Abstract
The radioprotective potential of bioflavonoid, rutin (RUT) and quercetin (QRT) was investigated in Swiss albino mice exposed to gamma radiation. The radioprotective potential of RUT and QRT was assessed in pre-treatment group of mice followed on radiation-induced changes in glutathione (GSH), glutathione-S-transferase (GST), superoxide dismutase (SOD), catalase (CAT), and lipid peroxidation (LPO) levels were also analyzed. Elevation in the GSH, GST, SOD, CAT, and decreased LPO levels were observed in RUT and QRT pretreated group when compared to the irradiated animals. Furthermore, it was observed that RUT and QRT treatment was found to inhibit various free radicals generated in vitro, viz., 2,2-diphenyl-1-picrylhydrazyl(DPPH), O2, 2,2’-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS)+, and OH in a concentration-dependent manner. This study clearly demonstrates the free radical scavenging action of RUT and QRT, indicating that it may have its potential as a radioprotective agent. Furthermore, the presence of a phenolic group in RUT and QRT is known to contribute to scavenging the radiation-induced free radicals and inhibition of oxidative stress. Present findings demonstrate the potential of RUT and QRT in mitigating radiation-induced oxidative stress, which may be attributed to the inhibition of radiation-induced decline in the endogenous antioxidant levels and scavenging of radiation-induced free radicals.
Keywords: Antioxidant, free radicals, rutin, quercetin
Introduction
Radiation therapy is the prime treatment modality against various cancers. However, its use is limited due to the lethal effects of radiation on normal tissues.[1] Therefore, attempts were made earlier to improve the therapeutic effect of radiotherapy by keeping the normal tissue damage to acceptable level by using synthetic compounds like cysteine, cysteamine, and WR-2721.[2] However, the successful use of these radioprotectors in medical practice is not appreciated much due to their inherent systemic toxicity and their biological short half life. Therefore, the quest for effective, nontoxic compound with its optimal radioprotective capability is of immediate need that shifted the interest more on the naturally occurring dietary antioxidants. A number of dietary antioxidants, medicinal plant extracts, and their isolated constituents have been reported for their hepatoprotective, neuroprotective, anti-inflammatory, and also antioxidant or radical scavenging properties.[2–4] Several earlier studies on some of the medicinal plants[5–8] indicated the usefulness of these natural products in reducing the radiation-induced genotoxicity and animal mortality.
Oxidative stress and low antioxidant levels are implicated in the etiology of an inflammatory disease. Rutin (RUT) and quercetin (QRT) is potent dietary antioxidants, which were also found to display anti-inflammatory activities. Rutin has been shown to have antioxidant and anti-inflammatory actions.[9] Natural non-toxic bioflavonoid rutin (vitamin P) inhibited oxygen radical overproduction in both rheumatoid arthritis and Fanconi anemia in an equally efficient manner and therefore may be considered as a useful supporting pharmaceutical agent for the treatment of free radical pathologies.[10] The oxidative effects of ultraviolet A (UVA) light (320–400 nm) and the antioxidant effects of QRT were examined in rat blood. Exposure of rats to UVA light leads to oxidative stress reflected by increased MDA and reduced antioxidant enzyme levels. The administration of QRT appeared to be a useful approach to reduce the damage produced by UVA radiation.[11] The present study was undertaken to assess the effect of RUT and QRT given as a protective regimen against radiation-induced oxidative stress, assessed by alteration in intracellular antioxidant enzymes, lipid peroxidation (LPO), and also in vitro free radical quenching.
Materials and Methods
Animals
Four- to six-weeks old inbred mice of Swiss albino strain of either sex weighing 25–30 g were selected and kept in well-ventilated polypropylene cages under standard conditions of temperature (23 ± 2°C), humidity (50 ± 5%), and light (10 and 14 hours of light and dark, respectively). Animals were allowed food and water ad libitum. The animal experiments were carried with the prior approval from the Institutional Animal Ethics Committee. Animal care and handling was done according to the guidelines issued by the World Health Organization, Geneva, Switzerland and the Indian National Science Academy, New Delhi, India.
