Abstract
Background:
Iodine-rich diet is necessary for proper thyroid gland function. Subclinical hypothyroidism (SCH) is associated with serious complications. Substantial numbers of patients have risk of SCH getting converted into primary hypothyroidism.
Objectives:
The objectives of the present study are to survey dietary iodine intake pattern in ethnic population of Kashmir and to study the prevalence of SCH.
Materials and Methods:
A retrospective, cross-sectional referral hospital study was conducted. Sample size comprised of 2550 patients who were referred to Department of Biochemistry, Government Medical college, Srinagar diagnostic laboratory from OPD and IPD of associated SMHS hospital. Assessment of thyroid function over a period of one year from March 2010 to March 2011 in the serum has been performed by electro-chemiluminescence immunoassay method on ECLIA 2010 fully automatic analyzer. Interview cum questionnaire methods were used to record the patient history and dietary iodine intake pattern. Iodine status of these patients was assessed by measuring urinary iodine excretion.
Results:
Total patients were 2550 comprising of 44.6% males and 56.4% females. Subjects with elevated and normal thyroid stimulating hormone (TSH) levels in the serum were 30.51 and 69.4% respectively. About 550 patients (21.56%) had subclinical hypothyroidism which includes both males and females. Prevalence of SCH was more in females (81.8%) than in males (18.2%). Most of the patients presenting with SCH were in the age group of 20–65 years.
Conclusion:
The percentage of SCH amongst the study sample patients was 21.56%, which is much higher as compared to other parts of the world. The highest percentage of SCH was found in females (81.8%) as compared to males (18.2%). On the basis of the present study, we suggest that routine screening of selected populations, especially women between 20 and 65 years of age, may be advocated. Further community level awareness programs need to be organized wherein people in mountainous valley of Kashmir are motivated to take salt in iodized form and diet rich in iodine to ensure proper thyroid gland functioning.
Keywords: Dairy products, iodine rich food, strawberries, subclinical hypothyroidism, thyroid stimulating hormone, urinary iodine excretion
INTRODUCTION
Thyroid disorders are amongst the most common endocrine diseases in India.[1] The total burden of thyroid disorders in India is 42 million.[2] The prevalence and pattern of thyroid disorders depends on sex, age, ethnic and geographical factors and especially on iodine intake.[3] Subclinical hypothyroidism (SCH) defined as the clinical status of elevated serum TSH levels(>4.3 to 10 mU/l)) with normal levels of serum thyroxine (T4) and tri-iodothyronine (T3) and is a more common disorder than primary hypothyroidism with a prevalence of 1.4–7.8% in older populations, prevalence being and even greater among women.[4,5,6] In relation to the prevalence of SCH, the data of the published series ranges considerably between 3.4 and 10.8% of the general population.[7,8,9,10] Iodine is an essential dietary element which is required for the synthesis of the thyroid hormones, thyroxine (T4) and tri-iodothyronine (T3), regulates cellular oxidation and hence has effect on calorigenesis, thermoregulation and intermediary metabolism.[11,12] Daily requirement of iodine is normally met by a well-balanced diet and drinking water except in hilly areas and around the rivers and great lakes where iodine has been leached out of soil so that food grown in soil is iodine deficient, resulting in increased incidence of hypothyroidism in these iodine deficient areas.[13] The regions with heavy rainfall or snowfall are particularly likely to be iodine deficient as the superficial layer of soil (in which iodine is present) is washed away. In these areas, 60–75% of the iodine needs are met by the iodine present in the diet and the rest through the iodine content of water. The food grown in iodine-deficient regions can never provide enough iodine to the population and livestock living there.[14] Nowadays, about 800 million people are affected by iodine deficiency disorders that include goiter, hypothyroidism, mental retardation, and a wide spectrum of other growth and developmental abnormalities. Iodine supplementation, under form of iodized salt and iodized vegetable oil, produces dramatic improvements in many areas, even though iodine deficiency is still a problem not only for developing countries. In fact, certain subpopulations like vegetarians may not reach an adequate iodine intake even in countries considered as iodine-sufficient. Furthermore, iodine intakes are declining in many countries where, after endemic goiter eradication, the lack of monitoring of iodine nutrition can lead to a reappearance of goiter and other iodine deficiency disorder.[15] In mountainous-hill lock region like Kashmir valley, where soil is deficient in iodine mineral, iodized salt, which is made by the addition of small amounts of iodine to table salts in the form of sodium iodide, potassium iodide, should be given to people and the suspected patients with thyroid disorder. This helps to combat the iodine deficiency which causes goiter, hypothyroidism etc. Yogurt, cow's milk, straw berries, nuts, fish and dairy products are good sources of locally available iodine-containing food sources.[16] The aim of this study is to investigate the percentage of subclinical hypothyroidism and awareness regarding the role of dietary iodine and iodized salt in proper thyroid functioning in study sample comprising of patients, from ethnic population of mountainous valley of Kashmir.
