Abstract
The Rhizobium etli CNPAF512 fnrN gene was identified in the fixABCX rpoN2 region. The corresponding protein contains the hallmark residues characteristic of proteins belonging to the class IB group of Fnr-related proteins. The expression of R. etli fnrN is highly induced under free-living microaerobic conditions and during symbiosis. This microaerobic and symbiotic induction of fnrN is not controlled by the sigma factor RpoN and the symbiotic regulator nifA or fixLJ, but it is due to positive autoregulation. Inoculation of Phaseolus vulgaris with an R. etli fnrN mutant strain resulted in a severe reduction in the bacteroid nitrogen fixation capacity compared to the wild-type capacity, confirming the importance of FnrN during symbiosis. The expression of the R. etli fixN, fixG, and arcA genes is strictly controlled by fnrN under free-living microaerobic conditions and in bacteroids during symbiosis with the host. However, there is an additional level of regulation of fixN and fixG under symbiotic conditions. A phylogenetic analysis of the available rhizobial FnrN and FixK proteins grouped the proteins in three different clusters.
Soil bacteria belonging to the genera Rhizobium, Allorhizobium, Azorhizobium, Bradyrhizobium, Mesorhizobium, and Sinorhizobium (collectively referred to as rhizobia) elicit the formation of nodules on the roots of their leguminous hosts. In these specialized organs, the bacteria are released into the plant cells and differentiate into bacteroids that fix atmospheric nitrogen into ammonia that can be assimilated by the host plant. The nodules provide the microoxic conditions required for functioning of the oxygen-sensitive nitrogenase enzyme complex.
In rhizobia, NifA activates transcription of several nitrogen fixation genes in conjunction with σ54 RNA polymerase (12). In Rhizobium etli CNPAF512, NifA is strictly required for nitrogen fixation activity in nodules of Phaseolus vulgaris and controls the expression of several genes involved in nitrogen fixation, including nifH, iscN, and orf180-rpoN2 (13, 33, 34). Transcription of the R. etli nifA gene itself occurs independent of the oxygen status of the cell (33). Two rpoN genes encoding the alternative σ factor, σ54, have been characterized in R. etli and have been shown to be differentially regulated (34, 35). During free-living growth, RpoN1 is required for growth on several nitrogen and carbon sources (35). There is a severe decrease in nitrogen fixation after inactivation of rpoN2 (34), indicating the essential role of this gene in bacteroids (12). The NifA enhancer-binding protein controls transcription activation of rpoN2 under free-living microaerobic conditions and during symbiosis (34). Besides nifA, fixLJ regulatory genes were identified in R. etli CNPAF512. The fixL gene encodes a protein lacking heme-binding capacity (9, 10). FixLJ is involved in microaerobic nifH expression, but in contrast to the situation in Sinorhizobium meliloti and Azorhizobium caulinodans (25, 26), the expression of nifA is not dependent on FixLJ. Nitrogen fixation in the R. etli fixLJ mutant bacteroids is reduced (10).
In this paper, we describe identification of a third nitrogen fixation regulatory protein, FnrN, in R. etli CNPAF512. This protein is homologous to the oxygen-responsive transcriptional Fnr regulator of Escherichia coli involved in the regulation of genes with functions in anaerobic respiration (reviewed in references 30 and 53). E. coli Fnr and rhizobial FnrN and FixK proteins belong to the same family of homologous transcriptional regulators, the cyclic AMP receptor protein Crp-Fnr family, which is divided into three classes (15). The first class includes the Fnr protein of E. coli and homologous proteins involved in oxygen control of various cellular processes and is further divided into four subgroups (15, 59). It has been proposed that Fnr proteins that belong to class IB of this protein family sense the redox status with a strictly conserved cysteine-rich domain in the N terminus and an additional, conserved cysteine residue in the central part of the polypeptide. These cysteine residues may contribute to the formation of an iron-binding domain (15, 57). The cysteine motif of the class IB proteins differs from that of the proteins belonging to class IA, like E. coli Fnr (15, 57). In contrast to FnrN, rhizobial FixK regulators (class IC of the Crp-Fnr family) lack the N-terminal and central cysteine residues. It has been speculated that the activity of these proteins is not directly controlled by oxygen (3, 15, 27). The oxygen regulation of S. meliloti fixK occurs at the transcriptional level through the FixLJ system (8, 20, 48). Both FnrN and FixK regulators contain a helix-turn-helix motif in the C-terminal region that is involved in DNA binding (49). The promoter regions of the target genes that are bound by FixK or FnrN dimers contain a conserved motif (TTGA-C--GATCAA-G), called the anaerobox (15). Vollack et al. (59) proposed a change in the tripartite classification (15) and suggested an additional subgroup, class ID, comprising the Dnr proteins. Together with the FixK-like class IC proteins, these proteins lack the N-terminal cysteine motif.
Here, we describe identification, localization, and functional analysis of the R. etli CNPAF512 fnrN gene, whose product is a member of class IB of the Crp-Fnr family.
MATERIALS AND METHODS
Bacterial strains, plasmids, and culture conditions.
Bacterial strains and plasmids used in this study are listed in Table 1. E. coli was routinely cultivated in Luria-Bertani medium (36) at 37°C. R. etli CNPAF512 and mutant strains were grown on TY (0.5% tryptone, 0.3% yeast extract, 7 mM CaCl2) or yeast extract-mannitol plates (58) at 30°C or in liquid defined acid minimal salts medium (40) supplemented with 10 mM mannitol and 10 mM NH4Cl. S. meliloti was grown on Luria-Bertani medium supplemented with 2.5 mM CaCl2 and 2.5 mM MgSO4. Antibiotics were added to the media at the following concentrations: nalidixic acid, neomycin, kanamycin, and gentamicin, 30 μg/ml; ampicillin, 100 μg/ml; and tetracycline, 1 μg/ml (final concentration) for R. etli or 10 μg/ml (final concentration) for E. coli.
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Relevant characteristics | Source or reference |
|---|---|---|
| Escherichia coli DH5α | F80dlacZΔM15 Δ(lacZYA-argF) recA endA hsdr supE | Gibco-BRL |
| Rhizobium etli strains | ||
| CNPAF512 | Nalr, wild type | 34 |
| FAJ1154 | NmrrpoN1::Ω-Km | 35 |
| FAJ1169 | NmrrpoN2::Ω-Km | 34 |
| FAJ1170 | Nmr SprrpoN1::Ω-Sp rpoN2::Ω-Km | 34 |
| FAJ1182 | NmrfnrN::Ω-Km, same orientation | This study |
| FAJ1183 | NmrfnrN::Ω-Km, opposite orientation | This study |
| Rp1000 | NmrnifA::aphII | 33 |
| RpFAJ1002 | NmrfixL::Ω-Km | 10 |
| RpFAJ1004 | NmrfixJ::Ω-Km | 10 |
| CMPG8007 | NmrfixO::mTn5gusA | This study |
| CMPG8169 | Nalr NmrfixG::mTn5gusA | 60 |
| CMPG8170 | Nalr NmrfixI::mTn5gusA | 60 |
| Sinorhizobium meliloti GMI347-CS112 | Smr Nmr Rfr Gmr GMI347 with pCS112 (pSUP202Gm containing the fixN-lacZ fusion of pGMI931) integrated into pSym | 6 |
| Plasmids | ||
| pUC18 | Apr, cloning vector | 38 |
| pUC18Not | Apr, cloning vector | 24 |
| pLAFR1 | Tcr, broad-host-range vector | 17 |
| pLAFR3 | Tcr, broad-host-range vector | 50 |
| pHP45Ω-Km | Apr Kmr | 14 |
| pJQ200-UC1 | GmrsacB | 43 |
| pWM6 | Apr NmruidA2 | 32 |
| pFAJ1172 | Tcr, fnrN gene in pLAFR1 | 34 |
| pFAJ1174 | Apr, a 4.2-kb NotI fragment containing fnrN cloned in pUC18Not | 34 |
| pFAJ1175 | Tcr, fnrN gene, rpoN2-gusA fusion, and orf180 gene in pLAFR1 | 34 |
| pFAJ1176 | Tcr, orf180-gusA fusion in pLAFR3 | 34 |
| pFAJ1178 | Kmr, pLAFR3 containing fnrN-gusA | This study |
| pFAJ1192 | Kmr, pLAFR3 containing fixG-gusA fusion | This study |
| pFAJ1193 | Kmr, pLAFR3 containing fixN-gusA fusion | This study |
| pFAJ1319 | Tcr, arcA-gusA fusion in pLAFR3 | 11 |
| pGMI931 | Tcr, S. meliloti fixN-lacZ | 7 |
DNA sequence analysis.
