Abstract
Objective:
Alteration in thyroid hormones are seen in critically ill patients admitted to intensive care units. Our objective was to study the thyroid hormone profile, prolactin and, glycosylated hemoglobin (HbA1c) at admission and analyze their correlation with mortality.
Materials and Methods:
In this single centre, prospective, observational study, 100 consecutive patients (52M; 48F) admitted to medical ICU irrespective of diagnosis were included. Patients with previous thyroid disorders and drugs affecting thyroid function were excluded. All participants underwent complete physical examination and a single fasting blood sample obtained at admission was analyzed for total triiodothyronine (T3), total thyroxine (T4), thyroid stimulating hormone (TSH), HbA1c, and prolactin. The patients were divided into two groups: Group 1 – survivors (discharged from the hospital) and Group 2 – nonsurvivors (patients succumbed to their illness inside the hospital). The data were analyzed by appropriate statistical methods and a P-value of <0.05 was considered significant.
Results:
The mean age of the participants was 58.7 ± 16.9 years and the mean duration of ICU stay was 3.3 ± 3.1 days. A total of 64 patients survived, whereas remaining 36 succumbed to their illness. The baseline demographic profile was comparable between survivors and nonsurvivors. Nonsurvivors had low T3 when compared with survivors (49.1 ± 32.7 vs. 66.2 ± 30.1, P = 0.0044). There was no significant difference observed between survivors and nonsurvivors with respect to T4, TSH, HbA1c, and prolactin.
Conclusion:
Our study showed that low T3 is an important marker of mortality in critically ill patients. Admission HbA1c, prolactin, T4, and TSH did not vary between survivors and nonsurvivors.
Keywords: Admission HbA1c, critically ill, medical ICU, thyroid hormone profile
INTRODUCTION
Stressful situations affect all endocrine axes and differ in the response during acute and chronic phases of stress. The physiological rationale behind these alterations is to change the internal milieu of the organism helping to fight the external threat to the homeostasis.[1] The endocrine alterations may overshoot the boundaries in some individuals leading to deleterious consequences. Previous attempts to correct these hormonal imbalances did not yield beneficial results in clinical trials.[2,3,4] Low circulating thyroid hormones with hypogonadism and hypercortisolism are the predominant changes in acute stress.[5] Few studies have reported that the low thyroid hormones are independent predictors of mortality in patients admitted to intensive care units (ICU), suggesting the inclusion of the thyroid profile in these scoring systems.[6,7,8] There appears a continuum of changes in critically ill patients, starting with low triiodothyronine (T3) followed by low thyroxine (T4) and lastly low thyroid stimulating hormone (TSH).[9]
Hyperglycemia is shown to be an independent predictor of mortality in intensive care patients.[10] The pendulum of intensive glycemic control in ICU is swinging with different trials giving conflicting opinions. The varying cutoff of glycemic control coupled with rapidity of change in glycosylated hemoglobin (HbA1c) lead to increase in mortality in recent studies.[11,12] Prolactin is a hormone with a variety of roles, and the secretion is increased in a variety of conditions including stressful situations. Hyperprolactinemia modulates immune response leading to deficiency in cell-mediated immunity.[13] There are no data available to assess the level of prolactin as a marker of the severity of the stress or indirectly leading to increase in mortality. Surprisingly, no data exist from India regarding the endocrinological parameters in critically ill patients and hence we conducted this prospective observational study to assess the thyroid hormone profile, prolactin levels, and HbA1c in relation to survival in patients admitted to medical ICU.
MATERIALS AND METHODS
Study population
A total of 100 consecutive patients admitted to medical ICU irrespective of the underlying diagnosis and diabetes state were included in this prospective, observational study. Admission into ICU was based on the presentation of the patient and underlying clinical conditions unrelated to the study objectives. Patients with a known history of thyroid disease, intake of drugs altering thyroid function, were excluded from the study. All the patients had a detailed clinical examination and were managed appropriate to their primary condition. The patients were divided into two groups for comparison: Group 1 – survivors (discharged from the hospital) and Group 2 – nonsurvivors (patients succumbed to their illness inside the hospital). An informed consent, to participate in the study, was obtained from the patients or relatives where appropriate, and the study protocol was approved by the local hospital ethics committee.
