Abstract
Antithyroid medications are one of the treatment options for Graves’ disease. Carbimazole is widely used as the drug of choice, except in pregnancy, where propythiouracil is preferred by many. It is generally well-tolerated. Its side-effects include allergy, upper gastrointestinal upset, a rare occurrence of granulocytosis, and others. Hepatitis is another rare, but serious side-effect. We report a healthy 30-year-old male patient with Graves’ disease, who developed cholestatic jaundice after Carbimazole therapy for four months. He made a full recovery after the drug was discontinued. An idiosyncratic mechanism seemed likely.
Keywords: Carbimazole, cholestatic jaundice, Graves’ disease, thyrotoxicosis
INTRODUCTION
Graves’ disease is the most common cause of hyperthyroidism. It accounts for approximately 80 – 90% of the cases. Treatment options include radioiodine, antithyroid medications, and surgery. The former is now considered the treatment of choice by most endocrinologists. Antithyroid medications are still widely used in younger patients with mild disease, and pregnant or lactating patients. It is usually continued for about 12 to 24 months. The chance of remission is around 40-50%. It has several side effects; the majority are mild and reversible such as allergic reaction, and upper GI disturbances. Other rare side effects include agranulocytosis- and vasculitis-like reaction, particularly with propylthiouracil. Hepatic toxicity is a rare, but serious side effect with both methimazole (and its pro-drug, carbimazole) and propylthiouracil (PTU).[1,2,3] Fatal cases have been documented with both drugs.[4] The hepatic histopathological findings with PTU are toxic hepatitis with necrosis,[5] whereas, they resemble cholestatic hepatitis with methimazole (and carbimazole).[6] We present a case of carbimazole-induced cholestatic hepatitis in a patient with Graves’ disease. We describe the clinical and biochemical findings in this patient and review the relevant literature.
CASE REPORT
A 30-year-old male patient presented to the outpatient clinic of the Endocrinology Department with chief complaints of a 6 kg weight loss over the last four months and bilateral prominent and bulging eyes. In addition he gave a history of palpitations and tremors. He also had complaints of frequent loose motions and heat intolerance. He was a known case of rheumatic heart disease with mitral stenosis under treatment with salt restriction, digoxin, and β blockers. In the last four months he was admitted thrice, with episodes of atrial fibrillation and congestive heart failure. He had no history of liver disease. He was neither a smoker nor an alcohol consumer.
On physical examination, he had fine tremors of both hands. His weight was 49 kg. Pulse rate was 120 beats / minute, regular, and blood pressure was 150 / 70 mmHg in the right arm supine position. Eye examination showed bilateral exophthalmos, with conjunctivitis and periorbital edema. There was diplopia on looking to both sides, indicating ophthalmoplegia amounting to stage IV NOSPECS classification. Hands were warm and moist. Thyroid was soft and diffusely enlarged with grade 3 goiter and audible bruit. The liver and spleen were not palpable. Cardiovascular system examination revealed loud first heart sound and mid-diastolic murmur, with delayed peaking at the mitral area. There was no evidence of pulmonary arterial hypertension or congestive heart failure at presentation. The other systemic examination, including per abdomen examination, was unremarkable.
His initial T4 was 22.4 μg / dl and TSH < 0.01 uIU / ml. He was diagnosed with Graves’ disease and started on propranolol with prednisolone for Graves’ ophthalmopathy. Carbimazole was also initiated at a dose of 30 mg per day, in two divided doses. He neither did a thyroid scan nor took I131 because of concern with iodine allergy and exacerbation of the eye manifestations. Three weeks later, FT4 was normal and TSH was < 0.03 uIU / ml. He complained of nausea and vomiting, and the accompanying attendants complained of yellowish discoloration of his eyes. Results of the liver function tests were as shown in Table 1. The laboratory findings were consistent with cholestatic jaundice. He had marked elevation of bilirubin and alkaline phosphatase. Aspartate and alanine aminotransferase were less markedly elevated. Prothrombin time was within normal limits. The test for antinuclear antibodies was negative. Abdominal ultrasound showed normal liver size and texture. The intrahepatic biliary ducts were normal and no obstruction was detected. Hepatitis serology was negative for hepatitis A, B, C, and E. As per the gastroenterologist's advice, a liver biopsy was done, which revealed cholestatic hepatitis with a paucity of bile ducts [Figure 1]. Carbimazole was stopped. He received radioactive iodine under the cover of prednisolone, to minimize the risk of eye sign deterioration. His liver functions normalized within two months. He developed post-radioiodine hypothyroidism and was started on thyroxine therapy. Upon re-evaluation after four and six months his liver functions were still normal.
Table 1.
Date wise liver function test results of the patient.