Chemicals
Drug preparation and mode of administration
RUT and QRT was purchased from Himedia Laboratories Pvt. Ltd., Mumbai, India. RUT and QRT powder was suspended in water using 0.5% w/v carboxy methyl cellulose (CMC) and was given once daily (5 ml/kg body weight (bw)), various doses of RUT and QRT 10-100 mg/kg bw orally once a day for 5 consecutive days. Radiation exposure was performed 1 hour after the last dose of RUT and QRT administration.
Other chemicals
RUT and QRT, glutathione, chloro-2,4-dinitrobenzene (CDNB), 5,5-dithiobis-2-nitrobenzoic acid, trichloroacetic acid (TCA), thiobarbituric acid (TBA), ethidium bromide, normal melting agarose, low melting agarose, and fetal bovine serum were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Acridine orange (AO) was purchased from BDH Chemicals Ltd, Poole, England. The other chemicals such as absolute alcohol, dimethyl sulphoxide, ethylene diamine tetraacetic acid, sodium bicarbonate, sodium chloride, potassium hydrogen phosphate, and hydrochloric acid were purchased from Qualigens Fine Chemicals (A Division of GlaxoSmithKline Pharmaceuticals), Mumbai, India.
Radiation exposure
Unanesthetized mice were restrained in a specially designed well-ventilated acrylic box and exposed to whole-body radiation from 60Co gamma tele-therapy facility (Theratron Atomic Energy Agency, Canada) at the Shirdi Sai Baba Cancer Hospital, Manipal, at a dose rate of 1.33 Gy/min and source to surface distance of 61 cm.
Estimation of liver antioxidant enzymes
To understand the mechanism of radioprotection, biochemical estimations were carried out. Animals were divided into groups of six animals each as follows:
Untreated Control group: The animals of this group were administered 0.1 ml/kg bw of CMC orally for 5 consecutive days.
RUT and QRT alone group: The animals of this group were administered optimum dose of RUT (10 mg/kg bw) and QRT (20 mg/kg bw) orally for 5 consecutive days.
Radiation alone group: These animals were administered 0.1 ml/kg bw of CMC orally once daily for 5 consecutive days. One hour after the last administration on the 5th day animals were exposed to 4.5 Gy gamma radiations.
RUT and QRT + Radiation group: The animals of this group were administered with optimal dose of RUT (10 mg/kg bw) and QRT (20 mg/kg bw) orally for 5 consecutive days, and the last dose of RUT and QRT was given just 1 hour before exposure to 4.5 Gy of gamma radiation.
Sample preparation (tissue homogenate)
All the animals were from above groups were euthanized at 12 hour post-irradiation time intervals and their livers were collected and processed as liver tissue was perfused with saline to remove any red blood cells and clots. Tissue was homogenized with the saline (0.9%) (1 g liver in 10 ml saline) with ice-cold PBS pH 8.0 using a homogenizer (Yamato LSC LH-21, Japan) and centrifuged at 12,000 rpm for 30 min at 4°C. Supernatant was collected and used for following biochemical estimations.
Protein estimation
Total protein contents were estimated by the modified method of Lowry et al.[12] The protein concentration of the test samples were calculated with reference to the standard graph and the results were expressed as milligram protein per gram of tissue weight.
Estimation of glutathione
Glutathione (GSH) contents were measured as total non-protein sulfhydryl (NPSH) group using the method of Moron et al.[13] with modifications. The absorbance was monitored for 2 min at 412 nm. The change in absorbance/min was determined and this value was converted to micromol GSH in comparison to a known standard.
Estimation of glutathione-S-transferase
Glutathione-S-transferase (GST) was determined according to the procedure of Habig et al.[14] Analysis of GST activity is based on enzyme catalyst condensation of GSH with the model substrate 1-chloro-2,4-dinitrobenzene (CDNB). The product obtained (2,4-nitrophenyle-glutathione) absorbs light at 340 nm. Results were expressed as micromole of product formed per minute per miiligram protein of the tissue.
Estimation of superoxide dismutase activity
Superoxide dismutase (SOD) activity was assayed by the nitroblue tetrazolium (NBT) method as described by Beauchamp et al.[15] Xanthine oxidase is used to generate a reproducible flux of O2●; NBT is used as an indicator of superoxide production. Specific activity of total SOD is expressed as units per milligram protein.
