Abstract
Background:
Thyroid dysfunction is a common occurrence in pregnancy and affects both maternal and fetal outcomes. There are limited data on prevalence of hypothyroidism during pregnancy from India. Therefore, this study was designed to evaluate the prevalence of thyroid dysfunction especially hypothyroidism during first trimester in a large public hospital in North India.
Materials and Methods:
All the consecutive first trimester pregnant women attending Lok Nayak and Kasturba Hospitals were enrolled in the study after institutional ethics approval and consent from the study subjects. The pregnant women with diagnosed thyroid disease and on thyroid medications were excluded from the study. Morning samples of study participants were analyzed for thyroid hormone profile which included free T3, free T4, TSH, and TPO Ab. In addition, all study participants were tested for CBC, LFT, KFT, and lipid profile.
Results:
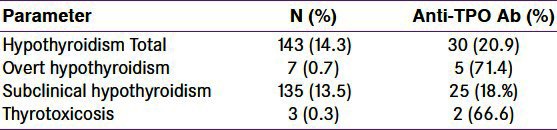
A total of 1000 women were enrolled for this prospective observational study. The mean (SD) age of study subjects was 25.6 (11.1) years, and mean (SD) gestational age was 10.3 (3.4) weeks. One hundred and forty-three (14.3%) subjects had TSH values more than 4.5 mIU/L above the cutoff used for definition of hypothyroidism. Out of these, 135 had normal free T4 and therefore labeled as subclinical hypothyroidism and 7 had low free T4 suggestive of overt hypothyroidism. TPO Ab was positive in 68 (6.82%) of total, 25 (18.5%) of subclinical and 5 (71%) of overt hypothyroid patients.
Conclusion:
Hypothyroidism, especially subclinical, is common in North Indian women during first trimester. Further countrywide studies are needed to evaluate the prevalence and etiology of hypothyroidism to prevent maternal and fetal adverse effects of hypothyroidism in India.
Keywords: Pregnancy, hypothyroidism, subclinical hypothyroidism, autoimmunity and India
INTRODUCTION
Hypothyroidism during pregnancy is deleterious to both mother and child. Children born to untreated or undertreated mothers have profound effect on future intellectual development.[1] Pregnancy has a profound impact on the thyroid gland and thyroid function. During pregnancy, the thyroid gland may enlarge by 10% in countries where iodine sources are sufficient, and to a greater extent in iodine-poor countries.[2] Production of thyroid hormones and iodine requirement each increases by approximately 50% during pregnancy.[3] Pregnancy is a stress test for the thyroid, resulting in hypothyroidism in women with limited thyroidal reserve or iodine deficiency.
Data from recently published studies have underscored the association between miscarriage and preterm delivery in women with normal thyroid function who test positive for thyroid peroxidase (TPO) antibodies.[4] Prenatal and postnatal adverse effects including attention deficit and hyperactivity syndrome have been reported in children born to hypothyroid mothers.[5,6] During the first trimester, approximately 1 in 10 pregnant women develop antibodies to TPO or to thyroglobulin, and hypothyroidism develops in roughly 16% of these women. The prevalence of hypothyroidism in pregnancy is around 2.5% according to the Western literature.[7] There are a few reports of prevalence of hypothyroidism during pregnancy from India with prevalence rates ranging from 4.8% to 11%.[8,9] Therefore, this study was carried out in a larger cohort of pregnant women during first trimester from a government hospital setting catering to majority of women from lower socioeconomic status.