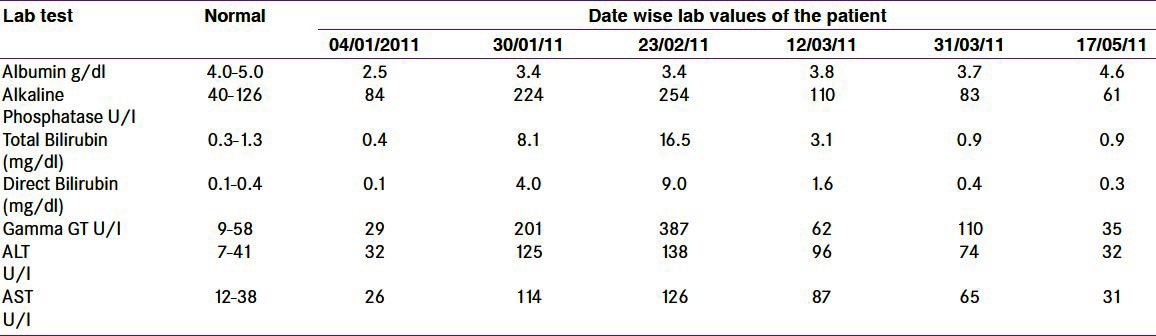
Figure 1.
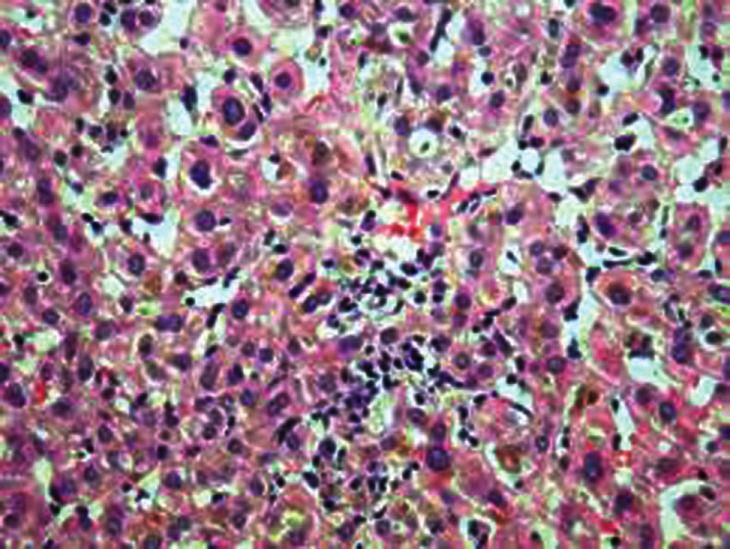
Liver biopsy revealing cholestatic hepatitis with paucity of bile ducts
DISCUSSION
Hyperthyroidism per se can affect the liver function tests. It causes mild elevation in the liver enzymes that normalizes with treatment.[7] In addition, cholestatic jaundice can occur solely due to severe hyperthyroidism.[8] All antithyroid drugs (methimazole, carbimazole, and propylthiouracil) can affect the liver on rare occasions. A cohort study on 50 patients by Liaw found that subclinical liver injury is common during PTU treatment.[5] Thirty percent of the patients had a transient, asymptomatic rise in the aminotransferase level, with no elevation in bilirubin after two months of therapy. Despite continuation of PTU, the liver enzymes normalized in most patients within five months. He was suggested cautious continuation of PTU in the absence of symptoms and hyperbilirubinemia. Despite this, PTU-induced liver disease can be severe and fatal.[9,10] Carbimazole and methimazole, on the other hand have been typically associated with cholestatic jaundice (mainly hyperbilirubinemia) without evidence of hepatic necrosis on liver biopsy.[2,6,11] Most patients recover on drug discontinuation. Nevertheless, there are occasional reports of severe and fatal cases with methimazole-induced liver disease.[12] A review of the literature has revealed around 22 cases of cholestatic jaundice due to both methimazole and carbimazole.[11] The mean time of onset after starting treatment is 36 days.[1] The majority of patients are female, reflecting the predominance of thyrotoxicosis in females. The proposed mechanism of carbimazole-induced cholestasis is not fully understood, but it is thought to be an allergic reaction.[13] A cross-reaction between PTU and carbimazole is still a concern, when a switch from one to another is considered, in spite of some reports to the contrary.[6] The hepatotoxic effect is dose-independent in carbimazole.[14]
Our patient developed significant hyperbilirubinemia within four weeks of starting carbimazole, despite normalization of the thyroxin level. It continued to rise for one more week after stopping the offending drug, until complete normalization over two months. The aminotransferase level was mildly elevated, reaching two-to-three times the normal.
Although the patient received steroids from the start for reasons discussed earlier, it did not affect the course of the liver disease. In fact, previous reports of using steroids in methimazole-induced hepatitis did not show any benefit.[15] This coincided with the fact that most patients recovered once the drug was stopped. Our patient showed a good response to drug withdrawal. He received radioiodine with no significant ocular complications.
