Abstract
The relationship between a loss of viability and several morphological and physiological changes was examined with Escherichia coli strain J1 subjected to high-pressure treatment. The pressure resistance of stationary-phase cells was much higher than that of exponential-phase cells, but in both types of cell, aggregation of cytoplasmic proteins and condensation of the nucleoid occurred after treatment at 200 MPa for 8 min. Although gross changes were detected in these cellular structures, they were not related to cell death, at least for stationary-phase cells. In addition to these events, exponential-phase cells showed changes in their cell envelopes that were not seen for stationary-phase cells, namely physical perturbations of the cell envelope structure, a loss of osmotic responsiveness, and a loss of protein and RNA to the extracellular medium. Based on these observations, we propose that exponential-phase cells are inactivated under high pressure by irreversible damage to the cell membrane. In contrast, stationary-phase cells have a cytoplasmic membrane that is robust enough to withstand pressurization up to very intense treatments. The retention of an intact membrane appears to allow the stationary-phase cell to repair gross changes in other cellular structures and to remain viable at pressures that are lethal to exponential-phase cells.
With increasing consumer demand for high-quality products with fresh characteristics, there has been an increasing interest in new mild methods of food preservation. One of the most promising is high hydrostatic pressure, and this is now being increasingly applied commercially for the processing of foodstuffs such as sauces, fruit juices, oysters, and meat products (27). The main advantage of this technology is that it can better preserve the taste, color, and original texture of the product (6, 14, 27). Also under study are combinations of high pressure with other methods, such as heat, low pH, or antimicrobial peptides (10, 21, 25). However, to exploit these new approaches fully, we need to better understand the effects of high pressure on microbes.
The inactivation of bacteria by high pressure has been investigated by numerous workers (14, 18, 32). It is generally acknowledged that bacterial spores are more resistant to high pressure than are vegetative cells and that gram-positive species are more resistant than gram-negative ones. However, there are many exceptions to this general rule, and great variation exists among strains (1, 4, 27, 29). For any given strain, resistance is affected by its physiological state and the composition of the suspending medium during pressurization. Cells in the stationary phase of growth are more resistant than those in the exponential phase (4, 24). Also, some studies on the influence of the pressurizing medium have been done, and they showed that complex media rich in ions, proteins, lipids, and carbohydrates are more protective than buffers (10, 12, 27, 35). However, despite much effort, the main cellular targets and events responsible for cell death have not yet been identified, and therefore it is difficult to understand, for instance, the wide variation in resistance within the same species or the influence of the composition of the pressurizing medium. Data published in the literature indicate that envelopes could be an important target for high-pressure inactivation (2, 16, 34). Several authors have reported damage to the cytoplasmic membrane, such as a loss of osmotic responsiveness, uptake of vital dyes (propidium iodide, ethidium bromide, or oxonol), or loss of intracellular material, after pressurization (4, 24, 33). The loss of function of some proteins, including the F1-F0 ATPase and multidrug efflux pumps, has also been described (34, 38, 39). Moreover, Ritz et al. (28) reported the loss of proteins from both the outer and inner membranes of Salmonella enterica serovar Typhimurium, as well as the formation of buds on the cell surface, after pressure treatments. However, it has also been reported that in some cases, pressure-killed stationary-phase cells retain a functional membrane (24), and this has led to the consideration of other structures inside the cell as potential key targets for inactivation. Some authors have reported similarities between cell inactivation and protein denaturation kinetics (19, 36), and changes in the conformation of various structures in the bacterial cell, such as the nucleoid and ribosomes, have been reported for electron microscopy and differential scanning calorimetry studies (20, 23, 26). However, it is still not clear which, if any, of these changes is responsible for the inactivation of the cell.
The aim of this work was to examine various morphological and physiological changes that occur in pressurized Escherichia coli cells that might be correlated with cell death. Changes in gross morphology, the loss of membrane integrity, the loss of osmotic responsiveness, changes in the conformation of nucleic acids, and protein coagulation were studied in exponential- and stationary-phase cells after exposure to different pressure treatments.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
E. coli J1, an acid-resistant commensal strain, was kindly provided by Ian Booth, University of Aberdeen, Aberdeen, United Kingdom. Stationary-phase cultures were prepared by inoculating 10 ml of tryptone soy broth (Oxoid, Basingstoke, United Kingdom) supplemented with 0.3% yeast extract (TSB-YE) with a loopful of growth from tryptone soy agar supplemented with 0.3% yeast extract (Oxoid) and incubating the resulting culture at 37°C for 6 h in a shaking incubator. Fifty microliters of this culture was inoculated into 50 ml of fresh TSB-YE and incubated for 18 h under the same conditions, which resulted in a stationary-phase culture containing approximately 5 × 109 cells/ml. Exponential-phase cells were prepared by inoculating 50 μl of the stationary-phase culture into 50 ml of fresh TSB-YE and incubating the culture for 2.5 to 3.0 h, until the optical density at 680 nm reached 0.3, corresponding to 108 cells/ml.
Pressure treatment.
Cells were centrifuged at 3,000 × g for 20 min at 4°C, and the pellets were resuspended in phosphate-buffered saline (PBS; pH 7.2). Cell suspensions (0.5 to 2.0 ml) were placed in sterile plastic pouches that were heat sealed and placed on ice before pressurization. Samples were pressure treated in a 300-ml pressure vessel (model S-FL-850-9-W; Stansted Fluid Power, Stansted, United Kingdom). The pressure-transmitting fluid was ethanol-castor oil (80:20). Cells were exposed to pressures from 100 to 600 MPa for 8 min. Pressure treatments were done at an ambient temperature (approximately 20°C), and the transient increase in temperature of the pressurization fluid caused by adiabatic heating was measured with a thermocouple. The maximum temperature reached during pressurization at 600 MPa was 43°C. After decompression, the pouches were removed from the unit and placed on ice before viable counts and other tests were performed.
Viable counts.
Samples were serially diluted in maximum recovery diluent (Oxoid) and plated onto tryptone soy agar-yeast extract supplemented with 0.1% (wt/vol) sodium pyruvate. Colonies were counted after 48 h of incubation at 37°C. Data presented are the mean values from at least three independent experiments, and error bars correspond to standard deviations of the means.
