (source: http://www.medscape.org/viewarticle/753421_transcript)
POTENTIAL CARCINOGENIC RISK
The mystery regarding the role of the long-acting insulin analogs in carcinogenesis remains to be unraveled. Insulin analogs having modifications in beta chain of insulin of B26 to B30 have increased affinity of binding towards IGF-1 receptor, which may play a role in mitogenic potential.[23] These insulin also show prolonged occupancy time of binding to insulin receptor, which may lead to abnormal activation of downstream pathways, that may lead to cell proliferation.[24,25] Although many evidences support the mitogenic potential of the insulin analogs, it may be amplified by the fact that diabetes, obesity, and insulin resistance itself can act as risk factor of many cancers, which may confound the results.[26]
Of all the analogs, glargine has the highest affinity toward IGF-1 receptor[25] and has greater concern. Several studies have identified the association of glargine with breast cancer risks.[27] Furthermore, a recent study demonstrated that the risk of cancer with glargine does not occur with its short-term use, rather it is associated with prolonged use of >5 years, in the context of using other forms of insulin therapy before starting glargine.[28] UKPDS and DIAGAMI-2 trial describes only increased mortality of cancer patients who are on insulin therapy and does not comment upon the incidence of cancer.[29,30] ORIGIN trial reassures the use of glargine and that it does not increase the overall cancer risks significantly.[31] We need more evidences to know the exact relation between the insulin analogs and cancer potential.
ROLE OF BASAL INSULIN IN SPECIAL SITUATIONS
Children
Glargine is the most frequent basal insulin used in children, but its safety is not yet established. Compared with glargine, detemir has shown more consistent pharmacokinetic profile in children.[2,14,32]
Pregnancy
Due to potential risks of embryo toxicity, teratogenicity, mitogenicity, and lack of adequate data, these long-acting agents are not approved for treatment in pregnancy.[2,33,34,35] Its role is yet to be clearly defined in pregnancy, although FDA now mentions this as a Class C indication in this situation.
EVOLVING BASAL ANALOGS
New formulations of glargine that are under development are LY2963016, which has completed phase III, and BIOD adjustable basal, which is in phase I. The advantage of the latter is that it can be mixed with other insulin preparations unlike conventional glargine preparations.[36,37] A protaminated form of insulin lispro is also under pipeline, which can be used as basal insulin.[38]
An unique ultra-long acting insulin FT-105 being developed, in which insulin is attached to polyglutamate peptide which in turn is attached to vitamin E, is under phase 1 clinical trials.[39] This formulation will cause aggregation of insulin particles that retards absorption.
Another novel basal analoge LY2605541 is presently undergoing phase 2 clinical trials.[2]
The formulation of combining basal insulin molecules with non-insulin such as GLP is also being investigated.[22] “Smart” insulin are specialized insulin preparations that release insulin according to concentration of glucose in tissue levels, thereby alleviating the risk of hypoglycemia.[36]
In summary, it would appear that glargine and detemir, which had largely replaced NPH insulin as basal insulin of choice today, will continue to be used as effective basal insulin in the near future. Their potential for increased cancer risk is yet to be established. Unless studies supporting clear links are reported, they will be continued to be used as basal insulin. However, both glargine and detemer may not provide full coverage of basal insulin for 24 h with single doses in some patients and may require a 2nd dose. Degludec appears to be more promising agent that can be used in thrice weekly doses besides providing full and more effective basal coverage for 24 h when given as once a day injection. If the consistency of the thrice weekly dose of degludec is proven by further studies, degludeg will certainly replace the existing agents as an ideal basal insulin in the future. With lot of newer basal insulin in the pipeline, especially smart insulin, which are very promising, degludec should expect tougher competition from future insulin also.