CONCLUSION
Hepatic toxicity is a rare, but serious side effect of antithyroid medications. Doctors dealing with thyroid patients should be aware of such complications. Routine liver function tests during therapy are not cost-effective, but must be performed when this complication is suspected. The drug must be withdrawn immediately and alternative therapy for hyperthyroidism, such as radioiodine, must be considered.
ACKNOWLEDGEMENTS
All the authors would extend their heartfelt thanks to Dr Jagadeesh Tangudu, M tech, MS, PhD and Mrs Sowmya Jammula, M Tech, for their immense and selfless contribution towards manuscript preparation, language editing and final approval of text.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Woeber KA. Methimazole- induced hepatotoxicity. Endocr Pract. 2002;8:222–4. doi: 10.4158/EP.8.3.222. [DOI] [PubMed] [Google Scholar]
- 2.Ozenne G, Manchon ND, Doucet J, Memet J, Schrub JC, Bercoff E. Carbimazole -induced acute cholestatic hepatitis. J Clin Gastroenterol. 1989;11:95–7. doi: 10.1097/00004836-198902000-00025. [DOI] [PubMed] [Google Scholar]
- 3.Westphal SA. Hepatotoxicity from propylthiouracil. South Med J. 1994;87:943–7. doi: 10.1097/00007611-199409000-00018. [DOI] [PubMed] [Google Scholar]
- 4.Williams KV, Nayak S, Becker D, Reyes J, Burmeister LA. Fifty years of experience with propylthiouracil-associated hepatotoxicity: What have we learned? J Clin Endocrinol Metab. 1997;82:1727–33. doi: 10.1210/jcem.82.6.4011. [DOI] [PubMed] [Google Scholar]
- 5.Liaw YF, Huang MJ, Fan KD, Li KL, Wu SS, Chen TJ. Hepatic injury during propylthiouracil therapy in patients with hyperthyroidism. A Cohort Study. Ann Intern Med. 1993;118:424–8. doi: 10.7326/0003-4819-118-6-199303150-00005. [DOI] [PubMed] [Google Scholar]
- 6.Arab DM, Malatjalian DA, Rittmaster RS. Severe cholestatic jaundice in uncomplicated hyperthyroidism treated with methimazole. J Clin Endocrinol Metab. 1995;80:1083–5. doi: 10.1210/jcem.80.4.7714072. [DOI] [PubMed] [Google Scholar]
- 7.Fong TL, McHutchinson JG, Reynolds TB. Hyperthyroidism and hepatic dysfunction. J Clin Gastroenterol. 1992;14:240–4. doi: 10.1097/00004836-199204000-00010. [DOI] [PubMed] [Google Scholar]
- 8.Yao JD, Gross JB, Ludwig J, Purnell DC. Cholestatic jaundice in hyperthyroidism. Am J Med. 1989;86:619–20. doi: 10.1016/0002-9343(89)90398-7. [DOI] [PubMed] [Google Scholar]
- 9.Ruiz JK, Rossi GV, Vallejos HA, Brenet RW, Lopez IB, Escribono AA. Fulminant hepatic failure associated with propylthiouracil. Ann Pharmacother. 2003;37:224–8. doi: 10.1177/106002800303700213. [DOI] [PubMed] [Google Scholar]
- 10.Limaye A, Ruffolo PR. Propylthiouracil-induced fatal hepatic necrosis. Am J Gastroenterol. 1987;82:152–4. [PubMed] [Google Scholar]
- 11.Mikhail NE. Methimazole induced cholestatic jaundice. South Med J. 2004;97:178–82. doi: 10.1097/01.SMJ.0000054690.98272.B1. [DOI] [PubMed] [Google Scholar]
- 12.Backer B, Shapiro B, Fig LM, Woodbury D, Sisson JC, Beierwaltes WM. Unusual complications of antithyroid drug therapy: Four cases reports and review of literature. Thyroidology. 1989;1:17–26. [PubMed] [Google Scholar]
- 13.Blom H, Stolk J, Schreuder HB, Von Blomberg-Vander Flier M. A case of carbimazole induced intrahepatic cholestasis. An immune-mediated reaction? Arch Intern Med. 1985;145:1513–5. [PubMed] [Google Scholar]
- 14.Ozenirler S, Tuncer C, Boztepe U. Propylthiouracil-induced hepatic damage. Ann Pharmacother. 1996;30:960–3. doi: 10.1177/106002809603000909. [DOI] [PubMed] [Google Scholar]
- 15.Becker CF, Gorden P, Robbins J. Hepatitis from methimazole during adrenal steroid therapy for malignant exophthalmos. JAMA. 1968;206:1787–9. [PubMed] [Google Scholar]