Cell staining.
Several fluorescent dyes were used to visualize different structures in the cells. Samples in PBS were mixed 1:1 (vol/vol) separately with the dye solutions, at the concentrations described below, and were immediately prepared for microscopic examination. Nucleoids were visualized by staining with 100 μM 4′,6-diamidino-2-phenylindole (DAPI) (Sigma-Aldrich, Poole, Dorset, United Kingdom). Cell envelopes were stained with a 165 μM solution of the lipophilic dye FM 4-64 (Molecular Probes, Eugene, Oreg.). Protein was stained with a 130 μM solution of fluorescein isothiocyanate (FITC) (Sigma-Aldrich). A solution of 83 μM acridine orange (Polysciences, Eppelheim, Germany) was used to simultaneously visualize double-stranded (green fluorescence) and single-stranded (red fluorescence) nucleic acids. Samples were prepared for microscopy as follows: 1 μl of either unstained or stained cell suspension was spread on a glass coverslip and immobilized by placement of the coverslip onto a glass slide coated with a very thin flat layer of 1% technical agar.
Examination of the ability of cells to plasmolyze.
To study the ability of the cells to respond to an osmotic shock, we spread 1 μl of sample onto a glass coverslip, gently placed the coverslip on a slide coated with 1% agar containing 0.75 M NaCl, and observed it under a microscope.
Microscopic examination.
Samples were examined under a Microphot-SA microscope (Nikon Corporation, Tokyo, Japan) equipped with phase-contrast optics and an epifluorescence unit. For unstained and plasmolyzed cells, phase-contrast optics alone were used. For cells stained with FM 4-64, FITC, and acridine orange, epifluorescence light with the appropriate filters was used, and for DAPI staining, cells were viewed simultaneously by phase-contrast and epifluorescence microscopy, as described by Hiraga et al. (13). In all cases, a ×100 objective was used with immersion oil, giving a total magnification of ×1,000. Images were captured with a 12-bit 1,392- by 1,040-pixel monochrome charge-coupled device camera (CoolSnap Procf) and were processed with Image-Pro Plus, v. 4.5 (Media Cybernetics, Silver Spring, Md.).
Quantification of protein released after pressurization.
Suspensions of exponential- and stationary-phase cells grown as described above were adjusted to a final concentration of approximately 2 × 109 cells/ml in PBS and were pressure treated at either 200 MPa for 8 min (exponential- and stationary-phase cells) or 600 MPa for 8 min (stationary-phase cells only). Five hundred microliters of the pressurized samples was centrifuged at 3,000 × g for 20 min at 4°C to pellet whole cells. Pellets were resuspended in 500 μl of sterile PBS, and the protein concentrations in both fractions (supernatant and cells) were quantified by the Bradford assay (Sigma), with bovine serum albumin as the standard.
RESULTS
Resistance to high hydrostatic pressure.
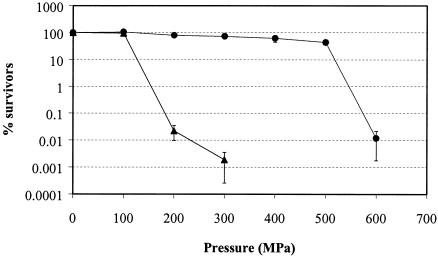
The inactivation of exponential- and stationary-phase cells of E. coli J1 after treatment for 8 min at different pressures up to 600 MPa is shown in Fig. 1. Stationary-phase cells were much more resistant to high pressure than were exponential-phase cells. For example, after 8 min at 300 MPa, >70% of stationary-phase cells remained viable compared to <0.01% of exponential-phase cells. No decrease in viability was observed after pressurization at 100 MPa for either exponential- or stationary-phase cells. Pressures higher than 100 MPa caused a sharp decrease in the viability of exponential-phase cells, while stationary-phase cells survived up to 500 MPa with little loss of viability (viability reduction of 57%). Treatment at 600 MPa inactivated 99.99% of stationary-phase cells. We cannot discount a combined effect of pressure plus heat at the higher pressures tested. Although nonlethal temperatures are reached during adiabatic heating due to the pressure increase, i.e., 43°C at 600 MPa and 40°C at 500 MPa, other authors have demonstrated that pressure treatments at moderately high temperatures are more bactericidal than those at low temperatures (7).
FIG. 1.
Pressure resistance of E. coli J1 during the exponential (▴) and stationary (•) phase. Data shown are percentages of survivors after 8 min of treatment at different pressures. Each point corresponds to the mean of at least three independent experiments. The initial inoculum levels were 5 × 109 cells/ml for stationary-phase cells and 108 cells/ml for exponential-phase cells.
In the next series of experiments, exponential-phase and stationary-phase cells were compared after treatments that resulted in approximately the same degree of inactivation (4- to 5-log decrease). Exponential-phase cells were treated at 200 and 300 MPa, and stationary-phase cells were treated at 600 MPa. We found that the various changes in exponential-phase cells were evident after treatment at 200 MPa, but since the appearance of the cells treated at 200 MPa was essentially similar to that of those treated at 300 MPa, either pressure was chosen for illustrative purposes for the figures.
Morphological changes visible under phase-contrast optics.