Biochemical measurements
Fasting venous blood samples were collected immediately on admission to ICU from all patients and were subjected for hormone analyses. Samples were tested for total T3, total T4, TSH, prolactin, and glycosylated hemoglobin in addition to the standard ICU protocol tests. The normal reference range for thyroid hormones in our laboratory is given below: TSH (0.3–4.6 mIU/L), T3 (67–156 ng/ dL), T4 (4.5–11.6 μg/dL), prolactin (0–15 ng/mL males, 0–25 females). Any deviation of the hormone results from the normal ranges is considered to be abnormal (low or elevated). Thyroid and prolactin hormones were estimated by solid phase competitive chemiluminescence and HbA1c using the high performance liquid chromatography method. The interassay and intraassay variation in all hormonal assays was less than 6%. We did not repeat the hormonal assays a second time in the ICU or prior to discharge in survivors. Free thyroid hormones were not measured in the study group to prevent the variability associated with these hormones.
Statistical analysis
Summary data are presented as mean values ± SD and comparison between groups was done by Mann–Whitney U-test. Fisher's exact test and Chi-square test were used to compare frequency of variables among two groups. P values were reported for all statistical tests and a value of <0.05 was considered to be significant.
RESULTS
The baseline profile of study population (48 females and 52 males) is given in Table 1. The mean age of the study population was 58.7 ± 16.9 years (range, 16–94) with average duration of ICU stay 3.3 ± 3.1 days (range, 1–18). Cardiovascular diseases constitute the majority, followed by infectious diseases and neurological disorders. There was a significant linear correlation of TSH with age in the entire study population [Figure 1]. HbA1c did not show such correlation with age in the entire study subjects (R2 = 0.00012, P = 0.9124).
Table 1.
Baseline profile of study participants
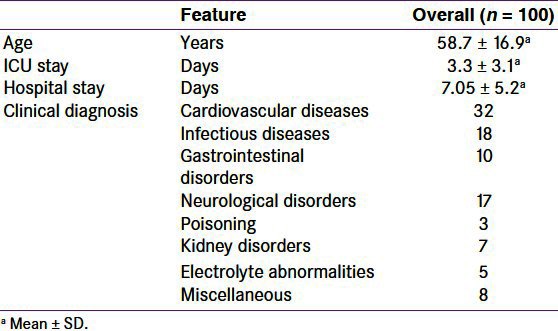
Figure 1.
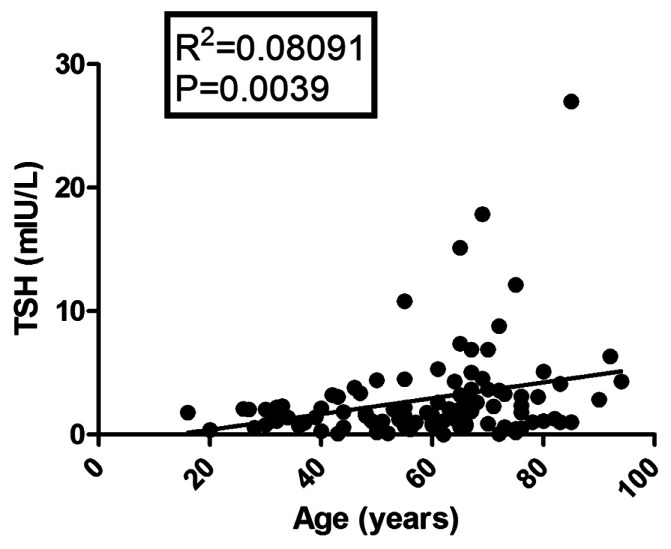
Correlation between TSH and age in entire study population.
Out of the 100 patients studied, 64 survived and discharged from the hospital whereas remaining 36 succumbed to their illness. The comparison between survivors and nonsurvivors is given in Table 2. Low T3 is the only significant difference in nonsurvivors when compared with all other studied parameters [Figure 2]. The distribution of thyroid hormone abnormalities in our study group are as follows: low T3 alone (45), low T3 and T4 (13), low T3, T4 and TSH (3), mixed patterns (16), and normal thyroid profile (23).
