Abstract
Background
The aim of the study was to investigate the effects of sesame oil on endothelial function and to detect the underlying mechanisms, both in the postprandial state and after long-term consumption.
Design
We enrolled 30 hypertensive men in a two phase study. In the first phase, 26 volunteers consumed 35 g of either sesame oil or control oil. Endothelial function, inflammatory activation and nitric oxide syntase (NOS) inhibition was assessed after a 12 hour fast and 2 hours after consumption of an oil-containing standardized meal. In the second phase, 30 volunteers consumed 35 g of sesame oil or control oil daily for 2 months and the above mentioned parameters were assessed at baseline, 15, 30 and 60 days.
Methods
Endothelial function was estimated by endothelium-dependent FMD (flow-mediated dilatation) of the brachial artery.
Results
Flow mediated dilatation (FMD) improved significantly both after acute (p=0.001) and long-term sesame oil consumption (p=0.015, p=0.005 and p=0.011 for 15, 30 and 60 days respectively). Intracellular adhesion molecule (ICAM) levels decreased significantly after only 60 days of daily sesame oil intake (p=0.014). By contrast, no changes were observed in the control group in either phase of the study.
Conclusions
This is the first study to show that sesame oil consumption exerts a beneficial effect on endothelial function and this effect is sustained with long-term daily use.
Keywords: blood pressure, sesame oil, FMD, endothelial function
Introduction
Sesame oil is a flavored oil with high nutritive value, containing, among other important nutrients, powerful antioxidant substances1. The most studied antioxidants in sesame oil (sesamin and sesamol), apart from their antioxidant activity, have been suggested to have favorable health effects, such as lowering fatty acid concentrations in liver and serum and lowering serum cholesterol levels1. Furthermore, sesame oil may even have anticarcinogenic and antihypertensive properties1-3 .
Despite several studies in rats4,5, only three studies in humans have investigated the possible effects of daily sesame oil consumption on conditions related to cardiovascular disease, hypertension, oxidative stress, lipid profile and inflammatory markers6-8. These studies suggest that substitution of sesame oil (~35 g of sesame oil daily) for another edible oil for 45 days greatly reduces arterial blood pressure in hypertensive subjects. Results from the above mentioned studies of Sankar et al. and from similar studies in rats have implied that sesame oil reduces oxidative stress and endothelin 1 (ET1), which causes vasoconstriction and increases nitric oxide (NO), the main endothelial derived vasodilator9.
Although these data suggest a favorable effect of sesame oil consumption on endothelial function, this has never been directly investigated in humans. It is possible, although not yet explored, that sesame oil may affect endothelial-dependent FMD, which is mainly mediated by NO release and additionally a prognostic factor for future cardiovascular events10,11. At the same time, there are almost no data assessing the possible mechanism behind NO modulation by sesame oil, namely induction of NO synthase, or inhibition of NO inhibitors like asymmetric dimethylarginine (ADMA)12. Finally, the effect of sesame oil consumption on other endothelium properties, such as suppression of endothelial activation, has not been examined13.
The aim of the present study was to investigate, for the first time in humans, the possible effects of sesame oil consumption on endothelial function, both in the postprandial state and after long-term intake.
Methods
Study population
Thirty hypertensive males (mean age 52.7 ± 10.4 years) participated in this study from a sample of consecutively screened individuals visiting an outpatient hypertension clinic at Alexandra University Hospital, Athens, Greece (Table 1). They were all recently diagnosed for hypertension by criteria of 24-hour BP monitoring (mean day blood systolic blood pressure >135 mmHg and diastolic blood pressure >85 mmHg)14 and were receiving antihypertensive medication (converting enzyme inhibitors, or angiotensin inhibitors plus diuretics). Exclusion criteria included diabetes, clinically overt coronary artery disease, metabolic syndrome or other metabolic diseases, renal diseases, medications other than those in the inclusion criteria and nutritional supplements. Volunteers on specific diets or on diets for reducing body weight were also excluded. All volunteers gave their informed consent before entering the study. The study protocol and the procedure for obtaining informed consent conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the local scientific ethics committee.