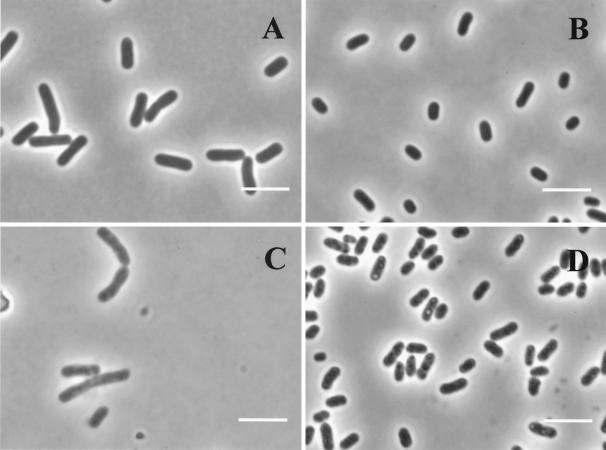
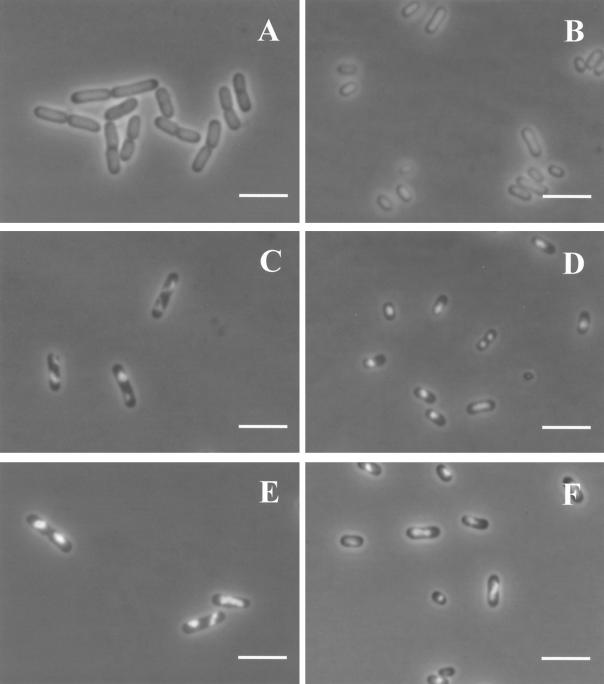
Figure 2 shows the effect of pressure on the appearance of cells viewed by phase-contrast microscopy. Untreated stationary-phase cells (Fig. 2B) were smaller and more rounded than those in the exponential phase (Fig. 2A), as reported by others, but were not spherical in form as occurs after starvation in a minimal medium (15). The appearances of exponential- and stationary-phase cells after pressure treatments at 300 and 600 MPa, respectively, are shown in Fig. 2C and D. Untreated cells of both types presented a dark homogeneous cytoplasm. Pressurization caused a general lightening of the cytoplasm, which was more evident in exponential-phase cells (Fig. 2C) than in stationary-phase ones (Fig. 2D). Exponential-phase cells showed a slight granularity of the cytoplasm, but in stationary-phase cells, distinct small rounded areas of high refractive ability were observed (Fig. 2D). An image analysis of the cells before and after pressurization showed no significant changes in size (cross-sectional area) for either exponential- or stationary-phase cells (data not shown).
FIG. 2.
Effect of pressure on general appearance of E. coli J1 by phase-contrast microscopy of exponential- and stationary-phase cells. (A) Untreated exponential-phase cells (100% viable cells); (B) untreated stationary-phase cells (100% viable cells); (C) exponential-phase cells treated with 300 MPa for 8 min (0.002% viable cells); (D) stationary-phase cells treated with 600 MPa for 8 min (0.01% viable cells). Bars, 2 μm.
Membrane integrity of pressure-treated cells.
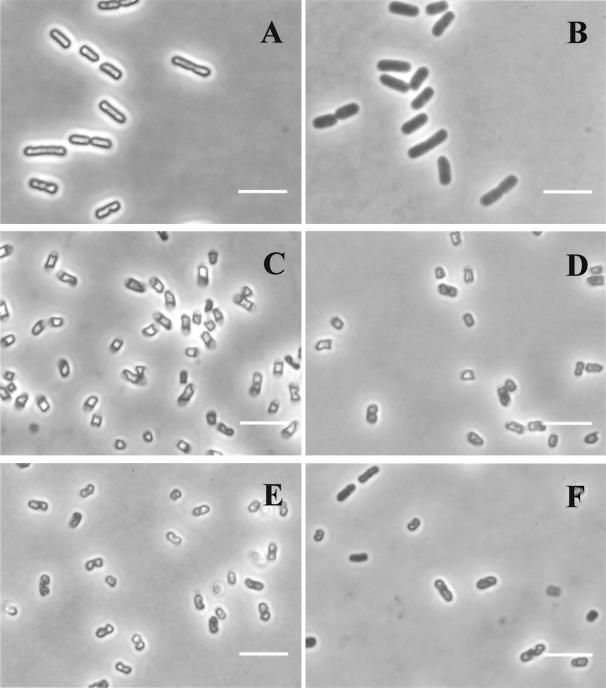
Figure 3 shows the osmotic responses of untreated and pressurized cells. Untreated cells that possessed an intact cytoplasmic membrane showed a very refractile cytoplasm when placed on agar containing 0.75 M NaCl which was caused by the loss of water and consequent concentration of the cytoplasm under hypertonic conditions (Fig. 3A and C). Exponential-phase cells appeared uniformly refractile and slightly shrunken in appearance (Fig. 3A), whereas in stationary-phase cells the refractile cytoplasm had apparently become separated from the poles of the cell (Fig. 3C). In cells with a nonfunctional membrane, water and salt can pass freely through the membrane, and there is no osmotic response and no condensation of the cytoplasm, which therefore appears dark gray. Exponential-phase cells pressurized at 100 MPa remained viable and retained the ability to respond to an osmotic challenge (data not shown), but those pressurized at 200 MPa, by which >99.99% of the population was killed, had completely lost the osmotic response (Fig. 3B). Stationary-phase cells maintained the ability to plasmolyze with all treatments up to 500 MPa (Fig. 3D and E, in which the percentages of viable cells were 79 and 43%, respectively). Only the cells pressurized at 600 MPa showed an effect of pressure on osmotic responsiveness, as can be seen in Fig. 3F, in which some of the cells do not show a refractile cytoplasm. However, although >99.99% of the cells pressurized at 600 MPa were dead, approximately 60% were still able to plasmolyze.
FIG. 3.
Effect of pressure on the osmotic response of E. coli J1. Phase-contrast photomicrographs of exponential- and stationary-phase cells placed on agar containing 0.75 M NaCl are shown. (A) Untreated exponential-phase cells (100% viable cells); (B) exponential-phase cells treated with 200 MPa for 8 min (0.02% viable cells); (C) untreated stationary-phase cells (100% viable cells); (D) stationary-phase cells treated with 200 MPa for 8 min (79% viable cells); (E) stationary-phase cells treated with 500 MPa for 8 min (43% viable cells); (F) stationary-phase cells treated with 600 MPa for 8 min (0.01% viable cells). Bars, 2 μm.
