Abstract
Online adaptive radiotherapy for bladder cancer is a novel radiotherapy technique that was found feasible in a pilot study at a single academic institution. In September 2010 this technique was opened as a multicenter study through the Trans-Tasman Radiation Oncology Group (TROG 10.01 bladder online adaptive radiotherapy treatment). Twelve centers across Australia and New-Zealand registered interest into the trial. A multidisciplinary team of radiation oncologists, radiation therapists and medical physicists represented the trial credentialing and technical support team. To provide timely activation and proper implementation of the adaptive technique the following key areas were addressed at each site: Staff education/training; Practical image guided radiotherapy assessment; provision of help desk and feedback. The trial credentialing process involved face-to-face training and technical problem solving via full day site visits. A dedicated “help-desk” team was developed to provide support for the clinical trial. 26% of the workload occurred at the credentialing period while the remaining 74% came post-center activation. The workload was made up of the following key areas; protocol clarification (36%), technical problems (46%) while staff training was less than 10%. Clinical trial credentialing is important to minimizing trial deviations. It should not only focus on site activation quality assurance but also provide ongoing education and technical support.
Keywords: Adaptive radiotherapy, clinical trial, credentialing, image-guided radiotherapy
Introduction
Radiotherapy treatment for muscle invasive bladder carcinomas requires large treatment margins of up to 3 cm to account for daily variations of the bladder size and position.[1–3] The use of large margins is common practice in radiotherapy departments where patient set-up and treatment verification is limited to skin/tattoo setup or bony anatomy matching prior to treatment delivery. With the advent of in-room 3D volumetric imaging systems such as computed tomography (CT) on rails, cone beam CT (CBCT) and Megavoltage CT (MVCT) pre-treatment soft tissue imaging has allowed verification of the bladder size and location prior to treatment delivery. Online adaptive radiotherapy for bladder cancers has been explored[4–9] to take full advantage of the imaging technology. This technique makes available a library of plans prior to each treatment session. By assessing the target volume a “best fit” plan can be used every day. Adaptive radiotherapy allows the reduction of treatment margins while maintaining dose coverage to the target.[5,9] Dose to normal tissue[5,10] small bowel[4] and intestinal cavity[9] can be reduced. However, online adaptive radiotherapy for bladder cancers is a complex approach involving multiple plans and interpretation of volumetric images under time pressure and as such only used by a few centers. Novel radiotherapy treatment techniques rely on close collaboration between radiation oncologists, radiation therapists and medical physicists as technical support, staff expertise and training allows success to this technique. These techniques which exceed convention may be introduced safely in the context of multicenter feasibility studies.[11–13]
At the Peter MacCallum Cancer Centre a pilot study of online adaptive radiotherapy for muscle invasive bladder cancer was conducted across two of its campuses. A total of 27 patients were enrolled into the trial over a 2-year period. This single institution pilot study found online adaptive radiotherapy to be feasible at both its 5-linac and 2-linac campus.[5] During the course of the pilot study two important tools were developed (1) education of radiation therapists in pelvic soft tissue anatomy (2) protocol with flexibility for multivendor sites.
The pilot study generated interest in many departments across Australian and New Zealand and in 2010 a multicenter feasibility study of bladder online adaptive radiotherapy treatment (BOLART) was opened through the Trans-Tasman Radiation Oncology Group (TROG) as TROG 10.01 (Clinical Trials identifier no NCT01142102).
The aim of this paper is to analyze the experience of the setup, activation and support of the TROG 10.01 multi-center feasibility trial during the first 15 months of when the study opened (September 2010-December 2011). The lessons learnt from this process will highlight the technical challenges in trials of advanced technologies as well as their ongoing management requirements.