Table 1.
Characteristics of study groups.
| Variables | Sesame oil group (N=17) Mean (SD) |
Control group (N=13) Mean (SD) |
p-value |
|---|---|---|---|
| Age (years) | 49.8 (8.4 | 56.8 (12) | 0.074 |
| BMI (kg/m2) | 27.7 (2.4) | 28.5 (2.9) | 0.473 |
| SBP (mmHg) | 126.8 (17.9) | 132.9 (17.7) | 0.378 |
| DBP (mmHg) | 78.3 (8.4) | 78.8 (10.8) | 0.873 |
| FMD (%) | 3.3 (1.5) | 4.1 (1.03) | 0.163 |
| ICAM-1 (ng/ml) | 762.9 (95.5) | 772.7 (186.3) | 0.358 |
| ADMA (μmol/L) | 0.45 (0.12) | 0.43 (0.03) | 0.673 |
p-value is for analysis with student’s t-test
BMI, body mass index, SBP, systolic blood pressure, DBP, diastolic blood pressure, FMD, flow mediated dilatation, ICAM, intracellular adhesion molecule, ADMA, asymetric dimethylarginine.
The authors of this manuscript have certified that they comply with the Principles of Ethical Publishing in the European Journal of Cardiovascular Prevention and Rehabilitation.
Experimental protocol
The study protocol was comprised of two sequential study phases: an acute and a chronic phase. In the first study phase, 26 of the 30 initially recruited volunteers were instructed to visit the Vascular Laboratory of Alexandra University Hospital of Athens, Greece, after a 12 hour fast from food, cigarettes and medication; and a 24 hour fast from alcohol and caffeinated beverages. After a 10 min resting period in the supine position in a quiet room with temperature a constant 20-25°C, subjects were assessed using ultrasound to assess endothelial function and blood samples were collected. Next, subjects received meals containing either sesame oil or the control oil (either olive oil or corn oil). The meal used for the study was a vegetable soup (containing either 35 g of sesame oil, or 35 g of either olive oil or corn oil, the oils mainly used in the typical diet) and 2 slices of white bread, to be consumed within 15 min. Ultrasound imaging and blood sample collection were repeated 2 hours after meal consumption. We used a 1:1 ratio to allocate subjects into the two study groups. In the control group, we also used a 1:1 ratio to allocate subjects into those receiving corn oil or olive oil. The number of participants and characteristics of the two study groups are shown in Table 1.
In the chronic phase of the study, all 30 subjects were enrolled. Seventeen subjects were instructed to consume 35 g of sesame oil daily for the next 60 days as alternative oil used for their salad, with no other alterations in their typical diet, their weight and physical activity. Subjects in the other group (N=13) were instructed not to change their diet, weight and physical activity in any way and to maintain their typical oil consumption (mostly olive oil and corn oil). Ultrasound imaging and blood sample collection were performed under fasting conditions at the first visit, and at 15, 30 and 60 days after the beginning of the second phase of the study. During the first and the fifth week of the study, all volunteers were given a 3 day food diary in order to assess their diet and ascertain that they made no alterations to it, which could affect the examined vascular parameters of the study.
Flow Mediated Dilatation Measurement
A 14.0 MHz multi-frequency linear array probe attached to a high-resolution ultrasound machine (Vivid 7 Pro, GE Healthcare, Little Chalfont, UK) was used to assess FMD. This method assessing endothelial function has been previously described15. In brief, measurements were obtained from the right brachial artery at a specific anatomic point. The measurement of the arterial diameter was performedmanually by two independent observers who were unaware of the study aims, at end-diastole, using electronic calipers. The procedure was guided by electrocardiographic assessment. After the initial measurement at resting conditions, a cuff fitted 8 cm distal to the brachial artery and near the wrist was inflated to 250-300 mmHg, altering arterial flow for 5 min. Then it was deflated, increasing arterial flow (reactive hyperemia). Afterwards, there was a continuous scan for 90 sec after cuff deflation, and the maximal resting diameter of the vessel at the same point was defined again (diameter during reactive hyperemia). FMD is defined as the percent change in arterial diameter (endothelial dependent vasodilation). The inter and intra observer variability for brachial diameter measurements in our laboratory is 0.1+0.12 and 0.08+0.19 mm respectively, while FMD variability measured on two different days was 1.1+1%.
