Abstract
Objectives
To explore oxygenation and ventilation status early after cardiac arrest in infants and children. We hypothesize that hyperoxia is common and associated with worse outcome after pediatric cardiac arrest.
Design
Retrospective cohort study.
Setting
Fifteen hospitals within the Pediatric Emergency Care Applied Research Network.
Patients
Children who suffered a cardiac arrest event and survived for at least 6 hours after return of circulation.
Interventions
None.
Measurements and Main Results
Analysis of 195 events revealed that abnormalities in oxygenation and ventilation are common during the initial 6 hours after pediatric cardiac arrest. Hyperoxia was frequent, affecting 54% of patients. Normoxia was documented in 34% and hypoxia in 22% of patients. These percentages account for a 10% overlap of patients who had both hyperoxia and hypoxia. Ventilation status was more evenly distributed with hyperventilation observed in 38%, normoventilation in 29%, and hypoventilation in 46%, with a 13% overlap of patients who had both hyperventilation and hypoventilation. Derangements in both oxygenation and ventilation were common early after cardiac arrest such that both normoxia and normocarbia were documented in only 25 patients (13%). Neither oxygenation nor ventilation status was associated with outcome. After controlling for potential confounders, arrest location and rhythm were significantly associated with worse outcome; however, hyperoxia was not (odds ratio for good outcome, 1.02 [0.46, 2.84]; p = 0.96).
Conclusions
Despite recent resuscitation guidelines that advocate maintenance of normoxia and normoventilation after pediatric cardiac arrest, this is uncommonly achieved in practice. Although we did not demonstrate an association between hyperoxia and worse outcome, the small proportion of patients kept within normal ranges limited our power. Preclinical data suggesting potential harm with hyperoxia remain compelling, and further investigation, including prospective, large studies involving robust recording of physiological derangements, is necessary to further advance our understanding of this important topic.
Keywords: hypercarbia, hyperoxia, hyperventilation, hypocarbia, hypoxia, resuscitation
Cardiac arrest (CA) affects approximately eight per 100,000 children in North America annually and causes high mortality and morbidity (1). Out-of-hospital (OH) CA has reported survival to hospital discharge of 2% to 12%, with intact neurological function in only 2% to 4% (1–3). In-hospital (IH) CA has slightly better outcomes with reported survival of approximately 25% and favorable neurological outcome in approximately 18% (4, 5). Treatment after CA is largely supportive and directed toward minimizing end-organ injury. Aside from rapid, effective cardiopulmonary resuscitation (CPR) and application of extracorporeal membrane oxygenation (ECMO) in select populations, no specific therapy has been shown to improve survival or outcomes after CA in children.
Supplemental oxygen therapy remains central to care during and after resuscitation. An association between hypoxia and poor outcome after CA has long been accepted. However, more recently, concerns about potentially detrimental effects of hyperoxia have been raised. Neonatal resuscitation reports show worse long-term outcomes with greater inspired concentrations of oxygen and higher oxygen saturations during resuscitation (6, 7). Similarly, some adult studies link hyperoxia with poorer outcomes (8, 9), although others do not (10).
The 2010 American Heart Association (AHA) Neonatal Resuscitation Guidelines recommend beginning resuscitation with room air for term infants (or a blended mixture of oxygen and air for preterm infants) with subsequent titration of inspired oxygen fraction based on pulse oximetry values (11). Similarly, the 2010 AHA Adult Advanced Cardiac Life Support Guidelines recommend initial resuscitation with 100% oxygen and then titration of inspired oxygen fraction after return of circulation (ROC) to target oxygen saturation greater than or equal to 94% (12, 13). Although investigations of hyperoxia and outcomes after CA in children are lacking, the 2010 AHA Pediatric Advanced Life Support Guidelines similarly recommend initial resuscitation with 100% oxygen and consideration of titrating oxygen delivery after ROC to achieve oxygen saturation greater than or equal to 94% (14).
Ventilatory support, another mainstay of resuscitation and supportive care, has also been linked with altered outcomes after CA. Hyperventilation reduces cerebral blood flow and is associated with hypotension during resuscitation (15). The 2010 AHA Adult Advanced Cardiac Life Support and Post-Cardiac Arrest Care Guidelines advocate avoiding hypocarbia (12, 13). Similarly, the 2010 AHA Pediatric Advanced Life Support Guidelines state that hyperventilation has no benefit and may be harmful (14). Reports of hyperventilation after CA in children are lacking; however, hyperventilation has been associated with adverse outcomes in children after stroke or traumatic brain injury (16–18) and in neonates after resuscitation from birth asphyxia (19).
In this study, we explore oxygenation and ventilation status early after CA in infants and children. Our primary hypothesis is that hyperoxia is common and associated with worse outcome. To test this hypothesis, we conduct a retrospective cohort study involving children who suffered CA yet survived for at least 20 mins after ROC.
Methods
We queried a pediatric CA database that was generated during planning for two simultaneous multicenter, randomized controlled trials of therapeutic hypothermia after pediatric CA (the THAPCA Trials, http://www.thapca.org/) (20). The database, previously described in detail (21–23), was populated by retrospective review of pediatric CA events that occurred between July 1, 2003, and December 31, 2004, at 15 hospitals within the Pediatric Emergency Care Applied Research Network. All children 24 hours to 18 years of age (inclusive) who experienced CA with ROC for greater than or equal to 20 mins and were treated at a participating center were eligible for database inclusion. Patients cared for in a Neonatal Intensive Care Unit or who experienced planned CA as part of cardiac surgery were excluded from the initial cohort. The study populating the database was granted approval with waiver of consent at all participating studies. The current study met exemption criteria for the institutional review board, as it utilized an existing database that contained only de-identified data.
