Abstract
Context:
Polycystic ovarian syndrome (PCOS), the most common endocrinopathy of women in the reproductive age group seems to be adversely affected by associated thyroid dysfunction. Both pose independent risks of ovarian failure and pregnancy related complications.
Aims:
The present study from Eastern India is, therefore, aimed to investigate the prevalence and etiology of different thyroid disorders in PCOS subjects.
Settings and Design:
Cross-sectional hospital based survey-single centre observational case-control study.
Materials and Methods:
This prospective single-center study recruited 106 female patients with hypertrichosis and menstrual abnormality among which 80 patients were defined as having PCOS according to the revised 2003 Rotterdam criteria and comprised the study population. Another 80 age-matched female subjects were studied as the control population. Thyroid function and morphology were evaluated by measurement of serum thyroid stimulating hormone (TSH), free thyroxine levels (free T3 and free T4), anti-thyroperoxidase antibody (anti-TPO Ab), clinical examination and ultrasound (USG) of thyroid gland.
Statistical Analysis Used:
It was done by Student's t-test and Chi-square test using appropriate software (SPSS version 19).
Results:
This case-control study revealed statistically significant higher prevalence of autoimmune thyroiditis, detected in 18 patients (22.5% vs. 1.25% of control) as evidenced by raised anti-TPO antibody levels (means 28.037 ± 9.138 and 25.72 ± 8.27 respectively; P = 0.035). PCOS patients were found to have higher mean TSH level than that of the control group (4.547 ± 2.66 and 2.67 ± 3.11 respectively; P value < 0.05). There was high prevalence of goiter among PCOS patients (27.5% vs. 7.5% of control, P value > 0.001). On thyroid USG a significantly higher percentage of PCOS patients (12.5%; controls 2.5%) had hypoechoic USG pattern also compatible with the diagnosis of autoimmune thyroiditis.
Conclusions:
High prevalence of thyroid disorders in PCOS patients thus points towards the importance of early correction of hypothyroidism in the management of infertility associated with PCOS.
Keywords: Anti-thyroperoxidase antibody, autoimmune thyroiditis, hypothyroidism, polycystic ovarian syndrome
INTRODUCTION
Polycystic ovarian syndrome (PCOS) is the most common form of chronic anovulation associated with androgen excess; perhaps occurring in 5-10% of reproductive women.[1] PCOS is viewed as a heterogeneous disorder of multifactorial etiology. It is also associated with increased metabolic and cardiovascular risk factors.[2] These risks are linked to insulin resistance and compounded by the common occurrence of obesity, although insulin resistance is also present in non-obese woman with PCOS. During the reproductive years, PCOS is associated with important reproductive morbidity including infertility, irregular uterine bleeding and increased pregnancy loss.
Dysfunction and anatomic abnormalities of the thyroid are among the most common diseases of the endocrine gland. Abnormalities in the supply of thyroid hormone to the peripheral tissue are associated with alteration in a number of metabolic processes. Early stages of thyroid dysfunction (before symptoms are obvious) can lead to subtle change in ovulation and endometrial receptivity, which may have profound effect on fertility. Infantile hypothyroidism if untreated, leads to sexual immaturity. Untreated juvenile hypothyroidism causes a delay in the onset of puberty followed by anovulatory cycles. In adult woman, severe hypothyroidism may be associated with diminished libido and failure of ovulation. Primary ovarian failure can also be seen in patients with Hashimoto's thyroiditis as a part of autoimmune polyglandular syndrome. Rarely, in primary hypothyroidism, secondary depression of pituitary function may lead to ovarian atrophy and amenorrhoea. Pregnancy complications are associated with overt and subclinical hypothyroidism, although the impact has varied among different studies.