DNA sequencing was performed with both strands of overlapping pUC18 subclones by using Cy5-labeled universal and synthetic oligonucleotide primers. Double-stranded DNA was sequenced by the dideoxynucleotide chain termination method with an automated sequencer (ALF; Pharmacia-LKB, Uppsala, Sweden). Computer-assisted sequence analyses were performed by using the ContigExpress software package (Informax Inc.).
Partial DNA sequence analysis of mutants CMPG8007, CMPG8169, and CMPG8170.
The partial DNA sequences of the inactivated genes of transposon mutants CMPG8007, CMPG8169, and CMPG8170 were determined (60; this study). The corresponding insertions were cloned as XhoI fragments in the SalI site of pUC18. The DNA sequences bordering the transposon insertion were determined by using a primer annealing at the 5′ end of the gusA gene in the mini-Tn5 transposon (5′-CGGTACCTGACTAGCTAAGGAG-3′), reading outward from the gusA gene, or a synthetic primer based on the sequences obtained.
Construction of a rhizobial FnrN dendrogram.
The amino acid sequences of 26 different FnrN and FixK proteins were aligned by using the ClustalW program (52). For representation purposes the alignment was imported into the GeneDoc program.
A tree was constructed with the Treecon for Windows (version 1.3b) software package (56). The distances between the sequences were calculated by using the Poisson correction method (insertions and deletions were taken into account) with a bootstrap analysis with 1,000 replications. The tree topology was inferred by the neighbor-joining method. The Phylip package (version 3.6; http://evolution.genetics.washington.edu/phylip.html) was used to construct the most parsimonious tree from 1,000 bootstrapped samples. Fnr of E. coli (gi26108071) was used as an outgroup to root the tree.
β-Glucuronidase assays.
Qualitative and quantitative analyses of β-glucuronidase activity were performed as described elsewhere (33). Cultures of R. etli were grown aerobically and microaerobically (0.3% O2) at 30°C. Cultures were grown in liquid defined acid minimal salts medium (40) supplemented with 10 mM mannitol and 10 mM NH4Cl. The data were analyzed for statistical differences by using Tukey's test (P < 0.05).
Construction of an R. etli CNPAF512 fnrN mutant.
Plasmid pFAJ1174 was constructed previously and contains the R. etli rpoN2, orf180, and fnrN genes (34). This plasmid was digested with SalI (Fig. 1) and ligated with the 1.8-kb BamHI fragment from pHP45Ω-Km after blunting of both fragments, which inactivated the fnrN gene. The resulting 6-kb NotI fragment was cloned into the NotI site of pJQ200-UC1 (43), and the resulting construct was subsequently used to mutagenize R. etli CNPAF512 as previously described (10). Insertion of the mutation was verified by Southern blot hybridization by using the appropriate probes. The resulting R. etli fnrN mutants were designated FAJ1182 and FAJ1183; in these mutants the fnrN gene and the resistance gene read in the same direction and in opposite directions, respectively.
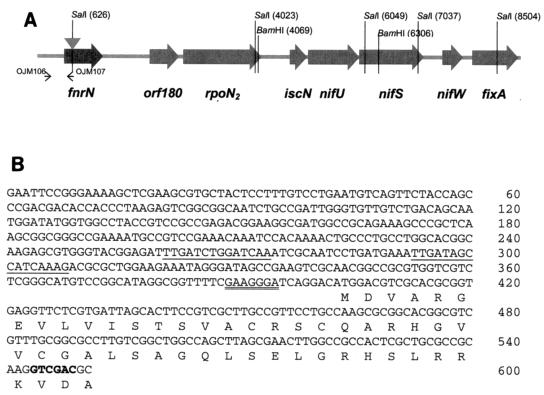
FIG. 1.
(A) Detailed physical map of the R. etli symbiotic region containing the fnrN gene. The insertion site for the Ω-Km interposon in the fnrN gene is indicated by a grey triangle. Oligonucleotide primers (small arrows) are described in Materials and Methods. (B) Promoter region of R. etli fnrN. Two putative anaeroboxes and a purine-rich DNA sequence resembling a ribosome-binding site are underlined once and twice, respectively. The SalI restriction site, used to insert the Ω-Km interposon, is indicated by boldface type.
Isolation and localization of fixN and fixG.
To localize the fixNOQP genes, a search for a cosmid clone carrying this region was performed. Based on database sequences (accession number U76906), primers were designed to amplify a 200-bp fixN fragment (primer OJM135 [5′-CTGATTAATTAAAGTAAGCGGGCGGTGCCAAAG-3′] and primer OJM137 [5′-CTGAGAATTCTTCTGTCGTGTAATTCATGATG-3′]). By using colony PCR four overlapping cosmid clones (p2D2, p2G7, p8C7, and p19H4) were isolated from a genomic library (34) containing the fixN region. Partial sequence analysis of several EcoRI and SalI fragments confirmed the presence of the fixN and fixG genes. This region was shown to be located close to the previously described nifHDK3 gene located on a 4.2-kb EcoRI fragment (33).
Construction of gusA fusions. (i) fnrN-gusA.
A 0.6-kb fnrN promoter fragment was amplified by PCR with Pwo DNA polymerase by using oligonucleotides OJM106 (5′-CTGAGGATCCGCGGCCGCTTTGTCCTGAATGTCAGTTC-3′; BamHI and NotI recognition sites are underlined) and OJM107 (5′-AAAGAGATCTCCGATCCTTGTGCAATGATCTC-3′; BglII recognition site is underlined). The resulting fragment was digested with BamHI and BglII and cloned into pFAJ1171 (55), which fused the first 59 codons of fnrN to the gusA gene. To facilitate cloning into pLAFR3, the Ω-Km cassette from pHP45Ω-Km was amplified by PCR by using primers OJM059 (5′-ACTTGGATCCACGCCTTCCTCTCCGAATGC-3′; BamHI site is underlined) and OJM060 (5′-ACTTGGATCCGAATTCCGTGCGCGTCAGCCAGTTGG-3′; BamHI and EcoRI sites are underlined), digested with BamHI, and inserted into the EcoRI site located downstream of gusA after blunting of both fragments. The resulting plasmid contained an intact EcoRI site bordering the 3′ end of the Ω-Km cassette. Finally, a BamHI-EcoRI fragment that was approximately 4 kb long and contained fnrN-gusA-Ω-Kmr was cloned into the broad-host-range vector pLAFR3, resulting in pFAJ1178.
(ii) fixN-gusA.
PCR amplification with Pwo DNA polymerase by using primers OJM151 (5′-CTGAGGATCCAGAAAGCAGCTGCGTCATAC-3′; BamHI site is underlined) and OJM152 (5′-CTGATCTAGACGCTGCGACCGCGATCACCATC-3′; XbaI site is underlined) yielded a fixN promoter fragment that was approximately 240 bp long. This fragment was cloned as a BamHI-XbaI fragment in pUCNot. The resulting construct was digested with XbaI and blunt end ligated to the 3.8-kb BamHI fragment from pWM6 containing a promoterless gusA-Kmr cassette. Finally, the 4-kb fragment was digested with NotI, blunted, and ligated in the blunted BamHI site of the broad-host-range plasmid pLAFR3. The resulting plasmid was designated pFAJ1193 (fixN-gusA).
(iii) fixG-gusA.