Materials and Methods
BOLART is a radiotherapy technique that relies on daily pre-treatment decision making by radiation therapists to choose a “plan-of-best-fit” from a library of four plans. The credentialing process for the trial requires a department’s ability to perform the following functions
Ability to CT simulate
Ability to create 3D conformal radiotherapy planning using 3 or more fields. Intensity modulated radiotherapy was not allowed in the trial due to concerns regarding possible requirement of extra treatment time
Ability to export planning contours to the record and verification system
Ability to perform pre-treatment soft tissue verification imaging using CBCT or CT on rails
Ability to export these images to the planning system where they can be registered with the planning scan to generate multiple plans
Ability to schedule multiple radiotherapy plans and a treatment record of dose accumulation from different plans
Show an understanding of the treatment protocol by demonstrating appropriate decision making procedures.
The protocol provided participating centers with an understanding of the intent of the adaptive radiotherapy process as well as guidelines on how this can be achieved within their department’s equipment and framework.
A multidisciplinary trial credentialing team was formed from staff at the trial management center. To ensure adherence to the protocol the quality assurance team engaged each center in the following areas:
Education and training: Half day on site training and discussion of technical and protocol requirements of the trial
Image guided radiotherapy (IGRT) assessment: Half day on site evaluation of image guided procedure
Help desk development: Direct E-mail/telephone contact to credentialing team for technical or protocol support. In addition to the help desk support, feedback in the form of flyer updates, troubleshooting forums and questionnaires were created and sent out to each department during the trial recruitment period.
Results/Discussion
The TROG 10.01 center credentialing opened in September 2010 and up until December 2011 a total of 12 centers from Australia and New Zealand completed the credentialing program. While trial specific QA were developed for this trial, emphasis was made on the education and development of new work processes for participating centers. Key areas of training and technical support for the trial are described.
Education and training
In the initial pilot study radiation therapist credentialing for treatment using the BOLART technique was done via a full day training program.[14] The training program consisted of presentations on pelvic CT/CBCT anatomy, physics of CBCT, radiotherapy contouring/planning. These presentations were given by radiologists/oncologists/medical physicists and radiation therapists. It was not feasible to provide training workshops for every participating center, instead an online e-Learning training program (based on the workshop presentations) was developed to train radiation therapists in Australia and New Zealand. Each of the participating centers was asked to enroll radiation therapists, oncologists, physicists, and trial co-coordinators onto the e-Learning website to ensure that a basic level of understanding of the trial is present.
As a follow up to the e-Learning training the credentialing team organized a face-to-face half-day presentation/lecture for each participating site. At this visit it was important that the collaboration between radiation therapists/medical physicist and radiation oncologist be established as on several occasions it was found that the medical physicists were not readily integrated in the site visit process.
The visit provided formal presentations covering three areas: (1) BOLART overview and update of trial (2) CBCT physics and dose and (3) Radiotherapy technical aspects.
A BOLART overview/update of the trial opened up each site visit. This was a more comprehensive presentation of the quarterly electronic newsletter sent out. New trial developments, recruitment update and a brief overview of the credentialing requirements ensured staff were aware of their current activation status. The CBCT physics presentation was requested by radiation therapists new and old to CBCT imaging. As this was a trial that allowed up to 47 CBCT scans to be used over the course of treatment it was important for radiotherapy/physics staff to understand the physics behind the imaging modality as well as to investigate individual methods of reducing dose. A number of methods were shown including the use of copper filters with the Varian on Board Imager™[15] as well as the use of patient specific field apertures on the OBI™ (Varian Medical Systems, Palo Alto, USA); or Elekta xray volume imaging (XVI)™, (Elekta, Stockholm, Sweden).
The radiotherapy technical aspects was aimed at the radiation therapists involved in the trial. Simulation procedures, radiotherapy image registration requirements, plan scheduling and staff credentialing was discussed in detail to prompt individual centers on how to best manage these areas for the trial. Presentations were continually developed as more site visits had been performed to include new observations. In this way with each site visit recommendations on technical procedures can be made by the credentialing team to centers with similar vendor setup.
Image guided radiotherapy assessment
The image guidance requirements for this trial require daily pretreatment CBCT. Not only is image matching important in this setting but as well the decision making process that is required in choosing the best plan for treatment.