So, it is evident that both the conditions have profound effect on fertility and reproductive biology. More interestingly hypothyroidism can initiate, maintain or worsen the syndrome. Hence, in the past few years different studies from various parts of the world regarding thyroid disorders in PCOS patient, have tried to explore the PCOS-thyroid interface. Mostly the results showed higher incidence of elevated TSH levels and four times higher prevalence of autoimmune thyroiditis in PCOS subjects.[3] Again, routine screening for thyroid dysfunction in hyperandrogenic patient is of little value since the incidence of these disorders is not higher in hyperandrogenic patients than in normal women of child bearing age.[4] With this background, the present study has been contemplated to investigate the prevalence and etiology of different thyroid disorders in PCOS patients attending a tertiary care hospital.
MATERIALS AND METHODS
Every consecutive female patient in the age group of 13-45 years with hypertrichosis and menstrual abnormality (amenorrhoea or oligomenorrhoea) visiting the outpatient departments of General Medicine and Endocrinology between 2010 and 2012 was screened for this study. The Rotterdam classification was used to define PCOS in the event of: (1) menstrual anomalies like amenorrhoea (no cycles in the past 6 months), oligomenorrhoea (cycles lasting longer than 35 days), or long cycles, (2) clinical and/or biochemical hyperandrogenism, (3) ultrasound (USG) appearance of polycystic ovaries (multiple cysts >12 in number of 2-9 mm size). The presence of two of these three criteria was required to define PCOS once all other diagnosis, like congenital adrenal hyperplasia, virilising tumor, cushing syndrome and prolactinoma was ruled out.[5] Clinical hyperandrogenism was defined as hypertrichosis (Ferriman–Gallwey score >7) and/or acne, and/or androgenic pattern of alopecia.[6,7] Biochemical hyperandrogenemia was defined by elevated testosterone. A luteinizing hormone (LH)-to-follicle stimulating hormone (FSH) ratio above 2 was considered elevated. Transabdominal pelvic USG was performed to detect the presence of cystic ovaries. Other reasons for hyperandrogenism were excluded by adrenocorticotropin-stimulated 17-OH progesterone, dexamethasone suppression test and/or 24-h urine cortisol excretion. Women of the same age group visiting the outpatient clinics with problems unrelated to PCOS or thyroid dysfunction and with normal menses served as control population.
Detailed clinical history, elaborate clinical examination and Laboratory investigations like Blood glucose (fasting and 2 h post 75 g glucose), serum LH, FSH, thyroid stimulating hormone (TSH), free thyroxine levels (free T3 and free T4), anti-thyroperoxidase antibody (anti-TPO Ab), free testosterone measured by an automated immune-enzyme assay systems were performed in both PCOS and control population.
Normal serum levels of different hormones and peptide were defined as (1) freeT3-2.4-4.2 pg/ml, (2) free T4-0.7-1.24 ng/dl, (3) TSH-0.34-4.25 mIU/ml, (4) anti-TPO taken antibody as <35 IU/ml, (5) prolactin-1.9-25 ng/ml, (6) dehydroepiandrosterone-sulphate-12-535 mcg/dl, (7) 17-OH progesterone-15-70 ng/dl, (8) free testosterone-3-19 pg/ml. LH and FSH were measured on the 2nd day of menstruation.
USG of the thyroid was done in presence of goiter. USG thyroid was performed using a 7.5 MHz transducer with duplex sonography. The thyroid was considered hypoechoic when its signal was equal or below the echogenicity of the surrounding neck muscles. Fine needle aspiration cytology (FNAC) of thyroid nodule was done in nodular goiter. Statistical analysis was done by Student's t-test and Chi-square test using appropriate software (SPSS version 19).