Table 2.
Parameters between survivors and nonsurvivors
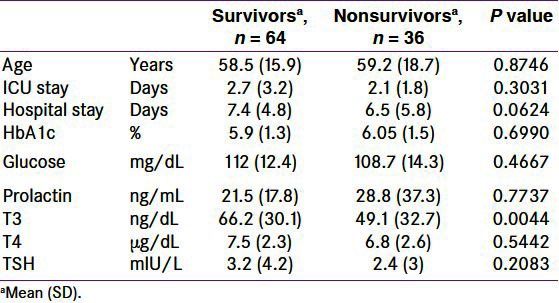
Figure 2.
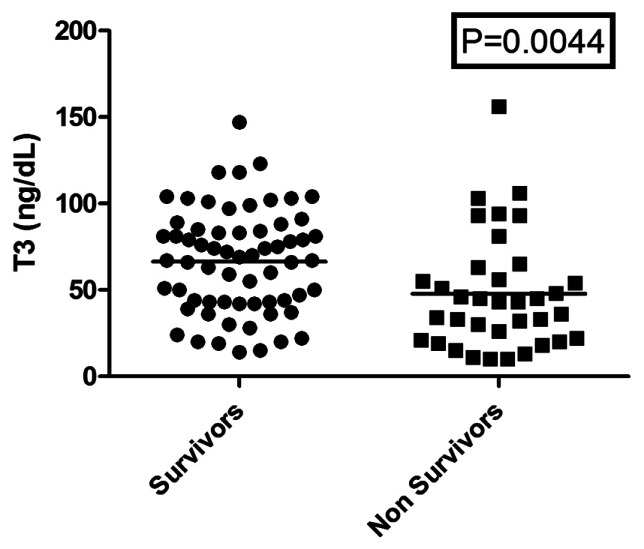
T3 levels between survivors and nonsurvivors.
A total of 61 patients had low T3 (defined as less than 65 ng/dL) and out of them 16 also had low T4 (defined as less than 4.5 μg/dL). The mortality rate was not different between patients with low T3 alone (19 nonsurvivors out of 45) and low T3 and T4 [9 nonsurvivors out of 16 (which includes 13 patients of low T3 and T4 and 3 patients of low T3, T4 and TSH)] at presentation (P = 0.5697). However out of 39 patients with normal T3, only 8 patients succumbed to their illness (P = 0.0111) when compared with 28 nonsurvivors in patients with low T3. There was no significant difference observed in the profile between survivors and nonsurvivors with respect to T4, TSH and prolactin. Prolactin and HbA1c levels did not predict the mortality in ICU patients.
DISCUSSION
Our study demonstrated that low T3 is an important marker of the severity of the illness and predicts mortality in ICU. The same was not seen when we combine low T4 along with low T3. Other hormonal parameters did not show any significant difference between survivors and nonsurvivors. Our study showed low T3 (61%) is the commonest abnormality followed by lowT4 (14%) and low TSH (7%). Previous data from pediatric ICU patients from Mumbai showed low T3 in 80%, low T4 in 50%, and low TSH in 6.7% patients,[14] and it was conducted in 30 critically ill children and controls of less than 12 years age admitted in pediatric ICU. Two samples were collected from all patients, first at admission and second sample at the time of discharge from ICU or death. This study showed that mean T3 and T4 levels were significantly lower in critically ill children than controls. The combination of low T3 and T4 together increased the mortality risk by 30 times. Our study differs in the age of the study population (adults), number of study samples (single sample at admission only), and lack of the control group from the previous study explaining the discrepancy in observed data.[14]
Euthyroid sick syndrome is the term used to describe thyroid hormonal changes in critically ill patients due to nonthyroidal illness. Low T3 is the earliest manifestation followed by low T4 and finally low TSH, indicating a continuum of changes in the spectrum.[15] A similar study involving pediatric ICU patients showed that low T3 is a good predictor of mortality, and the risk is increased by 30 times when it is associated with low T4.[14] However, that trend was not observed in our study, and this could be due to difference in age and number of study population and performing a single sample estimation instead of serial monitoring in our study.
