Abstract
For the head-and-neck cancer bilateral irradiation, intensity-modulated radiation therapy (IMRT) is the most reported technique as it enables both target dose coverage and organ-at-risk (OAR) sparing. However, during the last 20 years, three-dimensional conformal radiotherapy (3DCRT) techniques have been introduced, which are tailored to improve the classic shrinking field technique, as regards both planning target volume (PTV) dose conformality and sparing of OAR’s, such as parotid glands and spinal cord. In this study, we tested experimentally in a sample of 13 patients, four of these advanced 3DCRT techniques, all using photon beams only and a unique isocentre, namely Bellinzona, Forward-Planned Multisegments (FPMS), ConPas, and field-in-field (FIF) techniques. Statistical analysis of the main dosimetric parameters of PTV and OAR’s DVH’s as well as of homogeneity and conformity indexes was carried out in order to compare the performance of each technique. The results show that the PTV dose coverage is adequate for all the techniques, with the FPMS techniques providing the highest value for D95%; on the other hand, the best sparing of parotid glands is achieved using the FIF and ConPas techniques, with a mean dose of 26 Gy to parotid glands for a PTV prescription dose of 54 Gy. After taking into account both PTV coverage and parotid sparing, the best global performance was achieved by the FIF technique with results comparable to that of IMRT plans. This technique can be proposed as a valid alternative when IMRT equipment is not available or patient is not suitable for IMRT treatment.
Keywords: Head and neck, 3DCRT techniques, dosimetric comparison
Introduction
Head and neck (HN) treatment is considered among the most difficult to plan because of patient anatomy, multiple targets with different dose prescriptions, large extension of the treatment region, and the number of structures at risk. Moreover, doses up to 70–72 Gy with a conventional fractionation may be prescribed. To overcome planning difficulties, highly sophisticated techniques such as intensity-modulated radiation therapy (IMRT), intensity-modulated arc therapy, or volumetric modulated arc therapy (VMAT) have been developed, which yield much better results than does three-dimensional conformal radiotherapy (3DCRT), especially in the sparing of the organs-at-risk (OARs). However, these techniques cannot be universally used, due to unavailability of adequate equipment, organization, or patient status. Therefore, 3DCRT is still widely used to treat HN cancers, in spite of its evident limitations when compared with highly modulated techniques.
In 3DCRT, the 3-field classic technique (two lateral opposed fields abutted to an anterior low-neck field) seems the simplest to be used. However, this technique requires the addition of electron beams to match the photon fields in order to spare the spinal cord when delivering doses larger than 45-50 Gy. In practice, this match is difficult to obtain with sufficient accuracy, as it cannot be guided by the commonly available imagers. So, in case of mismatch, there is a potential of significant over/under dosage of target. Moreover, parotid glands may receive too high doses, which could induce serious complications.
Some more advanced 3DCRT treatment planning techniques have been developed to improve dose distribution to planning target volumes (PTVs) and OARs, such as, in order of time, Bellinzona technique,[1] Forward-Planned Multisegments (FPMS),[2,3] and field-in-field (FIF) technique.[4]
In this paper, we performed a planning study to compare these 4 techniques with the aim to determine the most effective technique for sparing parotid glands and spinal cord while keeping adequate dose coverage of PTV’s.
Materials and Methods
Thirteen consecutive patients with advanced cancer of oropharynx and nasopharynx were included in this comparative planning study. Planning CT was acquired in 3-mm slices using a Lightspeed General Electric scanner.
Patients were immobilized in a commercial thermoplastic head and shoulders mask with 5 fixation points. The primary gross tumor volume (GTV) and the clinical target volume (CTV) were contoured by radiation oncologists. The GTV was defined as the gross extent of the tumor. The CTV included the GTV and potential direct routes of microscopic spread. PTV was defined as CTV with a uniform margin of 5 mm, thus taking into account organ motion and set-up errors. In addition, a nodal target volume was outlined to irradiate node levels at risk; it included retropharyngeal nodes plus bilateral levels I-V or II-V (roughly, from the base of the skull to the supraclavicular region) according to the primary tumor site and to the stage.