MATERIALS AND METHODS
The study was conducted at the Department of Biochemistry Government Medical College Srinagar from March 2010 to March 2011. All patients referred from Out-Patient Department (OPD) and Inpatient Department (IPD) of Govt. medical college Srinagar and its associated Shri-Mahraja Hari Singh Hospital (SMHS) hospital, a major referral Hospital of Kashmir valley to the diagnostic Biochemistry laboratory of Govt. medical college Srinagar for the evaluation of thyroid function. All patients were examined by an endocrinologist. A total of 2550 patients were selected for the study.
Exclusion criteria
Patients with ischemic heart disease, cerebrovascular and neurological diseases, diabetes mellitus, chronic renal impairment, known psychological illnesses, previous history of thyroid disease or previous thyroxine therapy, asthma and pregnancy were excluded.
Sample collection
About 3–5 ml of venous blood was collected and centrifuged to separate serum from the cells as soon as the clot was formed.
Measurement of thyroid hormone profile
Serum aliquots were stored at 4°C to be run in batches. The samples were allowed to thaw prior to assay, mixed thoroughly. Hemolyzed and lipemic samples were rejected. Bi level i.e. high and low control was run with each batch. Thyroid function test (TFT) comprising of T3, T4 and TSH levels was carried out by electrochemiluminescence immunoassay method using a fully automatic analyzer ECLIA 2010(Roche Diagnostic Germany). Patients with thyroid hormone evaluation picture of elevated serum TSH levels(>4.3 to ≥ 10 mIU/ml) with normal levels of serum thyroxine (T4) and tri-iodothyronine (T3) were categorized as subclinical hypothyroidism (SCH) if similar levels were observed in repeated thyroid profile after a lapse of three months.
Urinary iodine estimation
The collected urine samples were stored in a refrigerator at 4°C until analyzed for iodine content using the standard laboratory method.[17] On the basis of urinary iodine excretion, subjects were classified into four groups with UIE less than 2, 2–4.9, 5–9.9 and 10 μgdl, indicating severe, moderate, mild and no iodine deficiency status, respectively.
Statistical analysis
Data were extracted and analyzed by software package for social science version 11.5 (Chicago IL). Data were represented as percentage, frequency, ratio, mean and standard error. Frequency bar Figure and Tables were prepared in Microsoft excel software program.
RESULTS
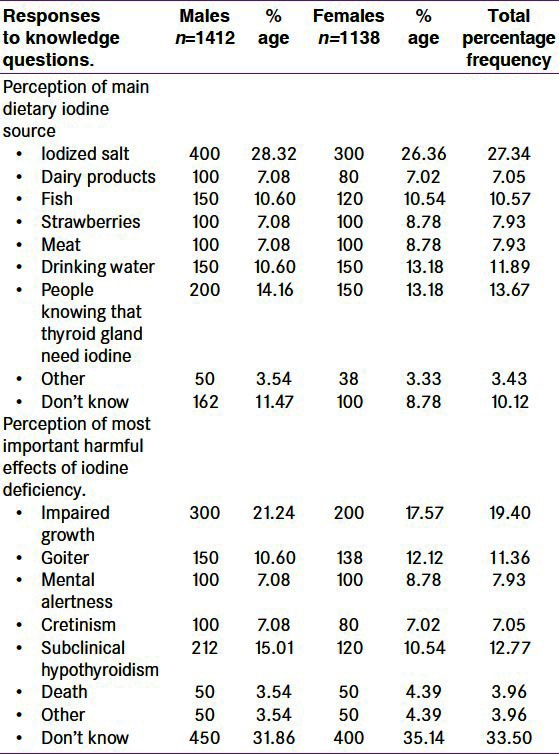