Footnotes
Source of Support: Nil
Conflict of Interest: No
REFERENCES
- 1.Keen H, Glynne A, Pickup JC, Viberti GC, Bilous RW, Jarrett RJ, et al. Human insulin produced by recombinant DNA technology: Safety and hypoglycaemic potency in healthy men. Lancet. 1980;2:398–401. doi: 10.1016/s0140-6736(80)90443-2. [DOI] [PubMed] [Google Scholar]
- 2.Borgono CA, Zinman B. Insulins: Past, present, and future. Endocrinol Metab Clin North Am. 2012;41:1–24. doi: 10.1016/j.ecl.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 3.Meneghini L, Liebl A, Abrahamson MJ. Insulin detemir: A historical perspective on a modern basal insulin analogue. Prim Care Diabetes. 2010;4:S31–42. doi: 10.1016/S1751-9918(10)60007-1. [DOI] [PubMed] [Google Scholar]
- 4.Leahy JL. Intensive insulin therapy in type 1 diabetes mellitus. In: Leahy JL, Cefalu WT, editors. Insulin Therapy. New York: Marcel Dekker; 2002. pp. 87–112. [Google Scholar]
- 5.Binder C, Lauritzen T, Faber O, Pramming S. Insulin pharmacokinetics. Diabetes Care. 1984;7:188–99. doi: 10.2337/diacare.7.2.188. [DOI] [PubMed] [Google Scholar]
- 6.Sheldon B, Russell-Jones D, Wright J. Insulin analogues: An example of applied medical science. Diabetes Obes Metab. 2009;11:5–19. doi: 10.1111/j.1463-1326.2008.01015.x. [DOI] [PubMed] [Google Scholar]
- 7.Arnolds S, Kuglin B, Kapitza C, Heise T. How pharmacokinetic and pharmacodynamic principles pave the way for optimal basal insulin therapy in type 2 diabetes. Int J Clin Pract. 2010;64:1415–24. doi: 10.1111/j.1742-1241.2010.02470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Campbell RK, White JR, Levien T, Baker D. Insulin glargine. Clin Ther. 2001;23:1938–57. doi: 10.1016/s0149-2918(01)80148-x. [DOI] [PubMed] [Google Scholar]
- 9.Yki-Jarvinen H, Dressler A, Ziemen M. HOE 901/300s Study Group. Less nocturnal hypoglycemia and better post-dinner glucose control with bedtime insulin glargine compared with bedtime NPH insulin during insulin combination therapy in type 2 diabetes. HOE 901/3002 Study Group. Diabetes Care. 2000;23:1130–6. doi: 10.2337/diacare.23.8.1130. [DOI] [PubMed] [Google Scholar]
- 10.Riddle MC, Rosenstock J, Gerich J. Insulin Glargine 4002 Study Investigators. The treat-to-target trial: Randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetic patients. Diabetes Care. 2003;26:3080–6. doi: 10.2337/diacare.26.11.3080. [DOI] [PubMed] [Google Scholar]
- 11.Russell-Jones D, Khan R. Insulin-associated weight gain in diabetes–causes, effects and coping strategies. Diabetes Obes Metab. 2007;9:799–812. doi: 10.1111/j.1463-1326.2006.00686.x. [DOI] [PubMed] [Google Scholar]
- 12.Barlocco D. Insulin detemir. Novo Nordisk. Curr Opin Investig Drugs. 2003;4:449–54. [PubMed] [Google Scholar]
- 13.Havelund S, Plum A, Ribel U, Jonassen I, Vølund A, Markussen J, et al. The mechanism of protraction of insulin detemir, a long-acting, acylated analog of human insulin. Pharm Res. 2004;21:1498–504. doi: 10.1023/b:pham.0000036926.54824.37. [DOI] [PubMed] [Google Scholar]
- 14.Danne T, Datz N, Endahl L, Haahr H, Nestoris C, Westergaard L, et al. Insulin detemir is characterized by a more reproducible pharmacokinetic profile than insulin glargine in children and adolescents with type 1 diabetes: Results from a randomized, double-blind controlled trial. Pediatr Diabetes. 2008;9:554–60. doi: 10.1111/j.1399-5448.2008.00443.x. [DOI] [PubMed] [Google Scholar]
