Abstract
Objective
The aim of this study was to investigate the plasma concentrations of vitamin D and its association with plasma lipid profiles.
Methods
Plasma vitamin D3 and lipid concentrations were measured in 119 female cynomolgus monkeys (pre-menopausal, n = 49; ovariectomized, n = 70) consuming approximately 1,000 IU per day of vitamin D3. In a subset of the ovariectomized monkeys (n = 23), vitamin D3 was remeasured after 6 months. The concentrations of vitamin D3 were analyzed as a continuous variable and were divided at the median into high (≥48 ng/mL) versus low (<48 ng/mL) groupings.
Results
Among the 119 monkeys, the range of vitamin D3 concentrations was 24.0 to 95.2 ng/mL (mean ± SD, 48.5 ± 12.7 ng/mL). Plasma vitamin D3 concentration was positively associated with high-density lipoprotein cholesterol (HDL-C; P = 0.003). Monkeys in the high vitamin D3 group had a significantly greater plasma HDL-C concentration (57.9 mg/dL) than did those in the low vitamin D3 group (47.1 mg/dL; P = 0.001). Although the difference was not significant (P = 0.120), the monkeys in the high vitamin D3 group had a decreased total plasma cholesterol–to–-C ratio compared with those in the low vitamin D3 group (5.4 and 6.2, respectively), potentially putting them at lower risk of atherosclerosis development.
Conclusions
Given that the monkeys all consumed a diet replete in vitamin D3, it seems that individual differences in vitamin D absorption or metabolism may have determined whether the monkeys had high or low concentrations of vitamin D3. Lower vitamin D3 was associated with a more atherogenic lipid profile, a major risk factor for progressing to coronary artery atherosclerosis in monkeys and human beings.
Keywords: Cardiovascular/coronary heart disease, Vitamin D concentrations, High-density lipoprotein, Lipid profiles, Menopause
It has been known for many years that vitamin D is necessary in maintaining bone health by promoting intestinal absorption of calcium and phosphorus.1 Vitamin D is also essential in the prevention of rickets2 and osteoporosis.3 For these reasons, the prevalence of vitamin D deficiency is an important health issue. According to the Third National Health and Nutrition Examination Survey, vitamin D deficiency is present in 25% to 57% of American adults.4 Additional studies indicate similar deficiencies in children, adolescents, and older persons.5,6 In the past, a deficiency has been defined as having a plasma 25-hydroxyvitamin D (25(OH)D) level lower than 15 ng/mL. A higher risk of deficiency is posed on individuals with darker skin pigmentation and individuals who live in higher latitudes because of decreased sun exposure.7 Current data suggest that plasma 25(OH)D concentrations should be greater than 30 ng/mL and, ideally, should be 36 to 40 ng/mL for improved health outcomes.8 Achieving these levels may require increasing daily vitamin D intake to 600 to 800 IU per day,8–10 as opposed to previous dietary standards that suggested 400 IU per day for individuals younger than 70 years and 600 IU for individuals 70 years or older.11
The results of more recent studies have suggested that additional benefits of vitamin D include improved overall health and chronic disease prevention. Vitamin D has been associated with the prevention of rectal cancer,12 prostate cancer,13 and breast cancer.14 In addition to cancer prevention, vitamin D has links to prevention of autoimmune diseases, such as multiple sclerosis15 and rheumatoid arthritis,16 along with type 1 diabetes17 and type 2 diabetes.18
Coronary heart disease (CHD) also has been linked to a deficiency in vitamin D. Well-documented risk factors for CHD include tobacco use, hypercholesterolemia, hypertension, obesity, diabetes mellitus, sex-specific age, and a family history of CHD.19,20 The findings of a recent study showed an inverse relationship between low plasma 25(OH)D concentrations (<15 ng/mL) and first-time cardiovascular events, with an increased hazard ratio of 1.6 to 2.1.21 The results of other studies have shown a higher incidence of hypertension and CHD in higher latitudes, suggesting that those with lower exposure to sunlight and, hence, lower vitamin D concentrations may have a higher risk of heart disease.22,23 CHD,24 myocardial infarction,25 sudden cardiac death,26 stroke,27 peripheral arterial disease,28 greater carotid intima-media thickness, 29 and hypertension30 have been associated with low plasma 25(OH)D levels.
Despite an extensive search, we were able to find only a few articles analyzing the relationship between vitamin D and plasma cholesterol. The results of several previous studies suggested that higher vitamin D concentrations were associated with worsening cholesterol parameters,31,32 whereas a few newer studies have suggested improved parameters.21,22 One report found that higher concentrations of vitamin D may be associated with a lower ratio of total plasma cholesterol (TPC) to high-density lipoprotein cholesterol (HDL-C).21 The results of another investigation showed lower triglyceride (TG) concentrations in those with higher vitamin D measurements. 33 We identified two reports suggesting an association between higher vitamin D concentrations and increased HDL-C34,35 and a lower prevalence of metabolic syndrome,34 major determinants of CHD risk. However, these studies showing cholesterol improvement have potential confounding factors, including compliance, recall bias, variations in ethnicity, and other factors associated with population-based studies.21,33 For these reasons and new suggestions that vitamin D supplementation is mostly unnecessary,9,10 along with the Institute of Medicine’s challenge to continue targeted research related to vitamin D,10 the objective of this research was to investigate the relationship between vitamin D and plasma lipid concentrations. More specifically, we sought to evaluate whether, and to what extent, low plasma concentrations of vitamin D are associated with a more atherogenic plasma lipid profile in a cohort of female cynomolgus monkeys.