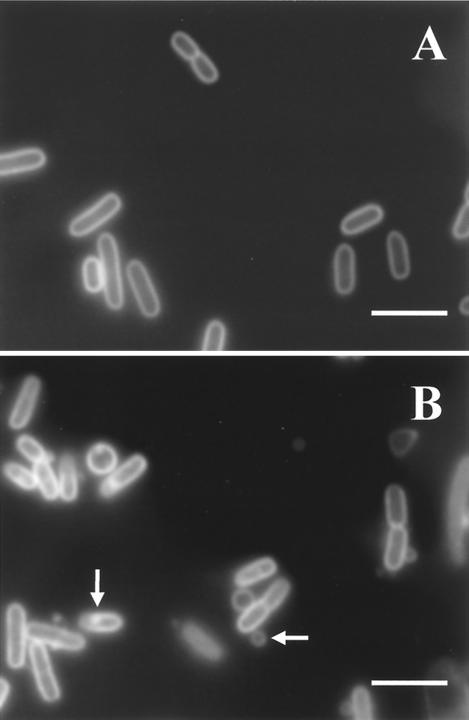
Figure 4 shows the appearances of untreated and pressurized (300 MPa) exponential-phase cells stained with FM 4-64, a lipophilic dye that binds to both outer and cytoplasmic membranes, but preferentially to the cytoplasmic membrane (9). Pressurization caused a visible disruption of the envelopes of exponential-phase cells, including the formation of vesicles of a lipidic material coming out from the cells and also an appearance of membrane thickening and the formation of local structures resembling invaginations protruding into the cytoplasm. However, for stationary-phase cells, no structural changes in the membrane were detected within the range of pressures studied (data not shown).
FIG. 4.
Effect of pressure on membrane integrity of exponential-phase cells of E. coli J1. Fluorescence photomicrographs of cells stained with the lipophilic membrane probe FM 4-64 are shown. (A) Untreated cells (100% viable cells); (B) cells pressurized at 300 MPa for 8 min (0.002% viable cells). Bars, 2 μm. Arrows show internal and external vesicles.
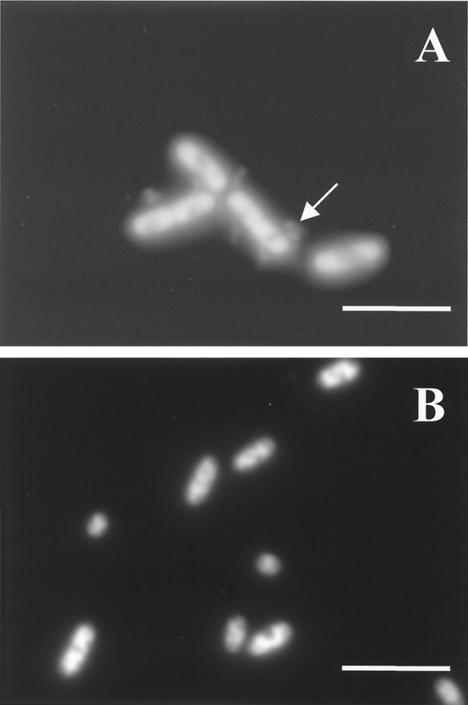
Effect of pressure on the nucleoid.
Figure 5 shows phase-contrast-fluorescence micrographs of untreated and pressurized cells stained with DAPI, a permeative intercalating probe that binds to DNA. Staining with DAPI revealed gross changes in the nucleoid conformation for both types of cells. In untreated exponential- or stationary-phase cells (Fig. 5A and B), the fluorescence was evenly distributed inside the cytoplasm. However, pressures higher than 100 MPa caused condensation of the nucleoid, which was very dramatic in exponential-phase cells pressurized at 200 MPa or more (Fig. 5C and E). Condensation of the nucleoid also occurred in stationary-phase cells, starting at 200 MPa, and became progressively more intense at increasing pressures (Fig. 5D and F). In pressure-treated cells, the nucleoids were often skewed across the cell or pulled to one side next to the membrane.
FIG. 5.
Effect of pressure on nucleoid configuration of E. coli J1. Phase-contrast-fluorescence photomicrographs of exponential- and stationary-phase cells stained with DAPI are shown. (A) Untreated exponential-phase cells (100% viable cells); (B) untreated stationary-phase cells (100% viable cells); (C) exponential-phase cells treated with 300 MPa for 8 min (0.002% viable cells); (D) stationary-phase cells treated with 300 MPa for 8 min (73% viable cells); (E) exponential-phase cells treated with 400 MPa for 8 min (<0.0001% viable cells); (F) stationary-phase cells treated with 400 MPa for 8 min (61% viable cells). Bars, 2 μm.
Effect of pressure on RNA distribution.
For the examination of RNA conformations, cells were stained with acridine orange, which binds to single- or double-stranded nucleic acids, giving red or green fluorescence, respectively. DNA thus appears green and RNA appears red. When we used this dye, a long-pass filter (590 nm) was placed in the light path to allow for selective visualization of the red-staining RNA. Untreated cells stained rather poorly with acridine orange, but the red fluorescence (RNA) was evenly distributed throughout the cytoplasm, with no evident areas of higher intensity (data not shown). In pressure-treated exponential-phase cells, the red-stained material had a condensed appearance (Fig. 6A). It was also noticeable that a considerable amount of red-stained material was released into the extracellular medium. This was observed only for exponential-phase cells (Fig. 6A), and no release of material was observed for pressurized stationary-phase cells (Fig. 6B).
FIG. 6.
Effect of pressure on RNA configuration of E. coli J1. Fluorescence photomicrographs of exponential- and stationary-phase cells stained with acridine orange are shown. (A) Exponential-phase cells treated with 200 MPa for 8 min (0.02% viable cells); (B) stationary-phase cells treated with 600 MPa for 8 min (0.01% viable cells). Bars, 2 μm. The arrow indicates stained material released from the cell.