The database contains: 1) patient characteristics (e.g., demographics, chronic preexisting conditions); 2) event characteristics (e.g., location of CA, first recorded cardiac rhythm, presence and type of vascular access); 3) etiology of CA; 4) hospital course (e.g., use of ECMO, therapeutic hypothermia); 5) physiological and laboratory data; 6) Pediatric Cerebral Performance Category (PCPC) scores at baseline (pre-CA) and at hospital discharge; and 7) survival to hospital discharge. Both IH and OH CA events are included. CA is defined as receiving CPR with greater than 1 min of chest compressions. IH CA is defined as chest compressions initiated in an emergency department or other hospital setting and OH CA as chest compressions initiated prior to hospital arrival. ROC includes either spontaneous or assisted (e.g., ECMO) circulation. Duration of OH CA was poorly captured, but the number of epinephrine doses administered during CPR is available as a surrogate marker.
For the current study, we excluded: 1) subjects with a diagnosis related to cyanotic heart disease including any single ventricle physiology; 2) subjects lacking documentation of both an indwelling arterial catheter and at least one recorded Pao2 value during the first 6 hrs post-CA; 3) subjects who died within the first 6 hours post-CA; and 4) subjects missing PCPC scores at baseline or at hospital discharge. We defined the following fields: preexisting condition as any identified chronic illness, CA etiology as respiratory or cardiac (nonexclusive), and rhythm during CA as presence of asystole, ventricular fibrillation/ventricular tachycardia, other, or missing (hierarchical). The location of CA was further refined for IH to have occurred in an ICU (IH-ICU) or another location (IH-other).
In the database, physiological and laboratory data are recorded as minimum and maximum values during select time periods. Because the risk of injury due to hyperoxia may be greatest early after reperfusion (24), our primary exposure of oxygenation status was from the period 0 to 6 hours post-CA. If there was only one value provided, it was assigned as both the minimum and the maximum values. The cutoff for hyperoxia, Pao2 greater than 200 mm Hg (26.7 kPa), was chosen as a compromise between neonatal (6, 7) and adult (8–10) reports, which used cutoffs ranging from a pulse oximetry reading greater than 94% to a Pao2 greater than 300 mm Hg (40 kPa); however, we also explored cutoffs of 100 mm Hg (13.3 kPa) and 300 mm Hg (40 kPa) by sensitivity analyses. The cutoff for hypoxia, Pao2 less than 50 mm Hg (4.0 kPa), was chosen to roughly correspond to a pulse oximetry reading less than 80%. Other physiological and laboratory variables were categorized as follows: hyperventilation as Paco2 less than 30 mm Hg (4.0 kPa); hypoventilation as Paco2 greater than 50 mm Hg (6.7 kPa); hypotension as systolic blood pressure less than fifth percentile for age (Pediatric Advanced Life Support criteria); hypothermia as temperature less than 35°C (excludes therapeutically induced hypothermia, which comprises a separate variable); fever as temperature greater than 38°C (25, 26); hyperglycemia as serum glucose concentration greater than 240 mg/dL (13.32 mmol/L) (27); and hypoglycemia as serum glucose concentration less than 40 mg/dL (2.22 mmol/L). Overall oxygenation status in the first 6 hrs post-CA was defined as hyperoxia (with no hypoxia), hypoxia (with no hyperoxia), both hyperoxia and hypoxia, and normoxia throughout interval. Overall ventilation status was similarly defined.
Our primary outcome variable was survival to hospital discharge with good neurological outcome. Good neurological outcome was defined by discharge PCPC score 1–2 (normal or mild disability) or no change in PCPC score between pre-CA and discharge. Conversely, poor neurological outcome was defined by discharge PCPC 3–6 (moderate or severe disability or death) or for those with an abnormal PCPC score pre-CA, any worsening. Hospital mortality was evaluated as a secondary outcome.
Statistical Analysis
For continuous variables, medians and interquartile ranges (25th–75th percentile) are reported. For categorical variables, counts and percentages are reported. The association of each variable with outcome was examined using the Wilcoxon's rank sum or Kruskal-Wallis test for continuous variables and chi-square or Fisher's exact test for categorical variables. We also described the association of oxygenation and ventilation status with key patient and CA characteristics. For the association of hyperoxia with outcome, sensitivity analyses were performed using cutoffs of Pao2 greater than 100 (13.3 kPa) and Pao2 greater than 300 (40.0 kPa), in addition to the defined cutoff of Pao2 greater than 200 (26.7 kPa).
Multivariable logistic regression was used to evaluate the potential association between early (0–6 hrs) derangements in oxygenation and ventilation with outcome after pediatric CA. Factors that might confound the association between outcome and oxygenation and/or ventilation were examined. All variables with a p value less than 0.15 in univariable analysis were eligible for inclusion in the logistic regression model. Forward stepwise selection was applied, and factors were removed from the model if p values were greater than 0.05. In addition, based on reported associations with outcome after pediatric CA, we decided a priori to include the following in all models regardless of statistical significance: location of CA, patient age, number of epinephrine doses, and cardiac rhythm during CA. Similarly, based on our primary hypothesis, we decided a priori to include hyperoxia regardless of statistical significance. All analyses were performed using SAS 9.2 for Windows (SAS Institute, Cary, NC).
Results
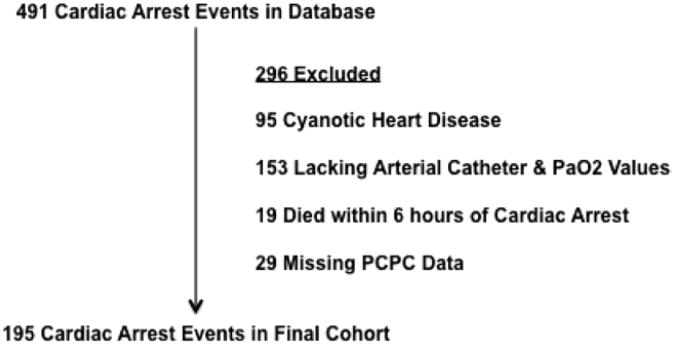
Four hundred ninety-one pediatric CA events were available for analysis. After exclusions, a final cohort of 195 remained (Fig. 1). Hyperoxia occurred in 54% of the cohort, normoxia in 34%, and hypoxia in 22%. These percentages account for a 10% overlap of patients who had both hyperoxia and hypoxia. Ventilation status was more evenly distributed with hyper-ventilation observed in 38%, normoventilation in 29%, and hypoventilation in 46%. Patients with both hyperventilation and hypoventilation account for a 13% overlap. Derangements in both oxygenation and ventilation, such as hyperoxia and hypocarbia, were common. Surprisingly, both normoxia and normocarbia were maintained during the initial 6 hrs post-CA in only 25 of the 195 patients (13%).