METHODS
Animals and diets
This study used a total of 119 female cynomolgus monkeys (Macaca fascicularis), which were imported from the Institute Pertanian Bogor in West Java, Indonesia (the Indonesian Primate Center, ie, the Pusat Studi Satwa Primata). To increase the translational value of the study and to recreate the variations in human exposures (hormonal, dietary, and sun exposure), the monkeys studied represented both premenopausal and surgically postmenopausal subjects, these monkeys were made to consume a moderately atherogenic diet for a relatively long (26 mo) and a relatively short (4 mo) period, and were housed indoors, either with (indoor-outdoor) or without (indoor) access to an outdoor run. All of the monkeys were determined to be adult (ie, middle aged and older), and all consumed the same amount of vitamin D3 (a woman’s equivalent of 1,000 IU/d). Of the total 119 monkeys, 49 were premenopausal and 70 were postmenopausal (ovariectomized). All monkeys were socially housed with 2 to 5 monkeys per pen. The indoor-outdoor housing group consisted of 50 ovariectomized monkeys. The indoor housing group (49 pre-menopausal, 20 ovariectomized monkeys) was housed in a facility with large tinted windows along both sides of the building to allow light but to screen out some of the direct ultraviolet sunlight. All monkeys were fed a controlled diet that was prepared in the animal diet laboratory of the Wake Forest University Primate Center and was formulated to be equivalent for cholesterol, macronutrient content (ie, protein, fat, and carbohydrate), and vitamin D3.
All procedures involving animals in this study were conducted in compliance with state and federal laws, standards of the US Department of Health and Human Services, and guidelines established by the Wake Forest University Institutional Animal Care and Use Committee, where the animals were housed.
Vitamin D measurement
Plasma vitamin D3 concentrations were measured in all of the 119 female cynomolgus monkeys (premenopausal, n = 49; ovariectomized, n = 70) at a single time point. A subset of the ovariectomized monkeys had vitamin D3 remeasured after 6 months (n = 23). All vitamin D assays were done at The Reading Hospital and Medical Center. Frozen samples (500 µL) were transported to The Reading Hospital and Medical Center and protected from direct sunlight, a technique that has been shown to yield stable results.36 We used high-performance liquid chromatography (LC)/tandem mass spectrometry (MS) for the 25(OH)D assay, with a determination for vitamin D3. Our approach used the Shimadzu LC–MS/MS technology. The LC-MS prepares the sample to be ionized, through the physical separation capabilities of LC, for mass analysis by injection into the AB Sciex 3200 Q Trap mass spectrometer.
Plasma lipids
Plasma lipid and lipoprotein levels and body weight (BW) were measured at the time of vitamin D3 measurement. All lipid measurements were done in the clinical chemistry laboratory of the Wake Forest University Primate Center. The determinations included TPC, HDL-C, non–HDL-C (very–low-density and low-density lipoprotein cholesterol), and plasma TG levels. Cholesterol and TG analyses were done using enzymatic methods on the COBAS FARA II analyzer, with protocols and reagents supplied by Boehringer Mannheim. The clinical chemistry laboratory was fully standardized in this method and is in the continuing surveillance phase of the Centers for Disease Control and Prevention Lipid Standardization Program. HDL-C concentrations were determined using the heparin-manganese precipitation procedure, 37 which is described in detail in the Manual of Laboratory Operations: Lipid Research Clinics Program.38 In addition to the baseline measurements, 23 of the monkeys had follow-up plasma lipid measurements after receiving an additional 6 months of the moderately atherogenic diet.
Statistical analysis
Descriptive statistics including medians, means, and SDs were used to describe the BW data. Arithmetic means were used to describe measured values including TG, HDL-C, TPC, and non–HDL-C levels. For calculated values (eg, TPC/HDL-C ratios), geometric means were calculated. Vitamin D3 concentrations and plasma lipids were assessed for normality of distribution and were analyzed as continuous variables. Vitamin D3 values were dichotomized into higher and lower concentrations using the median vitamin D3 concentration of 48 ng/mL.
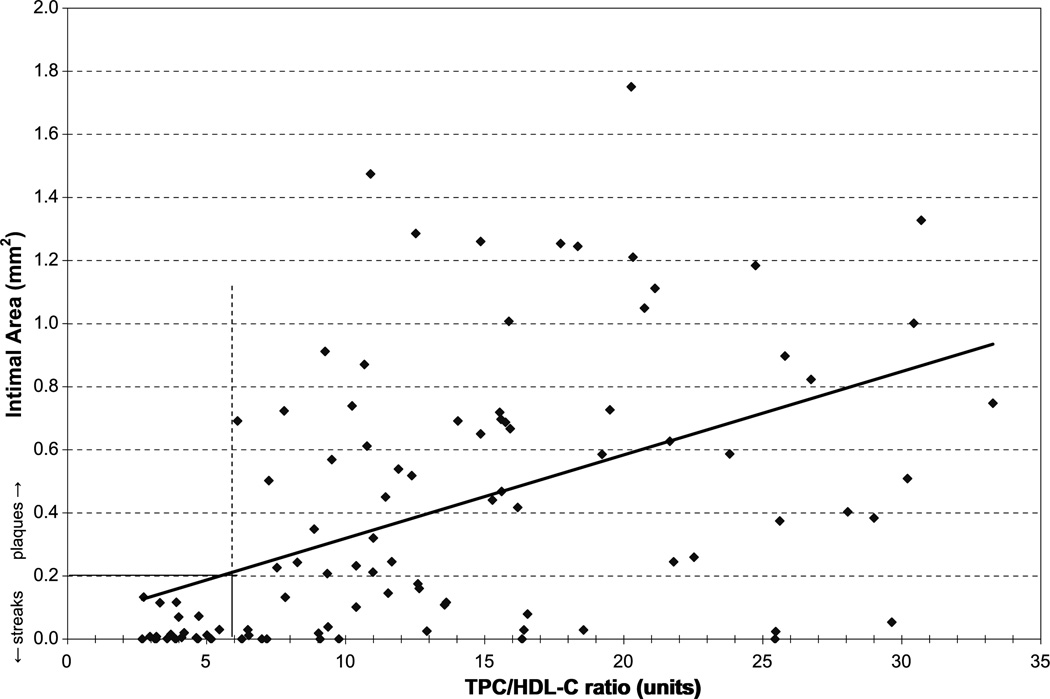
To determine the pathologic significance of TPC/HDL-C ratios for monkeys in the upper half of the distribution of plasma concentrations of vitamin D compared with those in the lower half of the distribution (TPC/HDL-C ratio, 6.2 vs 5.4, respectively), we used a separate database from a study reported previously.39 In that study, data regarding the relationship between plasma TPC/HDL-C ratio and the extent of atherosclerosis found in the iliac arteries of 103 participants were available. Iliac artery plaque size (intimal area) was expressed as a cross-sectional area in millimeters squared (mm2). Based on the pathologic convention that intimal areas of 0.2 mm2 or less equate with fatty streaks and intimal areas greater than 0.2 mm2 are considered plaques, we determined the relationship between plasma TPC/HDL-C ratios and the individual cases that had fatty streaks versus those with plaques (Fig. 1).