To ensure familiarity with the adaptive matching process a site visit with an IGRT specific phantom was developed to assess familiarity with CBCT acquisition and the plan of the day matching process. A phantom designed with accompanying CT dataset and contours was used to evaluate IGRT matching skills.[16]
All participating centers were required to download the phantom CT dataset and associated digital imaging and communication in medicine (DICOM) compatible structures. This dataset was imported into a planning system and exported to their record and verification system for CBCT acquisition and image review with trial defined structure contours. All participating centers were able to successfully import this plan into their planning system except for the centers using a Pinnacle (Philips, Milpitas, USA) treatment planning system. Due to DICOM license restrictions on Pinnacle software, importing of trial datasets was not possible. For two of the centers with this problem, a new CT scan of the phantom was done on the day of the site visit with the associated plans re-created for the machine matching process.
During the CBCT and the machine matching process radiation therapists were encouraged to review their matching process and decide on the plan that would best fit the bladder phantom. Imaging and matching scenarios based on excessively large bladder volumes were included in the practical assessment. Based on the matching results a discussion over prolonged treatment time and the effect of bladder filling was held at the console in order to gauge the level of knowledge about protocol requirements.
Help desk development
The multidisciplinary credentialing team provided a dedicated help desk to answer technical, operational/clinical queries relating to the trial. A documented total of 116 queries/problems were raised via E-mail/phone. Less than 30% of these queries were made during a center’s credentialing period. Resolution was predominantly done via E-mail (83.6%) followed by telephone (11.2%) and when possible additional on-site visits (5.2%).
Figure 1 shows a breakdown of the areas of support that arose through the help desk. Protocol related questions made up a majority of the workload (36%) however, areas that required technical management made up a total of 46% (CBCT registration/radiotherapy planning/IGRT matching/data submission/case report forms). Questions relating to staff training requirements (technical problems of e-Learning web access not included in this) made up less than 10% of the work. Patient specific issues as well as review of in-house documentation each contributed 5% or less. The nature of the problems and solutions developed are discussed below.