Blood Assays
Aliquots of blood were collected at each time point, by a physician. From each blood sample, 10 ml were aliquoted in a vacutainer, for serum separation. The clotted blood samples were stored at 4 °C and 30 minutes after collection were centrifuged, at 3,500 rpm, 4 °C, for 10 minutes using a bench centrifuge. The obtained serum was further aliquoted and stored at −80°C for further analysis.
We used the blood samples collected to analyze levels of asymmetric dimethylarginine (ADMA) and ICAM-1. Serum ADMA concentration was determined by using a competitive 96-well plate ADMA-ELISA kit (Immunodiagnostik AG, Bensheim, Germany) following the supplier’s protocol. ADMA shows negligible cross reactivity with L-arginine (<0.02%) and other endogenous derivatives of L-arginine. Coefficients of variation are 6.2-7.9% and 6.2-10.5% for inter-assay and intra-assay, respectively. The ELISA assay can accurately measure ADMA concentrations over the full range of physiologically-relevant concentrations (i.e., 0.05-2 μmol/L). The specificity of ADMA ELISA kit is 100% and the sensitivity is 0.05 μmol/L. Serum ICAM-1 was determined using a commercially available sandwich ELISA kit (Bender Medsystems, Vienna, Austria). Diluted serum samples and a horseradish-peroxidase labeled secondary monoclonal anti-sICAM-1 antibody were applied to the ELISA plates coated with a mouse monoclonal anti-sICAM-1 antibody according to the manufacturer’s instructions. Color was developed with 3,3′ 5′ 5-tetramethylbenzidine and absorbance was measured at 450 nm against standard curves. Mean values were reported. The optical density of each sample was determined using a microplate reader (Expert 96, Asys Hitech, Eugendorf, Austria). The sensitivity of sICAM-1 was 2.10 ng/ml. Intra-assay and inter-assay coefficient of variations were 4.1% and 7.7%, respectively.
Statistical analysis
Continuous variables are presented as mean ± standard deviation (SD). All continuous variables were tested for normal distribution by one-sample Kolmogorov-Smirnov test and normal probability (Q-Q) plots. ANCOVA adjusting for baseline differences for the dependent variable (FMD, ADMA, ICAM) was used to assess differences in changes between groups in the first phase of the study while adjustment for possible confounders was also performed with this test. ANOVA for repeated measures was used to assess changes in the second phase of the study in each group. To compare effects between groups in this phase of the study, the same test was used to assess interactions between dependent variables and groups as well as between groups. ANCOVA for repeated measures was performed to adjust for possible confounders in the chronic phase of the study. In both phases of the study, differences in measured variables between specific time points were evaluated by grouped comparisons using two-samples and paired samples student’s t-tests (adjusted P-value < 0.017 for the number of measurements was considered statistically significant). The study design provided 90% power to detect an intervention effect of 2% absolute change for FMD for both the acute and chronic phase of the study. Statistical analyses were performed using SPSS 18 for Windows (SPSS Inc, Chicago, IL).
Results
From the subjects who initially agreed to participate, i.e.18 for the intervention and 18 controls, 17 intervention and 13 controls finally completed the chronic experiment. Baseline characteristics of the study groups are shown in Table 1. Results from the 3 day food records of all participants in the study showed no differences between and within groups in the first and the fifth week of the long-term phase of the study in consumption of energy, protein, carbohydrates, total fat, monounsaturated fatty acids, polyunsaturated fatty acids, saturated fatty acids, trans fatty acids, alcohol, total fiber, soluble fiber, insoluble fiber and caffeine (data not shown).