Total cases were 2550 including 1138 (44.6%) males and 1412 (55.4%) females. Among them, 550 cases (out of total 2550 cases) had SCH which includes 100 (18.2%) males and 450 (81.8%) females [Figure 1 and Tables 1 and 2]. Thus, in our observation, we confirmed that 21.56% of patients were suffering from SCH, in which males are 18.2% and females are 81.2%. The TSH value above 4.3 to 10 mU/l and free T4 within normal range was taken as criteria for SCH. We used an interview cum questionnaire method to know the responses of respondents about diet containing iodine sources and the harmful effects of iodine deficiency [Table 3]. The data indirectly revealed the low level of awareness of the health benefits of iodized salt and diet containing iodine mineral in the study area. 27.34% of people ranging from 26.63% to 28.32% in our observation, knew that iodized salt is the most important or main source of iodine in the diet of people of valley. Lower percentage of respondents (7– 10%) believed that strawberries and meat were the most important dietary source of iodine. Only a few people considered dairy products, drinking water or other sources to be the primary source of iodine. Moreover, many of the respondents were unable to answer the question. When asked which part of the body (‘gland in the body’) needs iodine to produce hormones, 13.5% of respondents correctly identified the thyroid gland [Table 3]. The remaining 11% of respondents replied either that they did not know or they did not know what iodine is. Respondents’ perceptions of the most important harmful effect of inadequate iodine intake on the health are also summarized in Table 3. The data show that 19.40% of respondents considered impaired growth as the most important health consequence of iodine deficiency, while 11.36% considered goiter, 7.93% considered weak mental alertness and 7.05% considered cretinism as the most important consequence. 12.77% of the respondents mentioned subclinical hypothyroidism and 3.96% considers death or other consequences. About 33.50% of respondents were unaware of harmful effects of iodine deficiency.
Figure 1.
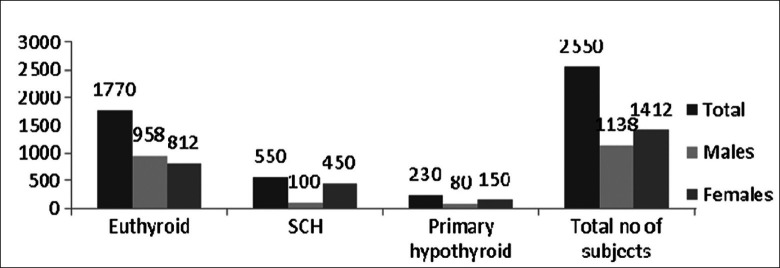
Sex distribution of patients
Table 1.
Classification of patient status based on serum thyroid hormone levels
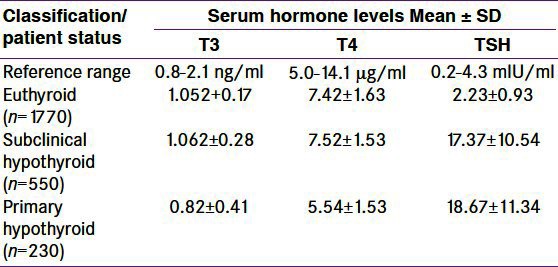
Table 2.
Frequency of subclinical hypothyroidism (n=2550), within population
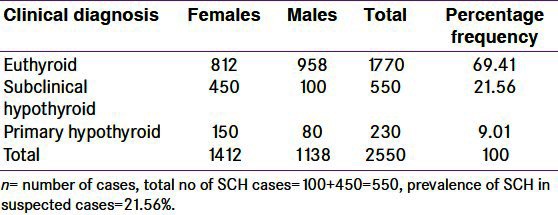
Table 3.
Frequency (%) of responses to iodine-related knowledge questions, by suspected population (n=2550)
Urinary iodine excretion
The iodine status of the subjects was found by measuring the Urinary iodine excretion (UIE). Severe, moderate and mild iodine deficient status was found in 5, 9, and 28% of cases. The average UIE in the severe, moderate and mild iodine deficient groups and in normal population was 5.5±0.25%, 9.5±0.75%, 28.5±2.5% and 58.5±3.5%. Thyroid status: euthyroid i.e., all parameters are in normal range (T3=0.8-21ng/ml, T4=5.0-14.1 μg/dl, TSH=0.2-4.3 mIU/ml), primary hypothyroid i.e. patients with TSH >4.3 mIU/ml and T3 <0.8 ng/ml or T4 <5.0 μg/ml, subclinical hypothyroidism i.e. patients with TSH >4.3 mIU/ml and T3, T4 in normal reference range [Table 1].