Figure 1.
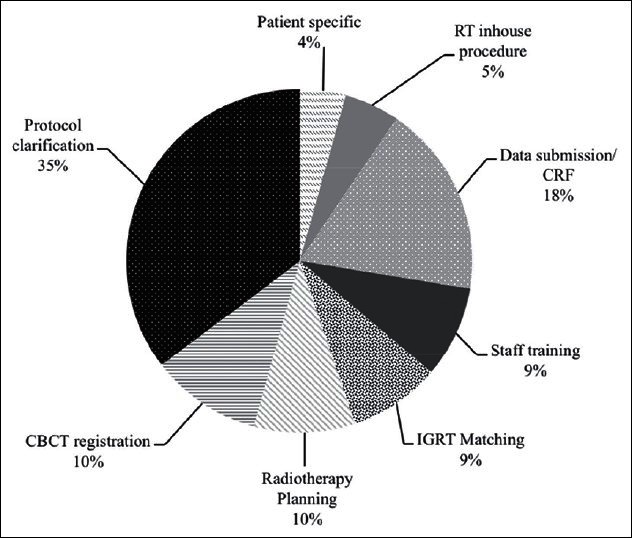
Breakdown of areas of diffi culty that were handled by credentialing team during and after credentialing process. Total numbers of lodged queries were 116 over a 15-month period
CBCT registration
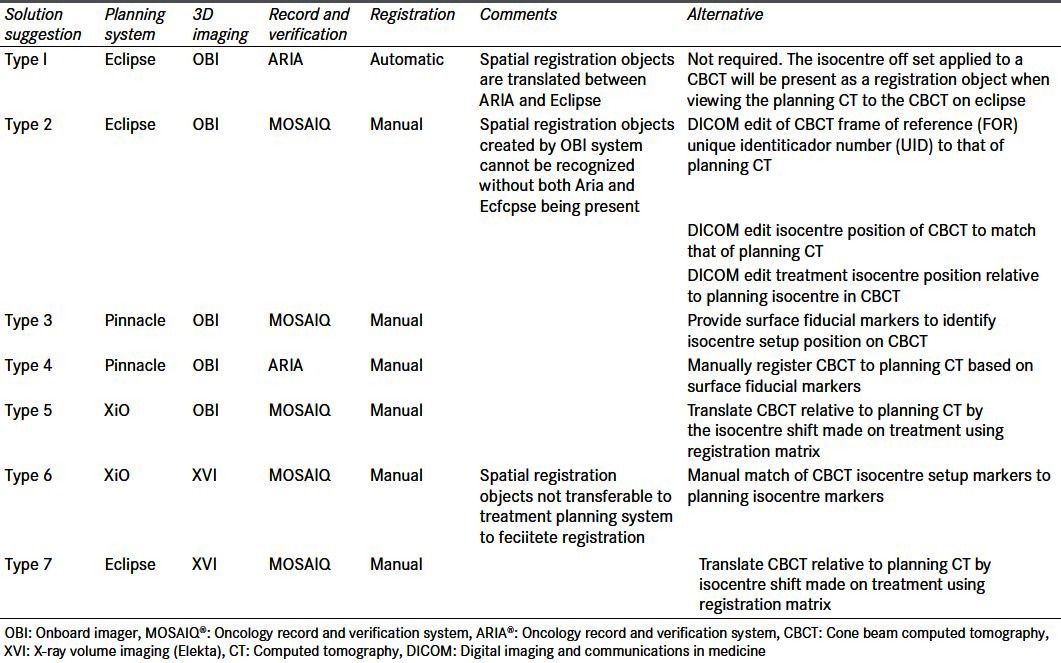
The online adaptive BOLART protocol requires the export of CBCT datasets back to treatment planning system for bladder centroid registration to the CT planning dataset. The recommendation made by the credentialing team was to use the pre-treatment isocentre shift as a surrogate for centroid localization. Seven types of multi-vendor systems were identified for treatment planning system/image acquisition and record and verification systems. A number of departments experienced problems with importing and registering the datasets. In these instances solutions were explored and investigated during the site visit [Table 1].
Table 1.
Multi-vendor systems encountered during site visit with registration problems identifi ed and solutions recommended for participating centers. Software version: On Board Imager® v1.3–1.4 (varian medical systems, Palo Alto, USA); XVI v4.5 (Elekta, Stockholm, Sweden); Mosaiq v1.6–v2.0 (Elekta, Stockholm, Sweden); Aria 8.6–8.8 (Varian Medical Systems, Palo Alto, USA); Eclipse v8.6–8.9 (varian medical systems, Palo Alto, USA); Pinnacle 8.0 (Philips, Milpitas, USA)
Radiotherapy planning
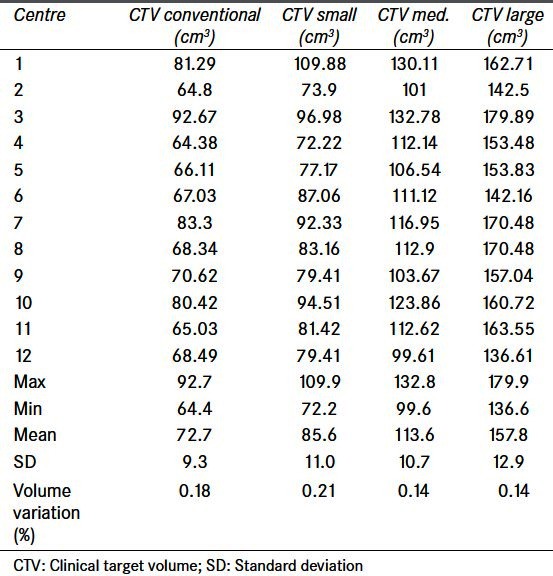
The BOLART technique is based on the creation of three clinical target volume (CTV) sizes for three radiotherapy plans to target three bladder volumes: Small, medium, and large. These adaptive plan formula can be found in our previous publication.[5] Initially the creation of the average CTV was different for all participating centers as there were numerous interpretations as to how an average CTV can be drawn from six superimposing CTVs. At the time of credentialing no known features on commercial planning systems allowed a systematic method of creating a contour averaged between multiple contours. To ensure consistency in creating an averaged volume an amendment was made to the protocol with a method described. This method while based on manual observations has been shown to provide greater consistency[14] and in this study showed volume variation similar to that used to derive small and large CTV structures [Table 2].