FIG. 1.
Progression of atherosclerosis as the TPC/HDL-C ratio increases. Plaques are defined as iliac artery intimal areas greater than 0.2 mm2. This figure suggests that the TPC/HDL-C ratio at which the progression of atherosclerosis begins is 6. TPC/HDL-C, total plasma cholesterol/high density lipoprotein ratio.
Inferential statistics consisted of Student’s t tests to compare lipid profiles by vitamin D3 group (high vs low), all lipid and vitamin D3 values by premenopause and post-menopause status, and the type of housing. For the comparison of lipid profiles and vitamin D3 concentrations of the entire cohort (n = 119), Pearson’s correlation coefficient was used to generate r values for continuous variables. Correlations between variables and high and low vitamin D3 values were analyzed using Spearman ranked correlation coefficient to generate ρ values.
All analyses were conducted using an a priori α level of 0.05 such that results yielding P < 0.05 were deemed statistically significant. SPSS version 17.0 (SPSS Inc., Chicago, IL) was used for all analyses.
RESULTS
Housing condition
Housing condition (indoor vs indoor-outdoor) had a significant effect on plasma vitamin D3 concentrations (P < 0.001). The mean ± SD plasma vitamin D3 concentration for the monkeys housed indoors was 44.68 ± 9.14 ng/mL (range, 24–64 ng/mL), whereas that for monkeys with access to outdoor pens was 53.87 ± 14.96 ng/mL (range, 26–95 ng/mL).
Time consuming an atherogenic diet
As expected, the length of time the monkeys consumed an atherogenic diet may have influenced their HDL-C concentrations. The monkeys that consumed the diet for 26 months had a mean ± SD HDL-C concentration of 46.35 ± 15.33 mg/dL, whereas those that consumed the diet for 4 months had a mean ± SD HDL-C concentration of 61.04 ± 18.89 mg/dL (P < 0.001).
The entire cohort
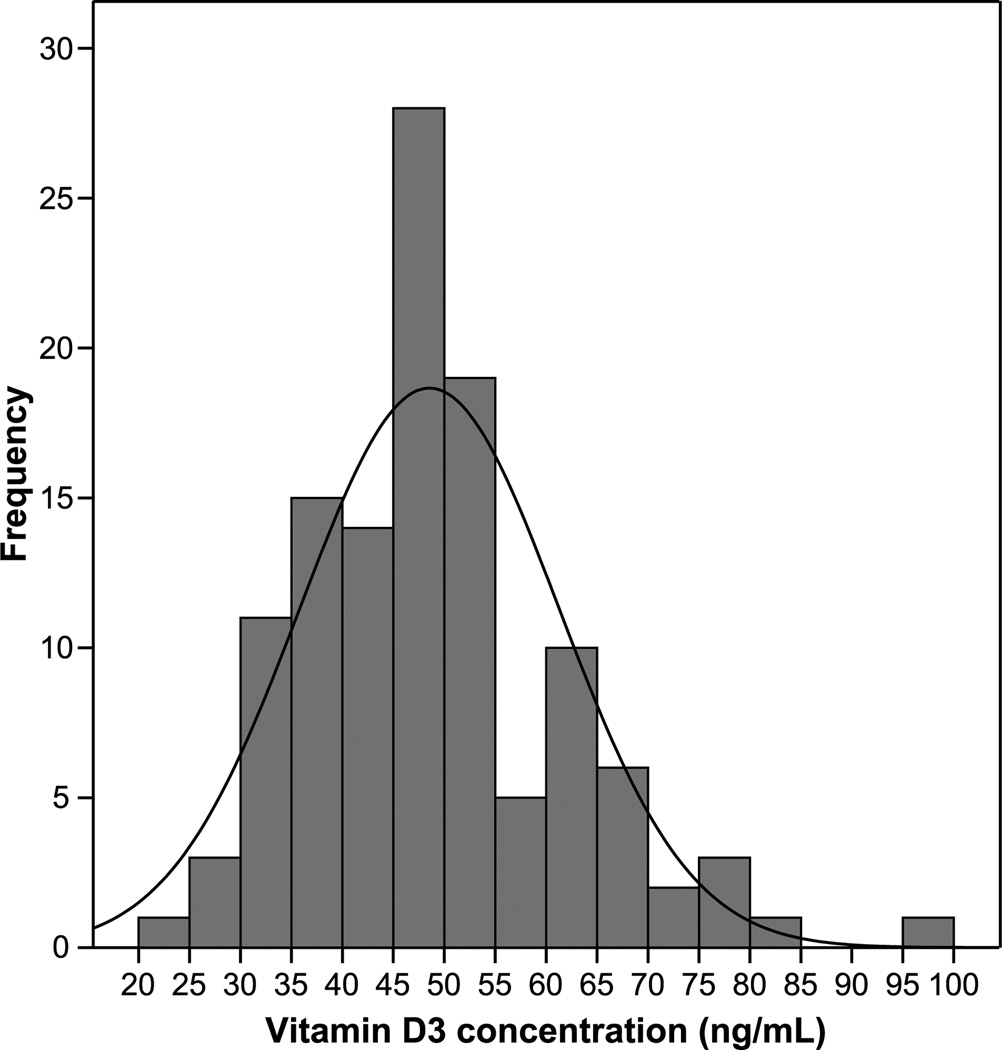
In the group of 119 monkeys fed an adequate and consistent quantity of vitamin D3, some with and some without exposure to sunlight, the range of vitamin D3 concentrations was 24.0 to 95.2 ng/mL (mean ± SD, 48.5 ± 12.7 ng/mL; Fig. 2). The BW distribution ranged from 2.12 to 4.46 kg (mean ± SD, 2.97 ± 0.46 kg).