MATERIALS AND METHODS
This study was conducted at the Maulana Azad Medical College and associated Lok Nayak and Katurba Hospitals, New Delhi. This study was conducted in the Department of Obstetrics and Gynaecology in collaboration with the Department of Medicine after clearance from Institutional Ethics Committee. The study period was from January 2011 to March 2011. All consecutive pregnant women who gave a written consent were included in this study. All patients were subjected to detailed history and clinical examination using a predesigned performa. Blood samples were collected in OPD setting between 0800 and 1100 hours. The serum urea, creatinine, bilirubin, aspartate aminotransferase, and alanine aminotransferase levels were checked to assess liver and renal function using standard autoanalyzers. Complete blood counts and lipid profile were also estimated. Estimation of free T3, free T4, and TSH was carried out using Advia Centaur XL Siemens kit dedicated equipment using CLIA technique, and anti-TPO was carried out by using Hycor kits by ELISA method in a NABL accredited lab. The intra-assay variability of free T4, free T3, TSH, and anti-TPO was 3.0, 2.4, 3.4, and 4.2%, and inter-assay variability for these parameters was 4.7, 2.7, 3.1, and 2.2%, respectively. The data were entered in Excel sheet and percentages of various outcome measures were calculated using SPSS for Windows version 12.
RESULTS
The baseline characteristics of study population are given in Table 1. The mean (SD) age of patients was 25.6 (11.1) years and mean gestational age was 9.2 (3.4) weeks. All the study subjects belonged to either poor or lower middle socioeconomic status. Blood pressure was normal in all the study subjects. The mean (SD) hemoglobin was 11.33 (1.31) g/dl. None of the subjects had kidney or liver dysfunction. The mean (SD) TSH, free T4, and TPO Ab were 3.6 (11.17) μIU/L,1.12 (0.96) ng/ml, and 14.06 (16.62) IU/L, respectively. These mean (SD) values are in the normal range. The parameters related to thyroid function including AbTPO are given in Table 2. One hundred and forty-three (14.3%) subjects had TSH values more than 4.5 μIU/L, the cutoff used for definition of hypothyroidism. Out of these subjects with high TSH, 135 had normal free T4 and therefore were labeled as subclinical hypothyroidism. Only seven of the study subjects had low free T4 along with high TSH and were grouped as overt hypothyroidism. TPO Ab was positive in 68 (6.82%) of total, 25 (18.5%) of subclinical and 5 (71%) of overt hypothyroid patients. Three subjects had TSH above 150 and this is represented as wide SD in TSH [Table 1].
Table 1.
Baseline characteristics of first trimester pregnant women
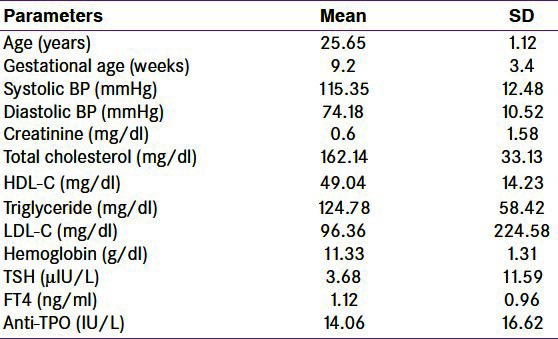
Table 2.
Thyroid dysfunction in first trimester pregnant women
DISCUSSION
This study was aimed to evaluate thyroid function during first trimester of pregnant women. The major findings are that 14.3% women attending a tertiary public hospital in Delhi have hypothyroidism and majority of these women have subclinical hypothyroidism.