Table 2.
Volume statistics for clinical target volume volume generation for conventional, small, medium and large adaptive plans. Data imported into Eclipse™ treatment planning system for further analysis. Despite the manual method used to create the clinical target volume medium, volume differences are similar to that used by boolean function to create small and large clinical target volumes
Radiation therapists from 12 departments were asked to import, register, and create adaptive volumes from a series of images provided by the clinical trial team. Contouring of the bladder CTV from the planning CT dataset by radiation oncologists showed some differences due to no contouring guidelines being set.
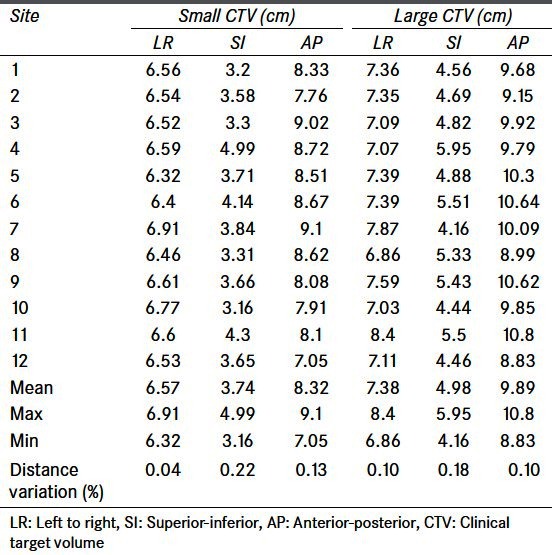
In terms of adaptive plan creation the dimensions of the small and large adaptive volumes varied greatest in the superior/inferior directions [Table 3]. Contributing factors to this included (1) a bony match registration rather than a bladder centroid registration was performed (2) structure contouring differences whereby amount of urethra as part of CTV contour varied. For the centers that used bony matching a discussion of the planning consequences was done during the site visit (as benchmarking was generally done prior to site visit).
Table 3.
Small and large adaptive clinical target volume dimensions as contoured and created by the 12 centers. Largest variation seen in the superior inferior direction which will be due to contouring differences as well as differences in registration in the superior/inferior direction
Electronic data submission/case report form
As part of the quality assurance process a real time review is required of the first two patients recruited by each participating center. The review occurs as soon as the adaptive plans are created and needs to be completed by fraction 10 of the patient’s treatment. An electronic submission of the planning CT and dosimetry is done in either DICOM or Radiation Therapy Oncology Group (RTOG) format to a central quality management system administered by the TROG group. To ensure that a timely review is made familiarity with the import/export process was reviewed during the credentialing process. Radiotherapy staff, who were responsible for data export for review encountered numerous problems with RTOG exporting procedures. Guidelines, developed by TROG, were made available to participating centers to ensure consistent and streamlined exporting of planning data. The most common problem to come out of the submission process was the absence of important critical structures. As part of the review, the credentialing team examined the creation of the adaptive CTV according to the protocol formula. In order to do this all CTV contours drawn from the CBCT and planning CT needed to be exported with the dataset submission. For many centers submission of planning data included only the structures relevant for the dose volume histogram. A checklist was developed by the TROG team to ensure submission of data included all relevant material as well as suggested naming of critical structures for evaluation. All planning data was reviewed by third party software[17] with DICOM/RTOG compatibility.
Protocol clarification
A large proportion of the help desk inquiries related to protocol requirements. The areas of the protocol that required further clarification include: Creation of the average radiotherapy plan, daily image verification requirements and treatment breaks. Prompted by the number of similar questions through the help desk the protocol went through two amendments, with changes used to clarify the first two radiotherapy points. Treatment breaks or the inability to use an adaptive plan was commonly dealt with through the help desk despite there being a dedicated section in the protocol regarding treatment breaks. The reason for direct inquiry to this area of treatment breaks could possibly be related to the fact that a point of violation in the trial is based on the inability to deliver adaptive radiotherapy more than 10% of the time. Inability to deliver radiotherapy can be due to either a complete hospital closure (i.e., government holidays, tropical storm) or temporarily no access to CBCT imaging. In the later situation the use of 2-dimensional planar imaging and conventional plan use is permitted however, there is a restriction to the total number of times that this can be done.