RESULTS
The present study was conducted among 80 patients of PCOS in the age group of 13-45 years. A total of 106 patients with hypertrichosis and menstrual abnormalities were screened. PCOS was diagnosed in 80 patients according to Rotterdam classification of PCOS and rest were eventually excluded from this study. Several clinical characteristics and laboratory parameters were compared among these study population and a control group of age matched healthy women emphasizing mainly on thyroid related investigations [Table 1, Figure 1]. In this study population maximum numbers of PCOS patients were in the age group of 15-20 years (32 patients; 40%) followed by 26-30 years (24 patients; 30%). Clinical Hirsuitism as per modified Ferriman–Gallwey score (>7) was present in 58 patients (72.5%; mean F-G score 18.65 ± 8.91) of PCOS while only 12 (15%; mean F-G score 9.57 ± 4.41) of control were hirsuit [Table 1, Figure 2]. 62 patients (77.5%) had oligomenorrhoea and 12 patients (15%) had amenorrhoea. Raised serum free testosterone level was detected in 60 patients (75%). 44 patients (55%) had elevated LH-to-FSH ratio above 2.0. 44 patients (55%) had BMI of more than 25, 64 patients (80%) had normal blood pressure, 14 patients (17.5%) had prehypertension and only 2 patients (2.5%) were hypertensive. Estimation of glycemic status of PCOS patients showed impaired fasting glucose (IFG) in 12 patients (15%), impaired glucose tolerance (IGT) in 22 patients (27.5%) and rest had normal status.
Table 1.
Statistical values of different variables and their correlation among polycystic ovarian syndrome subjects and controls
Figure 1.
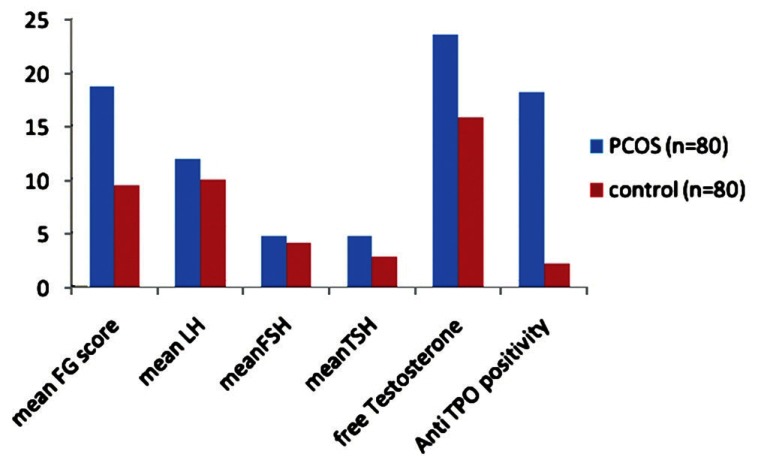
Bar diagram showing the difference in different laboratory parameters analyzed in polycystic ovarian syndrome patients and control group. Mean thyroid stimulating hormone and anti-thyroperoxidase levels were significantly elevated in the PCOS patients as compared to controls
Figure 2.
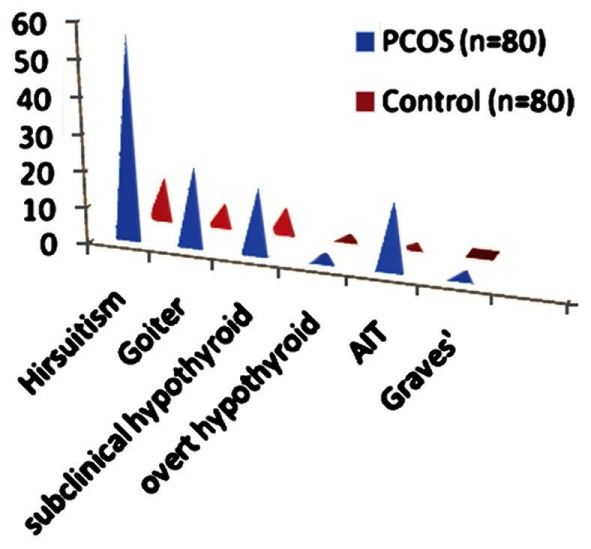
Higher prevalence of hirsuitism, goiter, hypothyroidism and autoimmune thyroiditis in polycystic ovarian syndrome subjects than in the control group
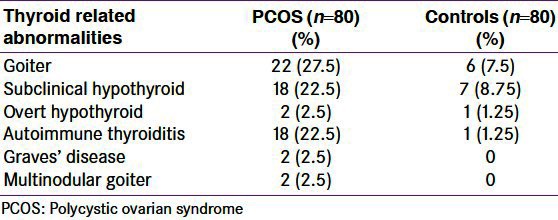
Biochemically thyroid disorders were detected in 22 (27.5%) out of 80 patients with PCOS as compared to only 9 of control population (11.25%; P < 0.05) [Table 2, Figure 2]. Subclinical hypothyroidism was detected in 18 patients (22.5%; 8.75% of control), 2 patients had clinically overt hypothyroidism (2.5%), and autoimmune thyroiditis was detected in 18 patients (22.5% vs. 1.25% of control) as evidenced