Abstract
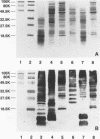
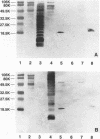
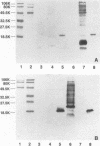
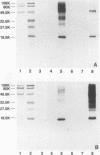
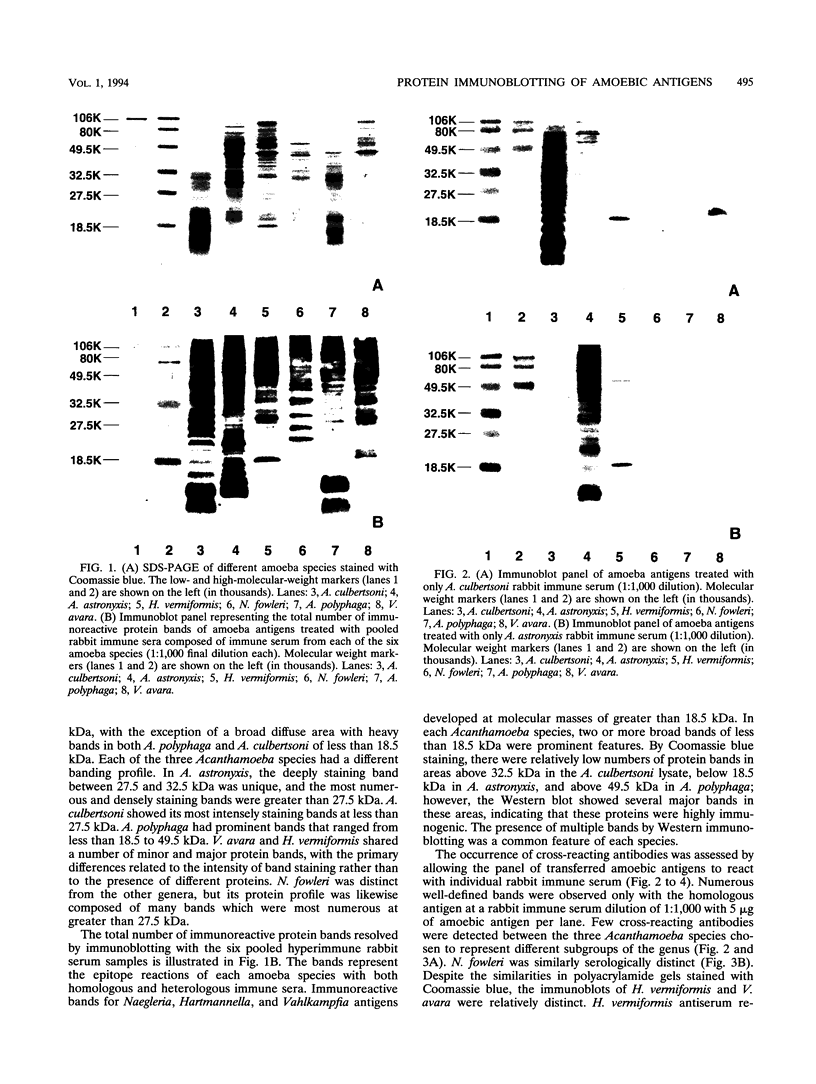
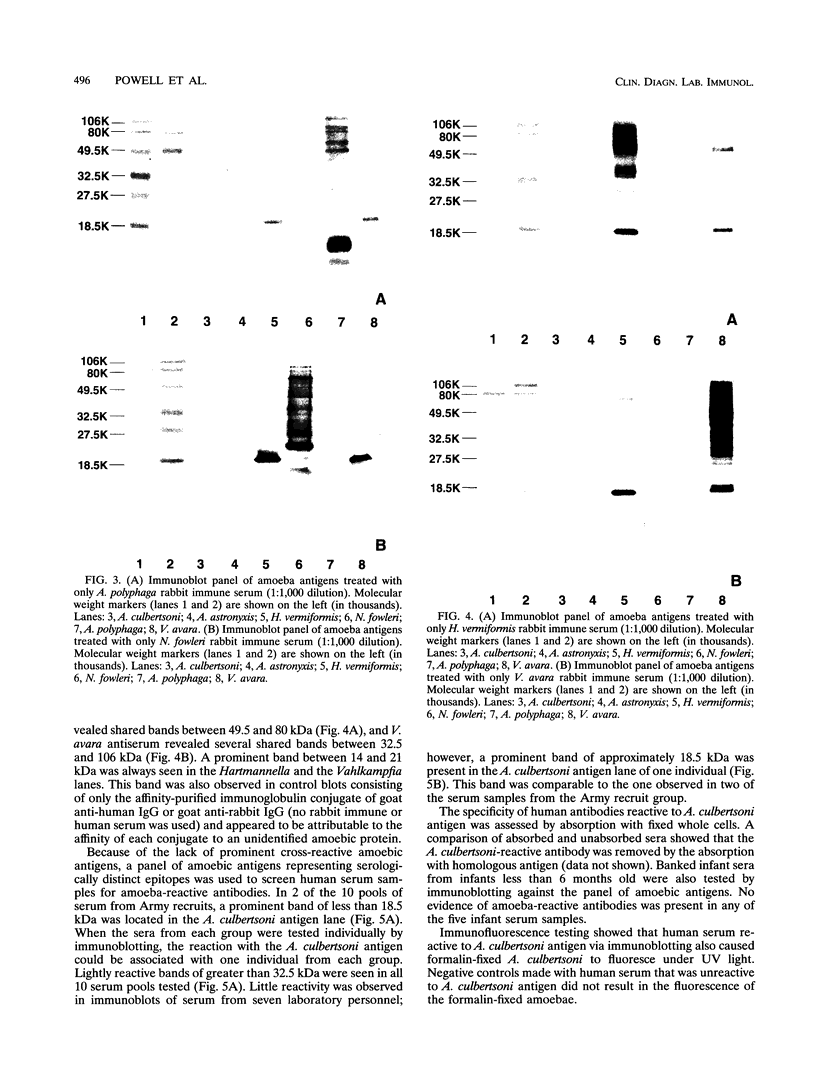
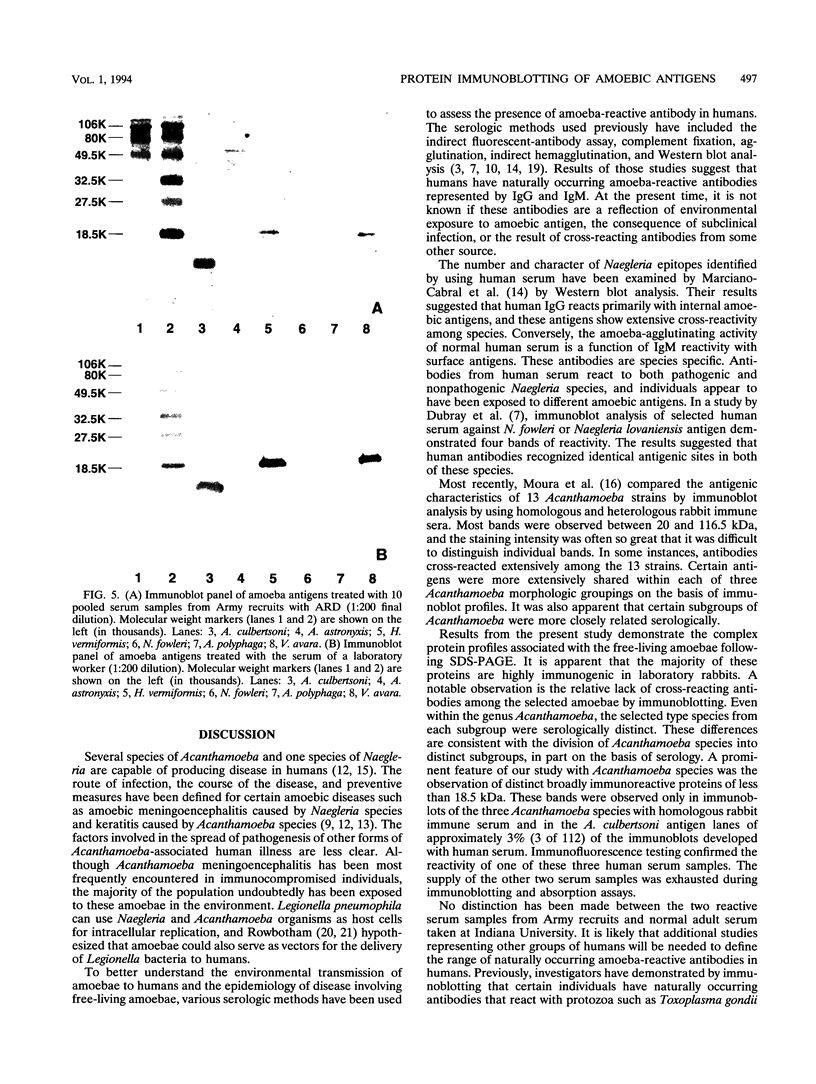
Prominent antigens of pathogenic and nonpathogenic free-living amoebae were identified by using polyclonal rabbit immune sera in immunoblot assays. The intent was to determine if prominent epitopes identified with rabbit immune sera could also be recognized by human sera. With rabbit sera, the development of immunoreactive bands was restricted to molecular masses of greater than 18.5 kDa for Naegleria, Hartmannella, and Vahlkampfia antigens. Two or more broad bands of less than 18.5 kDa were prominent features in three different Acanthamoeba species. Few cross-reactive antibodies could be detected between representative species of the three different subgroups of Acanthamoeba. Naegleria antigen was likewise serologically distinct, as were Hartmannella and Vahlkampfia antigens. The relative lack of cross-reacting antibodies between the pathogenic amoebae suggested that i would be desirable to use a panel of amoebic antigens to represent the range of serologically distinct antigens for assessing reactive antibodies in human sera. In pooled human sera (10 serum specimens per pool), the appearance of minimally reactive bands ranging from 32.5 to 106 kDa was a common feature of all six antigens. A prominent band of less than 18.5 kDa was identified in the Acanthamoeba culbertsoni antigen lane in 2 of the 10 human serum specimen pools. When sera from each of the two groups were tested individually by immunoblotting, the reaction with A. culbertsoni antigen could be associated with one individual. By using a panel of amoebic antigens, this method could prove useful in recognizing undiagnosed amoebic infections by revealing specific reactive antibodies.