A 230-bp fixG promoter fragment was obtained by performing PCR with Pwo DNA polymerase and primers OJM153 (5′CTGAAAGCTTGAGCCGATAGTTTCAGCTCC-3′; HindIII site is underlined) and OJM154 (5′-CTGATCTAGAACGCGAACGTGGTCAATGTC-3′; XbaI site is underlined). The resulting PCR fragment was cloned as a HindIII-XbaI fragment in pUCNot. The resulting construct was digested with XbaI and blunt end ligated to the 3.8-kb BamHI fragment from pWM6 containing a promoterless gusA-Kmr cassette. Finally, the 4-kb insert was removed as a NotI fragment and blunt end ligated in the BamHI site of the broad-host-range plasmid pLAFR3. The resulting plasmid was designated pFAJ1192 (fixG-gusA).
Plant culture and acetylene reduction assay.
Seeds of P. vulgaris cv. Limburgse vroege were sterilized by rinsing them with ethanol for 3 min and with 15% sodium hypochlorite for 13 min. Next, the seeds were washed 10 times in sterile water. Seeds were germinated for 3 days on water agar plates (15 g/liter) in the dark at 30°C. The seedlings were transferred to 250-ml conical bottles filled with 150-ml agar slants (1.2 g of agar/150 ml) of Snoeck medium, which is optimized for in vitro growth of common bean (46).
Plants were inoculated and grown essentially as described by Michiels et al. (34). For expression analysis during symbiosis, bacteroids from 3-week-old nodules were purified from plant material by differential centrifugation (34). The nitrogen fixation capacity was determined by the acetylene reduction assay as described by Michiels et al. (34). The acetylene reduction assay data and symbiotic expression data were analyzed for statistical differences by using Tukey's test (P < 0.05).
Nucleotide sequence accession number.
The nucleotide sequence of the R. etli fnrN gene locus has been deposited in the DDBJ-EMBL-GenBank nucleotide sequence databases under accession no. AJ005696.
RESULTS
Cloning and DNA sequence of R. etli fnrN.
Nucleotide sequence analysis of the upstream region of the previously identified orf180-rpoN2 operon (34) revealed the presence of an open reading frame (ORF) (Fig. 1A). This ORF encodes a 26-kDa protein that is very similar (80% or more identity) to the known FnrN proteins. The ORF was therefore designated fnrN. A putative ribosome-binding site is located 7 bp upstream from the proposed start codon (Fig. 1B). Two DNA sequence motifs, 5′-TTGATCTGGATCAAA-3′ and 5′-TTGATAGCCATCAAAG-3′, located 96 and 128 bp upstream of the ORF (Fig. 1B) strongly resemble (identical nucleotides are underlined) the consensus rhizobial FnrN- and FixK-binding site or anaerobox (TTGA-C--GATCAA-G) (15), suggesting that there is (auto)regulation by an Fnr-like protein. When this fnrN gene was used as a probe, no other signal was detected by Southern blot hybridization with genomic DNA.
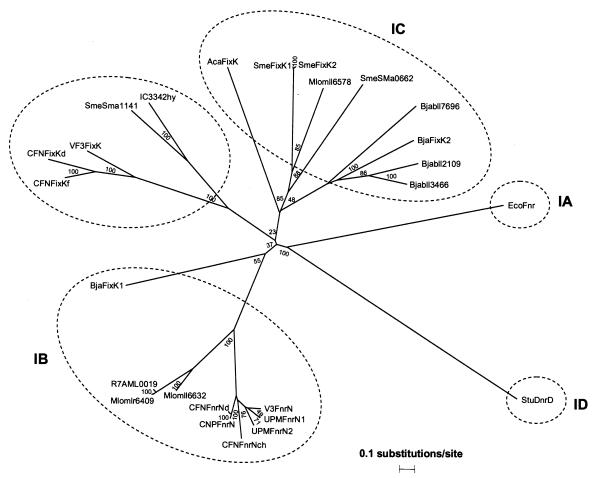
An alignment of rhizobial FnrN and FixK proteins was constructed (proteins were selected by using a threshold value of 30% amino acid identity with FnrN of R. etli CNPAF512). Based on this alignment, a phylogenetic tree was constructed (Fig. 2). The phylogenetic relationship illustrates that there are different groups of rhizobial FnrN and FixK proteins. The known FnrN proteins, together with FixK1 of Bradyrhizobium japonicum and several database entries for Mesorhizobium loti strain MAFF303099 and strain R7A, contain the cysteine signature, Cys-X2-Cys-X2-R-X4-Cys-X87-Cys-X-F, defined for class IB Fnr-related proteins (15) (data not shown) and form a cluster that is distinct from the more divergent group of FixK proteins. The FixK proteins are divided into two separated clusters, which are distinct from E. coli Fnr (a member of class IA of the Crp-Fnr family) and Pseudomonas stutzeri DnrD (a member of class ID) (Fig. 2). This tripartite division of the rhizobial FnrN and FixK proteins was confirmed by constructing a parsimonious tree (data not shown).
FIG. 2.
Phylogenetic relationships of rhizobial FnrN and FixK proteins. The following proteins were included in the analysis: R. etli CNPAF512 FnrN (CNPFnrN); R. etli CFN42 FnrNd (CFNFnrNd) (accession number gi12083693) (31); R. leguminosarum bv. viciae strain VF39 FnrN (VF3FnrN) (accession number gi120623) (6); R. leguminosarum bv. viciae UPM791 FnrN1 (UPMFnrN1) (accession number gi619722) (23); R. leguminosarum bv. viciae UPM791 FnrN2 (UPMFnrN2) (accession number gi2114416 (22); R. etli CFN42 FnrNchr (CFNFnrNch) (accession number gi3462873) (31); B. japonicum FixK1 (BjaFixK1) (accession number gi95165) (1); M. loti strain MAFF303099 mll6632 (Mlomll6632) (accession number gi13475537) (28); A. caulinodans FixK (AcaFixK) (gi38702) (27); B. japonicum FixK2 (BjaFixK2) (gi3021322) (37); S. meliloti FixK1 (SmeFixK1) (gi14523782) (3); S. meliloti FixK2 (SmeFixK2) (gi14523500) (2); R. etli CFN42 FixKf (CFNFixKf) (gi9857982) (21); R. etli CFN42 FixKd (CFNFixKd) (gi1679718) (47); R. leguminosarum bv. viciae VF39 FixK (VF3FixK) (gi1240048) (39); Rhizobium strain IC3342 orf4 (IC3342hy) (gi152262) (54); mlr6409 (Mlomlr6409) (gi13475362), mml6632 (Mlomll6632) (gi13475537), and mll6578 (Mlomll6578) (gi14026245) from M. loti strain MAFF303099 (28); M. loti strain R7A ML0019 (R7AML0019) (gi20803846) (51); SMa0662 (SmeSMa0662) (gi16262801) and Sma1141 (SmeSMa1141) (gi16263070) from S. meliloti (2); and bll7696 (Bjabll7696) (gi27382807), bll3466 (Bjabll3466) (gi27378577), and bll2109 (Bjabll2109) (gi27350363) from B. japonicum (29). Details of the construction are described in Materials and Methods. E. coli Fnr (EcoFnr) (gi26108071) and Pseudomonas stutzeri DnrD (StuDnrD) (gi4585795) were included to represent members of classes IA and IB, respectively, of the Crp-Fnr family. The numbers at the nodes indicate the percentages of trees in 1,000 bootstrap analyses that supported the topology.
Expression of R. etli fnrN in S. meliloti.
To investigate whether the cloned fnrN gene codes for an active protein, this gene was transferred into S. meliloti strain GMI347-CS112 (6). This S. meliloti fixJ mutant is unable to induce a chromosomally integrated, fixK-dependent fixN-lacZ fusion. Table 2 shows that the fixN-lacZ fusion in GMI347-CS112 was highly activated under microaerobic conditions (0.3% oxygen) in the presence of either pFAJ1172 or pFAJ1175. No expression was observed under aerobic conditions. These results indicate that the R. etli fnrN gene is actively expressed in S. meliloti and can functionally substitute for the fixK gene in expression of fixN. Since GMI347-CS112 is fixJ, expression of R. etli fnrN in S. meliloti occurs independent of the FixLJ system.
