Abstract
Cigarette smoking is a mixture of thousands of compounds, many of which are carcinogens, such as NNK [4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone]. Nicotine, as an addictive substance in cigarette, has been shown to promote growth of non-neuronal cells. It is unclear how nicotine cooperates with tobacco-related carcinogens during tumorigenesis. Here, by concurrent treatment of nicotine and NNK, we investigate the effect of the cooperation of these two compounds on cell growth and apoptosis in various different lung epithelial (RLE) or cancer (LKR) cells. We demonstrated that short-term nicotine exposure moderately activated mitogenic signaling pathways (such as PKC, ERK and Akt) and a mediocre protection against cisplatin-mediated apoptosis. In contrast, NNK strongly stimulated mitogenic signaling and rendered the cells a high resistance to cisplatin. The pre-ligation of nAChR by nicotine interfered with NNK-mediated mitogenic signaling and resistance to cisplatin, the magnitude of which was similar as that exposed to nicotine alone. Interestingly, a week after the exposure to nicotine or nicotine plus NNK, Bcl-2 expression was augmented, accompanied with the increased resistance to cisplatin-induced apoptosis. In comparison, long-term NNK treatment provided very little protection of the cells from cisplatin. We also showed that the combination treatment promoted more cells to grow in an anchorage-independent fashion than NNK exposure alone. Thus, the data suggest that through occupying nAChR, nicotine appears to modulate NNK-mediated signaling and persistently sustain pro-survival activities to promote transformation.
Keywords: nicotine acetylcholine receptor, nitrosamines, mitogenic pathways, lung cells
Introduction
Cigarette smoke is a major environmental risk factor for the development of many human diseases, including lung cancer. There are more than 4000 compounds in cigarette smoke, most of which are biohazards or carcinogens (1, 2). Emerging evidence demonstrates the correlation between tobacco smoking and the increased susceptibility to the onset of human lung, breast, pancreas, and esophagus malignancies (2–4). Tobacco-derived carcinogens NNK and N′-nitrosonornicotine (NNN) function directly on the genome by causing the formation of DNA adducts (5, 6). Studies also showed that NNK or NNN, through association with acetylcholine nicotine receptor (nAChR) on the surface membrane of non-neuronal cells, activates growth-related intracellular signaling pathways to promote tumorigenesis or transformation (2–7). Nicotine, as an addictive substance, not only functions in central nerve system but also in other types of cells (8–11). Although nicotine is not a conventional carcinogen, studies demonstrated that the interaction of nicotine with its receptor activates several intracellular, mitogenic-related signaling pathways to promote cell growth, angiogenesis or other cellular activities (8–11). In non-small cell lung cancer, nicotine ligation stimulates Src and Raf signaling, resulting in Rb phosphorylation and subsequent cell cycle progression (12, 13). Alterations of the expression of various genes related to cell migration and angiogenesis in different cell lines, including lung, breast and pancreatic cancer cells, occurred after cells were exposed to nicotine (3, 7, 10, 14–16). Furthermore, nicotine appeared to promote the formation of atherosclerotic plaques and tumors, which might play an important role in pathological angiogenesis (14, 15).
The physiological and pathological effects mediated by nicotine exerts via its receptor that express on a variety of non-neuronal cells, such as bronchial, mammary epithelial, pancreatic, esophageal, endothelial cells and keratinocytes (3, 5, 10, 17, 18). nAChR consists of nine α-subunits (α 2–10) and two β-subunits (β2 and 4) (20, 21). Upon ligation, the subunits of α 3, α 5, β 2 and β 4 form heteromeric channels in different combinations to transmit external signals (19–21). The homomeric channels are often formed by several α 7 or α 9 subunits. These hetero- or homomeric channels are highly Ca++ permeable, which allow Ca++ release from intracellular stores to the cytoplasma of cells (20, 21). In human epidermal cells, the interaction of nicotine and its receptor was shown to activate calmodulin-dependent protein kinase II, PKC, phosphodylinositol-3-kinase (PI3K)/Akt (13, 21, 22). In human non-small cell lung cancer cells, cell proliferation induced by nicotine involves the recruitment of β-arrestin to nAChR, which initiates a cascade of Src/Raf/Rb phosphorylation, leading to acceleration of cell cycle entry (12). Recently, we demonstrated that nicotine is able to activate PKC α, which in turn, promotes mammary epithelial or cancer cells migration or micro-invasion, via upregulating cdc42 activity (16). However, the treatment with nicotine does not stimulate mammary epithelial cells to form colonies in soft agar.
NNK and NNN are the derivatives of nicotine and strong tobacco-related carcinogens that are key factors for the genesis of lung, oral cavity, or esophagus cancer (8). The tumorigenic action of NNK is known to be through its binding to nAChR (8, 22–24). Studies have shown that the treatment with NNK altered growth kinetics of bronchial epithelial cells and promoted anchorage-independent growth (22–24). Using cultured cell lines, it was demonstrated that NNK can perturb cell cycle checkpoints, induce the formation of DNA adducts, and augment mutagenesis, which leads to the establishment of genetic instability (8). In the blood stream of smokers, the concentrations of nitrosamines are 5000–10,000 time less than that of nicotine (at pharmacological concentrations of 90–1000 nM) (8, 25). The interaction affinity of NNK or nicotine with the receptor is similar (8, 25).
Bcl-2 is a pro-survival factor. Through regulating the membrane permeability of the mitochondrial, Bcl-2 is able to interfere with apoptotic factors (such as Bax, cytochrome c) releasing to the cytosol, resulting in the suppression of cell death (26–28). It was noticed that heavy tobacco smoking correlated with the increase of Bcl-2 expression in patients with lung, head or neck cancer (29, 30). Many studies showed that nicotine activates various intracellular, growth-related signaling pathways (7, 8). Among these, nicotine-activated PKC phosphorylated Bcl-2 in lung cancer cells and further antagonized the apoptotic signaling (31, 32). All of these suggest that Bcl-2 is a possible target of nicotine to promote cell survival.