Figure 1.
Schematic of cohort selection. PCPC = Pediatric Cerebral Performance Category.
Demographics and CA characteristics were not statistically different across oxygenation groups (Table 1). Interestingly, intubation prior to CA did not influence oxygenation status; the proportions of patients intubated prior to CA were similar across groups (hyperoxia: 45%, normoxia or hypoxia: each 41%, hyperoxia and hyperoxia: 40%, p = 0.37). Post-CA features did not differ by oxygenation group with the following exceptions (Table 1). Ventilation status differed in that the majority of hyperoxic patients were hyperventilated, whereas normoxic or hypoxic patients were most commonly hypoventilated. Approximately one-third of either hyperoxic or normoxic patients were normoventilated compared with less than 10% of hypoxic patients. ECMO support and spontaneous hypothermia were less frequent among normoxic than hyperoxic and/or hypoxic patients. PICU and hospital days post-CA were similar across oxygenation groups.
Table 1. Cohort Characteristics by Oxygenation Status.
| Hyperoxia n = 87 (%) | Normoxia n = 66 (%) | Hypoxia n = 22 (%) | Hyperoxia and Hypoxia n = 20 (%) | p | |
|---|---|---|---|---|---|
| Demographics | |||||
| Age | |||||
| <2 yr | 43 (49) | 26 (39) | 10 (45) | 7 (35) | 0.78 |
| 2–11 yr | 28 (32) | 24 (36) | 9 (41) | 8 (40) | |
| 12–18 yr | 16 (18) | 16 (24) | 3 (14) | 5 (25) | |
| Gender | |||||
| Male | 59 (68) | 42 (64) | 13 (59) | 11 (55) | 0.69 |
| Female | 28 (32) | 24 (36) | 9 (41) | 9 (45) | |
| Preexisting condition | |||||
| Ye s | 56 (64) | 50 (76) | 18 (82) | 14 (50) | 0.28 |
| No | 31 (36) | 16 (24) | 4 (18) | 6 (30) | |
|
| |||||
| Cardiac arrest details | |||||
| Location | |||||
| In-hospital in an ICU | 31 (36) | 34 (52) | 11 (50) | 10 (50) | 0.34 |
| In-hospital not in an ICU | 26 (30) | 15 (23) | 3 (14) | 5 (25) | |
| Out of hospital | 30 (34) | 15 (23) | 8 (36) | 5 (25) | |
| Etiology | |||||
| Cardiac | 42 (48) | 35 (53) | 13 (59) | 13 (65) | 0.51 |
| Respiratory | 39 (45) | 36 (55) | 9 (41) | 6 (30) | 0.17 |
| Rhythm | |||||
| Asystole | 43 (49) | 22 (33) | 6 (27) | 14 (70) | 0.08 |
| Ventricular tachycardia/ventricular fibrillation | 7 (8) | 5 (8) | 3 (14) | 1 (5) | |
| Other | 27 (31) | 27 (41) | 9 (41) | 3 (15) | |
| Epinephrine doses | |||||
| 0 | 7 (8) | 7 (11) | 4 (18) | 0 (0) | 0.53 |
| 1 | 19 (22) | 17 (26) | 6 (27) | 3 (15) | |
| 2 | 14 (16) | 12 (18) | 4 (18) | 4 (20) | |
| 3 | 21 (24) | 11 (17) | 2 (9) | 3 (15) | |
| 4+ | 23 (26) | 18 (27) | 4 (18) | 9 (45) | |
|
| |||||
| Postarrest features | |||||
| Paco2 | |||||
| Hyperventilation | 29 (69) | 10 (15) | 6 (27) | 3 (15) | 0.001 |
| Normoventilation | 26 (30) | 25 (38) | 2 (9) | 4 (20) | |
| Hypoventilation | 19 (22) | 29 (44) | 8 (36) | 8 (40) | |
| Hyperventilation and hypoventilation | 13 (15) | 2 (3) | 6 (27) | 5 (25) | |
| Extracorporeal membrane oxygenation support | 11 (13) | 4 (6) | 4 (18) | 9 (45) | <0.001 |
| Spontaneous hypothermia | 46 (53) | 19 (29) | 13 (59) | 6 (30) | 0.009 |
| Hyperglycemia | 46 (53) | 31 (47) | 10 (45) | 9 (45) | 0.99 |
| Postarrest care | |||||
| PICU days, median (IQR) | 5 (1,16) | 6 (2,12.5) | 11 (4,28) | 6 (3,25.5) | 0.33 |
| Hospital days | 9 (1,29) | 7 (3,17) | 16 (4,28) | 7 (3,37) | 0.53 |
Column percentages are shown. Numbers may not sum to 100 due to rounding.
Demographics and CA characteristics were not statistically different across ventilation groups (data not shown). Intubation prior to CA was most common among hyperventilated patients (50%) and least common among normoventilated patients (37%), but the differences did not reach statistical significance (p = 0.18). Post-CA features also did not differ across ventilation groups with the exception of oxygenation status (data not shown). The associations for concurrent ventilation and oxygenation status are discussed above. PICU and hospital days post-CA did not differ by ventilation group.