DISCUSSION
In the present study, we studied 2550 subjects. Among whom, we observed 550 patients (21.56%) were suffering from subclinical hypothyroidism which is much higher as compared to the prevalence found in other epidemiological studies from other parts of the world which ranges from as low as 1% to an average of 4–8.5%. Also the females suffering SCH are 450(81.8%) and males are 100(18.2%). The results of the present study, though much higher, are in agreement with the data across the world in terms of high prevalence of SCH among females. As there are higher percentage of female subjects (55.4%) compared to males (44.6%), the number of female subjects was higher in both primary hypothyroid group and SCH groups compared to males as shown in Table 3. The highest percentage of SCH cases was thus found in the female age group of 20–65 years. Being a hospital based study, naturally the positive cases are expected more, but it gives an idea about the high incidence rates in the society as well. However, for getting a clear picture, an extensive epidemiological study is required. The people, living in goiter endemic zone like Kashmir surrounded by mountains and hills where soil is deficient in iodine mineral, are dependent upon external sources like iodized salts and diet rich in iodine. This is attributed to the fact that food grown in iodine deficient regions can never provide enough iodine to the population and livestock living there.[2,18] The urinary iodine level reflects an individual's iodine consumption as 90% of the body's iodine is excreted through urine, and thus the excretion of iodine is used as a biochemical marker of iodine intake.[19] Table 3 describes the iodine status in the community, it reveals the percentage of the people knowing diet containing iodine and awareness about iodized salt and its impact on health. Therefore, we suggest that an extensive epidemiological study and awareness regarding dietary iodine requirement should be carried out in the region. Role of iodine-rich diet as assessed through a questionnaire [Table 3] was sustained by the iodine status of the study sample revealed by UIE. Wherein, it was observed that around 60% of the population had normal iodine status and around 40% was iodine deficient (severe, moderate and mild). This clearly indicates that awareness regarding the role of dietary iodine and iodized salt in thyroid function correlated well with the actual iodine status of subjects.
CONCLUSION
A prevalence rate of 21.56% SCH was found among the study group which is quite high as compared to other epidemiological studies in various parts of the world where it ranges from 4 to 8.5%. The highest percentage of SCH was found especially in the female age group 20– 65 years. Considering the potential danger to progression to overt disease state and also SCH may itself be associated with serious complications, there needs to be proper guidelines to recommend the screening and treatment of SCH.[20,21] Studies have reported psychiatric problems such as panic disorder, anxiety and depression disorders a more common in SCH.[22] Further community level awareness programs need to be organized wherein people in mountainous valley of Kashmir are motivated to take salt in iodized form and diet rich in iodine to ensure proper thyroid gland functioning. Also, people should be educated that locally available food including yogurt, cow's milk, eggs, strawberries and fish are good sources of iodine. UIE depicts that around 60% study sample has normal iodine status and around 40% was iodine deficient (severe, moderate, mild). As it was a hospital-based study, further epidemiological, diet, environment and socio-economic development related studies are required. Meta-analysis may also be taken up for better comprehension of the hypothesis.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Laurberg P, Pedersen KM, Hreidarsson A, Sigfusson N, Iversen E, Knudsen PR. Iodine intake and the pattern of thyroid disorders: A comparative epidemiological study of thyroid abnormalities in the elderly in Iceland and in Jutland, Denmark. J Clin Endocr Metab. 1998;83:765–9. doi: 10.1210/jcem.83.3.4624. [DOI] [PubMed] [Google Scholar]