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Band R. N., Balamuth W. Hemin replaces serum as a growth requirement for Naegleria. Appl Microbiol. 1974 Jul;28(1):64–65. doi: 10.1128/am.28.1.64-65.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CULBERTSON C. G., SMITH J. W., COHEN H. K., MINNER J. R. Experimental infection of mice and monkeys by Acanthamoeba. Am J Pathol. 1959 Jan-Feb;35(1):185–197. [PMC free article] [PubMed] [Google Scholar]
- Cerva L. Acanthamoeba culbertsoni and Naegleria fowleri: occurrence of antibodies in man. J Hyg Epidemiol Microbiol Immunol. 1989;33(1):99–103. [PubMed] [Google Scholar]
- Cerva L., Serbus C., Skocil V. Isolation of limax amoebae from the nasal mucosa of man. Folia Parasitol (Praha) 1973;20(1):97–103. [PubMed] [Google Scholar]
- Culbertson C. G. Amebic meningoencephalitis. Antibiot Chemother (1971) 1981;30:28–53. doi: 10.1159/000398093. [DOI] [PubMed] [Google Scholar]
- Dubray B. L., Wilhelm W. E., Jennings B. R. Serology of Naegleria fowleri and Naegleria lovaniensis in a hospital survey. J Protozool. 1987 Aug;34(3):322–327. doi: 10.1111/j.1550-7408.1987.tb03183.x. [DOI] [PubMed] [Google Scholar]
- Dudding B. A., Top F. H., Jr, Winter P. E., Buescher E. L., Lamson T. H., Leibovitz A. Acute respiratory disease in military trainees: the adenovirus surveillance program, 1966-1971. Am J Epidemiol. 1973 Mar;97(3):187–198. doi: 10.1093/oxfordjournals.aje.a121499. [DOI] [PubMed] [Google Scholar]