The demographic profile of the study patients did not differ significantly between the survivors and nonsurvivors. Cardiovascular diseases constitute the common reason as we have a combined medical ICU and cardiac ICU in our hospital. This is followed by infectious diseases (complicated malaria, sepsis with multiorgan dysfunction syndrome) and neurological disorders (cerebrovascular accident, subarachnoid hemorrhage and intra cerebral hemorrhage). TSH showed a linear correlation with age in our study population [Figure 1]. This was explained earlier by many investigators that the age-related elevation in TSH could be due to increasing autoimmunity, environmental, and genetic factors.[16,17] Although insulin resistance and diabetes are also related to the age, our data did not show any correlation between HbA1c and age. This could be explained because of the selection bias of critically ill patients in the study which does not represent the general population.
Elevated prolactin is seen in 31 patients of the study group. Out of them, 14 survived and 17 succumbed to their illness. Prolactin is an immunomodulatory hormone involved in the endocrine response to the stress, and hyperprolactinemia is a common finding in initial phases. Blunted prolactin response during chronic stress is speculated to lead to increased susceptibility to infection.[18] Previous reports demonstrate that admission and fasting glucose is an important marker of short-term outcomes in hospitalized patients. HbA1c is a reflection of glucose concentration of preceding 3 months. Our data did not show any difference in outcomes based on HbA1c and glucose values. HbA1c does not vary with acute stress and admission hyperglycemia in ICU patients may be an indirect marker of severe stress.[19] The limitations of our study include small sample size, single blood sample for hormonal assay, lack of control group, and serial monitoring for the hormonal changes.
CONCLUSION
To conclude, our study suggests that low T3 is an important marker of mortality in critically ill patients. Low T4 and TSH did not increase the predictability. Admission HbA1c and prolactin did not vary between survivors and nonsurvivors. Further studies involving a large number of patients with serial evaluation for changes in the hormone profile are required to unravel the mystery of critical care endocrinology.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Van den Berghe G, de Zegher F, Bouillon R. Clinical review 95: Acute and prolonged critical illness as different neuroendocrine paradigms. J Clin Endocrinol Metab. 1998;83:1827–34. doi: 10.1210/jcem.83.6.4763. [DOI] [PubMed] [Google Scholar]
- 2.Haas NA, Camphausen CK, Kececioglu D. Clinical review: Thyroid hormone replacement in children after cardiac surgery: Is it worth a try? Crit Care. 2006;10:213. doi: 10.1186/cc4924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van den Berghe G, de Zegher F, Baxter RC, Veldhuis JD, Wouters P, Schetz M, et al. Neuroendocrinology of prolonged critical illness: Effects of exogenous thyrotropin-releasing hormone and its combination with growth hormone secretagogues. J Clin Endocrinol Metab. 1998;83:309–19. doi: 10.1210/jcem.83.2.4575. [DOI] [PubMed] [Google Scholar]
- 4.Brent GA, Hershman JM. Thyroxine therapy in patients with severe nonthyroidal illnesses and low serum thyroxine concentration. J Clin Endocrinol Metab. 1986;63:1–8. doi: 10.1210/jcem-63-1-1. [DOI] [PubMed] [Google Scholar]
- 5.Van den Berghe G. Endocrine evaluation of patients with critical illness. Endocrinol Metab Clin North Am. 2003;32:385–410. doi: 10.1016/s0889-8529(03)00005-7. [DOI] [PubMed] [Google Scholar]