What should be the normal upper limit of TSH in pregnancy? This has been a debate for a long time and it is established that an upper limit of TSH is 2.5 μIU/l. Recent guidelines proposed by the ATA and National Association of Clinical Biochemistry have stated that it is likely that in the future the upper limit of the serum TSH euthyroid reference range will be reduced to 2.5 mIU/L for all adults, because more than 95% of rigorously screened normal euthyroid volunteers have serum TSH values between 0.4 and 2.5 mIU/L.[10] However, the American Association of Clinical Endocrinologists and The Endocrine Society) consensus panel have continued to recommend that 4.5 mIU/L be maintained as the upper limit of normal, reasoning that although some individuals within the range of 2.6–4.5 mIU/L may have subclinical thyroid disease, there is a lack of evidence of adverse outcome in this group.[11] This was supported by a recent study from Reh et al. in a large cohort of first cycle IVF patients from 2005 through 2008.[12] Although lowering the TSH threshold to 2.5 mIU/L would result in a nearly fivefold increase in the number of women being classified as hypothyroid, the lack of differences in maternal clinical outcomes must be considered in the current controversy regarding the relative merits of lowering the upper limit of normal of TSH. Therefore, for this study we have taken upper limit of TSH as 4.5 μIU/l. Another confounding factor which affects thyroid function during pregnancy is human chorionic gonadotropin (hCG), a key pregnancy hormone having thyromimetic actions. Clinically, hCG levels peak between 6 and 12 weeks of pregnancy which correlates with reduced TSH level and hyperemesis gravidarum. Action of hCG is by cross reactivity of this hormone with TSH receptors.[13]
Thyroid dysfunction during pregnancy had been an important research area in clinical endocrinology due to the fact that thyroid dysfunction has immense impact on maternal and fetal outcomes.[14,15] More importantly, children born to hypothyroid mothers have poor intellectual function during later part of their life.[16] Therefore, majority of the developed countries have national neonatal screening program. The question whether to screen all pregnant women for hypothyroidism is still not resolved.[17] American Thyroid Association in its recently published guidelines have stated against universal screening of pregnant women for hypothyroidism.[1] There has been a wide geographic variation in prevalence of hypothyroidism during pregnancy. It varies from 2.5% from the West to 11% from India.[4,6] It seems that prevalence of hypothyroidism is more in Asian countries compared with the West.[18] In a large Chinese study which included 2899 pregnant women, the prevalence of hypothyroidism was significantly higher in the high-risk group than in the non-high-risk group (10.9 vs 7.0%, P = 0.008).
There are few Indian published studies on this pertinent topic. There are two published studies from South, one from Chennai and another from Hyderabad. Rao et al. included 163 non-pregnant women with recurrent pregnancy loss in a gestational age up to 12 weeks (2006) in Hyderabad.[19] Hypothyroidism was found in seven (4.12%) women with recurrent pregnancy loss and one in control group. The study demonstrates that hypothyroidism has a statistically significant relationship with recurrent pregnancy loss in the first trimester. Another study examined 500 pregnant women attending two government obstetrics and gynecology hospitals in Chennai during a period of 5 months in 2007 for thyroid function. Subclinical hypothyroidism was detected in 2.8%, among them TPO antibodies positivity was seen in 57.1%, whereas euthyroid women had significantly lower positivity (7%).[20]
In a more study, Sahu et al. have done thyroid function during second trimester in high-risk pregnant women and reported that prevalence of thyroid disorders, especially overt and subclinical hypothyroidism, was 6.47%.[9] Further, significant adverse effects on maternal and fetal outcome were seen emphasizing the importance of routine antenatal thyroid screening. In another study from India, authors have reported prevalence of hypothyroidism and thyroid autoimmunity as 4.8% and 12.4%, respectively.[8] In this study, we have reported the highest prevalence of hypothyroidism predominantly subclinical from India compared with previous studies.[9,19] This may be in line with the fact that in recent years physicians in India have noticed a rise in the number of autoimmune hypothyroidism in general population. In this study, majority of subjects with overt hypothyroidism and good number of subclinical hypothyroidism pregnant women had positive AbTPO suggesting autoimmunity as cause of hypothyroidism. One of the possible reasons is increased iodine intake in diet as suggested by one of the Chinese studies.[21] Other reasons may be presence of goitrogens in diet as reported from India.[22] Micronutrient deficiency such as selenium and iron deficiency may cause hypothyroidism and goiter.[23] Further, cutoffs used for TSH are also different in our study compared with other Indian and Chinese studies. In a study from China, the TSH value more than 4.8 μIUL was taken for the diagnosis of hypothyroidism.[17] With above reasons it is expected that the prevalence of hypothyroidism would have increased during pregnancy in India and Asia.