TABLE 2.
Expression of S. meliloti fixN-lacZ in S. meliloti and R. etli
| Straina | Plasmid | β-Glucuronidase activity (Miller units)b
|
|
|---|---|---|---|
| Aerobic conditions | Microaerobic conditions | ||
| GMI347-CS112 | 30 (3) | 11 (9) | |
| GMI347-CS112 | pFAJ1172 | 25 (3) | 969 (151) |
| GMI347-CS112 | pFAJ1175 | 21 (9) | 407 (76) |
| CNPAF512 | pGMI931 | 27 (4) | 1,033 (36) |
| FAJ1182 | pGMI931 | 29 (6) | 23 (3) |
| FAJ1183 | pGMI931 | 22 (2) | 8 (2) |
The S. meliloti strain used was GMI347-CS112 (fixJ mutant, chromosomal fixN-lacZ), and the R. etli strains used were CNPAF512 (wild type), FAJ1182, and FAJ1183 (fnrN mutant).
The values in parentheses are standard deviations. The data are the means of at least four replicates, and the results were confirmed independently in separate experiments.
Phenotypes of R. etli fnrN mutants.
To investigate fnrN function, R. etli fnrN mutant strains (FAJ1182 and FAJ1183) were constructed by site-specific mutagenesis (see Materials and Methods). The symbiotic phenotypes of these mutants were determined (Table 3). No effect of the mutation on nodule number was observed when the data were compared with data for the wild-type strain. Nitrogen fixation was expressed in terms of acetylene reduction activity. The acetylene reduction activities of both fnrN mutants were approximately 80% lower than the activity of the wild type.
TABLE 3.
Nitrogenase activities and nodulation of the R. etli wild type and fnrN mutants
| Strain | Relevant genotype | Acetylene reduction activity (μmol of ethylene/plant/h)a | No. of nodules/planta |
|---|---|---|---|
| CNPAF512 | Wild type | 9.5 (2.8) | 199 (31) |
| FAJ1182 | fnrN | 2 (1.0) | 204 (50) |
| FAJ1183 | fnrN | 2.3 (1.0) | NDb |
The data are the means of at least 10 replicates, and the results were confirmed independently in separate experiments. The values in parentheses are standard deviations.
ND, not determined.
Expression analysis of R. etli fnrN.
To study the regulation of R. etli fnrN, a translational fnrN-gusA fusion (pFAJ1178) was constructed (see Materials and Methods). This fusion was introduced into the R. etli wild type and into the following regulatory mutants: Rp1000 (nifA), FAJ1154 and FAJ1169 (rpoN), FAJ1182 and FAJ1183 (fnrN), RpFAJ1002 (fixL), and RpFAJ1004 (fixJ). Expression of the fusion was assayed under aerobic, microaerobic, and symbiotic conditions (Table 4). Expression of R. etli fnrN was induced during microaerobic growth and in bacteroids. The level of expression was reduced to aerobic background levels in both fnrN mutants, while it reached wild-type levels in R. etli nifA, rpoN, fixL, and fixJ mutants. The observed autoregulation is in agreement with the presence of two putative FnrN-binding sites in the fnrN promoter region. The R. etli FnrN protein is probably oxygen sensitive (see above), which could explain why positive autoregulation occurred only in microoxic environments.
TABLE 4.
Expression of R. etli fnrN-gusA (pFAJ1178) in R. etli wild-type and mutant backgroundsa
| Strain | Relevant genotype | β-Glucuronidase activity (Miller units)a
|
||
|---|---|---|---|---|
| Aerobic conditions | Microaerobic conditions | Symbiotic conditions | ||
| CNPAF512 | Wild type | 79 (33) | 474 (175) | 1,025 (169) |
| Rp1000 | nifA | 51 (29) | 380 (71) | 1,033 (99) |
| FAJ1182 | fnrN | 40 (6) | 59 (17) | 82 (12) |
| FAJ1183 | fnrN | 23 (5) | 66 (12) | 50 (22) |
| FAJ1154 | rpoN1 | 81 (20) | 267 (67) | 1,039 (145) |
| FAJ1169 | rpoN2 | 56 (38) | 451 (104) | 968 (131) |
| RpFAJ1002 | fixL | 51 (29) | 470 (108) | 1,008 (194) |
| RpFAJ1004 | fixJ | 60 (24) | 453 (131) | NDb |
The symbiotic expression data are the means of at least 10 replicates, and the results were confirmed independently in separate experiments. The free-living expression data are the means of at least four replicates. The values in parentheses are standard deviations.
ND, not determined.
Isolation and phenotypes of R. etli fixG, fixO, and fixI mutants.
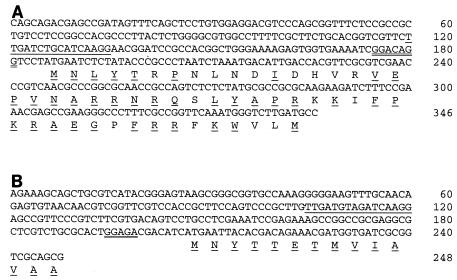
In a search for genes activated by low oxygen tension, three symbiotic mutants (CMPG8007, CMPG8169, and CMPG8170) were identified in an independent analysis based on screening of an R. etli CNPAF512::mTn5gusA library (60, 61; Moris, unpublished results). The partial DNA sequences of the inactivated genes of these mutants were determined as described in Materials and Methods. From the analysis of these partial sequences, the identities of the genes could be determined. The transposon insertion of mutant strain CMPG8169 was localized after codon 53 of the fixG gene. The sequence of the promoter and part of the fixG gene is shown in Fig. 3A. A sequence resembling an anaerobox was found 50 bp upstream from the presumptive ATG start codon. The presence of this sequence suggests that there was regulation by an Fnr-like protein. In mutant CMPG8170, the transposon was inserted after codon 377 of the fixI gene (the codon numbers are the Rhizobium leguminosarum bv. viciae fixI codon numbers). Analysis of the partial DNA sequence flanking the transposon in mutant CMPG8007 revealed that it was inserted after codon 23 of fixO. Expression of the R. etli fixG::gusA, fixI::gusA, and fixO::gusA fusions was clearly induced during symbiosis (Table 5). All three mutants displayed reduced acetylene reduction activity (27 to 95% reductions compared to the wild-type acetylene reduction activity) (60; Moris unpublished results). In order to test directly the fnrN-dependent expression of these genes, plasmid-borne fixN-gusA and fixG-gusA fusions were constructed.
FIG. 3.
DNA sequences of the promoter regions and 5′ ends of the fixG (A) and fixN (B) genes of R. etli CNPAF512. Putative anaeroboxes and ribosome-binding sites are underlined once and twice, respectively. The deduced amino acid sequence is indicated below the DNA sequence. Amino acids conserved in the R. leguminosarum bv. viciae FixG and FixN sequences are underlined.
TABLE 5.
Expression of fixG::gusA, fixI::gusA, and fixO::gusA in R. etlia
| Strain | Relevant genotype | β-Glucuronidase activity (Miller units)b
|
||
|---|---|---|---|---|
| Aerobic conditions | Microaerobic conditions | Symbiotic conditions | ||
| CMPG8169 | fixG | 35 (1) | 106 (7) | 182 (57) |
| CMPG8170 | fixI | 0 (0) | 56 (22) | 4,729 (1,058) |
| CMPG8007 | fixO | 27 (0.4) | 1,285 (186) | 1,931 (269) |
Cultures were grown in acid minimal salts medium supplemented with 10 mM succinate and 10 mM NH4Cl.
See Table 4, footnote a.
Regulation of R. etli fixN, fixG, and arcA expression.