Radiotherapy in-house procedures
Online adaptive radiotherapy was a new technique for all departments involved in the trial. Additional and amendments were drafted to fit in with local policies. The written procedures (while not a requirement) were submitted to the credentialing team for review. Common workplace documents developed included: Scheduling practices of multiple plans for treatment, daily image matching guidelines, CBCT dose reduction techniques and CBCT to planning CT registration.
Staff training
Online training for radiation therapists had been developed with a series of training modules (as discussed previously) however, a need for practical training was expressed by a number of centers. While the credentialing program does provide an onsite practical IGRT matching component, the time between center activation and recruitment of patients can be significant as bladder cancer radiotherapy is not very common.
Patient specific
A small number of questions/queries relating directly to patient specific problems. These mainly concerned a patient’s inability to void resulting in insufficient coverage using adaptive plans. Investigation of these cases revealed an infection, to which a recommendation of a course of antibiotic treatment with a short treatment break was made.
Feedback
Feedback was elicited for both participating centers and as well the credentialing team. A dedicated trial newsletter, telephone conference between credentialing team and activated centers ensures that any trial updates and recruitment motivation is maintained. Presentations about the trial at various national meetings ensured interest in the trial was maintained by active and non-active centers. An annual scientific meeting of the TROG provided a dedicated session for discussion of the BOLART trial amongst participating centers.
Eight of 12 centers responded to a questionnaire sent out post-site activation [Figure 2]. The site visit program of presentations and allocation of 2 h of IGRT matching on the machine was well received with a sufficient amount of time allocated for the practical component. This simulation of the treatment process was received positively by all centers as it provided a practical walkthrough of the adaptive treatment process. Sufficient resources were felt to be available within the department’s multidisciplinary team; however, it was felt that the credentialing team should be the main point of support with the main form of communication kept via E-mail and telephone.
Figure 2.
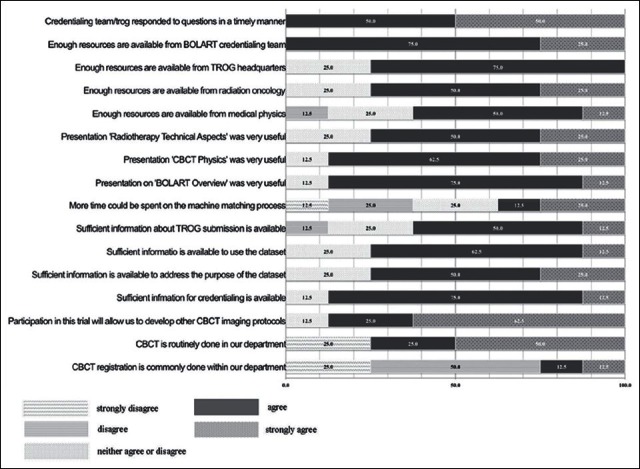
Survey responses from 8 of 12 centers
Centers felt that the credentialing program was developed with sufficient resources available to complete their credentialing with ongoing support for protocol management and staff training required post-activation. The majority of centers preferred e-Learning as the method of training with only two centers preferring e-Learning and workshop training.
Ongoing help desk support via E-mail was the most popular (62%) with E-mail/telephone the alternative. There was no preference for further on-site support.
The program is highly technical and despite the mixed CBCT experience of the centers general agreement was that participation in the trial will further develop other CBCT imaging protocols.