Results from the first phase of the study revealed that FMD increased significantly two hours after sesame oil consumption compared with baseline (p=0.001) (Table 2), while no significant changes were observed in the control group(p=0.249 for control group using corn oil and p=0.686 for control group using olive oil according to non – parametric tests). ANCOVA revealed that, compared with the control group, the changes observed in the sesame oil group were highly significant (F=8.76, p=0.007) (Table 2). This difference remained significant even after adjusting for age, BMI and BP changes (p=0.013). Neither ICAM-1 nor ADMA were modified in the postprandial phase.
Table 2.
Postprandial effects of sesame oil on FMD, ICAM-1 and ADMA.
| Variables | Control group (n=12) | Sesame oil group (N=14) | p1 | p2 | ||||
|---|---|---|---|---|---|---|---|---|
|
|
||||||||
| Baseline | Postprandial | Baseline | Postprandial | |||||
| Corn oil | Olive oil | Corn oil | Olive oil | |||||
| FMD (%) | 4.22 (1.31) | 3.88(0.73) | 2.82 (1.82) | 3.86(2.41) | 3.4 (1.6) | 5.7 (2.4) | 0.001 | 0.013 |
|
SBP
(mmHg) |
130(11.6) | 127(9.13) | 126.6(15.4) | 127.6(8.0) | 126(17.9) | 119.2(19) | 0.106 | 0.250 |
|
DBP
(mmHg) |
77(6.95) | 75.4(6.3) | 73.5(12.5) | 68.8(8.26) | 78.2(8.7) | 74.5(6.7) | 0.094 | 0.690 |
|
ICAM
(ng/ml) |
847.7 (231.1) | 750.3(163.7) | 856.3 (215.6) | 761.2(175.1) | 780.3 (204) | 829.9 (204.7) | 0.372 | 0.358 |
|
ADMA
(μmol/L) |
0.42 (0.03) | 0.45(0.02) | 0.40 (0.03) | 0.44(0.04) | 0.45 (0.13) | 0.42 (0.03) | 0.375 | 0.999 |
FMD, flow mediated dilatation, ICAM, intracellular adhesion molecule, ADMA, asymetric dimethylarginine. All variables are presented as mean (SD).
p1, p-value (student’s t-test) for comparison of baseline with postprandial value of the sesame oil group
p2, p-value (student’s t-test) for comparison of postprandial values between the control (as a whole) and the sesame oil group
In the second, chronic phase of the study, FMD increased significantly in the sesame oil group (F=5.13, p=0.004) while the control group showed a significant overall trend towards FMD deterioration (F=3.1, p=0.040, Figure 2). There were similar effects of both oils used in the control group (p=0.117 for control group using corn oil and p=0.801 for control group using olive oil according to non – parametric tests). Changes in the intervention group were significantly different by ANOVA for repeated measures, both in interaction between FMD and oil type (p=0.003) as well as in between volunteers’ effects (p=0.014). The above mentioned differences remained significant even after adjusting for age, BMI and BP changes (p=0.005 and p=0.042, respectively). In the sesame oil group, FMD increased significantly at all points compared with baseline (p=0.015, p=0.005 and p=0.011 for 15, 30 and 60 days, respectively) (Figure 1). Non parametric tests for each type of oil within the control group showed: p=0.117 for corn oil and p=0.801 for olive oil.
Figure 2.
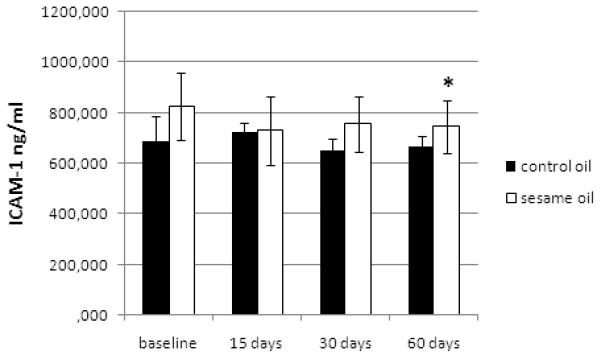
Long-term effects of sesame oil consumption on ICAM-1.