by raised anti-TPO antibody levels (means 28.037 ± 9.138 and 25.72 ± 8.27 respectively; P = 0.035). PCOS patients higher mean TSH level than control group (4.547 ± 2.66 and 2.67 ± 3.11 respectively; P < 0.05). There was high prevalence of goiter among PCOS patients (27.5% vs. 7.5% of control, P < 0.001). Only 2 patients but no control had features of Graves’ disease and another 2 patients had multinodular goiter where FNAC was suggestive of hypercellularity with colloid deposition. On thyroid USG a significantly higher percentage of PCOS patients (12.5%; controls 2.5%) had hypoechoic USG pattern compatible with the diagnosis of autoimmune thyroiditis.
Table 2.
Prevalence of different thyroid disorders in polycystic ovarian syndrome subjects and control group showing statistically significant higher rate of thyroid abnormalities among polycystic ovarian syndrome group, specially of autoimmune thyroiditis
DISCUSSION
In this study the age group of PCOS is maximum in 15-20 years and has decreased significantly after 30 years. This may be due to the fact that, menstrual symptoms begin from puberty itself which leads to early presentation in the OPD. In this study clinical hirsutism as detected by modified F.G. score was present in 72.5% of the patient. Raised serum free testosterone was detected in 75% patients. This finding also correlates with other studies stating that hirsutism affects 65-75% of white, black and south-east Asian woman.[8,9] Also hypertrichosis was present in 91% of 175 PCOS subjects and elevated testosterone was present in 69% of 175 patients as observed by Jassen, et al.[3] Najem, et al. in their study among 318 PCOS patients detected hirsutism among 91% patients.[10] In study by Marco c Amato, et al. done over 130 PCOS patients, 57% patients were observed to have hirsutism, 61% of the patients had elevated free testosterone.[11]
In this study patient having symptoms of menstrual disturbance in form of oligomenorrhoea or amenorrhoea was 92.5%. This is also reflected in other studies which report 60-85% patient suffering from gross menstrual dysfunction.[8] 93% PCOS patients had oligomenorrhoea or amenorrhoea as observed by Najem, et al.[10]
Obesity was detected in 55% patient of our study, which is also similar to other studies which states prevalence of obesity is approx 50%; in a Delhi based study with 33 PCOS subjects, obesity was observed in 46%. 57% prevalence of obesity among 318 PCOS patients as observed by Najem, et al., 54% overweight in study of Gomathi, et al.[10] However, obesity varies significantly with country of origin.