- 2.Gopalan C, Ramashastri BV. Hyderabad: Indian Council of Medical Research; 2002. Nutritive Value of Indian Foods, National Institute of Nutrition; p. 20. [Google Scholar]
- 3.Vanderpump MP, Turnbridge WM. Epidemiology and prevention of clinical and subclinical hypothyroidism. Thyroid. 2002;12:839–47. doi: 10.1089/105072502761016458. [DOI] [PubMed] [Google Scholar]
- 4.Cooper DS. Subclinical thyroid disease: A clinician's perspective. Ann Intern Med. 1998;129:135–8. doi: 10.7326/0003-4819-129-2-199807150-00016. [DOI] [PubMed] [Google Scholar]
- 5.Brabant G, Beck-Peccoz P, Jarzab B, Laurberg P, Orgiazzi J, Szabolcs I, et al. Is there a need to redefine the upper normal limit of TSH? 2006;154:633–7. doi: 10.1530/eje.1.02136. [DOI] [PubMed] [Google Scholar]
- 6.Gibbons V, Lillis S, Conaglen JV, Lawrenson R. Do general practitioners use thyroid stimulating hormone assay for opportunistic screening? N Z Med J. 2009;12:25–30. [PubMed] [Google Scholar]
- 7.Chu JW, Crapo LM. The treatment of subclinical hypothyroidism is seldom necessary. J Clin Endocrinol Metab. 2001;86:4591–9. doi: 10.1210/jcem.86.10.7961. [DOI] [PubMed] [Google Scholar]
- 8.Cooper DS. Subclinical hypothyroidism. N Engl J Med. 2001;345:260–5. doi: 10.1056/NEJM200107263450406. [DOI] [PubMed] [Google Scholar]
- 9.Kochupillai N. Clinical Endocrinology in India. Curr Sci. 2000;79:1061–7. [Google Scholar]
- 10.Delang F. The Disorders induced by iodine deficiency. Thyroid. 1994;4:107–28. doi: 10.1089/thy.1994.4.107. [DOI] [PubMed] [Google Scholar]
- 11.Umesh K, Preeti S. status of iodine content of salt and urinary iodine excretion levels in India. Pak J Nutr. 2003;2:361–73. [Google Scholar]
- 12.Surks MI, Ortiz E, Daniels GH, Sawin CT, Col NF, Cobin RH, et al. Subclinical thyroid disease: Scientific review and guidelines for diagnosis and management. JAMA. 2004;291:228–38. doi: 10.1001/jama.291.2.228. [DOI] [PubMed] [Google Scholar]
- 13.Sethi V, Kapil U. Iodine deficiency and development of brain. Indian J Pediatr. 2004;71:325–9. doi: 10.1007/BF02724099. [DOI] [PubMed] [Google Scholar]
- 14.Rafiq M. Prevalence survey of iodine deficiency disorders in 8-10 years old school children and use of iodized salt, Swat District NWFP Pakistan. UNICEF report. 1988 [Google Scholar]
- 15.Triggiani V, Tafaro E, Giagulli VA, Sabbà C, Resta F, Licchelli B, et al. Role of iodine, selenium and other micronutrients in thyroid function and disorders. Endocr Metab Immune Disord Drug Targets. 2009;18:277–94. doi: 10.2174/187153009789044392. [DOI] [PubMed] [Google Scholar]
- 16.Mason MB. Vitamins, trace minerals, and other micronutrients. In: Goldman L, Ausiello D, editors. Cecil Medicine. 23rd ed. Philadelphia, Pa: Saunders Elsevier; 2007. chap 237. [Google Scholar]
- 17.Dunn JT, Crutcheld H, Gutakunst R, Dunn D. Methods for measuring iodine in urine. International Council for Control of Iodine Deficiency Disorder. 1993:18–27. [Google Scholar]
- 18.Zargar AH, Shah JA, Masoodi SR, Laway BA, Shah NA, Mir MM. Prevalence of Goitre in school children in Baramulla (Kashmir valley) Indian J Pediatr. 1997;64:225–30. doi: 10.1007/BF02752453. [DOI] [PubMed] [Google Scholar]
- 19.Monika V, Rita SR. Dietary iodine intake and prevalence of iodine deficiency disorders in adults. J Nutr Environ Med. 2001;11:175–80. [Google Scholar]
- 20.Samuels MH. Subclinical thyroid disease in the elderly. Thyroid. 1998;8:803–13. doi: 10.1089/thy.1998.8.803. [DOI] [PubMed] [Google Scholar]
- 21.Parle JV, Cross FK, Jones SC, Sheppard MC. Prevalence and follow-up of abnormal thyrotrophin (TSH) concentrations in the elderly in the United Kingdom. Clin Endocr (Oxf) 1991;34:77–83. doi: 10.1111/j.1365-2265.1991.tb01739.x. [DOI] [PubMed] [Google Scholar]
- 22.Almeida C, Brasil MA, Costa AJ, Reis EA, Reuters V, Teixeira P, et al. Subclinical hypothyroidism: Psychiatric disorders and symptoms. Rev Bras Psiquiatr. 2007;29:157–9. doi: 10.1590/s1516-44462007000200013. [DOI] [PubMed] [Google Scholar]