Figure 1.
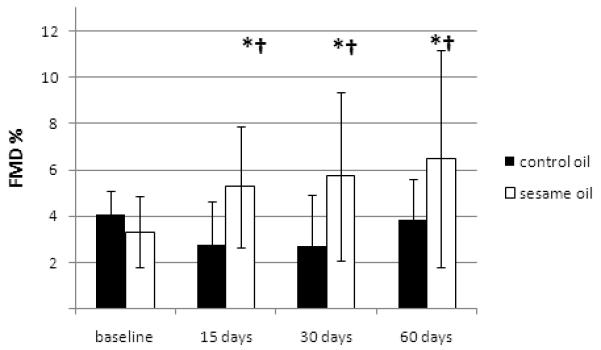
Long-term effects of sesame oil consumption on FMD.
ICAM-1 decreased at 60 days significantly in the sesame oil group (p=0.014) and marginally in the control group (p=0.054, Figure 2). No difference was observed in this change between the 2 groups by ANCOVA (p=0.572). ADMA showed no significant alterations during the long-term phase of the study.
Discussion
This is the first study to investigate the acute postprandial and long-term effects of sesame oil consumption on endothelial function in hypertensive men. The results suggest beneficial effects both on the endothelial vasodilatory capacity, assessed by FMD, and on the inhibition of endothelial activation, assessed by ICAM-1 levels.
Our findings imply that 35 g of sesame oil daily are enough to increase FMD levels two hours postprandially in a group of hypertensive volunteers. It is noteworthy that almost all of our volunteers (28/30) had baseline FMD values below normal (FMD <5%)15, which is expected for patients with hypertension16. Two hours after the meal, those in the sesame oil group showed remarkably increased FMD and 50% of the volunteers managed to exceed normal levels, whereas in the control group FMD values tended to further deteriorate (Table 2).
Postprandial deterioration of FMD values following a fatty meal containing olive oil or other oil types has been described previously17,18 and is usually attributed to postprandial hypertriglyceridemia, or to increased oxidative stress. Additionally, it has been suggested that the type of fat consumed in a meal is responsible for postprandial impairment of FMD. Compared with saturated fats, unsaturated fatty acids, being more prone to saturation and sensitive to oxidative stress, usually cause greater endothelial dysfunction19. Sesame oil, being rich in polyunsaturated fatty acids and antioxidant substances, may have managed to improve FMD levels despite a concomitant possible rise in triglycerides (not measured). We hypothesize that sesame oil antioxidant substances, like sesamin, may have induced activation of endothelial NO synthase, which is already described from experiments in human and rat aortic rings to lead to increased NO production and consequent vasodilation and FMD elevation. Concerning the possible mediating mechanisms of FMD improvement, ADMA remained unaltered during this short period, a fact that prevents us from suggesting a possible effect on this NOS inhibitor. Moreover, although ICAM levels may be affected even two hours following consumption of a test meal20, sesame oil had no effect on ICAM levels and therefore no relation to endothelial activation was found postprandially.
Although we cannot safely suggest a mediating mechanism(s) related to acute postprandial improvement in FMD levels, our findings strongly support the existence of direct beneficial effects of sesame oil on endothelial function comparable to drug-related improvements21 on a marker recognized as prognostic for future cardiovascular events. Such simple dietary interventions may be relatively easily applied in clinical practice, alone or in combination with other lifestyle modifications and antihypertensive drug treatment.