About 37% of the cohort survived with good neurological outcome (Table 2). Outcome did not differ by demographics except that a preexisting condition was more common among those who survived with good outcome. Good outcomes were relatively more common among IH CA, cardiac etiology of CA, no documented asystole, and fewer epinephrine doses during resuscitation. Outcome did not differ by oxygenation or ventilation status. In contrast, poor outcomes were associated with spontaneous hypothermia and hyperglycemia.
Table 2. Cohort Characteristics by Outcome.
| Total n = 195 | Good Outcome n (%) 73 (37) | Poor Outcome n (%) 122 (63) | p | |
|---|---|---|---|---|
| Demographics | ||||
| Age | ||||
| <2 yr | 86 | 31 (36) | 55 (64) | 0.93 |
| 2–11 yr | 69 | 27 (39) | 42 (61) | |
| 12–18 yr | 40 | 15 (38) | 25 (63) | |
| Gender | ||||
| Male | 125 | 48 (38) | 77 (62) | 0.71 |
| Female | 70 | 25 (36) | 45 (64) | |
| Baseline Pediatric Cerebral Performance Category | ||||
| 1–2 | 161 | 64 (40) | 97 (60) | 0.15 |
| 3–5 | 34 | 9 (26) | 25 (74) | |
| Preexisting condition | ||||
| Yes | 138 | 59 (43) | 79 (57) | 0.02 |
| No | 57 | 14 (25) | 43 (75) | |
|
| ||||
| Cardiac arrest details | ||||
| Location | ||||
| In-hospital in an ICU | 86 | 46 (53) | 40 (47) | <0.0001 |
| In-hospital not in an ICU | 49 | 19 (39) | 30 (61) | |
| Out of hospital | 58 | 7 (12) | 51 (88) | |
| Etiology | ||||
| Cardiac | 103 | 48 (47) | 55 (53) | 0.003 |
| Respiratory | 90 | 35 (39) | 55 (61) | 0.57 |
| Rhythm | ||||
| Asystole | 85 | 14 (16) | 71 (84) | <0.001 |
| Ventricular tachycardia/ventricular fibrillation | 16 | 9 (56) | 7 (44) | |
| Other | 66 | 40 (61) | 26 (39) | |
| Epinephrine doses | ||||
| 0 | 18 | 10 (56) | 8 (44) | 0.02 |
| 1 | 45 | 23 (51) | 22 (49) | |
| 2 | 34 | 15 (44) | 19 (56) | |
| 3 | 37 | 8 (22) | 29 (78) | |
| 4+ | 54 | 16 (30) | 38 (70) | |
|
| ||||
| Postarrest features | ||||
| Pao2 | ||||
| Hyperoxia | 87 | 30 (34) | 57 (66) | 0.57 |
| Normoxia | 66 | 29 (44) | 37 (56) | |
| Hypoxia | 22 | 8 (36) | 14 (64) | |
| Hyperoxia and hypoxia | 20 | 6 (30) | 14 (70) | |
| Paco2 | ||||
| Hyperventilation | 48 | 16 (33) | 32 (67) | 0.26 |
| Normoventilation | 57 | 27 (47) | 30 (53) | |
| Hypoventilation | 64 | 23 (36) | 41 (64) | |
| Hyperventilation and hypoventilation | 26 | 7 (27) | 19 (73) | |
| Spontaneous hypothermia | 84 | 20 (24) | 64 (76) | <0.001 |
| Hyperglycemia | 96 | 25 (26) | 71 (74) | 0.002 |
|
| ||||
| Survival | ||||
| PICU discharge | 89 | 73 (82) | 16 (18) | |
| Hospital discharge | 89 | 73 (82) | 16 (18) | |
Row percentages are shown.
Other physiological variables, including hypotension, ECMO support, hypoglycemia, fever, and therapeutic hypothermia, were not associated with outcome (data not shown). Interestingly, a greater documented change in body temperature during the initial 6 hrs post-CA was associated with poor outcome (median [IQR], 1.7 [0.6, 3.3] for poor vs. 1.1 [0.4, 1.8] for good outcome, p = 0.002). All patients who survived to PICU discharge survived to hospital discharge. Among survivors, 76% were discharged home, 17% were transferred to rehabilitation, and 7% were transferred to either another acute care facility or long-term care (data not shown).
Survival with good outcome was 34% for those with hyperoxia and 42% for those without (p = 0.23). This lack of association remained true in sensitivity analysis using cutoffs of 100 or 300 mm Hg (13.3 or 40 kPa). Hospital mortality was also similar between those with and without hyperoxia (56% and 52%, p = 0.60). Although OH CA, presence of asystole, greater number of epinephrine doses during CPR, development of spontaneous hypothermia, greater change in temperature during the first 6 hrs post-CA, and hyperglycemia were all associated with poor outcome in univariable analysis, only CA location and cardiac rhythm during CA remained significantly associated with outcome in the multivariable setting (Table 3). The adjusted odds of survival with good outcome were very similar for those with and without hyperoxia (odds ratio 1.02, 95% confidence interval 0.46, 2.27). This remained true when we excluded the 10 neonates in our cohort (odds ratio 0.925, 95% confidence interval 0.40, 2.12).
Table 3. Multivariable Logistic Regression Results For Good Outcome.