FIG. 2.
Frequency of vitamin D3 concentrations in cynomolgus monkeys (Macaca fascicularis) after controlled diet consumption.
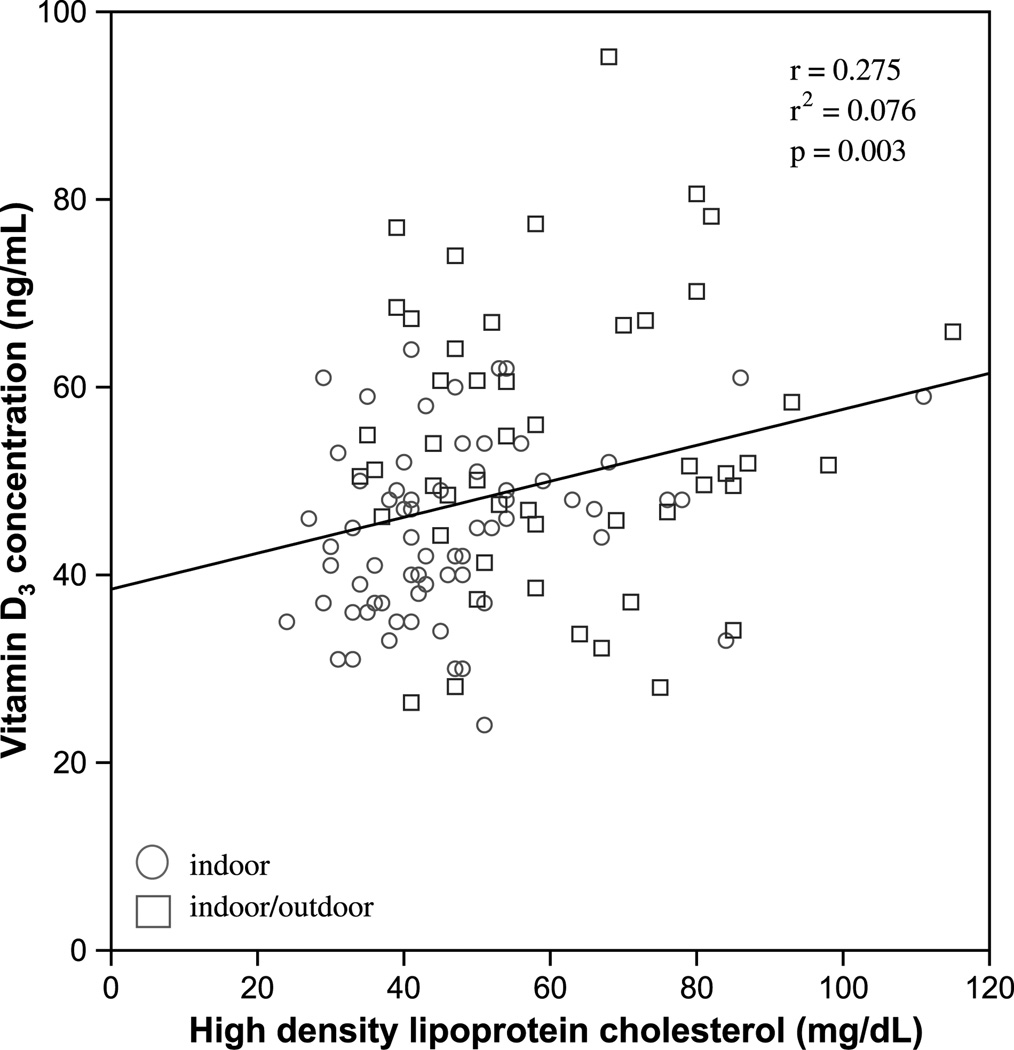
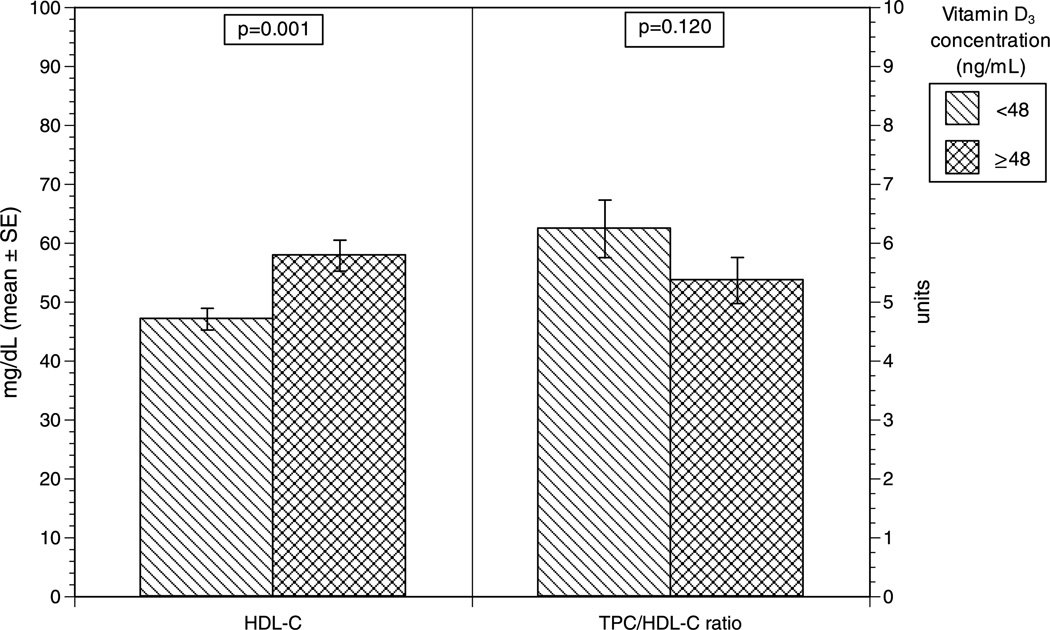
Analyzing vitamin D3 as a continuous variable, plasma vitamin D3 concentration was positively associated with HDL-C (r = 0.28, P = 0.003; Fig. 3). In contrast, there were no significant associations between vitamin D3 concentrations and any of the following: BW, TPC level, TG level, non–HDL-C level, or TPC/HDL-C ratio. Those with vitamin D3 concentrations of 48 ng/mL or higher, compared with those with vitamin D3 concentrations of less than 48 ng/mL, showed no significant differences in BW, TPC level, TG level, or non–HDL-C level. Conversely, monkeys with vitamin D3 concentrations of 48 ng/mL or higher had a significantly higher plasma HDL-C level than did those with vitamin D3 concentrations less than 48 ng/mL (57.9 vs 47.1 mg/dL, respectively; P = 0.001; Fig. 4). Although the difference was not significant (P = 0.120), the monkeys with vitamin D3 concentrations of 48 ng/mL or higher had a lower TPC/HDL-C ratio than did those with vitamin D3 concentrations less than 48 ng/mL (5.4 and 6.2, respectively; Fig. 4).