Glucose intolerance in this study was reflected as IFG in 15% and 27.5% had impaired postprandial glucose. This is also reflected in other studies where using 2 h glucose tolerance test with insulin 10% non-obese and 40-50% obese woman with PCOS had IGT.[9] Other studies show-impaired glucose tolerance and type 2 diabetes mellitus (T2DM) were present in 40% of PCOS; Huang Jia, et al. detected impaired glucose tolerance in 10.2% patients; Shaheen Ara Anwary, et al. observed raised (7.8 mmol/dl) blood sugar (2 h post 75 g glucose) in 30% PCOS and in study of Lergo, et al., IGT was detected in 31%, while T2DM was detected in 7.5%.[12,13] In study by Marco c Amato, et al. done over 130 PCOS patients, 12% had IFG and T2DM; Najem, et al. detected diabetes among 9% of 318 patients.[10,11]
In the present study, 17.5% patients were pre-hypertensive, 2.5% patients were detected to have hypertension. Other studies done previously also detected prevalence of raised blood pressure-Prevalence of hypertension was 4% in study conducted by Najem, et al. and pre-hypertension was detected in 8% as detected by Huang Jia, et al.[10,12] In study of Azevedo, et al., 18.6% patients were detected to have BP >130/85.[14] In study of Christiano, the prevalence of hypertension as a whole was 20.3% (n = 14).[15] Four patients showed isolated systolic hypertension and four showed isolated diastolic hypertension, while the other six showed both systolic and diastolic hypertension. The prevalence was higher among overweight and obese patients.
In the present study, 55% PCOS subjects had elevated level of LH/FSH (>2) while, 45% patients had LH/FSH <2. This has been also seen in other studies-Shaheen Ara Anwary, et al. found raised LH (>14) in 56% patients, Banaszewaska, et al. found raised LH/FSH ratio in 45.4% of their patients and Anlakesh, et al. detected a prevalence of raised LH/FSH in 64% of their 107 PCOS patients.[16,17] Three patients in the present study had oligomenorrhoea, hyperandrogenism but no polycystic ovary. These patients were also considered as having PCOS without morphology of polycystic ovary in the present study. In study conducted by Najem, et al. 74% had USG features of polycystic ovaries while Shaheen Ara Anwary, et al. found that 100% patients had polycystic ovaries.[10]
In this study goiter was present in 27.5% patient, subclinical hypothyroidism was present in 22.5% and clinical hypothyroidism was present in 2.5% cases. Among these hypothyroid patients, autoimmune hypothyroidism was present in 22.5% patient. Thus this prospective case-control revealed significant higher prevalence of thyroid disorders among young PCOS patients compared to age matched controls. These findings are very close to the study done by Janssen, et al. where they observed a prevalence of autoimmune thyroiditis (biochemically) in 26.9% of their 175 patients.[3] That was one of the first prospective multicenter studies done by Janssen, et al. from Germany on this very issue. Some other studies reported even higher prevalence of autoimmune thyroiditis in PCOS subjects. Didem Ozdemir, et al. among 107 patients found a prevalence of 30.5% Thyroid nodules were detected in 29 (27.1%) patients, 10 had solitary and 19 had multiple nodules. Thyroid pathologies were observed in half of the patients with PCOS.[18] Among the most recently held studies, Kachuei, et al. from Iran has also shown significantly higher prevalence of autoimmune thyroiditis and goiter in PCOS patients than that in control subjects (goiter 62.3% vs. 35.7%, P = 0.0001). The mean ± SD of serum anti-TPO Ab in PCOS patients and subjects in the control group was 216 ± 428 and 131 ± 364 IU/mL, respectively (P = 0.04) but serum levels of TSH and anti-Tg (thyroglobulin) antibody in both the groups were not much different.[19]
Lastly, review of Indian literature explores Ghosh, et al. who tried to evaluate the role of hypothyroidism in the causation of PCOS. Their comparative analysis suggested that hypothyroidism led to lowering of sex hormone binding globulin level and increment of testosterone level but not invariably directed towards estriol overproduction.[20] Two years later Wakim, et al. in their research on human reproductive biology also reestablished the hypothesis that hypothyroidism worsens PCOS by further decreasing sex hormone binding globulin levels, increasing the conversion of androstenedione to testosterone and aromatization to estradiol and reducing the metabolic clearance rates of androstenedione and estrone. Since thyroid hormones are involved in the gonadotropin induced estradiol and progesterone secretion by human granulosa cells, hypothyroidism would interfere with ovarian function and fertility.[21] Sridhar, et al. conducted a 30 months-duration study from Visakhapatnam to show how hypothyroidism was related to PCOS. Two women of primary hypothyroidism (2/13; 1.04%) presented with features of PCOS.[22] They opined that hypothyroid could initiate, maintain or worsen the syndrome and thereby correcting hypothyroid state, PCOS could be managed in a better way. This hypothesis seems to hold true even now but it lacks appropriate evidence based systematic analysis to establish the necessity of routine screening of all the PCOS patients for thyroid function and thyroid-specific autoantibodies even without clinical evidence of overt thyroid disorders because patients with anti-TPO and anti Tg autoantibodies are much likely to develop thyroid dysfunction later in the later life.