Conclusion
In Australia and New Zealand there are no formal guidelines for activation of highly technical multicenter clinical trials. The completion of credentialing items to activate centers is important in clinical trials, however, developing proper quality assurance items for the trial is an area that has led to much deliberation. Recommendations for trial specific quality assurance highlights its importance in the current climate of technology specific clinical trials.[18] Centre activation in a timely manner ensures recruitment targets are maintained for the trial. The role of technical support and face-to-face meetings, trial updates have been discussed in improving clinical trial recruitment.[19]
The activation of the 12 centers participating in the TROG 10.01 trial spanned an 18-month period. During this activation and ongoing support period three lessons were learnt:
Lesson 1 - Full day site visit is essential: A full day site visit dedicated to formal presentations in the morning and practical evaluation in the afternoon emphasizes trial importance, ensures multidisciplinary collaboration and gives an opportunity for the credentialing team to have a hands-on review of vendor specific issues
Lesson 2 - Dedicated help desk setup required: A multidisciplinary team to form the help desk ensures a broad knowledge base to solve any problems. E-mail, telephone and site visits are all methods that have been used to resolve any issues. This help desk workload will increase post-site activation and should be available until trial finishes
Lesson 3 - Feedback between credentialing team/active centers: Ensures flexible support and updates of trial database. Ongoing communication/quarterly trial updates and national presentations ensure interest and motivation is present among centers.
Bladder online adaptive radiotherapy is a novel and technically demanding radiotherapy treatment. The activation of a multicenter feasibility study of this technique generated new insights into multicenter activation and trial specific quality assurance. The role of the credentialing team is to not only provide quality assurance of trial parameters but to provide ongoing technical support and education.
Footnotes
Source of Support: Part funding NHMRC Project Grant 628527
Conflict of Interest: None declared.
References
- 1.Redpath AT, Muren LP. CT-guided intensity-modulated radiotherapy for bladder cancer: Isocentre shifts, margins and their impact on target dose. Radiother Oncol. 2006;81:276–83. doi: 10.1016/j.radonc.2006.10.017. [DOI] [PubMed] [Google Scholar]
- 2.Fokdal L, Honoré H, Høyer M, Meldgaard P, Fode K, von der Maase H. Impact of changes in bladder and rectal filling volume on organ motion and dose distribution of the bladder in radiotherapy for urinary bladder cancer. Int J Radiat Oncol Biol Phys. 2004;59:436–44. doi: 10.1016/j.ijrobp.2003.10.039. [DOI] [PubMed] [Google Scholar]
- 3.Turner SL, Swindell R, Bowl N, Marrs J, Brookes B, Read G, et al. Bladder movement during radiation therapy for bladder cancer: Implications for treatment planning. Int J Radiat Oncol Biol Phys. 1997;39:355–60. doi: 10.1016/s0360-3016(97)00070-9. [DOI] [PubMed] [Google Scholar]
- 4.Burridge N, Amer A, Marchant T, Sykes J, Stratford J, Henry A, et al. Online adaptive radiotherapy of the bladder: Small bowel irradiated-volume reduction. Int J Radiat Oncol Biol Phys. 2006;66:892–7. doi: 10.1016/j.ijrobp.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 5.Foroudi F, Wong J, Kron T, Rolfo A, Haworth A, Roxby P, et al. Online adaptive radiotherapy for muscle-invasive bladder cancer: Results of a pilot study. Int J Radiat Oncol Biol Phys. 2011;81:765–71. doi: 10.1016/j.ijrobp.2010.06.061. [DOI] [PubMed] [Google Scholar]