As fixG is generally linked with fixNOQP genes in rhizobia, a search for a cosmid clone carrying this region was performed (as described in Materials and Methods). The presence of the fixN and fixG genes was confirmed by partial sequence analysis of fragments of the identified cosmids. This region was shown to be located close to the previously described nifHDK3 gene located on a 4.2-kb EcoRI fragment (33). Analysis of the DNA sequence of the fixN promoter revealed the presence of an anaerobox 86 bp upstream from the ATG start codon (Fig. 3B). To study the regulation of expression of fixN and fixG, transcriptional gusA fusions with the promoters of both genes were constructed. The pFAJ1192 (fixG-gusA) and pFAJ1193 (fixN-gusA) fusions were introduced into the R. etli wild-type strain and into regulatory mutants, and expression of the fusions was analyzed under free-living aerobic and microaerobic conditions and during symbiosis (Table 6). Both fixN and fixG were induced under microaerobic and symbiotic conditions compared to the expression under aerobic conditions. A similar expression pattern was observed for R. etli mutant strains CMPG8169, CMPG8170, and CMPG8007 carrying fixG-gusA, fixI-gusA, and fixO-gusA insertions (Table 5). Clear differences in the extent of induction and the effect of mutations in regulatory genes on fixN and fixG expression were noticed (Table 6). During symbiosis, expression of pFAJ1192 was upregulated in rpoN, nifA, and fixL mutants compared to the wild-type expression, indicating that there was symbiosis-specific regulation. On the other hand, the β-glucuronidase activity of pFAJ1193 was lower in these mutants than in the wild-type strain during symbiosis. The β-glucuronidase activity of both fusions was reduced to aerobic background levels in the R. etli fnrN strain under all conditions tested (free-living microaerobic and symbiotic conditions). Based on these data, distinct and complex regulation of fixG and fixN expression during symbiosis is hypothesized. Similarly, expression of the S. meliloti fixN-lacZ fusion plasmid pGMI931 in R. etli was induced only under low oxygen tension (Table 2), and expression of this plasmid did not occur in the R. etli fnrN mutant.
TABLE 6.
Expression of R. etli fixG-gusA (pFAJ1192), fixN-gusA (pFAJ1193), arcA-gusA (pFAJ1319), rpoN2-gusA (pFAJ1175), and orf180-gusA (pFAJ1176) in R. etli wild-type and mutant backgrounds
| Straina | Plasmid | β-Glucuronidase activity (Miller units)b
|
||
|---|---|---|---|---|
| Aerobic conditions | Microaerobic conditions | Symbiotic conditions | ||
| CNPAF512 (wild type) | pFAJ1192 (fixG-gusA) | 31 (1) | 324 (42) | 134 (25) |
| FAJ1170 (rpoN1 rpoN2) | pFAJ1192 | 39 (1) | 345 (56) | 1,318 (190) |
| RP1000 (nifA) | pFAJ1192 | 15 (6) | 358 (38) | 979 (103) |
| FAJ1183 (fnrN) | pFAJ1192 | 43 (12) | 38 (15) | 27 (3) |
| RpFAJ1002 (fixL) | pFAJ1192 | 61 (12) | 263 (52) | 453 (152) |
| CNPAF512 (wild type) | pFAJ1193 (fixN-gusA) | 6 (1) | 16 (4) | 1,219 (153) |
| FAJ1170 (rpoN1 rpoN2) | pFAJ1193 | 6 (1) | 16 (2) | 604 (148) |
| RP1000 (nifA) | pFAJ1193 | 7 (2) | 22 (5) | 644 (70) |
| FAJ1183 (fnrN) | pFAJ1193 | 7 (1) | 8 (2) | 20 (14) |
| RpFAJ1002 (fixL) | pFAJ1193 | 8 (2) | 29 (4) | 403 (75) |
| CNPAF512 (wild type) | pFAJ1319 (arcA-gusA) | 0 (0) | 39 (6) | NDc |
| FAJ1182 (fnrN) | pFAJ1319 | 0 (0) | 0 (1) | ND |
| FAJ1183 (fnrN) | pFAJ1319 | 0 (0) | 0 (1) | ND |
| RpFAJ1002 (fixL) | pFAJ1319 | 0 (0) | 27 (6) | ND |
| CNPAF512 (wild type) | pFAJ1175 (rpoN2-gusA) | ND | 390 (49) | ND |
| FAJ1183 (fnrN) | pFAJ1175 | ND | 467 (56) | ND |
| FAJ1170 (rpoN1 rpoN2) | pFAJ1175 | ND | 7 (3) | ND |
| CNPAF512 (wild type) | pFAJ1176 (orf180-gusA) | ND | 49 (7) | ND |
| FAJ1183 (fnrN) | pFAJ1176 | ND | 52 (11) | ND |
| FAJ1170 (rpoN1 rpoN2) | pFAJ1176 | ND | 6 (3) | ND |
The arginine deiminase pathway in R. etli which is active in nitrogen-fixing bacteroids (11) is encoded by the arcABC genes, which are located 1 kb upstream from the R. etli fixLJ genes. Approximately 700 bp upstream from the R. etli arcA gene, we identified a sequence motif (TTGATCCGGCTCAATG) with strong similarity to an anaerobox (nucleotides conserved in the consensus are underlined) (15), suggesting that there was regulation by an Fnr-like protein, as it is the case in Pseudomonas aeruginosa (18). We tested whether inactivation of the R. etli fnrN gene affects expression of the R. etli arcA-gusA fusion plasmid pFAJ1319 (Table 6). The arcA-gusA fusion was induced under microaerobic conditions, and this induction was strictly dependent on the presence of a functional FnrN protein.
Finally, we tested whether a mutation in the fnrN gene affects expression of the rpoN2-gusA fusion pFAJ1175 and the orf180-gusA fusion pFAJ1176 (34). Microaerobic activation of these fusions depends on the presence of functional NifA and RpoN proteins. Expression of these genes was not reduced in the R. etli fnrN mutant (Table 6).
DISCUSSION
The primary structure of R. etli CNPAF512 FnrN reveals that there is a conserved cysteine cluster at the N terminus (15, 57) which is also present in other proteins encoded by fnrN-like genes belonging to class IB (data not shown). These proteins form a separate cluster, as revealed by the phylogenetic analysis of various rhizobial FnrN and FixK sequences (Fig. 2). The phylogenetic analysis also revealed a division of the FixK proteins into two distinct groups (Fig. 2). The FixK proteins of S. meliloti, A. caulinodans, and B. japonicum (FixK2) belong to the same cluster. This group of proteins was previously called class IC of the Crp-Fnr family (15). Null mutations in the fixK genes of these rhizobia eliminate nitrogen fixation (3, 15, 27). This cluster also includes homologous FixK proteins of M. loti, B. japonicum, and S. meliloti. In addition, there is a distinct group containing FixK proteins of R. leguminosarum bv. viciae VF39, R. etli CFN42, Rhizobium sp. strain IC3342, and S. meliloti (Fig. 2). In contrast to the symbiotic phenotype of S. meliloti, A. caulinodans, and B. japonicum fixK mutants, fixKd and fixKf mutants of R. etli CFN42 and fixK mutants of R. leguminosarum bv. viciae do not exhibit a severe reduction in nitrogenase activity (21, 39).
The phylogenetic tree shows that the sequence of R. etli CNPAF512 FnrN is most closely related to the sequence of FnrNd of R. etli CFN42. However, there are significant functional differences between these proteins. Besides the occurrence of a second copy of the fnrN gene (fnrNchr) in R. etli CFN42 (together with duplication of fixK genes), the methods of regulation differ (see below) (31). Knocking out the R. etli CNPAF512 fnrN gene causes a severe symbiotic defect beginning at the start of nitrogen fixation, whereas the CFN42 fnrNd gene has a role in the late stages of the symbiosis. Loss of the R. etli CNPAF512 FnrN protein in the bacteroids results in an almost 80% decrease in the nitrogen fixation activity compared to the wild-type nitrogen fixation activity. In addition, the fnrN gene is highly induced in bacteroids. These results indicate that R. etli CNPAF512 FnrN plays a key role during symbiosis with common bean plants.