Estimation of catalase in liver
Catalase (CAT) activity was determined by catalytic reduction of hydrogen peroxide using a standard method described by Aebi.[16] Results were expressed as micromole of product formed per minute per milligram protein of the tissue.
Estimation of LPO in liver
LPO was measured using the method of Buege and Aust.[17] Malonaldehyde (MDA) formed from the breakdown of poly-unsaturated fatty acids serves as a convenient index for determining the extent of the peroxidation reaction. Peroxidation of lipids generates MDA, which reacts with TBA to give a red species absorbing at 535 nm. Results were expressed as nanomole MDA per milligram total protein.
Free radical scavenging by RUT and QRT
DPPH scavenging activity
The effect of RUT and QRT on the DPPH radical was estimated according to the method Mensor et al.[18] The ability to scavenge the stable DPPH radical is measured by a decrease in the absorbance at 517 nm using spectrophotometer (Shimadzu UV-260, Shimadzu Corp, Tokyo, Japan). The measurement was repeated with three sets.
ABTS radical decolorisation assay
ABTS diammonium salt radical cation decolourisation test was performed using spectrophotometric method described by Miller et al.[19] The reaction mixtures were incubated at room temperature (28°C) for 30 min, and the absorbance was measured at 734 nm.
Hydroxyl radical scavenging activity
Hydroxyl radical scavenging assay was performed by the oxidation of deoxyribose using standard method described by Halliwell et al.[20] The absorbance was measured at 534 nm using spectrophotometer. Percent inhibition was calculated.
Superoxide radical scavenging activity
Superoxide scavenging activity of RUT and QRT was performed by photo-oxidation of riboflavin according to the method of Hyland et al.[21] The reaction mixture in a final volume of 3 ml and the absorbance was recorded at 513 nm. All tests were performed three times.
Statistical analysis
All data were expressed as mean ± SEM. The statistical significance between the treatments was evaluated by one-way ANOVA and with Bonforroni’s post hoc test using GraphPAD InStat, Software, USA.
Result
Biochemical parameters
The optimal dose of RUT (10 mg/kg bw) and QRT (20 mg/kg bw) were selected to assess the changes in radiation-induced liver antioxidant levels and LPO.
Glutathione activity
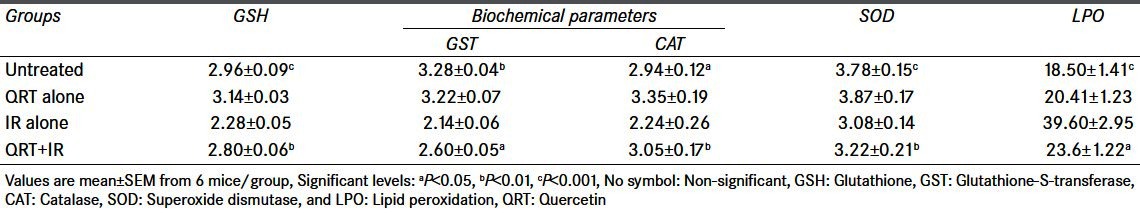
The glutathione (GSH) levels in the liver tissue for the control animals in RUT and QRT treated group were 2.76 ± 0.06 and 2.96 ± 0.09 μmol/g tissues, respectively. RUT and QRT treatment alone did not alter the GSH levels when compared with the untreated control. However, a significant decrease in GSH content was observed in irradiated animals. Whereas, treatment of mice with RUT (10 mg/kg bw) and QRT (20 mg/kg bw) 1 hour before exposure to 4.5 Gy of gamma radiation significantly normalized (P < 0.01) GSH content both RUT and QRT administered group compared with respective irradiated groups [Tables 1 and 2].
Table 1.