Both NNK and nicotine, through interacting with nAChR, promote growth-related signaling pathways and affect cell cycle checkpoints (12, 16, 33). However, studies demonstrate that nicotine itself does not directly cause cellular transformation or turmorigenesis (12, 16, 33). In order to mimic the function of tobacco mixture and to understand the interaction between nicotine and NNK, we in this study employed the combination treatment, in which nicotine was exposed 30 min prior NNK treatment. We showed that the treatment with nicotine for 1 h moderately affected the growth-related signaling pathways as well as cisplatin-induced apoptosis, which is in contrast with the strong effect of NNK on these biological activities. However, the pre-ligation with nicotine interfered with NNK-mediated mitogenic signaling and protection from cytotoxicity induced by cisplatin. We also demonstrated that the long-term exposure rat lung epithelial RLE or murine benign lung cancer LKR cells to nicotine or nicotine plus NNK for a week upregulated Bcl-2 expression, accompanied with the increasing of the resistance to cisplatin. The persistent treatment with NNK appeared to diminish its protection against cisplatin-mediated apoptosis. Furthermore, the combination treatment of nicotine plus NNK had stronger effect on the anchorage-independent growth in the cells than NNK treatment alone. Thus, our results indicate the complexity of nicotine in the regulation of transformational signaling.
Materials and Methods
Cells and Materials
Rat lung epithelial cells were purchased from ATCC (Manassas, VA, USA) and the abbreviated name as RLE. Murine lung cancer LKR cells were isolated from lung foci of K-ras transgenic mice (a gift from Dr. Jacks, MIT, Cambridge) and at early stages of transformation with a slow growth pattern. Human lung cancer H5800 cells were kindly provided by Dr. Yu (Boston University School of Medicine, Boston). Cells were cultured in Dulbecco’s modified Eagle’s (DME) medium with 10% fetal calf serum, 4 mM L-glutamine and antibiotics.
Nicotine, NNK, cisplatin and nAChR inhibitor mecamylamine hydrochloride (MCA) were purchased from Sigma-Aldrich, Inc. (St. Louis, MO, USA). Antibodies against Bcl-2, cytochrom c and PKC were purchased from BD Pharmingen (La Jolla, CA, USA) and an anti-AChR antibody was from Santa Cruz Biotech. Inc. (Santa Cruz, CA, USA). Antibodies against different phosphorylation status of ERK1/2, pAkt and JNK were bought from Cell Signaling Tech. Inc. (Danvers, MA, USA).
PCR and Real-Time PCR
Total RNAs were extracted from cultured cells with or without treatments using RNease Mini Kit (Qiagen, Valencia, CA) following the protocol provided by the manufacturer. Primers for the genes encoding the subunits of nAChR were designed with the assistance of the Primer Express software (Applied Biosystems, Foster City, CA, USA). For Real-Time PCR, the gene expression was normalized using β-actin as the control.
PKC Enzymatic Assay
After various treatments, cells were lysed in the lysis buffer (25 mM Tris-HCl, Ph 7.5, 1% NP-40, 20 mM MgCl2, 150 mM NaCl). The lysates were normalized for protein concentration. The equal amount of total proteins was immunoprecipitated with anti-pan-PKC antibody and the immunoprecipitates were analyzed for PKC activity using a PKC enzymatic kit (Millipore, Bilerica, MA, USA). Briefly, the immunoprecipitates were mixed with the substrate cocktail, the inhibitor cocktail, and the lipid activator in microcentrifuge tubes. After adding Mg++/ATP cocktail containing [γ-32P] ATP, the samples were incubated at 30°C for 10 min. and then spotted on P81 phosphocellulose papers. The radioactivity of the 32P-incorporating substrate was measured by a scintillation counter.
[3H]Thymidine incorporation
Cells were grown in Petri dishes until 60–70% confluence. Controls and treated cells were cultured in the medium containing 0.5% serum for 24 h. Subsequently, cells were grown in the medium containing 0.5% or 10% serum plus 4 μCi/ml of [3H]thymidine (Perkin Elmer Life Sciences, Waltham, MA) with or without nicotine treatment. The cells were labeled for 4 h at 37° C. After precipitation with cold 10% trichloroacetic acid, the cells were dissolved in 0.5 ml of 0.1 M NaOH overnight at 4° C. The amount of radioactivity in each sample was counted using a scintillation machine.
DNA Fragmentation Analysis
Following treatments, cells were harvested, fixed in 70% cold ethanol and stained with 1.0 μg/ml propidium iodide containing RNase (1.5 μg/ml). Subsequently, sub-G1 DNA contents of cells were analyzed by a FACScan machine and evaluated with DB FACStation software CellQuest.
Isolation of the Cytosol Fraction
Cells (1 × 108) were washed twice with 1 x PBS and resuspended in 1 ml of 1% Triton X-114 lysis buffer (34). Cell suspensions were transferred to a 1 ml-syringe and sheared by being passed 40 times through a 25-gauge needle. After centrifuged at 16,000 × g for 30 min, the supernatant was collected as the cytosolic fraction.
Immunoblotting
After treatments, cell lysates were prepared, and proteins were separated by SDS-PAGE gels. Membranes were incubated with the designated primary antibody overnight in a cold-room at 4° C. Bound primary antibodies were reacted with corresponding second antibodies for 2 h and detected by chemiluminescence.
Statistical Analysis
Three to five independent repeats were conducted in all experiments. Error bars represent these repeats. A Student’s T test was used and a p value of <0.05 was considered significant.
Results
Expression of nAChRs in murine lung cells upon the treatment with nicotine, NNK or both
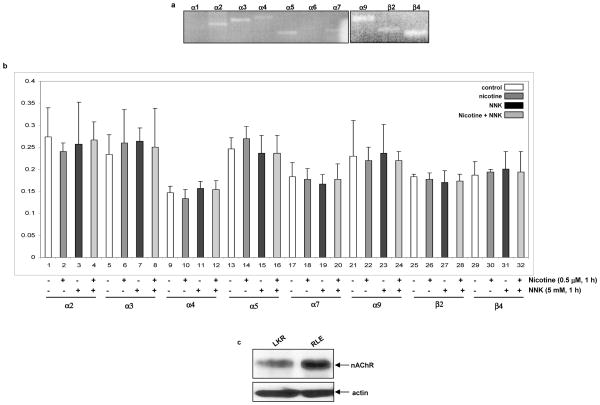
nAChRs are expressed not only on the surface of neuronal cells, but also of non-neuronal cells, such as mammary epithelial, vascular endothelial or esophageal cells (2–7). Through binding to the receptor, nicotine or nicotine derivative carcinogen NNK is able to affect various biological or patho-biological processes including angiogenesis, ischemia, or growth promotion (2–7). For the purpose to test the effect of the combination treatment of nicotine with NNK (in which nicotine was added for 15 min, prior to NNK treatment) on cell growth promotion, we first analyzed which subunit genes of nAChR are expressed in rat lung epithelial RLE cells by PCR (Fig. 1a). RLE cells expressed α2, α3, α4, α5, α7, α9, β2 and β4 of nAChR subunits, but not other subunits. We then tested whether the exposure to nicotine, NNK or the combination of two alters the levels of the gene expressions of these subunits by Real-Time PCR (Fig. 1b). After treated the cells with nicotine, NNK or both, the amount of the gene expression of these nAChR subunits was not changed. The expression of the subunits of nAChR in murine lung cancer LKR cells, with or without the treatments, was also examined by RT-PCR. A similar result was obtained from LKR cells (data not shown). Subsequently, the expression of nAChR protein was analyzed using immunoblotting in RLE and LKR cells using an anti-pan-nAChR antibody (Fig. 1c). The receptor was expressed in these two cell lines. It appears that the level of nAChR in RLE cells was higher than that in LKR cells, which may be related to the different origins of these two cell lines or malignant status of LKR cells. However, the treatment with nicotine or NNK did not affect the amount of the protein expression of nAChR (data not shown).