| Variable | Adjusted Odds Ratio (95% Confidence Interval) | p |
|---|---|---|
| Hyperoxia | 1.02 (0.46, 2.27) | 0.96 |
|
| ||
| Age category | 0.85 | |
| <2 yr | Reference | |
| 2–11 yr | 1.13 (0.45, 2.84) | |
| 12–18 yr | 0.78 (0.25, 2.46) | |
|
| ||
| Arrest location | 0.001 | |
| In-hospital in an ICU | 12.3 (3.28, 46.1) | |
| In-hospital not in an ICU | 9.87 (2.30, 42.4) | |
| Out of hospital | Reference | |
|
| ||
| Arrest rhythm | 0.003 | |
| Asystole | 0.23 (0.09, 0.57) | |
| Ventricular tachycardia/ventricular fibrillation | 1.29 (0.30, 5.47) | |
| Other | Reference | |
|
| ||
| Epinephrine doses | 0.32 | |
| 0 | Reference | |
| 1 | 1.73 (0.39, 7.68) | |
| 2 | 2.09 (0.41, 10.7) | |
| 3 | 0.54 (0.10, 2.86) | |
| 4+ | 1.32 (0.28, 6.19) | |
Discussion
Our most striking finding is that only 13% of pediatric patients were maintained both normoxic and normoventilated early post-CA. This is surprising, particularly given that current AHA pediatric resuscitation guidelines advocate maintaining normal oxygenation and ventilation (14). Our study includes mainly resuscitation efforts at large, experienced pediatric centers. In addition, the location of CA (IH-ICU, IH-other, or OH) and intubation prior to CA were not associated with the frequency of normoxia and normoventilation. This suggests that although maintaining normal oxygenation and ventilation status is advocated, it remains difficult to achieve in practice throughout a range of resuscitation settings. Likely, factors associated with both resuscitation practices and patient physiology contribute to this. For example, hyperoxia likely results from administration of high FiO2 in the absence of significant respiratory disease, whereas hypoxia likely stems from the presence of significant respiratory disease. Further, a study of resuscitation team performance during simulated pediatric CA events at a tertiary care, academic pediatric center revealed that hyperventilation was quite common during simulated resuscitation (28). A recent report of actual pediatric resuscitation events corroborated this, with hyperventilation being observed in 63% of resuscitations (29). Targeted efforts are needed to increase adherence with current oxygenation and ventilation guidelines.
Contrary to our primary hypothesis, we did not demonstrate an association between hyperoxia early post-CA and outcome. This differs from reports of worse outcomes in hyperoxic neo-nates with birth asphyxia (30–33) and from preclinical studies that suggest greater oxidative stress and worse functional and histological outcomes with hyperoxic vs. normoxic resuscitation (34–37). However, it falls in line with mixed reports of postresuscitation oxygenation status and outcomes in adults (8–10, 38).
The distribution of oxygenation status in our cohort differs from that reported in adults. Hyperoxia affected 54% of our cohort (includes isolated hyperoxia and combined hypoxia and hyperoxia) vs. 10% to 18% of adults (8, 10). Hypoxia occurred in 22% of our cohort vs. 63% to 74% of adults. Normoxia was maintained in 34% of our population vs. 16% to 19% of adults. These differences suggest that children likely have different responses to resuscitation and/or less severe hypoxic lung disease than adults. Given these differences, extrapolation of adult data regarding hyperoxia and outcomes after CA may not be appropriate.
We did not observe an association between ventilation status and outcome in our population. This differs from a study showing two-fold increased odds of poor outcome with hyperventilation (vs. normoventilation) after resuscitation from birth asphyxia (19) and from the reports of detrimental effects of hyperventilation in other types of pediatric acute cerebral injury (16–18).
Our report corroborates prior studies linking outcome after CA with arrest location (4, 39) or cardiac rhythm (5, 40, 41) and suggesting that post-CA hyperglycemia (27, 42) and spontaneous hypothermia (44) may be detrimental. Prior publications describe these associations in detail for the parent cohort of our study (21– 23). We also report a greater change in temperature during the first 6 hours post-resuscitation among those with poor outcome. Although it remains unclear whether spontaneous hypothermia is directly causal or merely associated with poorer outcome after CA, our findings support reports that the rate and degree of rewarming may be important (26, 43–45). Alternatively, our observations may simply reflect longer durations of CA, which could contribute to lower initial temperature, greater change with rewarming, and poorer outcomes. In either case, exacerbation of spontaneous hypothermia due to heat loss during transport and exacerbation of hyperglycemia should be avoided post-CA.
Strengths of our study include use of a robust, pediatric CA database that includes laboratory, physiological and outcome data from patients treated at 15 pediatric hospitals. All included subjects had arterial blood gas data to classify oxygenation and ventilation status. This allowed more precise discrimination of oxygenation or ventilation status than use of respiratory rate or pulse oximetery readings.
Our study has several limitations that may have hindered our ability to demonstrate an association between hyperoxia and outcome after pediatric CA. First, the unanticipated small percentage of patients who were maintained normoxic and normoventilated limited our study power. Second, availability of physiological data as only maximum and minimum values during specified time periods, rather than all tested values, precluded precise temporal assessments. In addition, our focus on the earliest period postresuscitation (0–6 hrs) may not represent the most crucial period of exposure. Longer periods or different timing of exposure may be necessary to affect outcomes. Exposure to suboptimal oxygenation or ventilation prior to hospital arrival may not have been well captured for OH-CA, which would introduce heterogeneity regarding the types and durations of exposures and the time between ROC and initial blood gas measurement. Similarly, our categories of CA etiology are broad, necessarily introducing some heterogeneity and limiting our ability to address effects of suboptimal oxygenation or ventilation by more specific CA etiology. Our selection of patients who had an arterial catheter placed also likely selected for greater severity of illness, which may limit our ability to discern more subtle effects of hyperoxia. Finally, the data were collected retrospectively, adding the multiple limitations inherent to any retrospective database, including missing data for some variables.
Conclusions
Our study reveals that although recent resuscitation guidelines advocate maintenance of normoxia and normoventilation after pediatric CA, this is uncommonly achieved in practice. We did not demonstrate an association between hyperoxia and outcome after pediatric CA. However, due to the small proportion of patients kept within normal ranges, our sample size was limiting. The preclinical data suggesting potential harm with hyperoxia remain compelling, and further investigation, including prospective, large studies involving more robust recording of physiological derangements, is necessary to further advance our understanding of this important topic.