Changes in GSH, GST, CAT, SOD, and LPO levels after exposure to 4.5 Gy with or without RUT given orally for 5 consecutive days
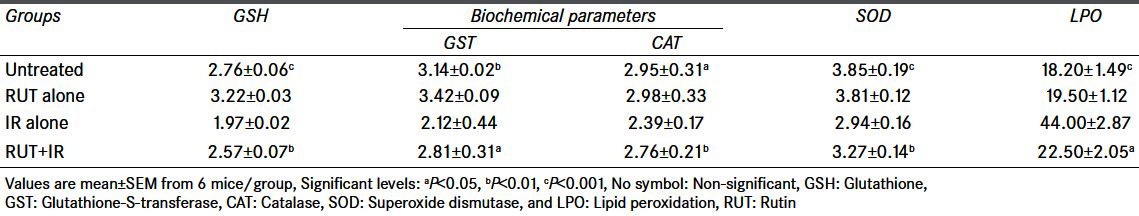
Table 2.
Changes in GSH, GST, CAT, SOD, and LPO levels after exposure to 4.5 Gy with or without QRT given orally for 5 consecutive days
GST activity
The GST activity in control mice liver was 3.14 ± 0.02 and 3.28 ± 0.04 μmol/g tissues at RUT and QRT treated group, respectively, RUT and QRT treatment by itself did not significantly alter the baseline GST levels. Whole-body irradiation of mice to 4.5 Gy resulted in declined GST activity. Whereas, RUT and QRT administered 1 hour prior to 4.5 Gy gamma radiation significantly normalized GST activity at 12 hours post treatment when compared with the respective irradiation groups [Tables 1 and 2].
SOD activity
In control mice liver, the mean SOD activity was 3.85 ± 0.19 and 3.78 ± 0.15 μmol/g tissue, respectively. RUT and QRT treatment by itself did not significantly alter the baseline SOD levels. Whole-body irradiation of mice to 4.5 Gy resulted in declined SOD activity. Whereas, RUT and QRT administered 1 hour prior to 4.5 Gy gamma radiation resulted in a significant (P < 0.01) normalized in SOD activity at 12 hours post treatment when compared with the respective irradiation groups [Tables 1 and 2].
CAT activity
In control mice liver, the mean CAT activity was 2.95 ± 0.31 and 2.94 ± 0.12 in RUT and QRT treated groups, respectively. RUT and QRT treatment by itself did not significantly alter the baseline CAT levels. Whole-body irradiation of mice to 4.5 Gy resulted in declined CAT activity. Whereas, RUT and QRT administered 1 hour prior to 4.5 Gy gamma radiation resulted in a significant (P < 0.01) normalized in CAT activity in 12 hours post treatment when compared with the respective irradiation groups [Tables 1 and 2].
LPO
Administration of RUT and QRT by itself did not result in LPO in RUT and QRT treated groups. Animals exposed to 4.5 Gy gamma radiation showed significantly (P < 0.001) increased thiobarbituric acid reactive substances (TBARS) (44.00 ± 2.87, 39.60 ± 2.95) in RUT and QRT treated groups as against the control values (22.50 ± 2.05, 23.6 ± 1.22). However, there was a significant inhibition of LPO products (TBARS) observed in RUT, and QRT was administered before 4.5 Gy of gamma radiation [Tables 1 and 2].
Table 1 and 2 shows the comparison between Irradiated (IR) alone against groups Untreated, Rutin + Irradiation and QRT + Irradiation. Values are mean ± SEM from 6 mice/group. Significant levels are, a = P < 0.05; b = P < 0.01; c = P < 0.001. No symbol = non-significant.
Free radical scavenging activity
DPPH scavenging activity
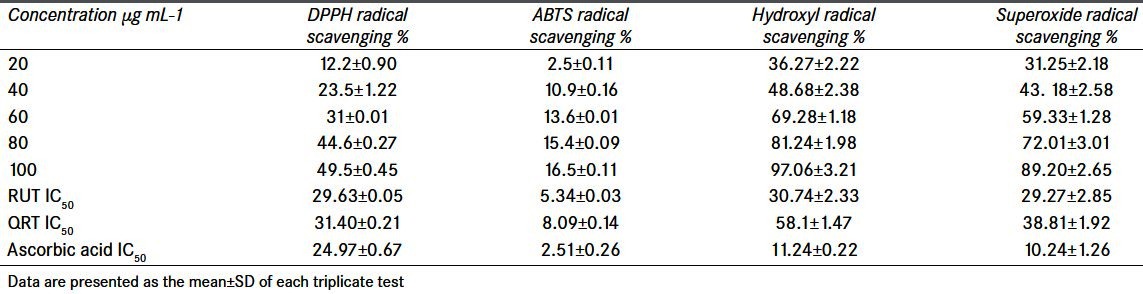
As it is evident in Table 3, the IC50 value of the RUT for DPPH radical was found to be 29.63 μg/ml, and, at the concentration of 80 μg/ml, RUT exhibited 44% inhibition and the scavenging activity was plateau thereafter at higher concentrations. Whereas the IC50 value of QRT was found to be 31.4 μg/ml.