As OAR’s, spinal cord and parotids were contoured on all CT images.
For each patient, treatment plans were generated using the superposition algorithm of a CMS XIO (Computerized Medical System, St. Louis, MO) planning system and 6 MV (in some cases mixed with 18 MV) photon beams from an ELEKTA Synergy linear accelerator equipped with an 80-leaf MLC, with 1-cm leaf width projected at the isocenter. The prescription dose was 54 Gy, 2 Gy/fraction, 5 fractions/week to a reference point in the PTV, which could fulfill most of the ICRU 50 recommendations. The reference point was selected in a clinically relevant region of the PTV with a low dose gradient. Other dose points in PTV were usually added in order to check dose homogeneity.
In addition, an adequate coverage of the neck nodal target by the 46-Gy (85% of the prescription dose) isodose was requested.
In the clinical setting, this treatment is followed by a boost with shrinking fields (usually two opposed lateral or oblique fields) to deliver a dose up to approximately 70 Gy to the primary cancer and to limit the dose to critical structures. In the present work, only the first 54 Gy treatment was considered. However, due to the larger extension of the irradiated volumes, the most dose to OARs is delivered in this first phase of treatment (see, for instance, the results presented in a previous study[3]), hence the request to reduce it as much as possible to allow the delivery of boost dose without limitations due to OAR’s toxicity. In particular, for the spinal cord, a maximum dose constraint of 45 Gy is adopted for the first phase and 50 Gy for the total treatment.
The main planning goals were to keep the dose to the targets as homogeneous as possible and minimizing OAR’s doses. In detail, no more than 1% of PTV would have received ≥110% (maximum dose) of the prescribed dose or ≤90% (minimum dose) of the prescribed dose. As for the node target, minimum dose could be as low as 85% of the prescribed dose to give priority to the achievement of the constraint of 45 Gy as maximum point dose in the spinal cord.
Four different treatment plans were obtained for each individual patient using the following planning techniques: The Bellinzona technique,[1] FPMS,[2] the 3-D ConPas,[3] and the FIF technique,[4] which are described as follows. All the four techniques use one isocenter point. All plans have been constructed and optimized by the same person and assessed firstly by visual inspection of the dose distributions and then by analysis of Dose-Volume Histograms (DVHs) of PTV, spinal cord, and parotids. The DRR’s relative to the same patient [Figures 1–4] are a representation of the plans obtained in our study for each of the four techniques.
Figure 1.
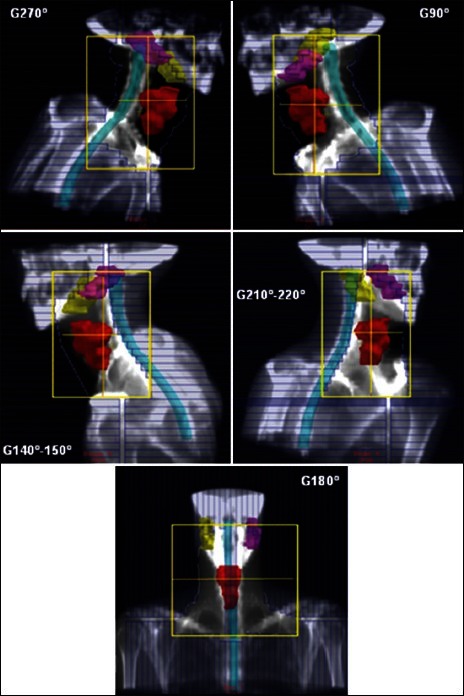
DRR’s of typical treatment fields obtained in our study for RB technique. The elective PTV, the spinal cord, and the parotids are represented. MLC shape takes into account the nodal target, not illustrated in the figure. For more details on field setup, see the Materials and Methods section
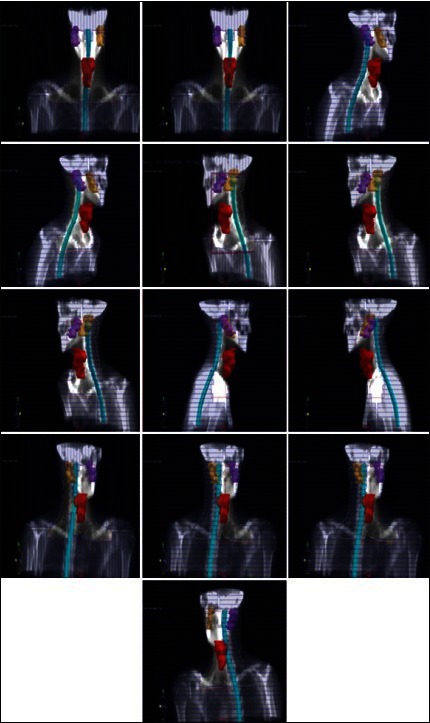
Figure 4.