Acknowledgments
We thank the following participating children's hospital, university affiliation, and site investigators: Children's Hospital Medical Center, University of Cincinnati, Cincinnati, Ohio (R. Brilli); Children's Hospital of Buffalo, SUNY-Buffalo, Buffalo, NY (B. Fuhrman); Children's Hospital of Michigan, Wayne State University, Detroit, MI (K. Meert); Children's Hospital of New York, Columbia University, New York, NY (C. Schleien); Children's Hospital of Philadelphia, University of Pennsylvania, Philadelphia, PA (V. Nadkarni).
Children's Hospital of Pittsburgh, University of Pittsburgh, Pittsburgh, PA (R. Clark); Children's Hospital of Wisconsin, Medical College of Wisconsin, Milwaukee, WI (K. Tieves); Children's National Medical Center, George Washington University, Washington D.C. (H. Dalton); C.S. Mott Children's Hospital, University of Michigan, Ann Arbor, MI (F. Moler); Helen DeVos Children's Hospital, Michigan State University, Grand Rapids, MI (R. Hackbarth); Primary Children's Medical Center, University of Utah, Salt Lake City, UT (K. Statler Bennett); St. Louis Children's Hospital, Washington University St. Louis, MO (F. Levy/D. Jaffe); Golisano Children's Hospital, University of Rochester, Rochester, NY (E. van der Jagt); The Johns Hopkins Hospital, Johns Hopkins University, Baltimore, MD (H. Shaffner); University of California at Davis, Sacramento, CA (R. Pretzlaff).
We acknowledge the efforts of the following individuals participating in PECARN at the time this study was initiated.
PECARN Steering Committee: N. Kuppermann, Chair; E. Alpern, J. Chamberlain, J. M. Dean, M. Gerardi, J. Goepp, M. Gorelick, J. Hoyle, D. Jaffe, C. Johns, N. Levick, P. Mahajan, R. Maio, K. Melville, S. Miller (deceased), D. Monroe, R. Ruddy, R. Stanley, D. Treloar, M. Tunik, A. Walker.
MCHB/EMSC liaisons: D. Kavanaugh, H. Park.
Central Data Management and Coordinating Center (CDMCC): M. Dean, R. Holubkov, S. Knight, A. Clark.
Data Analysis and Management Subcommittee (DAMS): J. Chamberlain, Chair; M. Brown, H. Corneli, J. Goepp, R. Holubkov, P. Mahajan, K. Melville, E. Stremski, M. Tunik.
Grants and Publications Subcommittee (GAPS): M. Gorelick, Chair; E. Alpern, J. M. Dean, G. Foltin, J. Joseph, S. Miller (deceased), F. Moler, R. Stanley, S. Teach.
Protocol Concept Review and Development Subcommittee (PCRADS): D. Jaffe, Chair; K. Brown, A. Cooper, J. M. Dean, C. Johns, R. Maio, N. C. Mann, D. Monroe, K. Shaw, D. Teitelbaum, D. Treloar.
Quality Assurance Subcommittee (QAS): R. Stanley, Chair; D. Alexander, J. Brown, M. Gerardi, M. Gregor, R. Holubkov, K. Lillis, B. Nordberg, R. Ruddy, M. Shults, A. Walker.
Safety and Regulatory Affairs Subcommittee (SRAS): N. Levick, Chair; J. Brennan, J. Brown, J. M. Dean, J. Hoyle, R. Maio, R. Ruddy, W. Schalick, T. Singh, J. Wright.
Supported, in part, by the federal grants (HD044955 and HD050531) to Dr. Moler; and the Emergency Medical Services for Children (EMSC) program of the Maternal and Child Health Bureau, Health Resources and Services Administration, US Department of Health and Human Services (U03MC00001, U03MC00003, U03MC00006, U03MC00007, and U03MC00008) to the Pediatric Emergency Care Applied Research Network (PECARN).
Footnotes
The authors have not disclosed any potential conflicts of interest.
For information regarding this article, kim.bennett@hsc.utah.edu
References
- 1.Atkins DL, Everson-Stewart S, Sears GK, et al. Resuscitation Outcomes Consortium Investigators. Epidemiology and outcomes from out-of-hospital cardiac arrest in children: The Resuscitation Outcomes Consortium Epistry-Cardiac Arrest. Circulation. 2009;119:1484–1491. doi: 10.1161/CIRCULATIONAHA.108.802678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Donoghue AJ, Nadkarni V, Berg RA, et al. CanAm Pediatric Cardiac Arrest Investigators. Out-of-hospital pediatric cardiac arrest: An epidemiologic review and assessment of current knowledge. Ann Emerg Med. 2005;46:512–522. doi: 10.1016/j.annemergmed.2005.05.028. [DOI] [PubMed] [Google Scholar]
- 3.Gerein RB, Osmond MH, Stiell IG, et al. OPALS Study Group. What are the etiology and epidemiology of out-of-hospital pediatric cardiopulmonary arrest in Ontario, Canada? Acad Emerg Med. 2006;13:653–658. doi: 10.1197/j.aem.2005.12.025. [DOI] [PubMed] [Google Scholar]
- 4.Berg M, Nadkarni V, Zuercher M, et al. In-Hospital Pediatric Cardiac Arrest. Pediatr Clin North Am. 2008;55:861–872. doi: 10.1016/j.pcl.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 5.Nadkarni VM, Larkin GL, Peberdy MA, et al. National Registry of Car-diopulmonary Resuscitation Investigators. First documented rhythm and clinical outcome from in-hospital cardiac arrest among children and adults. JAMA. 2006;295:50–57. doi: 10.1001/jama.295.1.50. [DOI] [PubMed] [Google Scholar]
- 6.Davis PG, Tan A, O'Donnell CP, et al. Resuscitation of newborn infants with 100% oxygen or air: A systematic review and meta-analysis. Lancet. 2004;364:1329–1333. doi: 10.1016/S0140-6736(04)17189-4. [DOI] [PubMed] [Google Scholar]
- 7.Rabi Y, Rabi D, Yee W. Room air resuscitation of the depressed newborn: A systematic review and meta-analysis. Resuscitation. 2007;72:353–363. doi: 10.1016/j.resuscitation.2006.06.134. [DOI] [PubMed] [Google Scholar]