Table 3.
Radical scavenging activity of rutin and quercetin at different concentrations
Total antioxidant activity
The total antioxidant capacity of the RUT was calculated from the decolourization of ABTS·+ upon interaction with the RUT that suppressed the absorbance of the ABTS·+ radical and the results are expressed as percentage inhibition of absorbance as shown in Table 3. RUT resulted in a concentration-dependent increase in free radical scavenging ability against ABTS·+ in a concentration-dependent manner, with a saturation point reaching a concentration of 80 μg/ml. The IC50 value of the RUT was found to be 5.34 μg/ml. QRT also exhibited dose-dependent scavenging up to 100 μg/ml. The IC50 value of QRT was found to be 8.09 μg/ml.
Hydroxyl radical scavenging assay
Hydroxyl radicals are the major active species causing lipid oxidation. This assay shows the ability of different concentrations of RUT to scavenge OH·, as shown in Table 3. RUT resulted in a concentration-dependent increase in free radical scavenging ability against OH· in a concentration-dependent manner, with a saturation point reaching a concentration of 80 μg/ml with an IC50 value of 30.74 μg/ml. The concentration of QRT needed for 50% inhibition was found to be 58.1 μg/ml.
Superoxide radical scavenging assay
Data in Table 3 shows the ability of RUT to quench superoxide radicals in the reaction mixture. The IC50 value of the RUT for super oxide radical was found to be 29.27 μg/ml, and, at the concentration of 80 μg/ml, RUT exhibited 72% inhibition and plateau thereafter at higher concentrations. QRT at concentrations ranging from 10 to 100 μg/ml inhibited the production of superoxide anion radicals. The IC50 value of QRT on superoxide radical scavenging activity was found to be 38.81 μg/ml.
Discussion
Radiation-induced reactive oxygen/nitrogen species (ROS/RNS) like nitric oxide and superoxide radicals react to produce reactive peroxynitrite, which is known to induce cytotoxicity by interacting with biomolecules.[22] ROS also affects the antioxidant defense mechanisms by reducing the intracellular concentration of GSH as well as SOD, GST, and CAT activity. RUT and QRT administration prior to irradiation inhibited the decline in the intracellular antioxidant enzyme levels, viz., SOD, CAT, GSH, and GST.[23,24] These enzymes are known to be modulated in various diseases caused by free radical attack.[25] Thus, maintaining a balance between the rate of generation of radicals and scavenging of free radicals is an essential part of biological homeostasis. Therefore, it is of particular interest to note that SOD catalyses the breakdown of O2·ˉ to O2 and H2O2, prevents formation of OH·, and thereby has been implicated as an essential defense against the potential toxicity of oxidative species. The ROS scavenging activity of SOD is effective only when it is followed by the actions of other enzymes, because the dismutase activity of SOD generates H2O2, which needs to be further scavenged by CAT and GPX. RUT and QRT is potent scavenger of peroxynitrite and superoxide radicals.[26–28] This indicates that the RUT and QRT can effectively scavenge H2O2 formed by SOD. Taken together, these results also suggest that the antioxidant activity of RUT and QRT may be due to degradation of H2O2 and other peroxides. Therefore, possible underlying mechanism of action by RUT and QRT due to the modulation of peroxynitrite and superoxide effects on tissues could provide a reasonable explanation for the enhanced radioprotection in RUT and QRT pretreated mice.