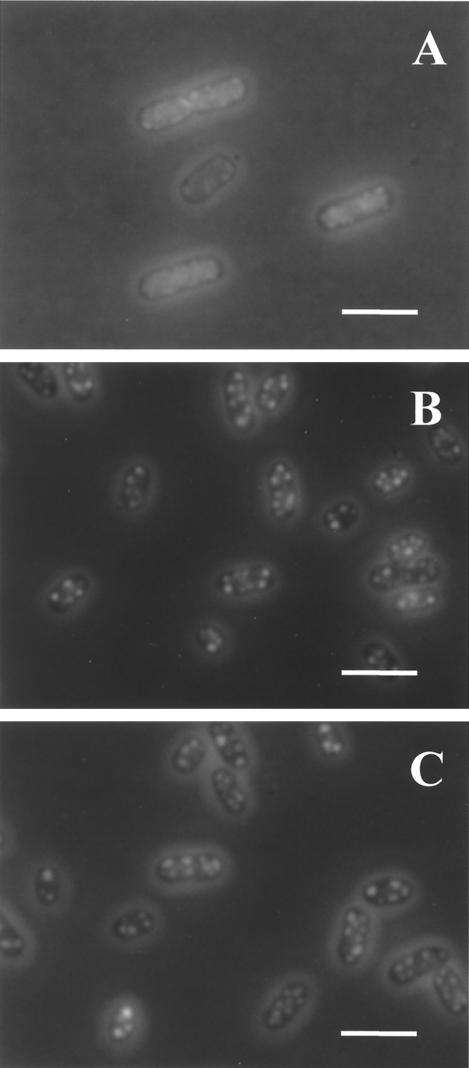
Effect of pressure on intracellular protein.
Figure 7 shows cells stained with FITC, a fluorescent dye that binds specifically to proteins. Untreated cells had an evenly distributed low-intensity fluorescence within the cytoplasm (data not shown). Pressurization caused the condensation of proteins in both exponential- and stationary-phase cells. In exponential-phase cells, clumps of aggregated protein were distributed inside the whole cell but were preferentially close to the membrane (Fig. 7A). In stationary-phase cells, protein aggregates were bigger and more intensely stained (Fig. 7B and C). The protein aggregates appeared in the whole population of cells at pressures as low as 200 MPa, and their appearance did not change appreciably at higher pressures. The amount of protein released from cells after pressurization was also measured, and the results are shown in Table 1. Exponential-phase cells lost a much larger proportion of cellular protein to the external medium than did stationary-phase cells. No attempt was made to solubilize protein aggregates present in the cell, which may have led to an underestimate of the protein content of pressure-treated cells and probably explains why the amount of cytoplasmic protein apparently decreased more than the amount lost from the cell.
FIG. 7.
Effect of pressure on cellular protein of E. coli J1. Phase-contrast-fluorescence photomicrographs of exponential- and stationary-phase cells stained with FITC are shown. (A) Exponential-phase cells treated with 200 MPa for 8 min (0.02% viable cells); (B) stationary-phase cells treated with 300 MPa for 8 min (73% viable cells); (C) stationary-phase cells treated with 600 MPa for 8 min (0.01% viable cells). Bars, 2 μm.
TABLE 1.
Protein content of whole cells and pressurizing medium (supernatant) of untreated and pressurized exponential- and stationary-phase cells of E. coli J1
| Phase | Sample type | Protein content (μg/ml) with indicated treatment
|
||
|---|---|---|---|---|
| Untreated | 200 MPa | 600 MPa | ||
| Exponential | Supernatant | 0 | 37 | NDa |
| Cells | 188 | 113 | NDa | |
| Stationary | Supernatant | 0 | 3 | 12 |
| Cells | 192 | 129 | 115 | |
ND, not determined.
DISCUSSION
In an attempt to distinguish between events that are associated with a loss of viability and those that are not, we examined several morphological and physiological changes that occur when cells of E. coli are exposed to different pressures and compared these changes to the loss of viability of exponential- and stationary-phase cells. The results are summarized in Table 2.
TABLE 2.
Morphological and physiological changes of E. coli strain J1 cells exposed to different pressures for 8 min
| Event | Pressure (MPa) at onset of event
|
|
|---|---|---|
| Exponential- phase cells | Stationary- phase cells | |
| Loss of osmotic responsiveness | 100-200 | 600 (partial) |
| Visible changes in membrane structure (blebs, invaginations) | 100-200 | Not observed |
| Loss of RNA from cell | 100-200 | Not observed |
| Condensation of the nucleoid | 100-200 | 100-200 |
| Formation of protein aggregates | 100-200 | 100-200 |
| Cell death (>90%) | 100-200 | 500-600 |
The pressure for the onset of rapid inactivation was between 100 and 200 MPa for exponential-phase cells and between 500 and 600 MPa for stationary-phase cells, a difference of 400 MPa. This difference in resistance is very similar to that seen for exponential- and stationary-phase cells of E. coli O157 strain C9490 (24). In general, the resistance of exponential-phase cells varies very little between strains (4, 24), but large differences occur in stationary-phase cells, due mainly to differences in rpoS status (29), and this has a large effect on the relative resistances of exponential- and stationary-phase cells.
The main differences in the accompanying morphological and physiological changes were as follows: a complete loss of the plasmolysis response occurred in exponential-phase cells between 100 and 200 MPa, whereas only a partial loss was seen in stationary-phase cells at the much higher pressure of 600 MPa; and a visible perturbation of membrane structure and a loss of RNA occurred at 100 to 200 MPa in exponential-phase cells, but corresponding changes were absent from stationary-phase cells. Condensation of the nucleoid and the formation of protein aggregates occurred in both cell types with about 200 MPa of pressure.
In this work, we observed markedly different behaviors of the membranes of exponential- and stationary-phase cells toward pressurization. Membranes of exponential-phase cells showed physical disruption after pressurization, with the formation of vesicles, areas of engrossment, and also invaginations toward the cytoplasm (Fig. 4). The observed vesicles resembled those observed by Katsui et al. (17) with heat-treated cells, which were formed from lipids from the outer membrane. It is possible that vesicles observed in pressurized exponential-phase cells are from outer membrane materials, whereas engrossment areas, which occupy part of the cytoplasm, are from the cytoplasmic membrane (Fig. 4). The membranes of stationary-phase cells remained virtually intact. Pagán and Mackey (24) previously suggested that the role of the cytoplasmic membrane as a critical target for the inactivation of cells by high pressure was different in exponential- and stationary-phase cells, but the reasons for the apparent differences in membrane behavior in response to pressure are unknown.