DRR’s of typical treatment fields obtained in our study for FIF technique. The elective PTV, the spinal cord, and the parotids are represented. MLC shape takes into account the nodal target, not illustrated in the figure. For more details on field setup, see the Materials and Methods section
For each plan, a set of DVH parameters was analyzed to evaluate the performance of each technique. For PTV, mean dose, D95%, D99% (near-minimum dose), and D1% (near-maximum dose) were taken into account, whereas, for OAR’s, the maximum point dose and the mean dose to the spinal cord (SC) and the mean dose to the left and right parotid (LP and RP) glands were considered.
To assess the homogeneity of dose distribution in the PTV, an homogeneity index was defined as HI = (D1% - D99%)/mean dose. The lower (closer to 0) the HI, the better is the dose homogeneity. Also, to facilitate the comparison of various treatment plans, the RTOG conformity index (CI) was calculated: CI = VRI/TV, where VRI = 95% − isodose volume and TV = target volume. A CI = 1 corresponds to ideal conformation. A CI > 1 indicates that the irradiated volume is greater than the target volume and includes healthy tissues. A CI < 1 indicates that the target volume is only partially irradiated.
Moreover, to overcome possible bias arising from the selection of the reference point to which the dose had been prescribed and to allow a proper comparison of the dosimetric results of the four techniques, we normalized all doses to the PTV mean dose too. In this manner, it is possible to estimate all relevant doses in case of dose prescription to the PTV mean dose.
Revised Bellinzona (RB) planning method
The technique described in 1999 by Fogliata et al.,[1] has been revised to make use of MLC instead of blocks as originally proposed. It consists of a 5-field setup: A posterior field (G180°−T0°, i.e., gantry angle 180°, couch angle °) and two posterior-oblique fields (G210 χ 220°, T0°) and (G140 χ 150°, T°), all shielding the spinal cord completely, and two lateral fields (G270°−T5 χ 15° and G90°−T5 χ 15°) encompassing the whole target volume. The posterior field can be split in 2 separated fields in case the spinal cord cannot be completely shielded due to the constraints of the travel distances of MLC leaves. In the original paper, a dose of 54 Gy was prescribed to the PTV at the ICRU point. The digitally reconstructed radiograph images (DRR’s) of a typical RB plan obtained in our study are shown in Figure 1.
ConPas planning method
The ConPas technique, described in detail by Wiggenraad et al.,[3] is a 6–7-field isocentric technique, including two pairs of full-length parallel opposed oblique half-beams, a full-length AP beam, and an AP supraclavicular beam covering the caudal part of the PTV. The use of angles allowing partial blocking of those parts of parotids that lie below the cranial field borders represents the strength of this method. The planning procedure begins by placing the isocenter in the anterior part of the vertebral body halfway between the upper and lower limits of the PTV. Then, both oblique posterior beams are set up and turned into half-beams by closing the collimators on the side of the spinal cord. These two half-beams are most important with respect to parotid sparing.[3] Beam weights and wedge fractions are optimized in each beam. In the original paper, a dose of 46 Gy was prescribed to the ICRU point. The DRR’s of a typical ConPas plan obtained in our study are shown in Figure 2.