FIG. 3.
Vitamin D3 concentration as a function of baseline HDL-C (mg/dL) concentration in cynomolgus monkeys (Macaca fascicularis) after controlled diet consumption. Circle indicates indoor; square indicates indoor/outdoor. HDL-C, high-density lipoprotein cholesterol.
FIG. 4.
Mean HDL-C (mg/dL) concentration and TPC/HDL-C ratio for cynomolgus monkeys (Macaca fascicularis) with vitamin D3 concentrations of 48 ng/mL or greater and less than 48 ng/mL. HDL-C, high density lipoprotein cholesterol; TPC/HDL-C, total plasma cholesterol/high-density lipoprotein cholesterol.
The difference in TPC/HDL-C ratios between those with lower and those with higher vitamin D3 concentrations seemed large but did not meet statistical significance. A post hoc power analysis showed that a total sample size of 380 or 190 in each group (divided at the median) would afford 80% power to detect a difference in means of 0.88 (the difference between a group 1 TPC/HDL-C ratio of 6.2 and a group 2 TPC/HDL-C ratio of 5.4), assuming that the common SD was 3.0, using a two-group t test with a 0.05 two-sided significance level.
There were 23 ovariectomized monkeys fed the moderately atherogenic diet for 4 months that were followed prospectively with additional plasma lipid measurements 6 months later. As expected with additional months of diet, all variables, with the exception of TG, moved in the direction of a more atherogenic plasma lipid profile. However, the finding that monkeys with higher plasma vitamin D3 concentrations (high group) tended to have lower adverse changes in plasma lipids than did those with lower values (low group) was unexpected, although these relationships did not reach statistical significance. TPC increased by 35.3% versus 45.9% in the high versus low vitamin D concentration groups, respectively. Likewise, the non–HDL-C concentrations increased by 49.3% versus 63.9%, the HDL-C concentrations decreased by 12.1% versus 17.1%, and the TPC/HDL-C ratio increased by 2.9 versus 4.3 in the high versus low vitamin D concentration groups, respectively (Table 1).
TABLE 1.
Actual and percentage changes in lipid profiles after 6 months of a high-fat diet
| Low vitamin Da |
High vitamin Da |
|||
|---|---|---|---|---|
| Lipid parameter | Actual change | % change | Actual change | % change |
| TPC, mg/dL | 117.9 | 45.9 | 92.7 | 35.3 |
| TG, mg/dL | −5.5 | −10.6 | −3.3 | −7.7 |
| HDL-C, mg/dL | −9.8 | −17.1 | −7.3 | −12.1 |
| Non–HDL-C, mg/dL | 127.7 | 63.9 | 100 | 49.3 |
| TPC/HDL-C ratio | 4.3 | 87.9 | 2.9 | 61.6 |
TPC, total plasma cholesterol; TG, plasma triglyceride; HDL-C, high-density lipoprotein cholesterol; non–HDL-C, low-density and very–low-density lipoprotein cholesterol.
Low vitamin D is defined as a plasma vitamin D3 concentration less than the median (48 ng/mL), whereas high vitamin D is defined as a plasma vitamin D3 concentration greater than or equal to the median.
The TPC/HDL-C ratio for both the low and high plasma vitamin D cohorts was 4.8 (n = 23).After 6months of consuming a moderately atherogenic diet, the ratio was 9.1 in the low vitamin D group compared with 7.7 in the high vitamin D group. In our analysis of a separate database, from a study published previously, 39 we considered the significance of the TPC/HDL-C ratio. From that database, we determined that the progression of atherosclerosis (defined as an intimal area >0.2 mm2) begins at a TPC/HDL-C ratio of approximately 6, and there was a strong and highly significant correlation between intimal area and TPC/HDL-C ratio (r = 0.489, P < 0.001; Fig. 1). Taken together, these findings suggest that vitamin D–modulated differences in the TPC/HDL-C ratio may affect atherosclerosis progression and thus have clinical significance.