- 15.Rosenstock J, Davies M, Home PD, Larsen J, Koenen C, Schernthaner G. A randomised, 52-week, treat-to-target trial comparing insulin detemir with insulin glargine when administered as add on to glucose-lowering drugs in insulin-naive people with type 2 diabetes. Diabetologia. 2008;51:408–16. doi: 10.1007/s00125-007-0911-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heise T, Tack CJ, Cuddihy R, Davidson J, Gouet D, Liebl A, et al. A new-generation ultra-long-acting basal insulin with a bolus boost compared with insulin glargine in insulin-naïve people with type 2 diabetes: A randomized, controlled trial. Diabetes Care. 2011;34:669–74. doi: 10.2337/dc10-1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jonassen I, Havelund S, Ribel U. Insulin degludec is a new generation ultra-long acting basal insulin with a unique mechanism of protraction based on multihexamer formation. Diabetes. 2010;59:A11. [Google Scholar]
- 18.Jonassen IB, Havelund S, Ribel U, Hoeg-Jensen T, Steensgard DB, Johansen T, et al. Orlando, USA: American Diabetes Association-70th Scientific Sessions; 2010. Insulin Degludec is a new generation ultra-long acting basal insulin with a unique mechanism of protraction based on multi-hexamer formation. [Google Scholar]
- 19.Zinman B, Prillis-Tsimikar A, Cariou B, Handelsman Y, Rodbard HW, Johansen T, et al. Insulin degludec versus glargine in insulin naive patiens with type 2 diabetes: A randomised treat to target trial (BEGIN Once Long) Diabetes Care. 2012;35:2464–71. doi: 10.2337/dc12-1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Birkeland KI, Home PD, Wendisch U, Ratner RE, Johansen T, Endahl LA, et al. Insulin degludec in type 1 diabetes: A randomized controlled trial of a new-generation ultra-long-acting insulin compared with insulin glargine. Diabetes Care. 2011;34:661–5. doi: 10.2337/dc10-1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jonassen I, Hoeg-Jensen T, Havelund S, Ribel U. Ultra-long acting insulin degludec can be combined with rapid-acting insulin aspart in a soluble co formulation. J Pept Sci. 2010;16:32. [Google Scholar]
- 22.Kalra S, Baruah MP, Niazi AK. Degludec: A novel basal insulin. Recent Pat Enodocr Metab Immune Drug Discov. 2012;6:18–23. doi: 10.2174/187221412799015326. [DOI] [PubMed] [Google Scholar]
- 23.Slieker LJ, Brooke GS, DiMarchi RD, Flora DB, Green LK, Hoffmann JA, et al. Modifications in the B10 and B26-30 regions of the B chain of human insulin alter affinity for the human IGF-I receptor more than for the insulin receptor. Diabetologia. 1997:S54–61. doi: 10.1007/s001250051402. [DOI] [PubMed] [Google Scholar]
- 24.Werner H, Weinstein D, Bentov I. Similarities and differences between insulin and IGF-I: Structures, receptors, and signalling pathways. Arch Physiol Biochem. 2008;114:17–22. doi: 10.1080/13813450801900694. [DOI] [PubMed] [Google Scholar]
- 25.Yehezkel E, Weinstein D, Simon M, Sarfstein R, Laron Z, Werner H. Long-acting insulin analogues elicit atypical signalling events mediated by the insulin receptor and insulin-like growth factor-I receptor. Diabetologia. 2010;53:2667–75. doi: 10.1007/s00125-010-1899-1. [DOI] [PubMed] [Google Scholar]