Figure 1.
Expression of nAChR in rat lung epithelial RLE and murine lung cancer LKR cells. a. Total RNAs from the cells were isolated. Equal amount of RNAs was reverse-transcribed, and the expressions of nAChR subunits were identified by PCR. b. The cells were treated with nicotine (0.5 μM) or NNK (1 μM), or treated with nicotine for 30 min prior to adding NNK for 1 h, total RNAs were extracted. After reverse-transcription, real-time PCR was performed. The abundance of nAChRs was normalized to actin. Error bars represent the standard deviation over 3 independent experiments. c. Expression of nAChR in RLE and LKR cells.
Effect of the combination treatment with nicotine and NNK on intracellular growth-related activities
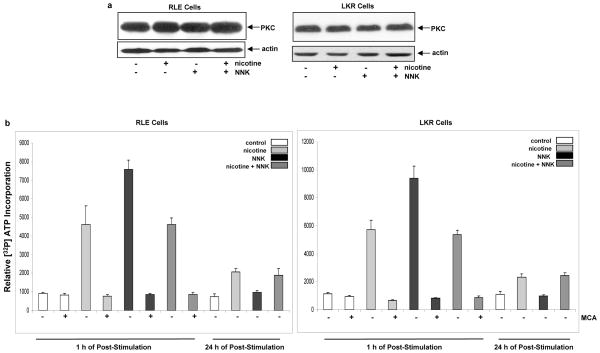
Ligation of nAChR with nicotine or NNK was shown to transiently activate various growth-related signaling pathways (8–11, 16, 35–37). Since the concentration of nicotine in tobacco smoking is 5,000–10,000 folds higher than NNK and probably has high chances to bind to the receptor (8, 25), we pre-treated the cells with nicotine for 30 min and then exposed to NNK to test the effect on intracellular mitogenic pathways. After the treatments, the expression of PKC in RLE (Fig. 2a, left panels) or LKR (Fig. 2a, right panels) was analyzed by immunoblotting. A similar level of PKC expression in the cells was recognized by a pan-anti-PKC antibody after the treatments. The activity of this kinase in RLE (Fig. 2a, left panel) or LKR (Fig. 2a, right panel) after the same treatments was measured, using a PKC-specific kinase activity assay. The cells were grown in the medium containing 0.2% serum for 24 h for the purpose of synchronization and then treated with nicotine, NNK or both. PKC activity was dramatically increased 1 h after NNK treatment (about 7–8 folds), and returned to the basal level 24 h following the treatment. Nicotine exposure moderately augmented PKC activity (about 3–4 folds), but was sustained at a moderate level until 24 h after the exposure. Interestingly, the pre-exposure to nicotine, prior to the addition of NNK, elicited a similar pattern of PKC activation at 1 h or 24 h of the post-treatment as that stimulated by nicotine alone. In the presence of MCA (a nAChR inhibitor), the induction of PKC activity by nicotine, NNK or both was abrogated, indicating that the activation of PKC is through nicotine receptor.
Figure 2.
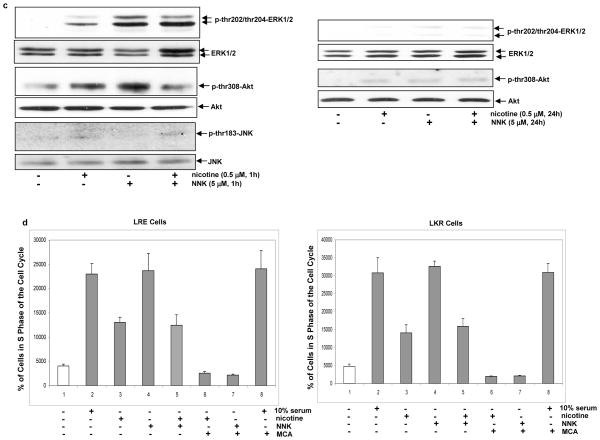
Activation of intracellular signaling pathways and entry of cell cycle in RLE or LKR cells treated with nicotine, NNK or both. a. The cells were treated with nicotine, NNK or both for 1 h, cell lysates were extracted and immunoblotting analysis was performed to detect PKC expression. β-actin was used to determine equal loading of total protein per lane. b. After the same treatments as described above for 1 h or 24 h in the presence or absence of MCA (50 nM), cells lysates were prepared for PKC activity assay. Error bars represent the standard deviation over 3 independent experiments. c. The cells were treated with nicotine, NNK or both for 1 h or 24 h, cell lysates were extracted and immunoblotting was performed to detect phosphorylated ERK1/2, Akt and JNK. ERK1/2, Akt and JNK were used for the determination of equal loading of total proteins per lane. d. the cells were grown in the medium containing 0.2% serum for 24 h, and then treated with nicotine, NNK or both in the presence or absence of MCA. [3H] incorporation was measured by a scintillation counter. Error bars represent the standard deviation over 5 independent experiments.
We then tested how the combination treatment of nicotine and NNK affected other signaling pathways in RLE cells. After the treatments for 1 h or 24 h, the phosphorylation status of ERK1/2, Akt or JNK was analyzed by immunoblotting. ERK1/2 was moderately phosphorylated 1 h after nicotine exposure and strongly activated by NNK (Fig. 2c, left panels). Consistently, the pre-nicotine exposure prior to NNK caused a moderate elevation of ERK1/2 phosphorylation, the magnitude of which was similar as that induced by nicotine exposure alone. Twenty-four hours after the treatments, the expression of phophorylated ERK1/2 was almost undetectable (Fig. 2c, right panels). The patterns of Akt phosphorylation in response to 1 h or 24 h of the treatments were similar as that of ERK1/2. The stress-related kinase JNK was not activated by the treatments for 1 h. Again, nicotine appeared to transiently interfere with NNK-mediated mitogenic pathways.