- 6.Peeters RP, Wouters PJ, van Toor H, Kaptein E, Visser TJ, Van den Berghe G. Serum 3,30,50-triiodothyronine (rT3) and 3,5,30-triiodothyronine/rT3 are prognostic markers in critically ill patients and are associated with postmortem tissue deiodinase activities. J Clin Endocrinol Metab. 2005;90:4559–65. doi: 10.1210/jc.2005-0535. [DOI] [PubMed] [Google Scholar]
- 7.Slag MF, Morley JE, Elson MK, Crowson TW, Nuttall FQ, Shafer RB. Hypothyroxinemia in critically ill patients as a predictor of high mortality. JAMA. 1981;245:43–5. [PubMed] [Google Scholar]
- 8.Lim DJ, Herring MK, Leef KH, Getchell J, Bartoshesky LE, Paul DA. Hypothyroxinemia in mechanically ventilated term infants is associated with increased use of rescue therapy. Pediatrics. 2005;115:406–10. doi: 10.1542/peds.2004-0192. [DOI] [PubMed] [Google Scholar]
- 9.Docter R, Krenning EP, de Jong M, Hennemann G. The sick euthyroid syndrome: Changes in thyroid hormone serum parameters and hormone metabolism. Clin Endocrinol (Oxf) 1993;39:499–518. doi: 10.1111/j.1365-2265.1993.tb02401.x. [DOI] [PubMed] [Google Scholar]
- 10.Umpierrez GE, Isaacs SD, Bazargan N, You X, Thaler LM, Kitabchi AE. Hyperglycemia: An independent marker of in-hospital mortality in patients with undiagnosed diabetes. J Clin Endocrinol Metab. 2002;87:978–82. doi: 10.1210/jcem.87.3.8341. [DOI] [PubMed] [Google Scholar]
- 11.van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, et al. Intensive insulin therapy in the critically ill patients. N Engl J Med. 2001;345:1359–67. doi: 10.1056/NEJMoa011300. [DOI] [PubMed] [Google Scholar]
- 12.Skyler JS, Bergenstal R, Bonow RO, Buse J, Deedwania P, Gale EA, et al. Intensive glycemic control and the prevention of cardiovascular events: Implications of the ACCORD, ADVANCE, and VA Diabetes Trials: A position statement of the American Diabetes Association and a Scientific Statement of the American College of Cardiology Foundation and the American Heart Association. J Am Coll Cardiol. 2009;53:298–304. doi: 10.1016/j.jacc.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 13.Russell DH. New aspects of prolactin & immunity: A lymphocyte-derived prolactin-like product & nuclear protein kinase C activation. Trends Pharmacol Sci. 1989;10:40–4. doi: 10.1016/0165-6147(89)90106-5. [DOI] [PubMed] [Google Scholar]
- 14.Suvarna JC, Fande CN. Serum thyroid hormone profile in critically Ill children. Indian J Pediatr. 2009;76:1217–21. doi: 10.1007/s12098-009-0250-7. [DOI] [PubMed] [Google Scholar]
- 15.Lodha R, Vivekanandhan S, Sarthi M, Arun S, Kabra SK. Thyroid function in children with sepsis and septic shock. Acta Paediatr. 2007;96:406–9. doi: 10.1111/j.1651-2227.2007.00135.x. [DOI] [PubMed] [Google Scholar]
- 16.Imaizumi M, Sera N, Ueki I, Horie I, Ando T, Usa T, et al. Risk for progression to overt hypothyroidism in an elderly Japanese population with subclinical hypothyroidism. Thyroid. 2011;21:1177–82. doi: 10.1089/thy.2010.0411. [DOI] [PubMed] [Google Scholar]
- 17.Kumar HK, Yadav RK, Prajapati J, Reddy CV, Raghunath M, Modi KD. Association between thyroid hormones, Insulin resistance and Metabolic syndrome. Saudi Med J. 2009;30:907–11. [PubMed] [Google Scholar]
- 18.Russell DH, Kibler R, Matrisian L, Larson DF, Poulos B, Magun BE. Prolactin receptors on human T- and Blymphocytes: Antagonism of prolactin-binding by cyclosporin. J Immunol. 1985;134:3027–31. [PubMed] [Google Scholar]
- 19.Cakmak M, Cakmak N, Cetemen S, Tanriverdi H, Enc Y, Teskin O, et al. The value of admission glycosylated hemoglobin level in patients with acute myocardial infarction. Can J Cardiol. 2008;24:375–8. doi: 10.1016/s0828-282x(08)70600-7. [DOI] [PMC free article] [PubMed] [Google Scholar]