- Ferrante A. Free-living amoebae: pathogenicity and immunity. Parasite Immunol. 1991 Jan;13(1):31–47. doi: 10.1111/j.1365-3024.1991.tb00261.x. [DOI] [PubMed] [Google Scholar]
- Kenney M. The Micro-Kolmer complement fixation test in routine screening for soil ameba infection. Health Lab Sci. 1971 Jan;8(1):5–10. [PubMed] [Google Scholar]
- Lawande R. V., Abraham S. N., John I., Egler L. J. Recovery of soil Amebas from the nasal passages of children during the dusty harmattan period in Zaria. Am J Clin Pathol. 1979 Feb;71(2):201–203. doi: 10.1093/ajcp/71.2.201. [DOI] [PubMed] [Google Scholar]
- Ma P., Visvesvara G. S., Martinez A. J., Theodore F. H., Daggett P. M., Sawyer T. K. Naegleria and Acanthamoeba infections: review. Rev Infect Dis. 1990 May-Jun;12(3):490–513. doi: 10.1093/clinids/12.3.490. [DOI] [PubMed] [Google Scholar]
- Marciano-Cabral F. Biology of Naegleria spp. Microbiol Rev. 1988 Mar;52(1):114–133. doi: 10.1128/mr.52.1.114-133.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marciano-Cabral F., Cline M. L., Bradley S. G. Specificity of antibodies from human sera for Naegleria species. J Clin Microbiol. 1987 Apr;25(4):692–697. doi: 10.1128/jcm.25.4.692-697.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez A. J., Visvesvara G. S. Laboratory diagnosis of pathogenic free-living amoebas: Naegleria, Acanthamoeba, and Leptomyxid. Clin Lab Med. 1991 Dec;11(4):861–872. [PubMed] [Google Scholar]
- Moura H., Wallace S., Visvesvara G. S. Acanthamoeba healyi n. sp. and the isoenzyme and immunoblot profiles of Acanthamoeba spp., groups 1 and 3. J Protozool. 1992 Sep-Oct;39(5):573–583. doi: 10.1111/j.1550-7408.1992.tb04853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potasman I., Araujo F. G., Remington J. S. Toxoplasma antigens recognized by naturally occurring human antibodies. J Clin Microbiol. 