Next, we tested the role of the combination treatment of nicotine and NNK in growth promotion. [3H]thymidine incorporation assay was used to test if the treatments affected DNA uptake in RLE (Fig. 2d, left panel) or LKR cells (Fig. 2d, right panel). After being grown in the medium containing 0.2% serum for 24h, the cells were treated with nicotine, NNK, both or re-fed with 10% serum in the presence of [3H]thymidine. Rates of DNA synthesis were then measured. Under serum depletion condition, little [3H]thymidine was incorporated by the cells. A moderate amount of [3H]thymidine (about 2–3 folds) was taken by nicotine-treated cells under serum-starvation conditions. The higher rate of [3H]thymidine intake (4–5 folds) was seen in the cells treated with NNK or re-fed with 10% serum. However, the addition of nicotine prior to NNK treatment diminished the NNK-induced increase of [3H]thymidine incorporation into the genome. Moreover, the addition of MCA blocked [3H]thymidine incorporation in the cells treated with nicotine or NNK, but not the cells re-fed with 10% serum, indicating that the growth promotion by nicotine or NNK is through nAChR.
Effect of the combination treatment of nicotine and NNK on the cytotoxicity triggered by cisplatin
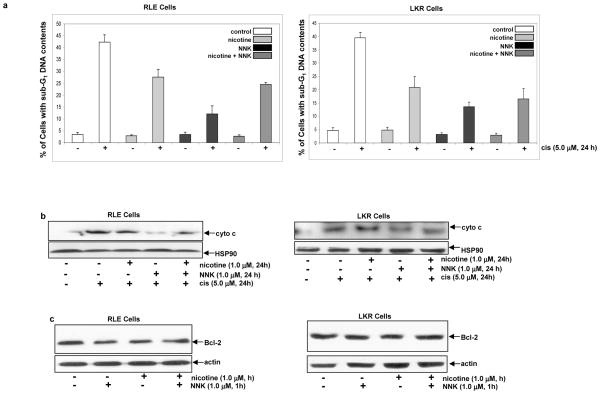
Apoptosis is an important mechanism to eliminate damaged or tumor cells (38). We then tested whether RLE or LKR cells, after the exposure to nicotine, NNK, or both, were susceptible to cytotoxicity triggered by cisplatin. Cisplatin is a common drug for anti-cancer therapy, especially for treating lung cancer (39, 40). Studies showed that nicotine, through regulating mitochondrial pro- or anti-apoptotic factors (such as Bax or Bcl-2), influences the sensitivity of cells to cisplatin or other anti-cancer drugs (39, 40). The cells pre-treated with nicotine, NNK or both for 1h, were exposed to cisplatin for 24 h and the percentages of DNA fragmentation was measured in RLE (Fig. 3a, left panel) or LKR cells (Fig. 3a, right panel). Cisplatin triggered more than 40% of control RLE or LKR cells to undergo apoptosis. In comparison, the cell, after being pre-treated with NNK, became very resistant to cisplatin and only about 10% of the cells were apoptotic. The pre-exposure to nicotine or nicotine plus NNK rendered the cells a moderate degree of the resistance to this anti-cancer drug, in which about 18%–20% of the cells underwent apoptosis. Since cisplatin functions through activating caspases or caspase-related apoptotic factors, the cytosol fractions were isolated from the cells with or without the treatments to determine the cytosolic cytochrome c releasing by immunoblotting (Fig. 3b). The addition of cisplatin caused a high level of cytochrome c releasing to the cytosol in the control cells, which is consistent with the result of DNA fragmentation analysis. The pre-treatment with NNK blocked the most of cisplatin-triggered cytochrome c releasing to the cytosol. A moderate amount of cytochrome c, after treated with cisplatin, was present in the cytosol isolated from the cells pre-exposed to nicotine or nicotine plus NNK. Thus, the results suggest that NNK strongly suppresses cytotoxicity induced by cisplatin. In comparison, nicotine only moderately blocks this drug-induced cytoxicity. However, the pre-ligation of nAChR with nicotine interferes with NNK-mediated anti-apoptotic function.
Figure 3.
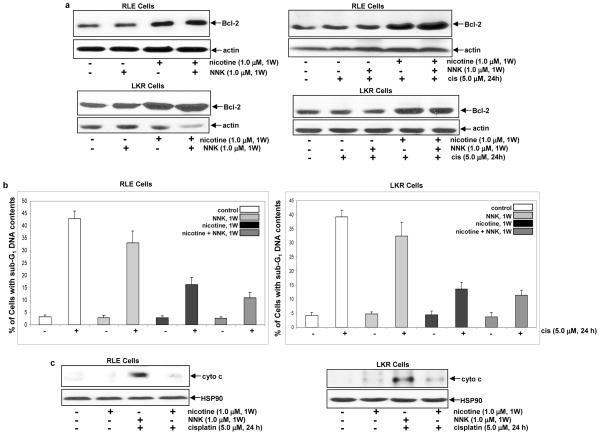
Effect of the treatments on cisplatin-initiated apoptosis and Bcl-2 expression. a. Following being exposed to nicotine, NNK or both, the cells were treated with cisplatin (5.0 μM) for 24 h and DNA fragmentation analysis was performed. Error bars represent the standard deviation over 5 independent experiments. b. After the same treatments as above, the cytosolic fraction was isolated and immunoblotted with an anti-cytochrome c antibody. Heat shot protein-90 (HSP90) was used for the determination of equal loading of total proteins per lane. c. Upon the treatments for 1 h, Bcl-2 expression was analyzed by immunoblotting. β-actin was used for the determination of equal loading of total proteins per lane.
Bcl-2 is a pro-survival factor and positively regulates anti-apopotitc activities, through either changing the permeability of the mitochondrial membrane or association with apoptotic factors (26–28). It was reported that nicotine could activate Bcl-2 and further desensitize cells to apoptotic stimuli (39). Thus, we examined whether Bcl-2 participated in the anti-apoptotc activity rendered by nicotine, NNK or both. Following the treatment with these agents, Bcl-2 expression in RLE or LKR cells was analyzed by immunoblotting (Fig. 3c). The level of Bcl-2 expression had no change following 1 h of the exposure to nicotine, NNK or both, indicating that Bcl-2 might not be a major player in the anti-apoptotic action elicited by the transient treatments.