DISCUSSION
Despite an abundance of data suggesting an association between low vitamin D3 and CHD risk,21–30 recent data have challenged this belief.9,10,40 A report from the Women’s Health Initiative looking at calcium and vitamin D supplementation and its link to the prevention of coronary artery calcification showed no significant effect. It should be noted, however, that the participants were receiving only 400 IU of vitamin D3 per day.40 In addition, plasma vitamin D concentrations were not known, compliance was known to be poor, and the participants were allowed to continue their baseline vitamin supplementation. Current data suggest that daily vitamin D intake should be at least 600 to 800 IU per day for better health outcomes.8–10
In the study presented here, a large variation in plasma vitamin D3 concentrations (24.0–95.2 ng/mL) was observed in a cynomolgus monkey population, in which all monkeys were consuming a diet nearly equivalent in cholesterol, protein, fat, carbohydrates, and vitamin D (1,000 IU human equivalents) at the time of sampling. We question whether these differences may be caused, at least in part, by genetic variations in vitamin D metabolism (although the time consuming each diet and environmental factors probably played a role as well). Although variations in absorption could also play a role, it would seem unlikely to have such a high prevalence of poor vitamin D absorption in this cohort of monkeys. The wide variation also raises the question on the usefulness of 1,000 IU of vitamin D supplementation in those with low levels. In other words, it is not known whether supplementing low vitamin D concentrations in general, or even with 1,000 IU, makes a clinically meaningful impact or whether low vitamin D concentrations are a marker for other processes (potentially unrelated to vitamin D supplementation). Therefore, this finding warrants further study related to vitamin D supplementation and its effects. It is interesting to note that despite a paucity of data or understanding of what vitamin D supplementation does, testing and aggressively treating decreased plasma vitamin D concentrations are becoming a more popular phenomenon.
The current study demonstrates an association between low plasma concentrations of vitamin D and a more atherogenic lipid profile. Therefore, the major findings propose an explanation for why vitamin D–deficient individuals may be at increased risk of CHD. In our sample, cynomolgus monkeys with higher plasma vitamin D concentrations had significantly higher HDL-C levels (Figs. 3 and 4). These findings suggest that low HDL-C levels, one of the well-recognized risk factors for CHD, may be causally linked to a deficiency in vitamin D in the monkey model. The monkeys with higher plasma concentrations of vitamin D also had a lower TPC/HDL-C ratio (Fig. 4). TPC/HDL-C ratios greater than 6.0, based on our additional analysis, are shown to be highly atherogenic in this model (Fig. 1). An increasingly higher TPC/HDL-C ratio, as shown in Figure 1, is correlated with greater plaque size, suggesting a worsening risk with increasing TPC/HDL-C ratios. The fact that monkeys in the high vitamin D group had TPC/HDL-C ratios less than 6 and those in the low vitamin D range had ratios greater than 6, although not statistically significant, has clinical implications. These findings, in combination with greater HDL-C values in the high vitamin D cohort (P = 0.001), provide evidence for a more favorable lipid profile in those monkeys with higher vitamin D concentrations.
A subset of 23 monkeys, followed prospectively for 6-month changes in their lipid profiles, revealed interesting findings (Table 1). Although none of the differences were statistically significant (largely because of the small sample size), it is noteworthy that the changes, evaluated as both actual and percentage differences, were all lower in the monkeys with higher baseline vitamin D concentrations. A post hoc power analysis determined the need for at least 38 monkeys to have 80% power to be able to detect a statistically significant difference in these parameters. Although the findings are not statistically significant, the consistency of the finding along with the degree of disparity in the percentage change may imply clinical relevance. This could imply that higher vitamin D concentrations may help abate the development of an atherogenic lipid profile despite a high-fat diet.
A study of human subjects that determines whether and to what extent HDL-C levels increase and TPC/HDL-C ratios decrease with vitamin D supplementation would be clinically beneficial. In addition, it would be helpful to analyze the role of further supplementation and, more specifically, the response of cholesterol measurements when vitamin D supplementation is sequentially increased. With adequate and maximal dosing, what is the vitamin D response, and does it vary widely among individuals? Further studies should also analyze whether patients who are hypercholesterolemic benefit from vitamin D assessment, both in lipid parameters and measures of atherosclerosis.
The variation in HDL-C and vitamin D3 concentrations based on diet and housing, respectively, is not surprising. We expected a worsening lipid profile in those monkeys with a larger exposure to a moderately atherogenic diet. Likewise, we expected higher vitamin D3 concentrations in those monkeys with outdoor exposure. Differences in the time consuming the diet and housing arrangements helped create a more realistic translational model that gave the necessary distribution of study variables. The small population size of cynomolgus monkeys could have prohibited our ability to see significant differences because of a low power and, hence, a β (type II) error. Studies to confirm these results in a human cohort would be helpful. However, the cynomolgus monkeys have been shown to be a reliable model in studying the potential causes of postmenopausal atherosclerosis,41 a model in which standardized dosing, compliance, sun exposure, time of dosing, food intake, and plasma measurements can all be controlled.
CONCLUSIONS
Despite the limitations mentioned here, the clinical relevance of our findings is important. To the best of our knowledge, this is the first report suggesting that lower HDL-C concentrations and a worsening lipid profile over time are associated with low vitamin D concentrations. It was also done in a way that controlled sun exposure, diet, and vitamin D3 intake. Given that all monkeys consumed a diet that was replete in vitamin D3, it seems that individual differences (presumably genetic) in vitamin D absorption or metabolism may have determined whether the monkeys had high or low plasma concentrations of vitamin D3. Lower concentrations of vitamin D3 are associated with significantly lower concentrations of HDL-C, a higher TPC/HDL-C ratio, and an enhancement of the adverse lipid effects of a moderately atherogenic diet, all of which are established risk factors for progressive coronary artery atherosclerosis in both the monkey model and in women. The results add to existing knowledge by identifying vitamin D deficiency as a potential cause of atherogenic lipid parameters and increased CHD.
Acknowledgments
Funding/support: This study was supported in part by the research budget of The Reading Hospital and Medical Center and the National Institute on Aging 2R01 AG027847 (SEA).
Footnotes
The data from this study were presented in oral abstract form at The North American Menopause Society 21st Annual Meeting in Chicago, IL, on October 8, 2010. These data and results, however, have not been published in manuscript form and have not been submitted previously to another journal.