Figure 2.
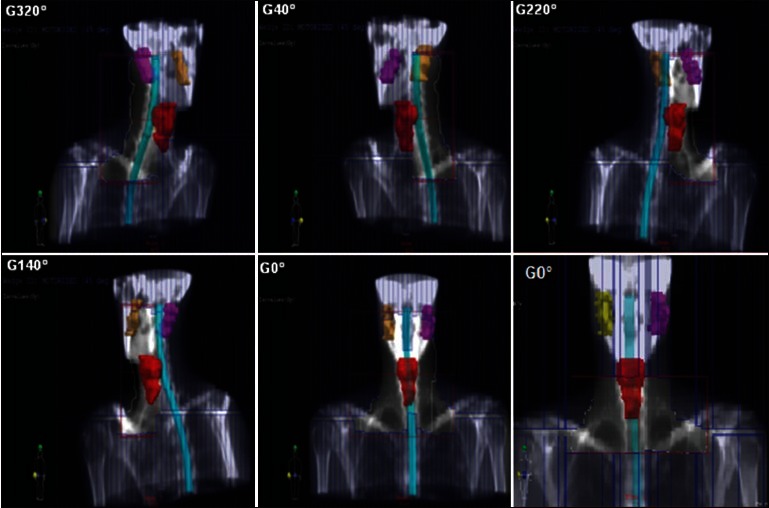
DRR’s of typical treatment fields obtained in our study for ConPas technique. The elective PTV, the spinal cord, and the parotids are represented. MLC shape takes into account the nodal target, not illustrated in the figure. For more details on field setup, see the Materials and Methods section
FPMS planning method
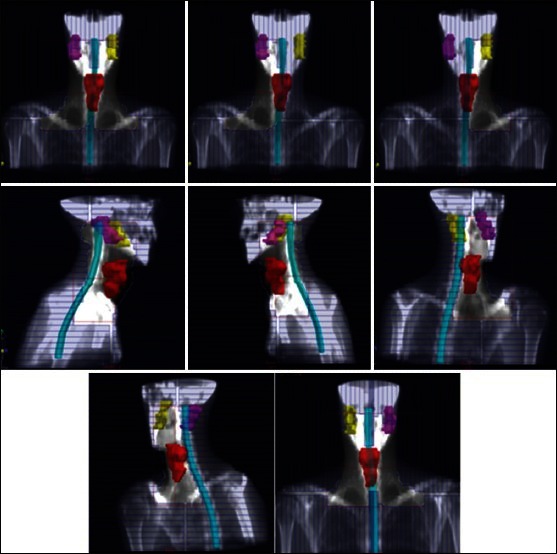
The FPMS technique, described in detail in Lee et al.,[2] aims at treating the primary tumor and the upper neck nodes of HN cancers with 7 gantry angles Figure 3 including an anterior, 2 lateral, 2 anterior oblique, and 2 posterior oblique fields, for a total of 13 MLC-shaped segments. Four of the 7 beam angles contain multiple segments and conformed to maximize the coverage of the target while minimizing the normal tissue exposure. Depending on the case, up to 3 segments can be included at a given angle. The treatment planning is based on a careful design of each segment and optimization of the associated weights. As in the original paper,[2] we used both 6 MV and 18 MV beams as well as wedges. No detail is given about dose normalization in that paper; however, it is said that the GTV was prescribed to 70 Gy at the 88% isodose line, whereas the CTV received a dose of 59.4 Gy at the 75% isodose line. The DRR’s of a typical FPMS plan obtained in our study are shown in Figure 3.
Figure 3.