- 26.Gerstein HC. Does insulin therapy promote, reduce, or have a neutral effect on cancers? JAMA. 2010;303:446–7. doi: 10.1001/jama.2010.60. [DOI] [PubMed] [Google Scholar]
- 27.Jonasson JM, Ljung R, Talback M, Haglund B, Gudbjörnsdòttir S, Steineck G. Insulin glargine use and short-term incidence of malignancies: A population-based follow-up study in Sweden. Diabetologia. 2009;52:1745–54. doi: 10.1007/s00125-009-1444-2. [DOI] [PubMed] [Google Scholar]
- 28.Suissa S, Azoulay L, Dell’Aniello S, Evans M, Vora J, Pollak M. Long-term effects of insulin glargine on the risk of breast cancer. Diabetologia. 2011;54:2254–62. doi: 10.1007/s00125-011-2190-9. [DOI] [PubMed] [Google Scholar]
- 29.Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352:837–53. [PubMed] [Google Scholar]
- 30.Malmberg K, Rydén L, Wedel H, Birkeland K, Bootsma A, Dickstein K, et al. Intense metabolic control by means of insulin in patients with diabetes mellitus and acute myocardial infarction (DIGAMI 2): Effects on mortality and morbidity. Eur Heart J. 2005;26:650–61. doi: 10.1093/eurheartj/ehi199. [DOI] [PubMed] [Google Scholar]
- 31.Gerstein HC, Bosch J, Dagenais GR, Díaz R, Jung H, et al. ORIGIN Trial Investigators. Basal insulin and cardiovascular and other outcomes in dysglycemia. N Engl J Med. 2012;367:319–28. doi: 10.1056/NEJMoa1203858. [DOI] [PubMed] [Google Scholar]
- 32.Heise T, Nosek L, Ronn BB, Endahl L, Heinemann L, Kapitza C, et al. Lower within-subject variability of insulin detemir in comparison to NPH insulin and insulin glargine in people with type 1 diabetes. Diabetes. 2004;53:1614–20. doi: 10.2337/diabetes.53.6.1614. [DOI] [PubMed] [Google Scholar]
- 33.Graves DE, White JC, Kirk JK. The use of insulin glargine with gestational diabetes mellitus. Diabetes Care. 2006;29:471–2. doi: 10.2337/diacare.29.02.06.dc05-2042. [DOI] [PubMed] [Google Scholar]
- 34.Gallen IW, Jaap A, Roland JM, Chirayath HH. Survey of glargine use in 115 pregnant women with type 1 diabetes. Diabet Med. 2008;25:165–9. doi: 10.1111/j.1464-5491.2007.02339.x. [DOI] [PubMed] [Google Scholar]
- 35.Torlone E, Di Cianni G, Mannino D, Lapolla A. Insulin analogs and pregnancy: An update. Acta Diabetol. 2009;46:163–72. doi: 10.1007/s00592-009-0130-7. [DOI] [PubMed] [Google Scholar]
- 36.Owens DR. Insulin preparations with prolonged effect. Diabetes Technol Ther. 2011:S5–14. doi: 10.1089/dia.2011.0068. [DOI] [PubMed] [Google Scholar]
- 37.Simon AC, DeVries JH. The future of basal insulin supplementation. Diabetes Technol Ther. 2011:S103–8. doi: 10.1089/dia.2010.0251. [DOI] [PubMed] [Google Scholar]
- 38.Chacra AR, Kipnes M, Ilag LL, Sarwat S, Giaconia J, Chan J, et al. Comparison of insulin lispro protamine suspension and insulin detemir in basal-bolus therapy in patients with Type 1 diabetes. Diabet Med. 2010;27:563–9. doi: 10.1111/j.1464-5491.2010.02986.x. [DOI] [PubMed] [Google Scholar]
- 39.Chan YP, Meyrueix R, Kravtzoff R, Nicolas F, Lundstrom K. Review on Medusa: A polymer-based sustained release technology for protein and peptide drugs. Expert Opin Drug Deliv. 2007;4:441–51. doi: 10.1517/17425247.4.4.441. [DOI] [PubMed] [Google Scholar]