ACKNOWLEDGMENT
We are indebted to every member of the Department of Medicine, NRSMCH, Kolkata for their heartfelt support.
Footnotes
Source of Support: Nil
Conflict of Interest: No
REFERENCES
- 1.Dunaif A. Insulin resistance and the polycystic ovary syndrome: Mechanism and implications for pathogenesis. Endocr Rev. 1997;18:774–800. doi: 10.1210/edrv.18.6.0318. [DOI] [PubMed] [Google Scholar]
- 2.Legro RS, Kunselman AR, Dodson WC, Dunaif A. Prevalence and predictors of risk for type 2 diabetes mellitus and impaired glucose tolerance in polycystic ovary syndrome: A prospective, controlled study in 254 affected women. J Clin Endocrinol Metab. 1999;84:165–9. doi: 10.1210/jcem.84.1.5393. [DOI] [PubMed] [Google Scholar]
- 3.Janssen OE, Mehlmauer N, Hahn S, Offner AH, Gärtner R. High prevalence of autoimmune thyroiditis in patients with polycystic ovary syndrome. Eur J Endocrinol. 2004;150:363–9. doi: 10.1530/eje.0.1500363. [DOI] [PubMed] [Google Scholar]
- 4.Balen AH, Anderson RA. Policy & Practice Committee of the BFS. Impact of obesity on female reproductive health: British Fertility Society, Policy and Practice Guidelines. Hum Fertil (Camb) 2007;10:195–206. doi: 10.1080/14647270701731290. [DOI] [PubMed] [Google Scholar]
- 5.Dewailly D, Hieronimus S, Mirakian P, Hugues JN. Polycystic ovary syndrome (PCOS) Ann Endocrinol (Paris) 2010;71:8–13. doi: 10.1016/j.ando.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 6.Ferriman D, Gallwey JD. Clinical assessment of body hair growth in women. J Clin Endocrinol Metab. 1961;21:1440–7. doi: 10.1210/jcem-21-11-1440. [DOI] [PubMed] [Google Scholar]
- 7.Carmina E, Lobo RA. A comparison of the relative efficacy of antiandrogens for the treatment of acne in hyperandrogenic women. Clin Endocrinol (Oxf) 2002;57:231–4. doi: 10.1046/j.1365-2265.2002.01594.x. [DOI] [PubMed] [Google Scholar]
- 8.Carmina E, Lobo RA. Treatment of hyperandrogenic alopecia in women. Fertil Steril. 2003;79:91–5. doi: 10.1016/s0015-0282(02)04551-x. [DOI] [PubMed] [Google Scholar]
- 9.Azziz R, Carmina E, Dewailly D, Diamanti-Kandarakis E, Escobar-Morreale HF, Futterweit W, et al. The Androgen Excess and PCOS Society criteria for the polycystic ovary syndrome: The complete task force report. Fertil Steril. 2009;91:456–88. doi: 10.1016/j.fertnstert.2008.06.035. [DOI] [PubMed] [Google Scholar]
- 10.Najem F, Elmehdawi R, Swalem A. Clinical and Biochemical Characteristics of Polycystic Ovary Syndrome in Benghazi-Libya; A Retrospective study. Libyan J Med. 2008;3:71–4. doi: 10.4176/080122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amato MC, Galluzzo A, Merlino S, Mattina A, Richiusa P, Criscimanna A, et al. Lower insulin sensitivity differentiates hirsute from non-hirsute Sicilian women with polycystic ovary syndrome. Eur J Endocrinol. 2006;155:859–65. doi: 10.1530/eje.1.02290. [DOI] [PubMed] [Google Scholar]