DRR’s of typical treatment fields obtained in our study for FPMS technique. The elective PTV, the spinal cord, and the parotids are represented. MLC shape takes into account the nodal target, not illustrated in the figure. For more details on field setup, see the Materials and Methods section
FIF planning method
This technique, described in detail by Portaluri et al.,[4] uses mainly 11 fields, from 6 gantry angles, (0°, 280°, 80°, 180°, 135°, and 220°) with a mean of two fields per angle.[4] The unique isocenter point is placed behind the first cervical vertebral body. The dosimetric calculation is performed using a forward-planning treatment system. In the original paper, the prescribed dose range was 44–64 Gy (mean, 52 Gy; median, 50 Gy) to the isocenter for the PTV1. The DRR’s of a typical FIF plan obtained in our study are shown in Figure 4.
Results
The data were collected from DVH’s generated for each patient for each treatment technique. The results of statistical analysis of PTV coverage and OAR’s doses are presented in Tables 1 and 2 as a function of planning technique for both types of dose prescription, i.e., to the reference point and to the mean dose to the PTV. Absolute dose values in Table 1 correspond to the former prescription, whereas PTV percentage doses in Table 2 are obtained after normalizing, patient by patient, dose distribution to the mean dose to the PTV actually obtained in the planning with each technique. As for PTV, absolute mean doses do not differ significantly among the different techniques, ranging from 52.5 Gy to 53.5 Gy (97% and 99% of the prescribed dose of 54 Gy, respectively). The HI’s are quite similar (0.17–0.20, though presenting large CV%) and fairly low, indicating good homogeneity of dose in the target volume. Also, the differences among the RTOG CI’s are negligible, with all techniques providing values close to 1, corresponding to ideal conformation.
Table 1.
Comparison of the dosimetric results obtained with the four different treatment planning techniques for a dose prescription to ICRU point. Mean and coeffi cient-of-variation values for the 13-patient samples are reported
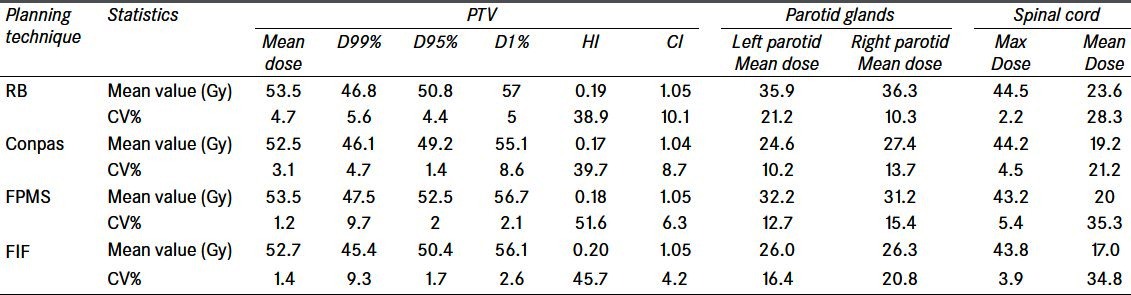
Table 2.