Long-term treatment with nicotine or nicotine plus NNK reduces the susceptibility of RLE cells to cisplatin-mediated apoptosis
We previously demonstrated that short- or long-term nicotine exposure preferentially activated different sets of intracellular signaling pathways that influence different growth-related processes (41). Therefore, we tested the effect of the persistent treatment of nicotine, NNK or both on apoptosis. RLE or LKR cells were treated with these agents for 1 week and the expression of Bcl-2 was tested (Fig. 4a, left panels). The long-term treatment with nicotine or nicotine plus NNK augmented Bcl-2 expression to about 2–3 folds, and however, NNK exposure for 1 week played no role on the expression of this pro-survival factor. Following the treatment with nicotine, NNK or both for 1 week, cisplatin was added into the cell cultures for 24 h and Bcl-2 expression was subsequently analyzed by immunoblotting (Fig. 4a, right panels). The addition of cisplatin did not further increase Bcl-2 expression in nicotine- or nicotine plus NNK-treated cells. The induction of apoptosis triggered by cisplatin in RLE or LKR cells was also tested (Fig. 4b). The persistent NNK exposure slightly, but not significantly, reduced the susceptibility of the cells to apoptosis initiated by cisplatin. However, the cells, following the persistent combination treatment, became highly resistant to cisplatin. The cells treated with nicotine for 1 week appeared to be slightly more sensitive to cisplatin than that exposed to these two compounds. The cytochrome c releasing to the cytosol in these two cells was examined (Fig. 4c). Consistently, a relatively large amount of cytochrome c was released to the cytosol after the addition of cisplatin in the cells treated with NNK for 1 week. Cisplatin was unable to induce the cytosolic cytochrome c releasing in the cells exposed to nicotine or nicotine plus NNK for 1 week. The data suggest that persistent nicotine exposure may, through upregulating Bcl-2, render the increased resistance of RLE or LKR cells to cisplatin. Since the persistent nicotine exposure partially blocked cisplatin-induced apoptosis and completely suppressed cytochrome c releasing, it indicates that multiple pathways might be involved in the execution of cisplatin-mediated apoptosis.
Figure 4.
Effect of the long-term treatments on Bcl-2 expression and cisplatin-induced apoptosis. a. Upon the treatments for 1 week, the cells were cultured in the medium containing cisplatin for 24 h. Subsequently, Bcl-2 expression was analyzed by immunoblotting. β-actin was used for the determination of equal loading of total proteins per lane. b. Following being exposed to nicotine, NNK or both for a week, the cells were cultured in the medium containing cisplatin for 24 h and DNA fragmentation analysis was performed. Error bars represent the standard deviation over 5 independent experiments. c. After the same treatments, the cytosolic fraction was isolated and immunoblotted with an anti-cytochrome c antibody. Heat shot protein-90 (HSP90) was used for the determination of equal loading of total proteins per lane.
Pre-treatment of nicotine exposure does not interfere with NNK-mediated long-term cell growth
It appears that the short-term nicotine exposure transiently interferes with the NNK-mediated signaling pathways, and the persistent exposure increases the resistance to cisplatin in NNK-treated cells. The effect of the treatments on anchorage-independent growth was examined using soft agar assay in RLE, LKR or human lung cancer H5800 cells that were isolated from lung foci of ras transgenic mice (Table 1). Untreated and nicotine-treated RLE cells did not form colonies in soft agar medium. NNK treatment permitted the cells to form colonies in soft agar. However, the more cells, after the exposure to the combination treatment of nicotine and NNK, were able to grow in an anchorage-independent fashion. LKR cells are relatively benign murine lung cancer cells and therefore, only a small number of murine lung cancer LKR cells were formed colonies in soft agar medium and the treatment with nicotine did not promote the anchorage independent growth of LKR cells. NNK stimulated LKR cells to form colonies in soft agar and the combination treatment further augmented this anchorage-independent growth. Human lung cancer H5800 cells were also used in the assay. As expected, certain number of this human lung cancer cells formed colonies in soft agar. However, nicotine exposure caused a moderate increase of the numbers of colony formation. Consistently, the combination treatment had the most effect on the colony formation of H5800 cells. It seemed that persistent nicotine exposure cooperates with NNK to promote anchorage-independent growth rates.
Table 1.
Colony formation of RLE, LKR or H5800 cells in soft agar upon the treatments.
| Cell Type | No. of seeded cells | No. of Colonies | SD |
|---|---|---|---|
| RLE | 5,000 | 0 | 0 |
| RLE (nicotine) | 5,000 | 0 | 0 |
| RLE (NNK) | 5,000 | 44 | ±0.2 |
| RLE (nicotine + NNK) | 5,000 | 67 | ±1.8 |
| LKR | 5,000 | 21 | ±3.5 |
| LKR (nicotine) | 5,000 | 26 | ±0.3 |
| LKR (NNK) | 5,000 | 76 | ±0.3 |
| LKR (nicotine + NNK) | 5,000 | 99 | ±0.3 |
| H5800 | 5,000 | 64 | ±4.8 |
| H5800 (nicotine) | 5,000 | 72 | ±5.2 |
| H5800 (NNK) | 5,000 | 135 | ±6.1 |
| H5800 (nicotine + NNK) | 5,000 | 151 | ±5.6 |
RLE (rat lung epithelial), LKR (murine lung cancer) and H5800 (human lung cancer) cells were cultured in soft agar in the presence or absence of nicotine, NNK or both for 2 weeks. Five microliter of fresh medium containing the drugs was added into the cultures every 4 days.
SD: standard deviation over 5 independent experiments.
Discussion
Emerging evidence demonstrates that tobacco smoke is an important risk factor of environment in the promotion of the onset of not only lung cancer but also malignancies in other organs (1–4). Nicotine exits high concentrations in the blood stream of smokers and has been shown to be able to directly influence cell growth-related activities in non-neuronal cells (1–4). Nitrosamines, such as NNK, are well-defined, cigarette-related carcinogens. In the lung, nitrosamines directly attacks the genome by promoting formation of DNA adducts in the cells. However, through binding to AChR, NNK or other nitrosamines are able to stimulate various key biological activities and further promote cell survival and tumorigenesis (1–4, 8). After NNK exposure, expressions of a diverse set of genes were altered, which subsequently regulate the processes of gene transcription, RNA processing and protein modification (2–7). Although nicotine is not a conventional carcinogen and plays no direct role in the genome, studies showed that nicotine promotes cell proliferation in various types of non-neuronal cells through binding to its receptor. In lung cancer, nicotine exposure facilitated the spread of the cancer in the body (16, 39, 42). Nicotine was also reported to stimulate the phosphorylation of μ- and m-calpains to enhance proteolytic activity and further to accelerate cell invasion (42). However, a little information is available for how nicotine interacts with tumorigenic signaling stimulated by tobacco-related carcinogens, such as NNK. Thus, this study aimed to investigate how pre-nicotine ligation with the receptor affects cellular transformational process induced by NNK.