- 6.Lalondrelle S, Huddart R, Warren-Oseni K, Hansen VN, McNair H, Thomas K, et al. Adaptive-predictive organ localization using cone-beam computed tomography for improved accuracy in external beam radiotherapy for bladder cancer. Int J Radiat Oncol Biol Phys. 2011;79:705–12. doi: 10.1016/j.ijrobp.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 7.Murthy V, Master Z, Adurkar P, Mallick I, Mahantshetty U, Bakshi G, et al. ‘Plan of the day’ adaptive radiotherapy for bladder cancer using helical tomotherapy. Radiother Oncol. 2011;99:55–60. doi: 10.1016/j.radonc.2011.01.027. [DOI] [PubMed] [Google Scholar]
- 8.Pos F, Remeijer P. Adaptive management of bladder cancer radiotherapy. Semin Radiat Oncol. 2010;20:116–20. doi: 10.1016/j.semradonc.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 9.Tuomikoski L, Collan J, Keyriläinen J, Visapää H, Saarilahti K, Tenhunen M. Adaptive radiotherapy in muscle invasive urinary bladder cancer: An effective method to reduce the irradiated bowel volume. Radiother Oncol. 2011;99:61–6. doi: 10.1016/j.radonc.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 10.Vestergaard A, Søndergaard J, Petersen JB, Høyer M, Muren LP. A comparison of three different adaptive strategies in image-guided radiotherapy of bladder cancer. Acta Oncol. 2010;49:1069–76. doi: 10.3109/0284186X.2010.501813. [DOI] [PubMed] [Google Scholar]
- 11.Kron T, Willis D, Bignell F, Martland J, Donnell S, May S, et al. Centre credentialing for Trans-Tasman Radiation Oncology Group trial 06.02: Multicentre feasibility study of accelerated partial breast irradiation. J Med Imaging Radiat Oncol. 2009;53:412–8. doi: 10.1111/j.1754-9485.2009.02097.x. [DOI] [PubMed] [Google Scholar]
- 12.Timmerman R, Galvin J, Michalski J, Straube W, Ibbott G, Martin E, et al. Accreditation and quality assurance for Radiation Therapy Oncology Group: Multicenter clinical trials using Stereotactic Body Radiation Therapy in lung cancer. Acta Oncol. 2006;45:779–86. doi: 10.1080/02841860600902213. [DOI] [PubMed] [Google Scholar]
- 13.Vicini F, Winter K, Straube W, Wong J, Pass H, Rabinovitch R, et al. A phase I/II trial to evaluate three-dimensional conformal radiation therapy confined to the region of the lumpectomy cavity for Stage I/II breast carcinoma: Initial report of feasibility and reproducibility of Radiation Therapy Oncology Group (RTOG) Study 0319. Int J Radiat Oncol Biol Phys. 2005;63:1531–7. doi: 10.1016/j.ijrobp.2005.06.024. [DOI] [PubMed] [Google Scholar]
- 14.Foroudi F, Wong J, Kron T, Roxby P, Haworth A, Bailey A, et al. Development and evaluation of a training program for therapeutic radiographers as a basis for online adaptive radiation therapy for bladder carcinoma. Radiography. 2010;16:14–20. [Google Scholar]
- 15.Roxby P, Kron T, Foroudi F, Haworth A, Fox C, Mullen A, et al. Simple methods to reduce patient dose in a Varian cone beam CT system for delivery verification in pelvic radiotherapy. Br J Radiol. 2009;82:855–9. doi: 10.1259/bjr/37579222. [DOI] [PubMed] [Google Scholar]
- 16.Kron T, Pham D, Roxby P, Rolfo A, Foroudi F. Credentialing of radiotherapy centres for a clinical trial of adaptive radiotherapy for bladder cancer (TROG 10.01) Radiother Oncol. 2012;103:293–8. doi: 10.1016/j.radonc.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 17.Ebert MA, Haworth A, Kearvell R, Hooton B, Coleman R, Spry N, et al. Detailed review and analysis of complex radiotherapy clinical trial planning data: Evaluation and initial experience with the SWAN software system. Radiother Oncol. 2008;86:200–10. doi: 10.1016/j.radonc.2007.11.013. [DOI] [PubMed] [Google Scholar]
- 18.Bekelman JE, Deye JA, Vikram B, Bentzen SM, Bruner D, Curran WJ, Jr, et al. Redesigning radiotherapy quality assurance: Opportunities to develop an efficient, evidence-based system to support clinical trials: Report of the National Cancer Institute Work Group on Radiotherapy Quality Assurance. Int J Radiat Oncol Biol Phys. 2012;83:782–90. doi: 10.1016/j.ijrobp.2011.12.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coles CE, Faivre-Finn C. Radiotherapy research trials in the UK: Secrets of success. Clin Oncol (R Coll Radiol) 2012;24:229–31. doi: 10.1016/j.clon.2012.02.002. [DOI] [PubMed] [Google Scholar]