- 12.Huang J, Ni R, Chen X, Huang L, Mo Y, Yang D. Metabolic abnormalities in adolescents with polycystic ovary syndrome in south China. Reprod Biol Endocrinol. 2010;17:8–14. doi: 10.1186/1477-7827-8-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Legro RS, Kunselman AR, Dodson WC, Dunaif A. Prevalence and predictors of risk for type 2 diabetes mellitus and impaired glucose tolerance in polycystic ovary syndrome: A prospective, controlled study in 254 affected women. J Clin Endocrinol Metab. 1999;84:165–9. doi: 10.1210/jcem.84.1.5393. [DOI] [PubMed] [Google Scholar]
- 14.Azevedo MF, Costa EC, Oliveira AI, Silva IB, Marinho JC, Rodrigues JA, et al. Elevated blood pressure in women with polycystic ovary syndrome: Prevalence and associated risk factors. Rev Bras Ginecol Obstet. 2011;33:31–6. [PubMed] [Google Scholar]
- 15.Barcellos CR, Rocha MP, Hayashida SA, Mion Junior D, Lage SG, Marcondes JA. Impact of body mass index on blood pressure levels in patients with polycystic ovary syndrome. Arq Bras Endocrinol Metabol. 2007;51:1104–9. doi: 10.1590/s0004-27302007000700013. [DOI] [PubMed] [Google Scholar]
- 16.Banaszewska B, Spaczyński RZ, Pelesz M, Pawelczyk L. Incidence of elevated LH/FSH ratio in polycystic ovary syndrome women with normo-and hyperinsulinemia. Rocz Akad Med Bialymst. 2003;48:131–4. [PubMed] [Google Scholar]
- 17.Anlakash AH. Polycystic ovarian syndrome-the correlation between LH/FSH ratio and disease manifestation. Middle East Fertil Soc Jl. 2007;12:35–40. [Google Scholar]
- 18.Kachuei M, Jafari F, Kachuei A, Keshteli AH. Prevalence of autoimmune thyroiditis in patients with polycystic ovary syndrome. Arch Gynecol Obstet. 2012;285:853–6. doi: 10.1007/s00404-011-2040-5. [DOI] [PubMed] [Google Scholar]
- 19.Ozdemir D, Cuhaci N, Balkan F, Usluogullari A, Ersoy R, Cakir B. Prevalence of thyroid pathologies in patients with polycystic ovary syndrome. Eur Cong Endocrinol. 2011;26:92. [Google Scholar]
- 20.Ghosh S, Kabir SN, Pakrashi A, Chatterjee S, Chakravarty B. Subclinical hypothyroidism: A determinant of polycystic ovary syndrome. Horm Res. 1993;39:61–6. doi: 10.1159/000182697. [DOI] [PubMed] [Google Scholar]
- 21.Wakim AN, Polizotto SL, Burholt DR. Augmentation by thyroxine of human granulosa cell gonadotrophin-induced steroidogenesis. Hum Reprod. 1995;10:2845–8. doi: 10.1093/oxfordjournals.humrep.a135805. [DOI] [PubMed] [Google Scholar]
- 22.Sridhar GR, Nagamani G. Hypothyroidism presenting with polycystic ovary syndrome. J Assoc Physicians India. 1993;41:88–90. [PubMed] [Google Scholar]