In this study, we demonstrated that the treatment with NNK for 1 h transiently, but strongly upregulated several major intracellular signaling pathways, such as PKC, ERK1/2 or Akt, which rapidly returned to the basal level 24 h after the treatment. In comparison, nicotine ligation had much less effect on the same pathways. Since tobacco smoke contains a high concentration of nicotine and relative low NNK, the combination treatment was designed by exposing the cells to nicotine for 30 minutes prior to NNK treatment. The pre-nicotine treatment transiently interfered with NNK-mediated mitogenic signaling. It is likely that nicotine, by occupying nAChR, blocks NNK functions. We further showed that the short-term NNK exposure strongly protected RLE cells from cisplatin-mediated cytotoxicity. Interestingly, pre-nicotine ligation also reduced NNK-induced resistance of the cells to the anti-cancer drug. As a carcinogen, NNK strongly activates growth-related activities and suppresses cell death program, which not only promotes tumorigenesis but also resistance to anti-cancer drugs. The partial abrogation of NNK-mediated activities by nicotine indicates that a transient competition between nicotine and NNK for the occupancy of the receptor may exist in tobacco smoking.
Interestingly, the cells treated with NNK for a week became sensitive to cisplatin again. However, when being exposed persistently to nicotine or the combination of nicotine plus NNK, the cells maintained relatively resistant to cytotoxicity induced by the anti-cancer drug, which coincided with the increased expression of Bcl-2. Bcl-2 is a key regulator of cell death or of a process required for transformation and autoimmune disease (26–28). It was reported that nicotine exposure is able to upregulate Bcl-2 expression and activity to protect lung cancer cells against apoptosis (39). We previously demonstrated that the persistent, long-term nicotine treatment transiently stimulated PKC activity which was sustained at a level above the baseline for a long period (41). Here, we demonstrated that PKC activity was upregulated 1 h after the treatment with either nicotine or nicotine plus NNK, and maintained at a moderate level 24 following the treatments, which was not seen in the cells treated with NNK alone (figure 2b). The data suggest that the mitogenic stimulatory activity triggered by NNK is transient. The action triggered by nicotine appears to be sustained, through which affects pro-survival activities in cells, such as Bcl-2. Since the cells co-treated with nicotine plus NNK were more resistant to cisplatin than that treated with nicotine alone, it is possible that besides upregulating Bcl-2, other pro-survival mechanisms are involved.
Rat lung epithelial RLE, murine lung cancer LKR as well as human lung cancer H5800 cells express nicotine receptor. The addition of MCA (a nAChR inhibitor) was shown to block the activation of NNK- or nicotine-mediated signaling pathways, which suggest that these cell growth promotion activities require the engagement of nicotine receptor. The results from our experiments further proved that NNK is a strong carcinogen that upregulates growth-related signaling pathways and allows normal rat lung epithelial cells to grow in soft agar or further promotes anchorage-independent growth in murine or human lung cancer cells. The pre-ligation of nAChRs by nicotine transiently interferes with or modulates NNK-mediated mitogenic signaling. In the long term, nicotine, probably through modifying NNK-mediated signaling, accelerates transformation process in not only normal epithelial cells but also cancer cells. Thus, our data demonstrate a complexity of nicotine as a major component in tobacco smoking in the regulation of lung cell growth or transformation.
Cell surface growth factors and adhesion molecules are often involved in the regulation of tumor development. Recently, the role of nicotine on cell growth started to be recognized (8). Long term exposure of nicotine was shown to activate Ras and its downstream signaling pathways in lung epithelial cells, resulting in the upregulation of cyclin D1 and subsequent entry of cell cycle following G1 stimulation (41). Upon nicotine exposure, the lung cells were unable to arrest in G1 phase of the cell cycle, which probably is in favor for the establishment of genetic instability. Our current study again demonstrated that NNK strongly stimulated multiple intracellular signaling pathways to support growth- or survival-related activities. Through binding to the receptor, NNK disrupted the G1 restriction point induced by serum starvation and pushed S phase transition of the cells in the cell cycle. In comparison, nicotine was relative less effect on cell cycle transition. The combination treatment of nicotine and NNK, like nicotine exposure alone, had a moderate effect on cell cycle progression during serum starvation. It suggests that the cell cycle progression mediated by NNK under serum-starvation was initiated by the receptor ligation, which was interfered by pre-ligation with nicotine. The results further support that through competing with the occupancy of the receptor nicotine is capable of modulating NNK signaling and sustains the receptor-induced growth-related activities, which often occurs in the cooperation between VEGF and other growth factors (43, 44). It is also possible that the interference of nicotine with NNK signaling is due to the differences of the affinity with the receptor or of the rates of the decay of these two compounds.
Many growth factors and their downstream effectors are aberrantly activated or overexpressed, which contribute to growth deregulation in cancer. It was reported that the addition of nicotine increases EGFR expression in colon cells (10). Therefore, nicotine exposure alone may, by modifying signaling, increase the sensitivity of lung epithelial or cancer cells to long-term mitogenic stimulation, and subsequently promote cell proliferation and metastasis. Since src has been shown to be responsible for the phosphorylation of nAChR, it would be interesting to determine if src acts as a mediator between nicotine and intracellular signaling pathways for effectively transmitting the signals.
RLE is a spontaneously immortalized, but non-transformed rat lung epithelial cell line derived from the lung tissue, and are suggested by ATCC to be normal lung epithelial cells, because these cells have normal numbers of chromosomes and can not grow in soft agar. Our investigation demonstrated that RLE cells express several subunits of nAChR, and nicotine or NNK treatment does not alter the expression level of these subunits. However, NNK engagement is highly mitogenic than nicotine ligation. Furthermore, the pre-nicotine exposure only partially interfered with NNK-mediated signaling. It is conceivable that besides binding to nAChR, NNK interacts with other membrane components (such as phospholipids or ion channels) to trigger a full mitogenic activation.
In summary, we have demonstrated that nicotine exposure is able to influence the activity of growth signaling pathways induced by NNK in rat lung epithelial RLE, murine lung cancer LKR as well as human lung cancer H5800 cells. The temporary interference of nicotine on and persistent cooperation with NNK signaling in lung cells reflects the complication of nicotine in promoting cell growth. Using the combination treatment of nicotine and NNK, we demonstrate a possible cooperation between nicotine and NNK in tobacco smoking and the possible role of nicotine in sustaining NNK-mediated cell growth promotion. The further understanding of the interaction of nicotine and NNK will provide molecular targets for better development of drugs or designing regimens to treat tobacco-related diseases.