In the present study, RUT and QRT alone did not significantly alter the LPO in unirradiated animals, but RUT and QRT pre-treatment significantly lowered the radiation-induced LPO in terms of MDA production in a dose-dependent manner. Therefore, inhibition of LPO by RUT and QRT is also of significance in protecting the cells from radiation-induced damage. Exposure of mice to gamma radiation reduced the GSH activity; this depletion of GSH in mice has been shown to cause inhibition of glutathione peroxidase activity and resultant increase in LPO.[29] GST catalyzes the antioxidant processes of thiol compounds, thereby protecting the cells from electrophiles, free radical-induced damage, and oxidative stress.[30] A similar correlation between the depletion of GSH and increase in LPO exists in the present investigation. Pre-treatment of mice with RUT and QRT significantly stalled the decline of GSH, GST, SOD, and CAT levels of liver, thereby rendering increased protection against radiation. Several earlier findings demonstrated efficient SOD and peroxyradical scavenging potential of RUT and QRT[26–28] as was also observed from the results of in vitro free radical scavenging assays. Furthermore, in the present study, RUT and QRT did not increase the GSH level above that of untreated control (base line) indicating its inability to promote the GSH synthesis pathway by itself. Taken together it may be proposed that the free radical scavenging ability of RUT and QRT may be one of the mechanisms for its radioprotective potential.
In the present study, the ability of RUT and QRT to scavenge free radicals using in vitro model systems was evaluated. OH· is the most reactive among ROS and it bears the shortest half-life compared with other ROS. The concentration of RUT and QRT needed for 50% inhibition for OH· was found to be 30.74 μg/ml and 58.1 μg/ml, respectively. Similarly, RUT and QRT at concentration from 20 to 100 μg/ml significantly inhibited the production of superoxide anion, DPPH and ABTS, indicating the ability of RUT and QRT as a free radical scavenger. These results also suggest that RUT and QRT may exert radioprotective effect by scavenging the free radicals. Similarly, earlier reports on plant extracts and natural compounds demonstrated a free radical scavenging mechanism as their potential to mitigate radiation-induced cellular damage.[31–33]
Present findings demonstrate the potential of a dietary compound, RUT and QRT, in mitigating radiation-induced oxidative stress. This study clearly demonstrates the free radical scavenging, indicating that it may have its potential as a radioprotective agent. Furthermore, the presence of a phenolic group in RUT and QRT is known to contribute hydrogen donation for scavenging the radiation-induced radicals, since the structure-activity relationship of these bioflavonoids acts as reducing agents of hydrogen or electron-donating agents could predict their potential to act as antioxidants and therefore it may be assumed that the observed radioprotective effect of RUT and QRT may be attributed to its potential to inhibit radiation-induced oxidative stress. These effects may be partly attributed to the scavenging of radiation-induced free radicals as well as by increasing the endogenous antioxidant levels. Therefore, further investigations on RUT and QRT may prove its potential application as a radioprotective agent or against any free radical medicated pathological conditions.
Acknowledgments
We acknowledge the financial support by Board of Research in Nuclear Sciences (BRNS, Mumbai) through Centre for Application of Radioisotopes and Radiation Technology (CARRT, Mangalore University, Mangalore). We also wish to thank Prof. Satish Rao (Head, Radiobiology Division, Manipal Life Sciences Centre), Prof. Vidhya Sagar (Head, Radiotherapy), and Prof. J.G.R. Solomon (Head, Medical Physics, Shirdi Saibaba Cancer Hospital, Manipal) for the use of the irradiation facility and for radiation dosimetry, respectively.
Footnotes
Source of Support: Board of Research in Nuclear Sciences (BRNS), Mumbai, India
Conflict of Interest: None declared.