The outcome of the second phase of the study confirms that sesame oil exerts sustained beneficial effects on FMD. Moreover, a parallel decrease in ICAM levels after two month was observed, implying additional chronic mediating mechanisms of sesame oil not evident in the acute phase of the study. Additionally, BP changes are known to affect FMD levels. In the present study, systolic BP decreased 15 days after beginning sesame oil consumption, but after 30 and 60 days, the decrease was marginally significant (p=0.016, and a p-value < 0.017 was considered statistically significant for grouped comparisons using independent and paired sample students’ t-tests). These results are quite different from previous studies by Sankar et al.6-8, allowing no speculation concerning the relation between FMD and BP levels in this study group.
Previous studies in hypertensives and diabetic hypertensives6-8 indicated that daily consumption for 45 days of 35 g of sesame oil improved the blood levels of several enzymatic and non-enzymatic antioxidants like glutathione peroxidase and catalase. Although we did not measure these antioxidants in the present study, previous studies have shown that endothelial dysfunction is commonly attributed to increased oxidative stress22 , and administration of antioxidant substances as nutritional supplements or through food and drink may counterbalance this phenomenon23-25.
On the other hand, a decrease in ICAM-1 levels throughout the long-term phase of the study revealed a potentially important, yet unknown, connection of sesame oil consumption to inflammatory markers and endothelial activation. It is already known that increased expression of cytokines is closely related to endothelial activation and increased levels of ICAM-1, which directly affects endothelial cells, leading to decreased production of NO and endothelial dysfunction26. Sesame oil consumption, even in small daily amounts, may interfere in this connection of endothelial activation and endothelial function, by decreasing ICAM-1 and consequently improving FMD.
There are some limitations in the present study. We have chosen not to use a placebo in the control group, but instead different kinds of oils. Since some kind of oil is regularly used in daily nutritional habits, this strategy allowed us to compare sesame oil as a novel nutritional element to other types of oils already present in daily nutrition. Therefore, we used a similar design with previous studies6-8 which randomly assigned control subjects to receive oils rich either in mono- or polyunsaturated fatty acids, aiming to compare the group effect of this combination of control oils against sesame oil. Finally, the possible postprandial effect on triglycerides by sesame oil or by the control oils could not be assessed as major possible mechanism acting on FMD after any oil consumption. Nevertheless, it is noteworthy that only 35 g of sesame oil can achieve sustained improvement in endothelial function in hypertensives and possibly interfere with inflammatory and endothelial activation pathways. Future studies of the effect of sesame oil on endothelial function, together with assessment of lipid changes, oxidative stress, inflammation and endothelial activation, are necessary to elucidate the possible vascular activity of this novel functional food. Finally, these results cannot be extrapolated to populations without hypertension.
Conclusions
The present study showed that 35 g of sesame oil increased FMD both acutely and after chronic consumption while endothelial activation was decreased after chronic consumption. Whether sesame oil consumption provides benefits additive to those achieved by other lifestyle modifications or antihypertensive treatment warrants further investigation.
Acknowledgments
We are indebted to the volunteers who participated in our study.
Funding Supported by grants from the Sealy Center on Aging, University of Texas Medical Branch at Galveston, the Department of Nutrition and Dietetics Graduate Program, Harokopio University Athens, the Hellenic Heart Foundation and the Haitoglou Bros SA.
Footnotes
Declaration of conflict of interest The authors declare that there is no conflict of interest.