The methods of regulation of the fnrN genes belonging to class IB are different in the different rhizobia. The expression of B. japonicum fixK1 is indirectly dependent on FixLJ through FixK2 (37). In R. etli CFN42, two copies of the fnrN genes (fnrNchr and fnrNd) were identified. Expression of these genes is differentially controlled by an unusual fixL gene under free-living microaerobic conditions without participation of a fixJ gene. fnrNd is positively regulated by FixL through FixKf. On the other hand, FixL negatively regulates fnrNchr independent of FixKf (31). In contrast to these observations, the free-living microaerobic and symbiotic induction of R. etli CNPAF512 fnrN was shown to be independent of fixLJ. Also, in R. leguminosarum bv. viciae VF39, fnrN is not controlled by FixK, but it has been proposed that FixK and FnrN act in parallel (39). In R. leguminosarum bv. viciae UPM791 no evidence for the presence of fixLJ orthologs has been found (22).
In S. meliloti and A. caulinodans no fnrN genes belonging to class IB were identified. The A. caulinodans and S. meliloti fixK genes are both regulated by FixLJ (3, 27). In addition, S. meliloti FixK induces the expression of fixT, whose product negatively regulates fixK expression by counteracting FixLJ activities (16, 19).
In microoxic conditions, R. etli CNPAF512 fnrN is autoactivated, resulting in a self-amplifying cascade. This autoregulation is consistent with the presence of anaeroboxes in the promoter region of fnrN. Positive autoregulation of fnrN also occurs in R. leguminosarum bv. viciae VF39 fnrN (4, 45). Likewise, in bacteroids of R. leguminosarum bv. viciae UPM791, autoregulation of fnrN1 and fnrN2 expression was observed. Analysis of the promoter region of the two fnrN genes revealed the presence of two anaeroboxes. Differential binding of FnrN1 on the anaeroboxes in the fnrN1 promoter has been reported, resulting in both positive and negative autoregulation (5). Furthermore, in the promoters of the R. etli CFN42 fnrN genes two putative Fnr-binding sites are present, and complex regulatory interactions between the two fnrN genes have been observed (31). In contrast, no autoregulation of B. japonicum FixK1 was observed (1).
Three target genes of FnrN have been identified in R. etli CNPAF512: fixN, which is part of the fixNOQP operon coding for a cbb3-type cytochrome oxidase (41); fixG, which is part of the fixGHIS operon and in B. japonicum is involved in the assembly and stability of the FixNOQP complex (42); and arcA, which encodes a protein with a function in the arginine deiminase pathway in R. etli (11). R. etli CNPAF512 FnrN is an essential positive regulator of fixG and fixN under free-living microaerobic conditions and in bacteroids and induces arcA under free-living microaerobic conditions. In contrast to the microaerobic regulation of fixG and fixN expression, the symbiotic expression of these genes is subject to an additional level of regulation. Besides the strict dependence on FnrN, symbiotic expression of these genes also involves fine-tuning by RpoN, NifA, and FixL (Table 6). Additional symbiotic regulatory mechanisms were also observed previously for the regulation of rpoN2 expression, and it was proposed that these mechanisms include an unknown symbiosis-specific mechanism (34). Positive regulation of fixN by FnrN is also observed in other rhizobia. FnrN (together with FixL) from R. leguminosarum bv. viciae VF39 induces fixNc and fixNd under free-living microaerobic conditions (44). The microaerobic expression of R. etli CFN42 fixNd is mainly activated by FnrNchr and FixL-FixKf (21, 31). In R. leguminosarum bv. viciae UPM791 no genes homologous to fixLJ or fixK were found, but fixN is controlled by the two fnrN genes under microoxic conditions (22). In contrast, in B. japonicum the fixNOQP operon is regulated by FixK2 (and consequently also by FixJ) but not by FixK1 (37, 41).
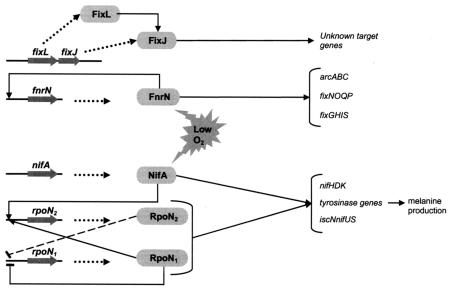
Taken together, the results presented here show that besides the previously identified regulatory cascades of nitrogen fixation genes controlled by NifA and FixL (10, 33), a third independent symbiotic regulator, FnrN, is operational in R. etli CNPAF512. This protein is involved in sensing a low-oxygen signal and in transducing the signal into a regulation cascade of a specific subset of nitrogen fixation genes (Fig. 4).
FIG. 4.
Schematic overview of the free-living microaerobic regulation of nif, fix, and other symbiotically important genes of R. etli CNPAF512. Only strictly dependent relationships are indicated. rpoN1 is essential during free-living growth, while rpoN2 is required for symbiosis. Transcription of rpoN2 is highly induced under free-living microaerobic conditions by RpoN1 and NifA. Expression of rpoN1 is negatively autoregulated (indicated by a blocked line); in addition, negative regulation of RpoN2 for rpoN1 expression is hypothesized (indicated by a blocked dashed line) (34). NifA and RpoN are strictly required for expression of iscNnifUS (13), for expression of nifHDK, and for the production of melanine (34). The target genes of FixLJ are unknown. FixLJ contributes to the regulation of nifH, but because of the strict dependence of nifH transcription on NifA and RpoN, it has been suggested that the regulation of FixLJ is indirect (10). The target genes of FnrN represented in this scheme were identified in this study.
Acknowledgments
We thank J. Batut and U. Priefer for providing plasmid pGMI931 and S. meliloti strain GMI347-CS112.
This work was supported by grants from the Research Council of Katholieke Universiteit Leuven (grant GOA/2003/09) and from the Fund for Scientific Research—Flanders (grant G.0108.01).
REFERENCES
- 1.Anthamatten, D., B. Scherb, and H. Hennecke. 1992. Characterization of a fixLJ-regulated Bradyrhizobium japonicum gene sharing similarity with the Escherichia coli fnr and Rhizobium meliloti fixK genes. J. Bacteriol. 174:2111-2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barnett, M. J., R. F. Fisher, T. Jones, C. Komp, A. P. Abola, F. Barloy-Hubler, L. Bowser, D. Capela, F. Galibert, J. Gouzy, M. Gurjal, A. Hong, L. Huizar, R. W. Hyman, D. Kahn, M. L. Kahn, S. Kalman, D. H. Keating, C. Palm, M. C. Peck, R. Surzycki, D. H. Wells, K. C. Yeh, R. W. Davis, N. A. Federspiel, and S. R. Long. 2001. Nucleotide sequence and predicted functions of the entire Sinorhizobium meliloti pSymA megaplasmid. Proc. Natl. Acad. Sci. 98:9883-9888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Batut, J., M. L. Daveran-Mingot, M. D. J. Jacobs, A. M. Garnerone, and D. Kahn. 1989. FixK, a gene homologous with fnr and crp from Escherichial coli, regulates nitrogen-fixation genes both positively and negatively in Rhizobium meliloti. EMBO J. 8:1279-1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clark, S. R. D., I. J. Oresnik, and M. F. Hynes. 2001. RpoN of Rhizobium leguminosarum bv. viciae strain VF39SM plays a central role in FnrN-dependent microaerobic regulation of genes involved in nitrogen fixation. Mol. Gen. Genet. 264:623-633. [DOI] [PubMed] [Google Scholar]