- 8.Kilgannon JH, Jones AE, Shapiro NI, et al. Emergency Medicine Shock Research Network (EMShockNet) Investigators. Association between arterial hyperoxia following resuscitation from cardiac arrest and in-hospital mortality. JAMA. 2010;303:2165–2171. doi: 10.1001/jama.2010.707. [DOI] [PubMed] [Google Scholar]
- 9.Kilgannon JH, Jones AE, Parrillo JE, et al. Emergency Medicine Shock Research Network (EMShockNet) Investigators. Relationship between supranormal oxygen tension and outcome after resuscitation from cardiac arrest. Circulation. 2011;123:2717–2722. doi: 10.1161/CIRCULATIONAHA.110.001016. [DOI] [PubMed] [Google Scholar]
- 10.Bellomo R, Bailey M, Eastwood GM, et al. Study of Oxygen in Critical Care (SOCC) Group. Arterial hyperoxia and in-hospital mortality after resuscitation from cardiac arrest. Crit Care. 2011;15:R90. doi: 10.1186/cc10090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perlman JM, Wyllie J, Kattwinkel J, et al. Neonatal Resuscitation Chapter Collaborators. Part 11: Neonatal resuscitation: 2010 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science With Treatment Recommendations. Circulation. 2010;122(16 Suppl 2):S516–S538. doi: 10.1161/CIRCULATIONAHA.110.971127. [DOI] [PubMed] [Google Scholar]
- 12.Neumar RW, Otto CW, Link MS, et al. Part 8: adult advanced cardiovascular life support: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122(18 Suppl 3):S729–S767. doi: 10.1161/CIRCULATIONAHA.110.970988. [DOI] [PubMed] [Google Scholar]
- 13.Peberdy MA, Callaway CW, Neumar RW, et al. Part 9: post-cardiac arrest care: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122(18 Suppl 3):S768–S786. doi: 10.1161/CIRCULATIONAHA.110.971002. [DOI] [PubMed] [Google Scholar]
- 14.Kleinman ME, Chameides L, Schexnayder SM, et al. Part 14: Pediatric advanced life support: 2010 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2010;122(18 Suppl 3):S876–S908. doi: 10.1161/CIRCULATIONAHA.110.971101. [DOI] [PubMed] [Google Scholar]
- 15.Aufderheide TP, Sigurdsson G, Pirrallo RG, et al. Hyperventilation-induced hypotension during cardiopulmonary resuscitation. Circulation. 2004;109:1960–1965. doi: 10.1161/01.CIR.0000126594.79136.61. [DOI] [PubMed] [Google Scholar]
- 16.Buunk G, van der Hoeven JG, Meinders AE. Cerebrovascular reactivity in comatose patients resuscitated from a cardiac arrest. Stroke. 1997;28:1569–1573. doi: 10.1161/01.str.28.8.1569. [DOI] [PubMed] [Google Scholar]
- 17.Muizelaar JP, Marmarou A, Ward JD, et al. Adverse effects of prolonged hyperventilation in patients with severe head injury: A randomized clinical trial. J Neurosurg. 1991;75:731–739. doi: 10.3171/jns.1991.75.5.0731. [DOI] [PubMed] [Google Scholar]
- 18.Skippen P, Seear M, Poskitt K, et al. Effect of hyperventilation on regional cerebral blood flow in head-injured children. Crit Care Med. 1997;25:1402–1409. doi: 10.1097/00003246-199708000-00031. [DOI] [PubMed] [Google Scholar]
- 19.Pappas A, Shankaran S, Laptook AR, et al. Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Hypocarbia and adverse outcome in neonatal hypoxic-ischemic encephalopathy. J Pediatr. 2011;158:752–758.e1. doi: 10.1016/j.jpeds.2010.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clinicaltrials.gov: Therapeutic Hypothermia to Improve Survival After Cardiac Arrest in Pediatric Patients. [Accessed November 1, 2011]; Available at: http://clinicaltrials.gov/ct2/results??term=THAPCA.
- 21.Meert KL, Donaldson A, Nadkarni V, et al. Pediatric Emergency Care Applied Research Network. Multicenter cohort study of in-hospital pediatric cardiac arrest. Pediatr Crit Care Med. 2009;10:544–553. doi: 10.1097/PCC.0b013e3181a7045c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moler FW, Donaldson AE, Meert K, et al. Pediatric Emergency Care Applied Research Network. Multicenter cohort study of out-of-hospital pediatric cardiac arrest. Crit Care Med. 2011;39:141–149. doi: 10.1097/CCM.0b013e3181fa3c17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moler FW, Meert K, Donaldson AE, et al. Pediatric Emergency Care Applied Research Network. In-hospital versus out-of-hospital pediatric cardiac arrest: A multicenter cohort study. Crit Care Med. 2009;37:2259–2267. doi: 10.1097/CCM.0b013e3181a00a6a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abdel-Rahman U, Risteski P, Tizi K, et al. Hypoxic reoxygenation during initial reperfusion attenuates cardiac dysfunction and limits ischemia-reperfusion injury after cardioplegic arrest in a porcine model. J Thorac Cardiovasc Surg. 2009;137:978–982. doi: 10.1016/j.jtcvs.2008.09.025. [DOI] [PubMed] [Google Scholar]
- 25.Bembea MM, Nadkarni VM, Diener-West M, et al. American Heart Association National Registry of Cardiopulmonary Resuscitation Investigators. Temperature patterns in the early postresuscitation period after pediatric inhospital cardiac arrest. Pediatr Crit Care Med. 2010;11:723–730. doi: 10.1097/PCC.0b013e3181dde659. [DOI] [PubMed] [Google Scholar]
- 26.Hickey RW, Kochanek PM, Ferimer H, et al. Hypothermia and hyperthermia in children after resuscitation from cardiac arrest. Pediatrics. 2000;106(1 Pt 1):118–122. doi: 10.1542/peds.106.1.118. [DOI] [PubMed] [Google Scholar]