References
- 1.Namiki M. Nutraceutical functions of sesame: a review. Crit Rev Food Sci Nutr. 2007;47:651–73. doi: 10.1080/10408390600919114. [DOI] [PubMed] [Google Scholar]
- 2.Hirata F, Fujita K, Ishikura Y, Hosoda K, Ishikawa T, Nakamura H. Hypocholesterolemic effect of sesame lignan in humans. Atherosclerosis. 1996;122:135–36. doi: 10.1016/0021-9150(95)05769-2. [DOI] [PubMed] [Google Scholar]
- 3.Shimizu S, Akimoto K, Shinmen Y, Kawashima H, Sugano M, Yamada H. Sesamin is a potent and specific inhibitor of delta 5 desaturase in polyunsaturated fatty acid biosynthesis. Lipids. 1991;26:512–6. doi: 10.1007/BF02536595. [DOI] [PubMed] [Google Scholar]
- 4.Nakano D, Itoh C, Ishii F, Kawanishi H, Takaoka M, Kiso Y, Tsuruoka N, Tanaka T, Matsumura Y. Effects of sesamin on aortic oxidative stress and endothelial dysfunction in deoxycorticosterone acetate-salt hypertensive rats. Biol Pharm Bull. 2003;26:1701–5. doi: 10.1248/bpb.26.1701. [DOI] [PubMed] [Google Scholar]
- 5.Nakano D, Itoh C, Takaoka M, Kiso Y, Tanaka T, Matsumura Y. Antihypertensive effect of sesamin. IV. Inhibition of vascular superoxide production by sesamin. Biol Pharm Bull. 2002;25:1247–9. doi: 10.1248/bpb.25.1247. [DOI] [PubMed] [Google Scholar]
- 6.Sankar D, Rao MR, Sambandam G, Pugalendi KV. A pilot study of open label sesame oil in hypertensive diabetics. J Med Food. 2006;9:408–12. doi: 10.1089/jmf.2006.9.408. [DOI] [PubMed] [Google Scholar]
- 7.Sankar D, Rao MR, Sambandam G, Pugalendi KV. Effect of sesame oil on diuretics or Beta-blockers in the modulation of blood pressure, anthropometry, lipid profile, and redox status. Yale J Biol Med. 2006;79:19–26. [PMC free article] [PubMed] [Google Scholar]
- 8.Sankar D, Sambandam G, Ramakrishna Rao M, Pugalendi KV. Modulation of blood pressure, lipid profiles and redox status in hypertensive patients taking different edible oils. Clin Chim Acta. 2005;355:97–104. doi: 10.1016/j.cccn.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 9.Lee CC, Chen PR, Lin S, Tsai SC, Wang BW, Chen WW, Tsai CE, Shyu KG. Sesamin induces nitric oxide and decreases endothelin-1 production in HUVECs: possible implications for its antihypertensive effect. J Hypertens. 2004;22:2329–38. doi: 10.1097/00004872-200412000-00015. [DOI] [PubMed] [Google Scholar]
- 10.Cohn JN, Quyyumi AA, Hollenberg NK, Jamerson KA. Surrogate markers for cardiovascular disease: functional markers. Circulation. 2004;109:IV31–46. doi: 10.1161/01.CIR.0000133442.99186.39. [DOI] [PubMed] [Google Scholar]
- 11.Mancini GB, Dahlof B, Diez J. Surrogate markers for cardiovascular disease: structural markers. Circulation. 2004;109:IV22–30. doi: 10.1161/01.CIR.0000133443.77237.2f. [DOI] [PubMed] [Google Scholar]
- 12.Boger RH, Maas R, Schulze F, Schwedhelm E. Asymmetric dimethylarginine (ADMA) as a prospective marker of cardiovascular disease and mortality-An update on patient populations with a wide range of cardiovascular risk. Pharmacol Res. 2009 doi: 10.1016/j.phrs.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 13.Chen YH, Lin SJ, Chen YL, Liu PL, Chen JW. Anti-inflammatory effects of different drugs/agents with antioxidant property on endothelial expression of adhesion molecules. Cardiovasc Hematol Disord Drug Targets. 2006;6:279–304. doi: 10.2174/187152906779010737. [DOI] [PubMed] [Google Scholar]