Acknowledgments
This work was supported by National Institutes of Health Grant RO1 CA124490 (to C. C) and by Flight Attendant Medical Research Institute.
References
- 1.Karnath B. Smoking cessation. Am J Med. 2002;112:399–405. doi: 10.1016/s0002-9343(01)01126-3. [DOI] [PubMed] [Google Scholar]
- 2.Hecht SS. Cigarette smoking and lung cancer: chemical mechanisms and approaches to prevention. Lancet Oncol. 2002;3:461–469. doi: 10.1016/s1470-2045(02)00815-x. [DOI] [PubMed] [Google Scholar]
- 3.Arredondo J, Chernyavsky AI, Grando SA. Nicotinic receptors mediate tumorigenic action of tobacco-derived nitrosamines on immortalized oral epithelial cells. Cancer Biol Ther. 2006;5:511–517. doi: 10.4161/cbt.5.5.2601. [DOI] [PubMed] [Google Scholar]
- 4.Bose C, Zhang H, Udupa KB, Chowdhury P. Activation of p-EKR1/2 by nicotine in pancreatic tumor cell line AR42J: effects on proliferation and secretion. Am J Physiol Gastrointest Liver Physiol. 2005;289:G926–G934. doi: 10.1152/ajpgi.00138.2005. [DOI] [PubMed] [Google Scholar]
- 5.Hecht SS, Abbaspour A, Hoffman D. A study of tobacco carcinogenesis. Cancer Lett. 1988;42:141–145. doi: 10.1016/0304-3835(88)90251-0. [DOI] [PubMed] [Google Scholar]
- 6.Sckido Y, Fong KM, Minna JD. Molecular genetics of lung cancer. Annu Rev Med. 2003;54:73–87. doi: 10.1146/annurev.med.54.101601.152202. [DOI] [PubMed] [Google Scholar]
- 7.Heeschen C, Weis M, Aicher A, Dimmeler S, Cooke JP. A novel angiogenic pathway mediated by non-neuronal nicotinic acetylcholine receptors. J Clin Inves. 2002;110:527–536. doi: 10.1172/JCI14676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Minna JD. Nicotine exposure and bronchial epithelial cell nicotinic acetylcholine receptor expression in the pathogenesis of lung cancer. J Clin Inves. 2003;111:31–90. doi: 10.1172/JCI17492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shi VY, Wu WK, Chu KM, et al. Nicotine induces cyclooxygenase-2 and vascular endothelial growth factor receptor-2 in association with tumor-associated invasion and angiogenesis in gastric cancer. Mole Cancer Res. 2005;3:607–615. doi: 10.1158/1541-7786.MCR-05-0106. [DOI] [PubMed] [Google Scholar]
- 10.Dagupta P, Rizwani W, Pilai S. Nicotine induces cell proliferation, invasion and epithelial-mesenchymal transition in a variety of human cancer cell lines. Int J Cancer. 2009;124:36–45. doi: 10.1002/ijc.23894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shi VY, Wu WK, Ye YN, et al. Nicotine promotes gastric tumor growth and neovascularization by activating extracellular signal-regulated kinase and cycloxygenase-2. Carcinogenesis. 2004;25:2487–2495. doi: 10.1093/carcin/bgh266. [DOI] [PubMed] [Google Scholar]
- 12.Dasgupta P, Rastogi S, Pillai S, et al. Nicotine induces cell proliferation by beta-arrestin-mediated activation of Src and Rb-Raf-1 pathways. J Clin Invest. 2006;116:2208–2217. doi: 10.1172/JCI28164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakayama H, Numakawa T, Ikeuchi T. Nicotine-induced phosphorylation of Akt through epidermal growth factor receptor and Src in PC12h cells. J Neurochem. 2002;83:1372–1379. doi: 10.1046/j.1471-4159.2002.01248.x. [DOI] [PubMed] [Google Scholar]
- 14.Heeschen C, Jang JJ, Weis M, et al. Nicotine stimulates angiogenesis and promotes tumor growth and atheroscierosis. Nat Med. 2001;7:833–839. doi: 10.1038/89961. [DOI] [PubMed] [Google Scholar]
- 15.Heeschen C, Weis M, Cooke JP. Nicotine promotes arteriogenesis. J Am Coll Cardiol. 2003;41:489–496. doi: 10.1016/s0735-1097(02)02818-8. [DOI] [PubMed] [Google Scholar]
- 16.Guo J, Ibaragi S, Zhu T, et al. Nicotine promotes mammary tumor migration via a signaling cascade involving PKC and cdc42. Cancer Res. 2008;68:8473–8478. doi: 10.1158/0008-5472.CAN-08-0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arredondo J, Nguyen VT, Chernyavsky AI, et al. A receptor-mediated mechanism of nicotine toxicity in oral keratinocytes. Lab Invest. 2001;81:1653–1668. doi: 10.1038/labinvest.3780379. [DOI] [PubMed] [Google Scholar]
- 18.Arredondo J, Chernyavsky AI, Marubio LM, et al. Receptor-mediated tobacco toxicity: regulation of gene expression through alpha3beta2 nicotinic receptor in oral epithelial cells. Am J Pathol. 2005;166:597–613. doi: 10.1016/s0002-9440(10)62281-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nguyen VT, Hall LL, Gallacher G. Choline acetyltransferase, acetylcholinesterase, and nicotinic acetylcholine receptors of human gingival and esophageal epithelia. J Dent Res. 2000;79:939–49. doi: 10.1177/00220345000790040901. [DOI] [PubMed] [Google Scholar]
- 20.Seguela P, Wadiche J, Dineley-Miller K, Dani JA, Patrick JW. Molecular cloning, functional properties, and distribution of rat brain alpha 7: a nicotinic cation channel highly permeable to calcium. J Neurosci. 1993;13:596–604. doi: 10.1523/JNEUROSCI.13-02-00596.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Castro NG, Albuquerque EX. alpha-Bungarotoxin-sensitive hippocampal nicotinic receptor channel has a high calcium permeability. Biophys J. 1995;68:516–524. doi: 10.1016/S0006-3495(95)80213-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schuller HM, Orloff M. Tobacco-specific carcinogenic nitrosamines. Biochem Pharmacol. 1998;55:1377–1384. doi: 10.1016/s0006-2952(97)00651-5. [DOI] [PubMed] [Google Scholar]