- 27.Beiser DG, Carr GE, Edelson DP, et al. Derangements in blood glucose following initial resuscitation from in-hospital cardiac arrest: A report from the national registry of cardiopulmonary resuscitation. Resuscitation. 2009;80:624–630. doi: 10.1016/j.resuscitation.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Niebauer JM, White ML, Zinkan JL, et al. Hyperventilation in pediatric resuscitation: Performance in simulated pediatric medical emergencies. Pediatrics. 2011;128:e1195–e1200. doi: 10.1542/peds.2010-3696. [DOI] [PubMed] [Google Scholar]
- 29.McInnes AD, Sutton RM, Orioles A, et al. The first quantitative report of ventilation rate during in-hospital resuscitation of older children and adolescents. Resuscitation. 2011;82:1025–1029. doi: 10.1016/j.resuscitation.2011.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saugstad OD, Ramji S, Soll RF, et al. Resuscitation of newborn infants with 21% or 100% oxygen: An updated systematic review and meta-analysis. Neonatology. 2008;94:176–182. doi: 10.1159/000143397. [DOI] [PubMed] [Google Scholar]
- 31.Smith KE, Keeney S, Zhang L, et al. The association of early blood oxygenation with child development in preterm infants with acute respiratory disorders. Int J Dev Neurosci. 2008;26:125–131. doi: 10.1016/j.ijdevneu.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vento M, Asensi M, Sastre J, et al. Resuscitation with room air instead of 100% oxygen prevents oxidative stress in moderately asphyxiated term neonates. Pediatrics. 2001;107:642–647. doi: 10.1542/peds.107.4.642. [DOI] [PubMed] [Google Scholar]
- 33.Vento M, Asensi M, Sastre J, et al. Oxidative stress in asphyxiated term infants resuscitated with 100% oxygen. J Pediatr. 2003;142:240–246. doi: 10.1067/mpd.2003.91. [DOI] [PubMed] [Google Scholar]
- 34.Hazelton JL, Balan I, Elmer GI, et al. Hyperoxic reperfusion after global cerebral ischemia promotes inflammation and long-term hippocampal neuronal death. J Neurotrauma. 2010;27:753–762. doi: 10.1089/neu.2009.1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koch JD, Miles DK, Gilley JA, et al. Brief exposure to hyperoxia depletes the glial progenitor pool and impairs functional recovery after hypoxic-ischemic brain injury. J Cereb Blood Flow Metab. 2008;28:1294–1306. doi: 10.1038/jcbfm.2008.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pilcher J, Weatherall M, Shirtcliffe P, et al. The effect of hyperoxia following cardiac arrest - A systematic review and meta-analysis of animal trials. Resuscitation. 2012;83:417–422. doi: 10.1016/j.resuscitation.2011.12.021. [DOI] [PubMed] [Google Scholar]
- 37.Angelos MG, Yeh ST, Aune SE. Post-cardiac arrest hyperoxia and mitochondrial function. Resuscitation. 2011;82(Suppl 2):S48–S51. doi: 10.1016/S0300-9572(11)70151-4. [DOI] [PubMed] [Google Scholar]
- 38.Kilgannon JH, Roberts BW, Reihl LR, et al. Early arterial hypotension is common in the post-cardiac arrest syndrome and associated with increased in-hospital mortality. Resuscitation. 2008;79:410–416. doi: 10.1016/j.resuscitation.2008.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Donoghue AJ, Nadkarni V, Berg RA, et al. CanAm Pediatric Cardiac Arrest Investigators. Out-of-hospital pediatric cardiac arrest: An epidemiologic review and assessment of current knowledge. Ann Emerg Med. 2005;46:512–522. doi: 10.1016/j.annemergmed.2005.05.028. [DOI] [PubMed] [Google Scholar]
- 40.Samson RA, Nadkarni VM, Meaney PA, et al. American Heart Association National Registry of CPR Investigators. Outcomes of in-hospital ventricular fibrillation in children. N Engl J Med. 2006;354:2328–2339. doi: 10.1056/NEJMoa052917. [DOI] [PubMed] [Google Scholar]
- 41.Donoghue A, Berg RA, Hazinski MF, et al. American Heart Association National Registry of CPR Investigators. Cardiopulmonary resuscitation for bradycardia with poor perfusion versus pulseless cardiac arrest. Pediatrics. 2009;124:1541–1548. doi: 10.1542/peds.2009-0727. [DOI] [PubMed] [Google Scholar]
- 42.Longstreth WT, Jr, Diehr P, Inui TS. Prediction of awakening after out-of-hospital cardiac arrest. N Engl J Med. 1983;308:1378–1382. doi: 10.1056/NEJM198306093082302. [DOI] [PubMed] [Google Scholar]
- 43.Benz-Woerner J, Delodder F, Benz R, et al. Body temperature regulation and outcome after cardiac arrest and therapeutic hypothermia. Resuscitation. 2012;83:338–342. doi: 10.1016/j.resuscitation.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 44.Lavinio A, Timofeev I, Nortje J, et al. Cerebrovascular reactivity during hypothermia and rewarming. Br J Anaesth. 2007;99:237–244. doi: 10.1093/bja/aem118. [DOI] [PubMed] [Google Scholar]
- 45.Steiner T, Friede T, Aschoff A, et al. Effect and feasibility of controlled rewarming after moderate hypothermia in stroke patients with malignant infarction of the middle cerebral artery. Stroke. 2001;32:2833–2835. doi: 10.1161/hs1201.99511. [DOI] [PubMed] [Google Scholar]
- 46.Linares G, Mayer SA. Hypothermia for the treatment of ischemic and hemorrhagic stroke. Crit Care Med. 2009;37(7 Suppl):S243–S249. doi: 10.1097/CCM.0b013e3181aa5de1. [DOI] [PubMed] [Google Scholar]