Recent work on the role of membrane fluidity on cellular pressure resistance (5) provided evidence that pressure resistance increases with membrane fluidity. However, membrane fluidity seems to be less important for stationary-phase cells than for exponential-phase ones, because unspecified changes, possibly linked to RpoS activity, occur during stationary phase that have a greater influence on pressure resistance than membrane fluidity and hence mask its effect (5, 29). In fact, according to the microscopic studies of Beney et al. (3) on phospholipid vesicles, the factor that determines whether gross structural perturbations of the membrane vesicles occur under pressure is the difference in compressibility between the membrane and the water inside. Those authors demonstrated that vesicles with a more compressible membrane lose part of their aqueous content during pressurization and change in shape (produce buds) upon decompression due to the excess of membrane in relation to the remaining aqueous content. However, vesicles containing cholesterol, which have less compressible membranes, undergo a smaller decrease in volume and do not change in shape upon decompression. Therefore, if bacterial cells were to behave somewhat like these vesicles, one could infer that exponential-phase cells would have more compressible membranes than those in stationary phase, and in consequence, would undergo more drastic changes in shape and form vesicles from the excess membrane material upon decompression. Conversely, stationary-phase cells would be expected to have less compressible membranes or ones that could resist the loss of material after the release of pressure.
When cells enter the stationary phase of growth, they develop a generalized resistance to a variety of stresses, partly due to the action of the alternative sigma factor σs, which controls the transcription of >50 genes (15). A range of morphological and physiological changes have been described that might possibly account for the increase in pressure resistance. The cell envelope (outer membrane, cell wall, and cytoplasmic or inner membrane) drastically changes upon entry into the stationary phase. There is an increase in cardiolipin, synthesized from phosphatidylglycerol (8), and the unsaturated fatty acids are converted into their cyclopropyl fatty acid derivatives (11). Stationary-phase cells also have a higher protein/lipid ratio in their membranes, which makes them less prone to lateral phase separation (37), and a higher degree of cross-linking among membrane proteins (22). Also, the accumulation of trehalose, the increased level of stabilizing polyamines, and the thickening of the wall during stationary phase (four to five layers of peptidoglycan compared to only two or three during growth) (15, 22) could contribute to the high level of stability of the cell envelopes of stationary-phase cells.
From the results obtained in this work, we can conclude that in exponential-phase cells the loss of viability is always accompanied by a loss of the physical integrity of the membrane, whereas in stationary-phase cells membranes can remain physically intact, even in dead cells. This leads to the suggestion that the inactivation of stationary-phase cells by high pressure may occur by a mechanism other than the permanent loss of membrane integrity.
One of the most obvious changes in both exponential- and stationary-phase cells was the condensation of the nucleoid (Fig. 5). The condensed nucleoids of pressure-treated cells were often irregularly positioned within the cell, unlike those seen in chloramphenicol-treated cells, which are generally rather symmetrical and central to the cell axis. This asymmetric appearance has also been observed with pressure-treated cells of S. enterica serovar Thompson examined by electron microscopy (20). Condensation of the nucleoid was also observed for the gram-positive organisms Listeria monocytogenes and Lactobacillus plantarum (20, 39). Exponential-phase cells showed a more drastic change in nucleoid shape than those in the stationary phase, possibly due to the absence of compacting DNA-binding proteins, such as Dps, which is synthesized in response to oxidative stress but is also synthesized during stationary phase under the complex control of the stationary-phase regulator σs (15). The difference in appearance of the nucleoid could also be explained by a different initial conformation of the DNA in exponential-phase cells, in which active transcription and translation are continuously taking place in the cytoplasm. There may also be attachments between some nascent proteins and the membrane. In any case, after 8 min at 400 MPa, about 60% of stationary-phase cells were still alive, but >90% of the cells contained a highly condensed nucleoid. We can conclude that in exponential-phase cells the loss of viability is correlated with changes in nucleoid conformation, but for stationary-phase cells this morphological change occurs at pressures below those which cause death. The most straightforward conclusion from these results is that changes in the nucleoid are not related to cell death in stationary-phase cells.
It is noteworthy that neither the loss of membrane integrity nor nucleoid condensation happened at 100 MPa. A pressure value between 100 and 200 MPa seemed to be the threshold for the initiation of nucleoid changes in both types of cells, despite the enormous difference in pressure sensitivity between them. Previous work (24) demonstrated that stationary-phase cell membranes become permeable during pressurization but are able to reseal afterwards. This transient permeabilization phenomenon happened only at pressures above 100 MPa. We can speculate, then, that some changes happening in the cells at pressures above 100 MPa could be indirectly related to the permeabilization of the membrane during pressurization and to consequent changes in the intracellular environment. For example, membrane damage could lead to the loss of magnesium ions, which are essential for the maintenance of nucleoid and ribosome structure and are known to protect cells against high-pressure inactivation (12).
Staining with acridine orange revealed further changes in the cytoplasm of the cells. Exponential-phase cells lost RNA to the extracytoplasmic medium (Fig. 6A). This leakage was never observed with stationary-phase cells. These results confirm that membranes of exponential-phase cells are much more pressure sensitive than are those of stationary-phase cells and also indicate that both cytoplasmic and outer membranes are extensively damaged, allowing the leakage of large molecules such as RNA from the cytoplasm. In stationary-phase cells, pressurization caused the appearance of strongly fluorescent areas of condensed RNA inside the cytoplasm, which could reflect changes in ribosome conformation. This would agree with calorimetric studies showing that pressure causes denaturation of the ribosome (23).