- 5.Colombo, M. V., D. Gutierrez, J. M. Palacios, J. Imperial, and T. Ruiz-Argueso. 2000. A novel autoregulation mechanism of fnrN expression in Rhizobium leguminosarum bv viciae. Mol. Microbiol. 36:477-486. [DOI] [PubMed] [Google Scholar]
- 6.Colonna-Romano, S., W. Arnold, A. Schluter, P. Boistard, A. Puhler, and U. B. Priefer. 1990. An Fnr-like protein encoded in Rhizobium leguminosarum biovar viciae shows structural and functional homology to Rhizobium meliloti FixK. Mol. Gen. Genet. 223:138-147. [DOI] [PubMed] [Google Scholar]
- 7.David, M., M. L. Daveran, J. Batut, A. Dedieu, O. Domergue, J. Ghai, C. Hertig, P. Boistard, and D. Kahn. 1988. Cascade regulation of nif gene expression in Rhizobium meliloti. Cell 54:671-683. [DOI] [PubMed] [Google Scholar]
- 8.de Philip, P., J. Batut, and P. Boistard. 1990. Rhizobium meliloti Fix L is an oxygen sensor and regulates R. meliloti nifA and fixK genes differently in Escherichia coli. J. Bacteriol. 172:4255-4262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.D'hooghe, I., J. Michiels, and J. Vanderleyden. 1998. The Rhizobium etli FixL protein differs in structure from other known FixL proteins. Mol. Gen. Genet. 257:576-580. [DOI] [PubMed] [Google Scholar]
- 10.D'hooghe, I., J. Michiels, K. Vlassak, C. Verreth, F. Waelkens, and J. Vanderleyden. 1995. Structural and functional analysis of the fixLJ genes of Rhizobium leguminosarum biovar phaseoli CNPAF512. Mol. Gen. Genet. 249:117-126. [DOI] [PubMed] [Google Scholar]
- 11.D'hooghe, I., C. Vander Wauven, J. Michiels, C. Tricot, P. de Wilde, J. Vanderleyden, and V. Stalon. 1997. The arginine deiminase pathway in Rhizobium etli: DNA sequence analysis and functional study of the arcABC genes. J. Bacteriol. 179:7403-7409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dombrecht, B., K. Marchal, J. Vanderleyden, and J. Michiels. 26 November 2002, posting date. Prediction and overview of the RpoN-regulon in closely related species of the Rhizobiales. Genome Biol. 3:research0076.1-0076.11. [Online.] http://genomebiology.com. [DOI] [PMC free article] [PubMed]
- 13.Dombrecht, B., M. Z. Tesfay, C. Verreth, C. Heusdens, M. C. Napoles, J. Vanderleyden, and J. Michiels. 2002. The Rhizobium etli gene iscN is highly expressed in bacteroids and required for nitrogen fixation. Mol. Genet. Genomics 267:820-828. [DOI] [PubMed] [Google Scholar]
- 14.Fellay, R., J. Frey, and H. Krisch. 1987. Interposon mutagenesis of soil and water bacteria: a family of DNA fragments designed for in vitro insertional mutagenesis of gram-negative bacteria. Gene 52:147-154. [DOI] [PubMed] [Google Scholar]
- 15.Fischer, H. M. 1994. Genetic regulation of nitrogen fixation in rhizobia. Microbiol. Rev. 58:352-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Foussard, M., A. M. Garnerone, F. Ni, E. Soupene, P. Boistard, and J. Batut. 1997. Negative autoregulation of the Rhizobium meliloti fixK gene is indirect and requires a newly identified regulator, FixT. Mol. Microbiol. 25:27-37. [DOI] [PubMed] [Google Scholar]
- 17.Friedman, A. M., S. R. Long, S. E. Brown, W. J. Buikema, and F. M. Ausubel. 1982. Construction of a broad host range cosmid cloning vector and its use in the genetic analysis of Rhizobium mutants. Gene 18:289-296. [DOI] [PubMed] [Google Scholar]
- 18.Galimand, M., M. Gamper, A. Zimmermann, and D. Haas. 1991. Positive Fnr-like control of anaerobic arginine degradation and nitrate respiration in Pseudomonas aeruginosa. J. Bacteriol. 173:1598-1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garnerone, A. M., D. Cabanes, M. Foussard, P. Boistard, and J. Batut. 1999. Inhibition of the FixL sensor kinase by the FixT protein in Sinorhizobium meliloti. J. Biol. Chem. 274:32500-32506. [DOI] [PubMed] [Google Scholar]
- 20.Gilles-Gonzalez, M. A., G. S. Ditta, and D. R. Helinski. 1991. A haemoprotein with kinase activity encoded by the oxygen sensor of Rhizobium meliloti. Nature 350:170-172. [DOI] [PubMed] [Google Scholar]
- 21.Girard, L., S. Brom, A. Dávalos, O. Lopez, M. Soberón, and D. Romero. 2000. Differential regulation of fixN-reiterated genes in Rhizobium etli by a novel fixL-fixK cascade. Mol. Plant-Microbe Interact. 13:1283-1292. [DOI] [PubMed] [Google Scholar]
- 22.Gutierrez, D., Y. Hernando, J. M. Palacios, J. Imperial, and T. Ruiz-Argueso. 1997. FnrN controls symbiotic nitrogen fixation and hydrogenase activities in Rhizobium leguminosarum biovar viciae UPM791. J. Bacteriol. 179:5264-5270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hernando, Y., J. M. Palacios, J. Imperial, and T. Ruiz-Argueso. 1995. The hypBFCDE operon from Rhizobium leguminosarum biovar viciae is expressed from an Fnr type promoter that escapes mutagenesis of the fnrN gene. J. Bacteriol. 177:5661-5669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Herrero, M., V. de Lorenzo, and K. N. Timmis. 1990. Transposon vectors containing non-antibiotic resistance selection markers for cloning and stable chromosomal insertion of foreign genes in gram-negative bacteria. J. Bacteriol. 172:6557-6567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hertig, C., R. Y. Li, A. M. Louarn, A. M. Garnerone, M. David, J. Batut, D. Kahn, and P. Boistard. 1989. Rhizobium meliloti regulatory gene fixJ activates transcription of R. meliloti nifA and fixK genes in Escherichia coli. J. Bacteriol. 171:1736-1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaminski, P. A., and C. Elmerich. 1998. The control of Azorhizobium caulinodans nifA expression by oxygen, ammonia and by the HF-I-like protein, NrfA. Mol. Microbiol. 28:603-613. [DOI] [PubMed] [Google Scholar]
- 27.Kaminski, P. A., K. Mandon, F. Arigoni, N. Desnoues, and C. Elmerich. 1991. Regulation of nitrogen fixation in Azorhizobium caulinodans: identification of a fixK like gene, a positive regulator of nifA. Mol. Microbiol. 5:1983-1991. [DOI] [PubMed] [Google Scholar]
- 28.Kaneko, T., Y. Nakamura, S. Sato, E. Asamizu, T. Kato, S. Sasamoto, A. Watanabe, K. Idesawa, A. Ishikawa, K. Kawashima, T. Kimura, Y. Kishida, C. Kiyokawa, M. Kohara, M. Matsumoto, A. Matsuno, Y. Mochizuki, S. Nakayama, N. Nakazaki, S. Shimpo, M. Sugimoto, C. Takeuchi, M. Yamada, and S. Tabata. 2000. Complete genome structure of the nitrogen-fixing symbiotic bacterium Mesorhizobium loti. DNA Res. 7:331-338. [DOI] [PubMed] [Google Scholar]
- 29.Kaneko, T., Y. Nakamura, S. Sato, K. Minamisawa, T. Uchiumi, S. Sasamoto, A. Watanabe, K. Idesawa, M. Iriguchi, K. Kawashima, M. Kohara, M. Matsumoto, S. Shimpo, H. Tsuruoka, T. Wada, M. Yamada, and S. Tabata. 2002. Complete genomic sequence of nitrogen-fixing symbiotic bacterium Bradyrhizobium japonicum USDA110. DNA Res. 9:189-197. [DOI] [PubMed] [Google Scholar]
- 30.Kiley, P. J., and H. Beinert. 1998. Oxygen sensing by the global regulator, FNR: the role of the iron-sulfur cluster. FEMS Microbiol. Rev. 22:341-352. [DOI] [PubMed] [Google Scholar]
- 31.Lopez, O., C. Morera, J. Miranda-Rios, L. Girard, D. Romero, and M. Soberón. 2001. Regulation of gene expression in response to oxygen in Rhizobium etli: role of FnrN in fixNOQP expression and in symbiotic nitrogen fixation. J. Bacteriol. 183:6999-7006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Metcalf, W. W., and B. L. Wanner. 1993. Evidence for a fourteen-gene, phnC to phnP locus for phosphonate metabolism in Escherichia coli. Gene 129:27-32. [DOI] [PubMed] [Google Scholar]