Comparison of the dosimetric results obtained with the four different treatment planning techniques for a dose prescription to the mean dose to PTV. Mean and coeffi cient-of-variation values for the 13-patient sample are reported. For PTV, doses are expressed as percentage of the mean dose, while, for OAR’s, absolute values are shown
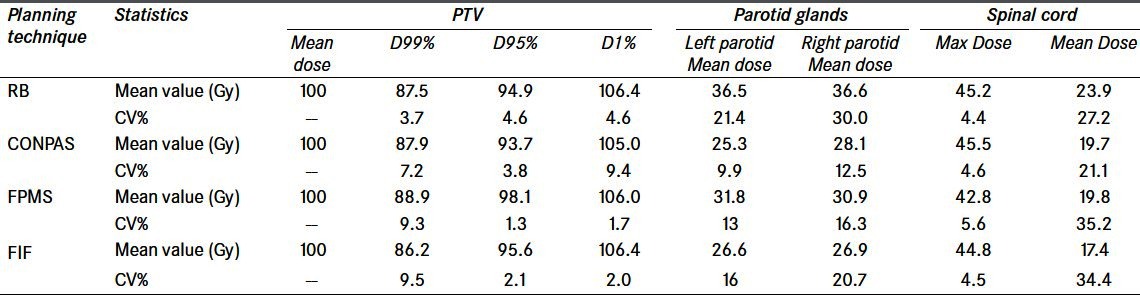
After normalizing to the mean dose to the PTV, the dosimetric results can be compared more properly as they are less sensitive to the selection of the reference point [Table 2]. PTV coverage is acceptable for all the four techniques, as D95% is always above 90% of the prescribed dose. In more detail, D95% is significantly highest for the FPMS technique (98%), lowest for the ConPas technique (94%), and about 95% for the other two techniques. Near-maximum doses, represented by D1%, are always below the ICRU recommended level of 107%,[5] while, in no case, the near-minimum doses (D99%) satisfy the recommended 95% level, as they range from 86.2% for the FIF technique to 88.9% for the FPMS technique, which is not a significant difference when considering their large CV%’s.
As for the OAR’s, for a prescription of 54 Gy to the PTV mean dose [Table 2], mean doses to parotids close to the tolerance level of 26 Gy[6] are obtained for the FIF and ConPas techniques, whereas they are significantly higher for FPMS (~32 Gy) and RB (~36 Gy). A significant finding is that the ConPas technique exhibited, in general, the lowest variability (CV%) for the OAR’s doses, most notable for parotid doses, the main objective for which the technique was built.
Finally, maximum and mean doses to the spinal cord are not significantly different for all techniques.
In Figures 5–7, DVHs are shown for PTV, parotids, and spinal cord, for all planning techniques applied to a patient from the sample. It can be seen that, in this case, the PTV dose coverage is almost similar for RB and FIF techniques, better for FMPS and worse for ConPas, whereas the dose-volume values for parotids and spinal cord seem best for Conpas.
Figure 5.
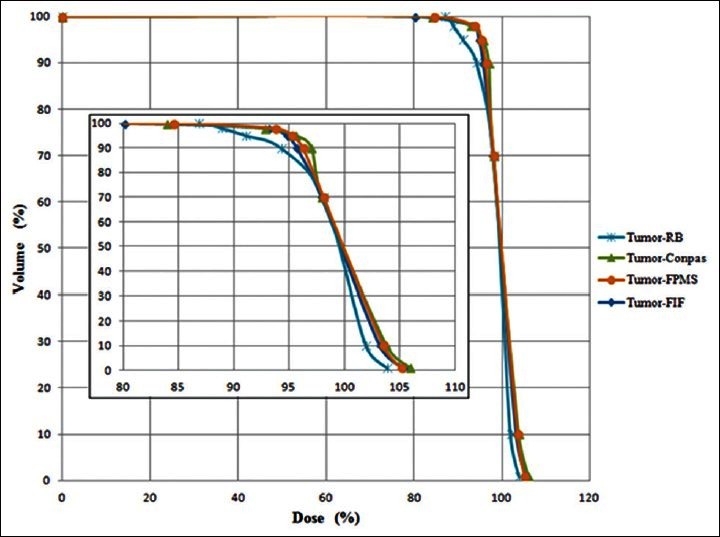
Comparison of dose-volume histograms obtained for PTV with the four planning techniques for one example patient. The inserted graph represents a zoom to evidence differences among the curves
Figure 7.