The strong point of this study is that we have included largest number of subjects in this study from India. This study also demonstrates a secular trend in prevalence of hypothyroidism in India when data from other previous studies were analyzed. However, there are few limitations of this study. We have not carried out thyroid examination using ultrasound, and apart from autoimmunity we have not evaluated other causes of hypothyroidism in these women.
This study concludes that there is a high prevalence of hypothyroidism (14.3%), majority being subclinical in pregnant women during first trimester from India and universal screening of hypothyroidism may be desirable in our country.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Stagnaro-Green A, Abalovich M, Alexander E, Azizi F, Mestman J, Negro R, et al. Guidelines of the American Thyroid Association for the Diagnosis and Management of Thyroid Disease During Pregnancy and Postpartum. Thyroid. 2011;21:1081–125. doi: 10.1089/thy.2011.0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van Raaij JM, Vermaat-Miedema SH, Schonk CM, Peek ME, Hautvast JG. Energy requirements of pregnancy in The Netherlands. Lancet. 1987;2:953–5. doi: 10.1016/s0140-6736(87)91431-0. [DOI] [PubMed] [Google Scholar]
- 3.Glinoer D. The regulation of thyroid function in pregnancy: Pathways of endocrine adaptation from physiology to pathology. Endocr Rev. 1997;18:404–33. doi: 10.1210/edrv.18.3.0300. [DOI] [PubMed] [Google Scholar]
- 4.Stagnaro-Green A. Thyroid antibodies and miscarriage: Where are we at a generation later? J Thyroid Res. 2011;2011:841949. doi: 10.4061/2011/841949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ghassabian A, Bongers-Schokking JJ, de Rijke YB, van Mil N, Jaddoe VW, de Muinck Keizer-Schrama SM, et al. Maternal Thyroid Autoimmunity During Pregnancy and the Risk of Attention Deficit/Hyperactivity Problems in Children. The Generation R Study. Thyroid. 2012;22:178–86. doi: 10.1089/thy.2011.0318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Männistö T, Vääräsmäki M, Pouta A, Hartikainen AL, Ruokonen A, Surcel HM, et al. Perinatal outcome of children born to mothers with thyroid dysfunction or antibodies: A prospective population-based cohort study. J Clin Endocrinol Metab. 2009;94:772–9. doi: 10.1210/jc.2008-1520. [DOI] [PubMed] [Google Scholar]
- 7.LeBeau SO, Mandel SJ. Thyroid disorders during pregnancy. Endocrinol Metab Clin North Am. 2006;35:117–36. doi: 10.1016/j.ecl.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 8.Nambiar V, Jagtap VS, Sarathi V, Lila AR, Kamalanathan S, Bandgar TR, et al. Prevalence and impact of thyroid disorders on maternal outcome in Asian-Indian pregnant women. J Thyroid Res. 2011;2011:429097. doi: 10.4061/2011/429097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sahu MT, Das V, Mittal S, Agarwal A, Sahu M. Overt and subclinical thyroid dysfunction among Indian pregnant women and its effect on maternal and fetal outcome. Arch Gynecol Obstet. 2010;281:215–20. doi: 10.1007/s00404-009-1105-1. [DOI] [PubMed] [Google Scholar]
- 10.Baloch Z, Carayon P, Conte-Devolx B, Demers LM, Feldt-Rasmussen U, Henry JF, et al. Laboratory medicine practice guidelines. Laboratory support for the diagnosis and monitoring of thyroid disease. Thyroid. 2003;13:3–126. doi: 10.1089/105072503321086962. [DOI] [PubMed] [Google Scholar]