The cytoplasm of pressure-treated stationary-phase cells contained numerous protein-containing aggregates throughout the cytoplasm, often close to the membrane (Fig. 7B and C). Further work will be needed to determine the types of protein contained in the aggregates and to establish whether other components, such as RNA, are also present. This coagulated protein was also observed in exponential-phase cells, but to a lesser extent. This may be explained partly by the fact that exponential-phase cells lose a much larger amount of protein to the extracellular medium (Table 1), but it could also possibly be caused by a different state of the cytoplasmic protein in the two types of cell. The amount and distribution of denatured protein in stationary-phase cells appeared to be similar in cells pressurized at nonlethal (300 MPa) and lethal (600 MPa) pressures, indicating again that this kind of damage is not lethal for the cell.
From all these data, we can conclude that exponential- and stationary-phase cells undergo several common changes, such as condensation of the nucleoid and the aggregation of protein, but there are some other changes that are specifically associated with exponential-phase cells and are correlated with the loss of viability of these cells. These include all of the changes related to membrane integrity, such as the formation of vesicles and irregularities in the membrane ultrastructure, the loss of osmotic responsiveness, and the loss of protein and RNA to the external medium. Other workers have also suggested that the cell membrane is a critical target for the inactivation of cells by high pressure and have suggested, for example, that death may be associated with the loss of specific membrane functions, such as ATPase activity or multidrug efflux systems (34, 38). From the results presented here, we propose a simpler explanation, at least for exponential-phase cells: we suggest that death results from a catastrophic and irreversible loss of physical membrane integrity.
In contrast, the ability of stationary-phase membranes to reseal after pressure treatment may allow a proportion of the cell population to maintain homeostasis and to reverse the deleterious effects, such as protein aggregation or nucleoid condensation, of pressure. The ability of stationary-phase cells to recover after pressure treatment may also be assisted by the presence of molecular chaperones, such as the DnaK protein, that have an essential role in stress resistance and are specifically induced during stationary phase (30, 31). However, after a certain pressure level, at about 600 MPa, the damage is irreparable in a majority of the population and there is a drastic decrease in viability. Further work is required to clarify the causes of this drastic decrease in the viability of stationary-phase cells.
Acknowledgments
We thank the European Commission for providing Pilar Mañas with a Marie Curie postdoctoral grant in the Quality of Life program.
REFERENCES
- 1.Alpas, H., N. Kalchayanand, F. Bozoglu, A. Sikes, C. P. Dunne, and B. Ray. 1999. Variation in resistance to hydrostatic pressure among strains of food-borne pathogens. Appl. Environ. Microbiol. 65:4248-4251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beney, L., and P. Gervais. 2001. Influence of the fluidity of the membrane on the response of microorganisms to environmental stresses. Appl. Microbiol. Biotechnol. 57:34-42. [DOI] [PubMed] [Google Scholar]
- 3.Beney, L., J. M. Perrier-Cornet, M. Hayert, and P. Gervais. 1997. Shape modification of phospholipid vesicles induced by high pressure: influence of bilayer compressibility. Biophys. J. 72:1258-1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benito, A., G. Ventoura, M. Casadei, T. Robinson, and B. Mackey. 1999. Variation in resistance of natural isolates of Escherichia coli O157 to high hydrostatic pressure, mild heat and other stresses. Appl. Environ. Microbiol. 65:1564-1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Casadei, M. A., P. Mañas, G. W. Niven, E. Needs, and B. M. Mackey. 2002. Role of membrane fluidity in pressure resistance of Escherichia coli NCTC 8164. Appl. Environ. Microbiol. 68:5965-5972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheftel, J. C. 1995. Review: high-pressure, microbial inactivation and food preservation. Food Sci. Technol. Int. 1:75-90. [Google Scholar]
- 7.Chen, H., and D. G. Hoover. 2003. Modelling the combined effect of high hydrostatic pressure and mild heat on the inactivation kinetics of Listeria monocytogenes Scott A in whole milk. Innovative Food Sci. Emerg. Technol. 4:25-34. [Google Scholar]
- 8.El-Khani, M. A., and R. J. Stretton. 1981. Effect of growth medium on the lipid composition of log and stationary phase cultures of Salmonella typhimurium. Microbios 31:161-170. [PubMed] [Google Scholar]
- 9.Fishov, I., and C. L. Woldringh. 1999. Visualization of membrane domains in Escherichia coli. Mol. Microbiol. 32:1166-1172. [DOI] [PubMed] [Google Scholar]
- 10.García-Graells, C., B. Masschalck, and C. W. Michiels. 1999. Inactivation of Escherichia coli in milk by high-hydrostatic pressure treatment in combination with antimicrobial peptides. J. Food Prot. 62:1248-1254. [DOI] [PubMed] [Google Scholar]
- 11.Grogan, D. W., and J. E. Cronan. 1997. Cyclopropane ring formation in membrane lipids of bacteria. Microbiol. Mol. Biol. Rev. 61:429-441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hauben, K. J. A., K. Bernaerts, and C. W. Michiels. 1998. Protective effect of calcium on inactivation of Escherichia coli by high hydrostatic pressure. J. Appl. Microbiol. 85:678-684. [DOI] [PubMed] [Google Scholar]
- 13.Hiraga, S., H. Niki, T. Ogura, C. Ichinose, H. Mori, B. Ezaki, and A. Jaffé. 1989. Chromosome partitioning in Escherichia coli: novel mutants producing anucleate cells. J. Bacteriol. 171:1496-1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoover, D. 1997. Minimally processed fruits and vegetables: reducing microbial load by non-thermal physical treatments. Food Technol. 51:66-71. [Google Scholar]
- 15.Huisman, G. W., D. A. Siegele, M. M. Zambrano, and R. Kolter. 1996. Morphological and physiological changes during stationary phase, p. 1672-1682. In F. C. Neidhardt et al. (ed.), Escherichia coli and Salmonella: cellular and molecular biology. ASM Press, Washington, D.C.