Financial disclosure/conflicts of interest: None reported.
REFERENCES
- 1.Pérez-López FR. Vitamin D: secosteroid hormone and human reproduction. Gynecol Endocrinol. 2006;22:1–12. doi: 10.1080/09513590601045629. [DOI] [PubMed] [Google Scholar]
- 2.Gartner LM, Greer FR. Prevention of rickets and vitamin D deficiency: new guidelines for vitamin D intake. Pediatrics. 2003;111:908–910. doi: 10.1542/peds.111.4.908. [DOI] [PubMed] [Google Scholar]
- 3.Lips P, Duong T, Black D, et al. A global study of vitamin D status and parathyroid function in postmenopausal women with osteoporosis: baseline data from the multiple outcomes of raloxifene evaluation clinical trial. J Clin Endocrinol Metab. 2006;86:1212–1221. doi: 10.1210/jcem.86.3.7327. [DOI] [PubMed] [Google Scholar]
- 4.Looker AC, Dawson-Hughes B, Calvo MS, Gunter EW, Sahyoun NR. Serum 25-hydroxyvitamin D status of adolescents and adults in two seasonal subpopulations from NHANES III. Bone. 2002;30:771–777. doi: 10.1016/s8756-3282(02)00692-0. [DOI] [PubMed] [Google Scholar]
- 5.Fuleihan GE, Nabulsi M, Choucair M, et al. Hypovitaminosis D in healthy schoolchildren. Pediatrics. 2001;107:e53. doi: 10.1542/peds.107.4.e53. [DOI] [PubMed] [Google Scholar]
- 6.Gloth MF, Gundberg CM, Hollis BW, Haddas JG, Tobin JD. Vitamin D deficiency in homebound elderly persons. JAMA. 1995;274:1683–1686. doi: 10.1001/jama.1995.03530210037027. [DOI] [PubMed] [Google Scholar]
- 7.Gordan CM, DePeter KC, Feldman HA, Grace E, Emans SJ. Prevalence of vitamin D deficiency among healthy adolescents. Arch Pediatr Adolesc Med. 2004;158:531–537. doi: 10.1001/archpedi.158.6.531. [DOI] [PubMed] [Google Scholar]
- 8.Bischoff-Ferrar HA, Giovannucci E, Willet WC, Dietrich T, Dawson-Hughes B. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr. 2006;84:18–28. doi: 10.1093/ajcn/84.1.18. [DOI] [PubMed] [Google Scholar]
- 9.Ross AC, Taylor CL, Yaktine AL, Del Valle HB, editors. Committee to Review Dietary Reference Intakes for Vitamin D and Calcium; Institute of Medicine. Dietary Reference Intakes for Calcium and Vitamin D. [Accessed January 21, 2011]. Available at: http://books.nap.edu/openbook.php?record_id=13050&page=R1. [Google Scholar]
- 10.Institute of Medicine, Committee to Review Dietary Reference Intakes for Vitamin D and Calcium. [Accessed January 21, 2011];Dietary reference intakes for calcium and vitamin D [brief report] 2010 Available at: http://www.iom.edu/~/media/Files/Report%20Files/2010/Dietary-Reference-Intakes-for-Calcium-and-Vitamin-D/Vitamin%20D%20and%20Calcium%202010%20Report%20Brief.pdf.
- 11.Vieth R, Bischoff-Ferrari H, Boucher BJ, et al. The urgent need to recommend an intake of vitamin D that is effective. Am J Clin Nutr. 2007;85:649–650. doi: 10.1093/ajcn/85.3.649. [DOI] [PubMed] [Google Scholar]
- 12.Otani T, Iwasaki M, Sasazuki S, Inoue M, Tsugame S. Plasma vitamin D and risk of colorectal cancer: the Japan Public Health CenterYBased Prospective Study. Br J Cancer. 2007;97:446–451. doi: 10.1038/sj.bjc.6603892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li H, Stampfer MJ, Hollis JB, et al. A prospective study of plasma vitamin D metabolites, vitamin D receptor polymorphisms, and prostate cancer. PLoS Med. 2007;4:e103. doi: 10.1371/journal.pmed.0040103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garland CF, Gorham ED, Mohr SB, et al. Vitamin D and prevention of breast cancer: pooled analysis. J Steroid Biochem Mol Biol. 2007;103:708–711. doi: 10.1016/j.jsbmb.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 15.Munger KL, Levin LI, Hollis BW, Howard NS, Ascherio A. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. JAMA. 2006;296:2832–2838. doi: 10.1001/jama.296.23.2832. [DOI] [PubMed] [Google Scholar]
- 16.Merlino LA, Curtis J, Mikuls TR, Cerhan JR, Criswell LA. Vitamin D: intake is inversely associated with rheumatoid arthritis. Arthritis Rheum. 2004;50:72–77. doi: 10.1002/art.11434. [DOI] [PubMed] [Google Scholar]
- 17.Zipitis CS, Akobeng AK. Vitamin D supplementation in early childhood and risk of type 1 diabetes: a systematic review and meta-analysis. Arch Dis Child. 2008;93:512–517. doi: 10.1136/adc.2007.128579. [DOI] [PubMed] [Google Scholar]
- 18.Pittas AG, Dawson-Hughes B, Li T, et al. Vitamin D and calcium intake in relation to type 2 diabetes in women. Diabetes Care. 2006;29:650–656. doi: 10.2337/diacare.29.03.06.dc05-1961. [DOI] [PubMed] [Google Scholar]
- 19.National Cholesterol Education Program. [Accessed January 21, 2011];Third report of the Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Available at: www.nhlbi.nih.gov/guidelines/cholesterol/profmats.htm.