- 11.Surks MI, Ortiz E, Daniels GH, Sawin CT, Col NF, Cobin RH, et al. Subclinical thyroid disease: Scientific review and guidelines for diagnosis and management. JAMA. 2004;291:228–38. doi: 10.1001/jama.291.2.228. [DOI] [PubMed] [Google Scholar]
- 12.Reh A, Grifo J, Danoff A. What is a normal thyroid-stimulating hormone (TSH) level? Effects of stricter TSH thresholds on pregnancy outcomes after in vitro fertilization. Fertil Steril. 2010;94:2920–2. doi: 10.1016/j.fertnstert.2010.06.041. [DOI] [PubMed] [Google Scholar]
- 13.Ballabio M, Poshyachindra M, Ekins RP. Pregnancy induced changes in thyroid function; role of human chorionic gonadotropin as a putative regulator of maternal thyroid. J Clin Endocrinol Metab. 1991;73:824–31. doi: 10.1210/jcem-73-4-824. [DOI] [PubMed] [Google Scholar]
- 14.Negro R, Schwartz A, Gismondi R, Tinelli A, Mangieri T, Stagnaro-Green A. Increased pregnancy loss rate in thyroid antibody negative women with TSH levels between 2.5 and 5.0 in the first trimester of pregnancy. J Clin Endocrinol Metab. 2010;95:E44–8. doi: 10.1210/jc.2010-0340. [DOI] [PubMed] [Google Scholar]
- 15.Casey BM, Dashe JS, Wells CE, McIntire DD, Byrd W, Leveno KJ, et al. Subclinical hypothyroidism and pregnancy outcomes. Obstet Gynecol. 2005;105:239–45. doi: 10.1097/01.AOG.0000152345.99421.22. [DOI] [PubMed] [Google Scholar]
- 16.Haddow JE, Palomaki GE, Allan WC, Williams JR, Knight GJ, Gagnon J, et al. Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child. N Engl J Med. 1999;341:549–55. doi: 10.1056/NEJM199908193410801. [DOI] [PubMed] [Google Scholar]
- 17.Negro R, Schwartz A, Gismondi R, Tinelli A, Mangieri T, Stagnaro-Green A. Universal screening versus case finding for detection and treatment of thyroid hormonal dysfunction during pregnancy. J Clin Endocrinol Metab. 2010;95:1699–707. doi: 10.1210/jc.2009-2009. [DOI] [PubMed] [Google Scholar]
- 18.Wang W, Teng W, Shan Z, Wang S, Li J, Zhu L, et al. The prevalence of thyroid disorders during early pregnancy in China: The benefits of universal screening in the first trimester of pregnancy. Eur J Endocrinol. 2011;164:263–8. doi: 10.1530/EJE-10-0660. [DOI] [PubMed] [Google Scholar]
- 19.Rao VR, Lakshmi A, Sadhnani MD. Prevalence of hypothyroidism in recurrent pregnancy loss in first trimester. Indian J Med Sci. 2008;62:357–61. [PubMed] [Google Scholar]
- 20.Gayathri R, Lavanya S, Raghavan K. Subclinical hypothyroidism and autoimmune thyroiditis in pregnancy - a study in South Indian subjects. J Assoc Physicians India. 2009;57:691–3. [PubMed] [Google Scholar]
- 21.Teng X, Shan Z, Chen Y, Lai Y, Yu J, Shan L, et al. More than adequate iodine intake may increase subclinical hypothyroidism and autoimmune thyroiditis: A cross-sectional study based on two Chinese communities with different iodine intake levels. Eur J Endocrinol. 2011;164:943–50. doi: 10.1530/EJE-10-1041. [DOI] [PubMed] [Google Scholar]
- 22.Marwaha RK, Tandon N, Gupta N, Karak AK, Verma K, Kochupillai N. Residual goitre in the postiodization phase: Iodine status, thiocyanate exposure and autoimmunity. Clin Endocrinol (Oxf) 2003;59:672–81. doi: 10.1046/j.1365-2265.2003.01895.x. [DOI] [PubMed] [Google Scholar]
- 23.Das S, Bhansali A, Dutta P, Aggarwal A, Bansal MP, Garg D, et al. Persistence of goitre in the post-iodization phase: Micronutrient deficiency or thyroid autoimmunity? Indian J Med Res. 2011;133:103–9. [PMC free article] [PubMed] [Google Scholar]