- 33.Michiels, J., I. D'hooghe, C. Verreth, H. Pelemans, and J. Vanderleyden. 1994. Characterization of the Rhizobium leguminosarum biovar phaseoli nifA gene, a positive regulator of nif gene expression. Arch. Microbiol. 161:404-408. [DOI] [PubMed] [Google Scholar]
- 34.Michiels, J., M. Moris, B. Dombrecht, C. Verreth, and J. Vanderleyden. 1998. Differential regulation of Rhizobium etli rpoN2 gene expression during symbiosis and free-living growth. J. Bacteriol. 180:3620-3628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Michiels, J., T. Van Soom, I. D'hooghe, B. Dombrecht, T. Benhassine, P. de Wilde, and J. Vanderleyden. 1998. The Rhizobium etli rpoN locus: DNA sequence analysis and phenotypical characterization of rpoN, ptsN, and ptsA mutants. J. Bacteriol. 180:1729-1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 37.Nellen-Anthamatten, D., P. Rossi, O. Preisig, I. Kullik, M. Babst, H. M. Fischer, and H. Hennecke. 1998. Bradyrhizobium japonicum FixK2, a crucial distributor in the FixLJ-dependent regulatory cascade for control of genes inducible by low oxygen levels. J. Bacteriol. 180:5251-5255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Norrander, J., T. Kempe, and J. Messing. 1983. Construction of improved M13 vectors using oligodeoxynucleotide-directed mutagenesis. Gene 26:101-106. [DOI] [PubMed] [Google Scholar]
- 39.Patschkowski, T., A. Schluter, and U. B. Priefer. 1996. Rhizobium leguminosarum bv viciae contains a second fnr/fixK-like gene and an unusual fixL homologue. Mol. Microbiol. 21:267-280. [DOI] [PubMed] [Google Scholar]
- 40.Poole, P. S., N. A. Schofield, C. J. Reid, E. M. Drew, and D. L. Walshaw. 1994. Identification of chromosomal genes located downstream of dctD that affect the requirement for calcium and the lipopolysaccharide layer of Rhizobium leguminosarum. Microbiology 140:2797-2809. [DOI] [PubMed] [Google Scholar]
- 41.Preisig, O., D. Anthamatten, and H. Hennecke. 1993. Genes for a microaerobically induced oxidase complex in Bradyrhizobium japonicum are essential for a nitrogen-fixing endosymbiosis. Proc. Natl. Acad. Sci. 90:3309-3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Preisig, O., R. Zufferey, and H. Hennecke. 1996. The Bradyrhizobium japonicum fixGHIS genes are required for the formation of the high-affinity cbb(3)-type cytochrome oxidase. Arch. Microbiol. 165:297-305. [DOI] [PubMed] [Google Scholar]
- 43.Quandt, J., and M. F. Hynes. 1993. Versatile suicide vectors which allow direct selection for gene replacement in Gram-negative bacteria. Gene 127:15-21. [DOI] [PubMed] [Google Scholar]
- 44.Schluter, A., T. Patschkowski, J. Quandt, L. B. Selinger, S. Weidner, M. Kramer, L. Zhou, M. F. Hynes, and U. B. Priefer. 1997. Functional and regulatory analysis of the two copies of the fixNOQP operon of Rhizobium leguminosarum strain VF39. Mol. Plant-Microbe Interact. 10:605-616. [DOI] [PubMed] [Google Scholar]
- 45.Schluter, A., T. Patschkowski, G. Unden, and U. B. Priefer. 1992. The Rhizobium leguminosarum FnrN protein is functionally similar to Eschericha coli FNR and promotes heterologous oxygen dependent activation of transcription. Mol. Microbiol. 6:3395-3404. [DOI] [PubMed] [Google Scholar]
- 46.Snoeck, C., C. Verreth, I. Hernandez-Lucas, E. Martinez-Romero, and J. Vanderleyden. 2003. Identification of a third sulfate activation system in Sinorhizobium sp. strain BR816: the CysDN sulfate activation complex. Appl. Environ. Microbiol. 69:2006-2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Soberón, M., O. Lopez, C. Morera, M. L. Girard, M. L. Tabche, and J. Miranda. 1999. Enhanced nitrogen fixation in a Rhizobium etli ntrC mutant that overproduces the Bradyrhizobium japonicum symbiotic terminal oxidase cbb3. Appl. Environ. Microbiol. 65:2015-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Soupène, E., M. Foussard, P. Boistard, G. Truchet, and J. Batut. 1995. Oxygen as a key developmental regulator of Rhizobium meliloti N2 fixation gene expression within the alfalfa root nodule. Proc. Natl. Acad. Sci. 92:3759-3763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Spiro, S. 1994. The FNR family of transcriptional regulators. Antonie Leeuwenhoek 66:23-36. [DOI] [PubMed] [Google Scholar]
- 50.Staskawicz, B., D. Dahlbeck, N. Keen, and C. Napoli. 1987. Molecular characterization of cloned avirulence genes from race 0 and race 1 of Pseudomonas syringae pv. glycinea. J. Bacteriol. 169:5789-5794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sullivan, J. T., J. R. Trzebiatowski, R. W. Cruickshank, J. Gouzy, S. D. Brown, R. M. Elliot, D. J. Fleetwood, N. G. McCallum, U. Rossbach, G. S. Stuart, J. E. Weaver, R. J. Webby, F. J. De Bruijn, and C. W. Ronson. 2002. Comparative sequence analysis of the symbiosis island of Mesorhizobium loti strain R7A. J. Bacteriol. 184:3086-3095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Unden, G., and J. Schirawski. 1997. The oxygen-responsive transcriptional regulator FNR of Escherichia coli: the search for signals and reactions. Mol. Microbiol. 25:205-210. [DOI] [PubMed] [Google Scholar]
- 54.Upadhyaya, N. M., K. F. Scott, W. T. Tucker, J. M. Watson, and P. J. Dart. 1992. Isolation and characterization of Rhizobium (IC3342) genes that determine leaf curl induction in pigeon pea. Mol. Plant-Microbe Interact. 5:129-143. [DOI] [PubMed] [Google Scholar]
- 55.Vande Broek, A., M. Lambrecht, K. Eggermont, and J. Vanderleyden. 1999. Auxins upregulate expression of the indole-3-pyruvate decarboxylase gene in Azospirillum brasilense. J. Bacteriol. 181:1338-1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Van de Peer, Y., and R. De Wachter. 1994. TREECON for Windows: a software package for the construction and drawing of evolutionary trees for the Microsoft Windows environment. Comput. Applic. Biosci. 10:569-570. [DOI] [PubMed] [Google Scholar]
- 57.Van Spanning, R. J., A. P. De Boer, W. N. Reijnders, H. V. Westerhoff, A. H. Stouthamer, and Van Der Oost J. 1997. FnrP and NNR of Paracoccus denitrificans are both members of the FNR family of transcriptional activators but have distinct roles in respiratory adaptation in response to oxygen limitation. Mol. Microbiol. 23:893-907. [DOI] [PubMed] [Google Scholar]
- 58.Vincent, J. M. 1970. A manual for the practical study of root-nodule bacteria. Blackwell Scientific Publications, Ltd., Oxford, United Kingdom.
- 59.Vollack, K. U., E. Hartig, H. Korner, and W. G. Zumft. 1999. Multiple transcription factors of the FNR family in denitrifying Pseudomonas stutzeri: characterization of four fnr-like genes, regulatory responses and cognate metabolic processes. Mol. Microbiol. 31:1681-1694. [DOI] [PubMed] [Google Scholar]
- 60.Xi, C., G. Dirix, J. Hofkens, F. C. Schryver, J. Vanderleyden, and J. Michiels. 2001. Use of dual marker transposons to identify new symbiosis genes in Rhizobium. Microb. Ecol. 41:325-332. [DOI] [PubMed] [Google Scholar]
- 61.Xi, C., M. Lambrecht, J. Vanderleyden, and J. Michiels. 1999. Bi-functional gfp- and gusA-containing mini-Tn5 transposon derivatives for combined gene expression and bacterial localization studies. J. Microbiol. Methods 35:85-92. [DOI] [PubMed] [Google Scholar]