- 16.Kato, M., and R. Hayashi. 1999. Effects of high pressure on lipids and biomembranes for understanding high-pressure-induced biological phenomena. Biosci. Biotechnol. Biochem. 63:1321-1328. [DOI] [PubMed] [Google Scholar]
- 17.Katsui, N., T. Tsuchido, R. Hiramatsu, S. Fujikawa, M. Takano, and I. Shibasaki. 1982. Heat-induced blebbing and vesiculation of the outer membrane of Escherichia coli. J. Bacteriol. 151:1523-1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Knorr, D. 1993. Effects of high-hydrostatic-pressure processes on food safety and quality. Food Technol. 47:156-161. [Google Scholar]
- 19.Ludwig, H., W. Scigalla, and B. Sojka. 1996. Pressure- and temperature-induced inactivation of microorganisms, p. 346-363. In J. L. Markley, D. B. Northrup, and C. A. Royer (ed.), High pressure effects in molecular biophysics and enzymology. Oxford University Press, Oxford, United Kingdom.
- 20.Mackey, B., K. Forestière, N. S. Isaacs, R. Stenning, and B. Brooker. 1994. The effect of high hydrostatic pressure on Salmonella thompson and Listeria monocytogenes examined by electron microscopy. Lett. Appl. Microbiol. 19:429-432. [Google Scholar]
- 21.Masschalck, B., R. Van Houdt, E. G. R. Van Haver, and C. W. Michiels. 2001. Inactivation of gram-negative bacteria by lysozyme, denatured lysozyme, and lysozyme-derived peptides under high hydrostatic pressure. Appl. Environ. Microbiol. 67:339-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mirelman, D., and R. C. Siegel. 1979. Oxidative deamination of aminolysine residues and formation of Schiff base cross linkages in cell envelopes of Escherichia coli. J. Biol. Chem. 254:571-574. [PubMed] [Google Scholar]
- 23.Niven, G. W., C. A. Miles, and B. M. Mackey. 1999. The effects of hydrostatic pressure on ribosome conformation in Escherichia coli: an in vivo study using differential scanning calorimetry. Microbiology 145:419-425. [DOI] [PubMed] [Google Scholar]
- 24.Pagán, R., and B. M. Mackey. 2000. Relationship between membrane damage and cell death in pressure-treated Escherichia coli cells: differences between exponential and stationary phase cells and variation among strains. Appl. Environ. Microbiol. 66:2829-2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pagán, R., S. Jordan, A. Benito, and B. M. Mackey. 2001. Enhanced acid sensitivity of pressure-damaged Escherichia coli O157 cells. Appl. Environ. Microbiol. 67:1983-1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park, S. W., K. H. Sohn, J. H. Shin, and H. J. Lee. 2001. High hydrostatic pressure inactivation of Lactobacillus viridescens and its effect on ultrastructure of cells. Int. J. Food Sci. Technol. 36:775-781. [Google Scholar]
- 27.Patterson, M. 1999. High-pressure treatment of foods, p. 1059-1065. In R. K. Robertson, A. Batt, and P. D. Patel (ed.), The encyclopedia of food microbiology. Academic Press, London, United Kingdom.
- 28.Ritz, M., M. Feulet, N. Orange, and M. Federighi. 2000. Effects of high hydrostatic pressure on membrane proteins of Salmonella typhimurium. Int. J. Food Microbiol. 55:115-119. [DOI] [PubMed] [Google Scholar]
- 29.Robey, M., A. Benito, R. H. Hutson, C. Pascual, S. F. Park, and B. M. Mackey. 2001. Variation in resistance to high hydrostatic pressure and rpoS heterogeneity in natural isolates of Escherichia coli O157:H7. Appl. Environ. Microbiol. 67:4901-4907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rockabrand, D., T. Arthur, G. Korinek, K. Livers, and P. Blum. 1995. An essential role for the Escherichia coli DnaK protein in starvation-induced thermotolerance, H2O2 resistance, and reductive division. J. Bacteriol. 177:3695-3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rockabrand, D., K. Livers, T. Austin, R. Kaiser, D. Jensen, R. Burgess, and P. Blum. 1998. Roles of DnaK and RpoS in starvation-induced thermotolerance of Escherichia coli. J. Bacteriol. 180:846-854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.San Martin, M. F., G. V. Barbosa-Canovas, and B. G. Swanson. 2003. Food processing by high hydrostatic pressure. Crit. Rev. Food Sci. Nutr. 42:627-645. [DOI] [PubMed] [Google Scholar]
- 33.Shigehisa, T., T. Ohmori, A. Saito, S. Taji, and R. Hayashi. 1991. Effects of high hydrostatic pressure on characteristics of pork slurries and inactivation of microorganisms associated with meat products. Int. J. Food Microbiol. 12:207-216. [DOI] [PubMed] [Google Scholar]
- 34.Smelt, J. P. P. M., A. G. F. Rijke, and A. Hayhurst. 1994. Possible mechanism of high-pressure inactivation of microorganisms. High Pressure Res. 12:199-203. [Google Scholar]
- 35.Smelt, J. P. P. M. 1998. Recent advances in the microbiology of high pressure processing. Trends Food Sci. Technol. 9:152-158. [Google Scholar]
- 36.Sonoike, K., T. Setoyama, Y. Kuma, and S. Kobayashi. 1992. Effect of pressure and temperature on the death rate of Lactobacillus casei and Escherichia coli, p. 297-301. In C. Balny, R. Hayashi, K. Heremans, and P. Masson (ed.), High pressure and biotechnology. Proceedings of the First European Seminar on High Pressure and Biotechnology. Colloque INSERM/John Libbey Eurotext Ltd., Le Grande Motte, France.
- 37.Souzu, H. 1986. Fluorescence polarization studies on Escherichia coli membrane stability and its relation to the resistance of the cell to freeze-thawing. I. Membrane stability in cells of different growth phase. Biochim. Biophys. Acta 861:353-360. [DOI] [PubMed] [Google Scholar]
- 38.Ulmer, H. M., H. Herberhold, S. Fahsel, M. G. Ganzle, R. Winter, and R. F. Vogel. 2002. Effect of pressure-induced membrane phase transitions on inactivation of HorA, an ATP-dependent multidrug resistance transporter, in Lactobacillus plantarum. Appl. Environ. Microbiol. 68:1088-1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wouters, P. C., E. Glaasker, and J. P. P. M. Smelt. 1998. Effects of high pressure on inactivation kinetics and events related to proton efflux in Lactobacillus plantarum. Appl. Environ. Microbiol. 64:509-514. [DOI] [PMC free article] [PubMed] [Google Scholar]