References
- 1.Stone HB, Coleman CN, Anscher MS, McBride WH. Effects of radiation on normal tissue: Consequences and mechanisms. Lancet Oncol. 2003;4:529–36. doi: 10.1016/s1470-2045(03)01191-4. [DOI] [PubMed] [Google Scholar]
- 2.Nair CK, Parida DK, Nomura T. Radioprotectors in radiotherapy. J Radiat Res. 2001;42:21–37. doi: 10.1269/jrr.42.21. [DOI] [PubMed] [Google Scholar]
- 3.Uma Devi P. Radioprotective, anticarcinogenic and antioxidant properties of the Indian holy basil, Ocimum sanctum (Tulasi) Indian J Exp Biol. 2001;39:185–90. [PubMed] [Google Scholar]
- 4.Devasagayam TP, Sainis KB. Immune system and antioxidants, especially those derived from Indian medicinal plants. Indian J Exp Biol. 2002;40:639–55. [PubMed] [Google Scholar]
- 5.Uma Devi P, Ganasoundari A, Rao BS, Srinivasan KK. In vivo radioprotection by ocimum flavonoids: Survival of mice. Radiation Research. 1999;151:74–8. [PubMed] [Google Scholar]
- 6.Rao BS, Shanbhoge R, Upadhya D, Jagetia GC, Adiga SK, Kumar P, et al. Antioxidant, anticlastogenic and radioprotective effect of Coleus aromaticus on Chinese hamster fibroblast cells (V79) exposed to gamma radiation. Mutagenesis. 2006;21:237–42. doi: 10.1093/mutage/gel023. [DOI] [PubMed] [Google Scholar]
- 7.Rao BS, Upadhya D, Adiga SK. Protection of ionizing radiation-induced cytogenetic damage by hydroalcoholic extract of Cynodon dactylon in Chinese hamster lung fibroblast cells and human peripheral blood lymphocytes. J Environ Pathol Tox. 2008;27:101–12. doi: 10.1615/jenvironpatholtoxicoloncol.v27.i2.30. [DOI] [PubMed] [Google Scholar]
- 8.Rao BN, Rao BS. Antagonistic effects of Zingerone, a phenolic alkanone against radiation-induced cytotoxicity, genotoxicity, apoptosis and oxidative stress in Chinese hamster lung fibroblast cells growing in vitro. Mutagenesis. 2010;25:577–87. doi: 10.1093/mutage/geq043. [DOI] [PubMed] [Google Scholar]
- 9.Khan MM, Raza SS, Javed H, Ahmad A, Khan A, Islam F, et al. Rutin protects dopaminergic neurons from oxidative stress in an animal model of Parkinson’s disease. Neurotox Res. 2012;22:1–15. doi: 10.1007/s12640-011-9295-2. [DOI] [PubMed] [Google Scholar]
- 10.Ostrakhovitch EA, Afanas’ev IB. Oxidative stress in rheumatoid arthritis leukocytes: Suppression by rutin and other antioxidants and chelators. Biochem Pharmacol. 2001;62:743–6. doi: 10.1016/s0006-2952(01)00707-9. [DOI] [PubMed] [Google Scholar]
- 11.Kahraman A, Inal ME. Protective effects of quercetin on ultraviolet a light-induced oxidative stress in the blood of rat. J Appl Toxicol. 2002;22:303–9. doi: 10.1002/jat.863. [DOI] [PubMed] [Google Scholar]
- 12.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;93:265–75. [PubMed] [Google Scholar]
- 13.Moron MS, Depierre JW, Mannervik B. Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochim Biophys Acta. 1979;582:67–78. doi: 10.1016/0304-4165(79)90289-7. [DOI] [PubMed] [Google Scholar]
- 14.Habig WH, Pabst MJ, Jakoby WB. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974;249:7130–9. [PubMed] [Google Scholar]
- 15.Beauchamp C, Fridovich I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971;44:276–87. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- 16.Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–6. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 17.Buege JA, Aust SD. Microsomal lipid peroxidation. Methods Enzymol. 1978;52:302–10. doi: 10.1016/s0076-6879(78)52032-6. [DOI] [PubMed] [Google Scholar]
- 18.Mensor LL, Menezes FS, Leitao GG, Reis AS, dos Santos TC, Coube CS, et al. Screening of Brazilian plant extracts for antioxidant activity by the use of DPPH free radical method. Phytother Res. 2001;15:127–30. doi: 10.1002/ptr.687. [DOI] [PubMed] [Google Scholar]