- 20.American Heart Association. Heart disease and stroke statistics–2005 update. Dallas, TX: American Heart Association; 2005. [Accessed January 21, 2011]. Available at: www.americanheart.org/downloadable/heart/1105390918119HDSStats2005Update.pdf. [Google Scholar]
- 21.Wang TJ, Pencina MJ, Booth SL, et al. Vitamin D deficiency and risk of cardiovascular disease. Circulation. 2008;117:503–511. doi: 10.1161/CIRCULATIONAHA.107.706127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grimes DS, Hindle E, Dyer T. Sunlight, cholesterol and coronary heart disease. QJM. 1996;89:579–589. doi: 10.1093/qjmed/89.8.579. [DOI] [PubMed] [Google Scholar]
- 23.Rostand SG. Untraviolet light may contribute to geographic and racial blood pressure differences. Hypertension. 1997;30:150–156. doi: 10.1161/01.hyp.30.2.150. [DOI] [PubMed] [Google Scholar]
- 24.Watson KE, Abrolat ML, Malone LL, et al. Active serum vitamin D levels are inversely correlated with coronary calcification. Circulation. 1997;96:1755–1760. doi: 10.1161/01.cir.96.6.1755. [DOI] [PubMed] [Google Scholar]
- 25.Giovannucci E, Liu Y, Hollis BW, Rimm EB. 25-Hydroxyvitamin D and risk of myocardial infarction in men. Arch Intern Med. 2008;168:1174–1180. doi: 10.1001/archinte.168.11.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pilz S, Marz W, Wellnitz B, et al. Association of vitamin D deficiency with heart failure and sudden cardiac death in a large cross-sectional study of patients referred for coronary angiography. J Clin Endocrinol Metab. 2008;93:3927–3935. doi: 10.1210/jc.2008-0784. [DOI] [PubMed] [Google Scholar]
- 27.Poole KE, Loverridge N, Barker PJ, et al. Reduced vitamin D in acute stroke. Stroke. 2006;37:243–245. doi: 10.1161/01.STR.0000195184.24297.c1. [DOI] [PubMed] [Google Scholar]
- 28.Melamed ML, Munter P, Michos ED, et al. Serum 25-hydroxyvitamin D levels and the prevalence of peripheral arterial disease. Arterioscler Thromb Vasc Biol. 2008;28:1179–1185. doi: 10.1161/ATVBAHA.108.165886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Targher G, Bertolini L, Padvoani R, et al. Serum 25-hydroxyvitamin D3 concentrations and carotid artery intima-media thickness among type 2 diabetic patients. Clin Endocrinol. 2006;65:593–597. doi: 10.1111/j.1365-2265.2006.02633.x. [DOI] [PubMed] [Google Scholar]
- 30.Forman JP, Giovannucci E, Homes MD, et al. Plasma 25-hydroxyvitamin D levels and risk of incident hypertension. Hypertension. 2007;49:1063–1069. doi: 10.1161/HYPERTENSIONAHA.107.087288. [DOI] [PubMed] [Google Scholar]
- 31.Lindén V. Vitamin D and serum cholesterol. Scand J Soc Med. 1975;3:83–85. doi: 10.1177/140349487500300206. [DOI] [PubMed] [Google Scholar]
- 32.Curčić VG, Curčić B. Effect of vitamin D on serum cholesterol and arterial blood pressure in infants. Nutr Metab. 1975;18:57–61. [PubMed] [Google Scholar]
- 33.Martins D, Wolf M, Pan D, et al. Prevalence of cardiovascular risk factors and the serum levels of 25-hydroxyvitamin D in the United States: data from the Third National Health and Nutrition Examination Survey. Arch Intern Med. 2007;167:1159–1165. doi: 10.1001/archinte.167.11.1159. [DOI] [PubMed] [Google Scholar]
- 34.Maki KC, Rubin MR, Wong LG, et al. Serum 25-hydroxyvitamin D is independently associated with high density lipoprotein cholesterol and the metabolic syndrome in men and women. J Clin Lipidol. 2009;3:289–296. doi: 10.1016/j.jacl.2009.07.003. [DOI] [PubMed] [Google Scholar]
- 35.Kazlauskaite R, Powell LH, Mandapakala C, Cursio JF, Avery EF, Calvin J. Vitamin D is associated with atheroprotective high-density lipoprotein profile in postmenopausal women. J Clin Lipidol. 2010;4:113–119. doi: 10.1016/j.jacl.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lewis JG, Elder PA. Serum 25-OH vitamin D2 and D3 are stable under exaggerated conditions. Clin Chem. 2008;54:1931–1932. doi: 10.1373/clinchem.2008.111526. [DOI] [PubMed] [Google Scholar]
- 37.Burstein M, Samaille J. On a rapid determination of the cholesterol bound to the serum α- and β-lipoproteins. Clin Chim Acta. 1960;5:609–617. doi: 10.1016/0009-8981(60)90075-9. [DOI] [PubMed] [Google Scholar]
- 38.US Dept of Health, Education, and Welfare. Manual of Laboratory Operations: Lipid Research Clinics Program. Vol 1. Lipid and Lipoprotein Analysis. Bethesda, MD: National Heart, Lung and Blood Institute; 1974. National Institutes of Health publication NIH 75-268. [Google Scholar]
- 39.Clarkson TB, Anthony MS, Morgan TM. Inhibition of postmenopausal atherosclerosis progression: a comparison of the effects of conjugated equine estrogens and soy phytoestrogens. J Clin Endocrinol Metab. 2001;86:41–47. doi: 10.1210/jcem.86.1.7151. [DOI] [PubMed] [Google Scholar]
- 40.Manson JE, Allison MA, Carr JJ, et al. Calcium/vitamin D supplementation and coronary artery calcification in the Women’s Health Initiative. Menopause. 2010;17:683–691. doi: 10.1097/gme.0b013e3181d683b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Clarkson TB, Mehaffey MH. Coronary heart disease of females: lessons learned from nonhuman primates. Am J Primatol. 2009;71:785–793. doi: 10.1002/ajp.20693. [DOI] [PubMed] [Google Scholar]