- 23.Plummer HK, Sheppard BJ, Schuller HM. Interaction of tobacco-specific toxicants with nicotinic cholinergic regulation of fetal pulmonary neuroendocrine cells: implications for pediatric lung disease. Exp Lung Res. 2000;26:121–135. doi: 10.1080/019021400269916. [DOI] [PubMed] [Google Scholar]
- 24.Schuller HM, Jull BA, Sheppard BJ, Plummer HK. Interaction of tobacco-specific toxicants with the neuronal alpha 7 nictoinic acetylcholine receptor and its associated mitogenic signal transduction pathway: potential role in lung carcinogenesis and pediatric lung disorders. Eur J Pharmacol. 2000;393:265–277. doi: 10.1016/s0014-2999(00)00094-7. [DOI] [PubMed] [Google Scholar]
- 25.Heusch WL, Maneckjee R. Signaling pathways involved in nicotine regulation of apoptosis of human lung cancer cells. Carcinogenesis. 1998;19:551–556. doi: 10.1093/carcin/19.4.551. [DOI] [PubMed] [Google Scholar]
- 26.Gottlieb RA. Mitochondria: execution central. FEBS Lett. 2009;482:6–12. doi: 10.1016/s0014-5793(00)02010-x. [DOI] [PubMed] [Google Scholar]
- 27.Adrain C, Martin SJ. The mitochondrial apoptosome: a killer unleashed by the cytochrome seas. Trends Biochem Sci. 2001;26:390–397. doi: 10.1016/s0968-0004(01)01844-8. [DOI] [PubMed] [Google Scholar]
- 28.Colin J, Gaumer S, Guenal I, Mignotte B. Mitochondria, Bcl-2 family protein and apoptosomes: of worms, flies and men. Front Biosci. 2009;14:4127–4137. doi: 10.2741/3517. [DOI] [PubMed] [Google Scholar]
- 29.Gallo O, Bianchi S, Porfirio B. Bcl-2 overexpression and smoking history in head and neck cancer. J Natl Cancer Inst. 1995;87:1024–1025. doi: 10.1093/jnci/87.13.1024. [DOI] [PubMed] [Google Scholar]
- 30.Assis GF, Ceolin DS, Marques ME, Salvadori DM, Ribeiro DA. Cigarette smoke affects apoptosis in rat tongue mucosa: role of bcl-2 gene family. J Mol Histol. 2005;36:483–489. doi: 10.1007/s10735-006-9023-z. [DOI] [PubMed] [Google Scholar]
- 31.Ruvolo PP, Deng X, Carr BK, May WS. A functional role for mitochondrial protein kinase Calpha in Bcl-2 phosphorylation and suppression of apoptosis. J Biol Chem. 1998;273:25436–25442. doi: 10.1074/jbc.273.39.25436. [DOI] [PubMed] [Google Scholar]
- 32.Kurinna S, Konopleva M, Palla SL, et al. Bcl-2 phosphorylation and active PKC alpha are associated with poor survival in AML. Leukemia. 2006;20:1316–1319. doi: 10.1038/sj.leu.2404248. [DOI] [PubMed] [Google Scholar]
- 33.Sato T, Abe T, Makamoto N, et al. Nicotine induces cell proliferation in association with cyclin D1 upregulation and inhibits cell differentiation in association with p53 regulation in a murine pre-osteoblastic cell line. Biochem Biophys Res Commun. 2008;377:126–130. doi: 10.1016/j.bbrc.2008.09.114. [DOI] [PubMed] [Google Scholar]
- 34.Denis GV, Yu Q, Ma P, et al. Bcl-2, via its BH4 domain, blocks apoptotic signaling mediated by mitochondrial Ras. J Biol Chem. 2003;278:5775–5782. doi: 10.1074/jbc.M210202200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Newton AC. Protein kinase C: structure, function, and regulation. J Biol Chem. 1995;270:28495–28498. doi: 10.1074/jbc.270.48.28495. [DOI] [PubMed] [Google Scholar]
- 36.Gomez DE, Skilton G, Alonso DF, Kazanietz M. The role of protein kinase C and novel phorbol ester receptors in tumor cell invasion and metastasis. Onclo Rep. 1999;6:1363–1370. doi: 10.3892/or.6.6.1363. [DOI] [PubMed] [Google Scholar]
- 37.Mai H, May WS, Gao F, Jin Z, Deng X. A functional role for nicotine in Bcl-2 phosphorylation and suppression of apoptosis. J Biol Chem. 2003;278:6369–6379. doi: 10.1074/jbc.M209044200. [DOI] [PubMed] [Google Scholar]
- 38.Cotter TG. Apoptosis and cancer: the genesis of a research field. Nat Rev Cancer. 2009;9:501–507. doi: 10.1038/nrc2663. [DOI] [PubMed] [Google Scholar]
- 39.Mai H, May SW, Gao F, Jin Z, Deng X. A functional role for nicotine in Bcl-2 phosphorylation and suppression of apoptosis. J Biol Chem. 2003;278:1886–1891. doi: 10.1074/jbc.M209044200. [DOI] [PubMed] [Google Scholar]
- 40.Xin M, Deng X. Nicotine inactivation of the proapoptotic function of Bax through phosphorylation. J Biol Chem. 2005;280:10781–10789. doi: 10.1074/jbc.M500084200. [DOI] [PubMed] [Google Scholar]
- 41.Guo J, Chu M, Abbeyquaye T, Chen CY. Persistent nicotine treatment potentiates DHFR amplification in rat lung epithelial cells as a consequence of Ras activation. J Biol Chem. 2005;280:30422–30431. doi: 10.1074/jbc.M504688200. [DOI] [PubMed] [Google Scholar]
- 42.Xu L, Deng X. Protein kianse Cτ promotes nicotine-induced migration and invasion of cancer cells via phosphorylation of μ and τ-calpains. J Biol Chem. 2006;281:4457–4466. doi: 10.1074/jbc.M510721200. [DOI] [PubMed] [Google Scholar]
- 43.Hata Y, Rook SL, Aiello LP. Basic fibroblast growth factor induces expression of VEGF receptor KDR through a protein kinase C and p44/p42 mitogen-activated protein kinase-dependent pathway. Diabetes. 1999;48:1145–1155. doi: 10.2337/diabetes.48.5.1145. [DOI] [PubMed] [Google Scholar]
- 44.Sanchez-Elsner T, Botella LM, Velasco B, et al. Synergistic cooperation between hypoxia and transforming growth factor-beta pathways on human vascular endothelial growth factor gene expression. J Biol Chem. 2001;276:38527–38535. doi: 10.1074/jbc.M104536200. [DOI] [PubMed] [Google Scholar]