1986 Dec;24(6):1050–1054. doi: 10.1128/jcm.24.6.1050-1054.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowbotham T. J. Current views on the relationships between amoebae, legionellae and man. Isr J Med Sci. 1986 Sep;22(9):678–689. [PubMed] [Google Scholar]
- Rowbotham T. J. Preliminary report on the pathogenicity of Legionella pneumophila for freshwater and soil amoebae. J Clin Pathol. 1980 Dec;33(12):1179–1183. doi: 10.1136/jcp.33.12.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visvesvara G. S., Balamuth W. Comparative studies on related free-living and pathogenic amebae with special reference to Acanthamoeba. J Protozool. 1975 May;22(2):245–256. doi: 10.1111/j.1550-7408.1975.tb05860.x. [DOI] [PubMed] [Google Scholar]
- Visvesvara G. S. Classification of Acanthamoeba. Rev Infect Dis. 1991 Mar-Apr;13 (Suppl 5):S369–S372. doi: 10.1093/clind/13.supplement_5.s369. [DOI] [PubMed] [Google Scholar]
- Wang S. S., Feldman H. A. Isolation of hartmannella species from human throats. N Engl J Med. 1967 Nov 30;277(22):1174–1179. doi: 10.1056/NEJM196711302772204. [DOI] [PubMed] [Google Scholar]
- Wenzel R. P., McCormick D. P., Smith E. P., Clark D. L., Beam W. E., Jr Acute respiratory disease: clinical and epidemiologic observations of military trainees. Mil Med. 1971 Dec;136(12):873–880. [PubMed] [Google Scholar]
- Wong M. M., Karr S. L., Jr, Balamuth W. B. Experimental infections with pathogenic free-living amebae in laboratory primate hosts: I. (B) A study on susceptibility to Acanthamoeba culbertsoni. J Parasitol. 1975 Aug;61(4):682–690. [PubMed] [Google Scholar]