- 14.Staessen JA, Asmar R, De Buyzere M, Imai Y, Parati G, Shimada K, Stergiou G, Redon J, Verdecchia P. Task Force II: blood pressure measurement and cardiovascular outcome. Blood Press Monit. 2001;6:355–70. doi: 10.1097/00126097-200112000-00016. [DOI] [PubMed] [Google Scholar]
- 15.Corretti MC, Anderson TJ, Benjamin EJ, Celermajer D, Charbonneau F, Creager MA, Deanfield J, Drexler H, Gerhard-Herman M, Herrington D, Vallance P, Vita J, Vogel R. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol. 2002;39:257–65. doi: 10.1016/s0735-1097(01)01746-6. [DOI] [PubMed] [Google Scholar]
- 16.Guntekin U, Gunes Y, Gunes A, Ceylan Y, Gumrukcuoglu HA, Yucel Y, Simsek H, Tuncer M. Noninvasive Assessment of Atherosclerosis in Patients with Isolated Hypertension. Echocardiography. 2009 doi: 10.1111/j.1540-8175.2009.00987.x. [DOI] [PubMed] [Google Scholar]
- 17.Vogel RA. The Mediterranean diet and endothelial function: why some dietary fats may be healthy. Cleve Clin J Med. 2000;67:232, 235–6. doi: 10.3949/ccjm.67.4.232. [DOI] [PubMed] [Google Scholar]
- 18.Vogel RA, Corretti MC, Plotnick GD. The postprandial effect of components of the Mediterranean diet on endothelial function. J Am Coll Cardiol. 2000;36:1455–60. doi: 10.1016/s0735-1097(00)00896-2. [DOI] [PubMed] [Google Scholar]
- 19.Berry SE, Tucker S, Banerji R, Jiang B, Chowienczyk PJ, Charles SM, Sanders TA. Impaired postprandial endothelial function depends on the type of fat consumed by healthy men. J Nutr. 2008;138:1910–4. doi: 10.1093/jn/138.10.1910. [DOI] [PubMed] [Google Scholar]
- 20.Burdge GC, Calder PC. Plasma cytokine response during the postprandial period: a potential causal process in vascular disease? Br J Nutr. 2005;93:3–9. doi: 10.1079/bjn20041282. [DOI] [PubMed] [Google Scholar]
- 21.Nagy L, Tarjan J, Samoczi M, Kovacs I, Takacs J. Effect of benazepril on endothelial function in previously untreated hypertensive patients. The Working Group of Cardiology of the Academic Committee of Veszprem, Hungary. Am J Ther. 1998;5:233–6. doi: 10.1097/00045391-199807000-00005. [DOI] [PubMed] [Google Scholar]
- 22.Matsuoka H. Endothelial dysfunction associated with oxidative stress in human. Diabetes Res Clin Pract. 2001;54(Suppl 2):S65–72. doi: 10.1016/s0168-8227(01)00337-0. [DOI] [PubMed] [Google Scholar]
- 23.Karatzi K, Karatzis E, Papamichael C, Lekakis J, Zampelas A. Effects of red wine on endothelial function: Postprandial studies vs clinical trials. Nutr Metab Cardiovasc Dis. 2009 doi: 10.1016/j.numecd.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 24.Karatzi K, Papamichael C, Karatzis E, Papaioannou TG, Voidonikola PT, Vamvakou GD, Lekakis J, Zampelas A. Postprandial improvement of endothelial function by red wine and olive oil antioxidants: a synergistic effect of components of the Mediterranean diet. J Am Coll Nutr. 2008;27:448–53. doi: 10.1080/07315724.2008.10719724. [DOI] [PubMed] [Google Scholar]
- 25.Plantinga Y, Ghiadoni L, Magagna A, Giannarelli C, Franzoni F, Taddei S, Salvetti A. Supplementation with vitamins C and E improves arterial stiffness and endothelial function in essential hypertensive patients. Am J Hypertens. 2007;20:392–7. doi: 10.1016/j.amjhyper.2006.09.021. [DOI] [PubMed] [Google Scholar]
- 26.Sprague AH, Khalil RA. Inflammatory cytokines in vascular dysfunction and vascular disease. Biochem Pharmacol. 2009;78:539–52. doi: 10.1016/j.bcp.2009.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]