- 19.Miller NJ, Castelluccio C, Tijburg L, Rice-Evans C. The antioxidant properties of the aflavins and their gallate esters-radical scavengers or metal chelators? FEBS Lett. 1996;392:40–4. doi: 10.1016/0014-5793(96)00780-6. [DOI] [PubMed] [Google Scholar]
- 20.Halliwell B, Gutteridge JM, Aruoma OI. The deoxyribose method: A simple “test-tube” assay for determination of rate constants for reactions of hydroxyl radicals. Anal Biochem. 1987;165:215–9. doi: 10.1016/0003-2697(87)90222-3. [DOI] [PubMed] [Google Scholar]
- 21.Hyland K, Voisin E, Banoun H, Auclair C. Superoxide dismutase assay using alkaline dimethylsulfoxide as superoxide anion-generating system. Anal Biochem. 1983;135:280–7. doi: 10.1016/0003-2697(83)90684-x. [DOI] [PubMed] [Google Scholar]
- 22.Nair U, Bartsch H, Nair J. Lipid peroxidation-induced DNA damage in cancer-prone inflammatory diseases: A review of published adduct types and levels in humans. Free Radic Biol Med. 2007;43:1109–20. doi: 10.1016/j.freeradbiomed.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 23.Turner ND, Braby LA, Ford J, Lupton JR. Opportunities for nutritional amelioration of radiation-induced cellular damage. Nutrition. 2002;18:904–12. doi: 10.1016/s0899-9007(02)00945-0. [DOI] [PubMed] [Google Scholar]
- 24.Weiss JF, Landauer MR. Protection against ionizing radiation by antioxidant nutrients and phytochemicals. Toxicology. 2003;189:1–20. doi: 10.1016/s0300-483x(03)00149-5. [DOI] [PubMed] [Google Scholar]
- 25.Halliwell B, Gutteridge JM. Free radicals in biology and medicine. 3rd. Oxford: Clarendon Press; 1999. Oxidative stress and antioxidant protection: Some special cases; pp. 530–3. [Google Scholar]
- 26.Olthof MR, Hollman PC, Vree TB, Katan MB. Bioavailabilities of quercetin-3-glucoside and quercetin-4’- glucoside do not differ in humans. J Nutr. 2000;130:1200–3. doi: 10.1093/jn/130.5.1200. [DOI] [PubMed] [Google Scholar]
- 27.Park JB, Levine M. Intracellular accumulation of ascorbic acid is inhibited by flavonoids via blocking of dehydroascorbic acid and ascorbic acid uptakes in HL-60, U937 and Jurkat cells. J Nutr. 2000;130:1297–302. doi: 10.1093/jn/130.5.1297. [DOI] [PubMed] [Google Scholar]
- 28.Guardia T, Rotelli AE, Juarez AO, Pelzer LE. Anti-inflammatory properties of plant flavonoids. Effects of rutin, quercetin, and hesperidin on adjuvant arthritis in rat. Farmaco. 2001;56:683–7. doi: 10.1016/s0014-827x(01)01111-9. [DOI] [PubMed] [Google Scholar]
- 29.Schafer FQ, Buettner GR. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic Biol Med. 2001;30:1191–212. doi: 10.1016/s0891-5849(01)00480-4. [DOI] [PubMed] [Google Scholar]
- 30.Grdina DJ, Murley JS, Kataoka Y. Radioprotectants: Current status and new directions. Oncology. 2002;63(Suppl 2):2–10. doi: 10.1159/000067146. [DOI] [PubMed] [Google Scholar]
- 31.Uma Devi P, Ganasoundari A. Radioprotective effect of leaf extract of Indian medicinal plant Ocimum sanctum. Indian J Exp Biol. 1995;33:205–8. [PubMed] [Google Scholar]
- 32.Goel HC, Prasad J, Singh S, Sagar RK, Kumar IP, Sinha AK. Radioprotection by a herbal preparation of Hippophae rhamnoides, RH-3, against whole body lethal irradiation in mice. Phytomedicine. 2002;9:15–25. doi: 10.1078/0944-7113-00077. [DOI] [PubMed] [Google Scholar]
- 33.Krishna A, Kumar A. Evaluation of radioprotective effects of Rajgira (Amaranthus paniculatus) extract in Swiss albino mice. J Radiat Res. 2005;46:233–9. doi: 10.1269/jrr.46.233. [DOI] [PubMed] [Google Scholar]