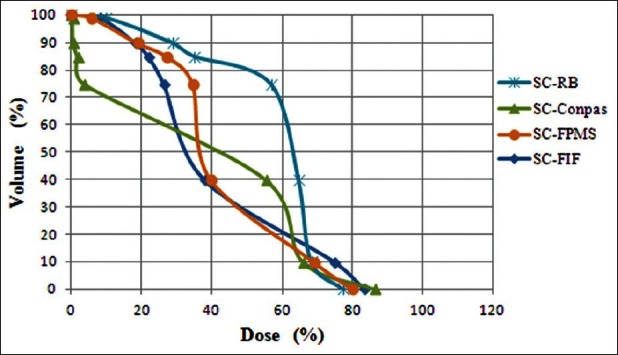
Comparison of dose-volume histograms obtained for spinal cord with the four planning techniques for one example patient
Figure 6.
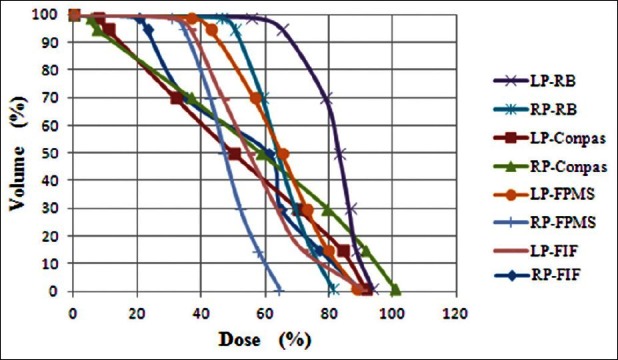
Comparison of dose-volume histograms obtained for parotid glands with the four planning techniques for one example patient. RP = right parotid; LP = left parotid
Discussion
The present work aimed to assess the potential benefits and limitations of four 3D-CRT techniques in the treatment of advanced HN cancer. The four planning techniques for HN cancer treatments we included in this comparison study make use of photon beams only and have a relatively easy handling advantage with respect to more sophisticated techniques such as IMRT or VMAT.
The comparison of our results with the original works illustrating the techniques seems to confirm the validity of our implementation.
In the paper illustrating the RB technique by Fogliata et al.,[1] the prescribed dose to the PTV was 54 Gy, while maintaining a maximum dose to the spinal cord of 75% of prescription (40.5 Gy). Average results for PTV were a minimum dose of 84.2%, mean dose of 100.7%, maximum dose of 112.8%; for the parotids, the median dose was 97%.
In the present work, applying this technique and the same type of dose normalization at a reference point, the minimum dose, the mean, and maximum dose obtained for the PTV are about 87%, 99%, and 105.5%, respectively. Moreover, our median dose to the parotids is approximately 67%, well below the above cited value. However, our better results could be due to differences in the two studies concerning the extension of PTV’s, which may include different portions of the glands.
The Conpas technique was developed for the treatment of bilateral HN cancer as an alternative to IMRT with the intent to spare the parotid glands. In the illustrating paper by Wiggenraad et al.,[3] the dose prescribed to the primary tumor was 46 Gy, the mean V95% was 91% and the mean parotid dose was about 50% (23.2 and 23.7 Gy) of the PTV dose. The work concluded that this technique, although demanding more planning and delivery time, enables good target coverage and relevant parotid sparing (mean dose <26 Gy for the entire 70 Gy treatment) without beam modulation. The same conclusions have been obtained with the present work results: V95% is 92% and the mean parotid dose is 48.5% of the prescribed dose of 54 Gy to a reference point in the PTV.
In the same way, the FPMS technique was developed as an alternative to IMRT by Lee et al.[2] Results for a sample of patients treated with FPMS technique were compared to the results for similar patients treated with IMRT. The dose prescribed to GTV was 70 Gy at the 88% isodose line, whereas the dose received by the CTV was 59.4 Gy at the 75% isodose line. Results showed that CTV D95% was 99% (58.9 Gy) of the prescribed dose and the mean dose to parotid glands was 32.0 Gy, i.e., 46% of the GTV dose and 54% of the CTV dose. Results of the present work using FPMS technique show that the PTV D95% is 97% of the dose prescribed to the reference point, and the mean parotid dose less than 32 Gy, about 60% of the prescribed dose, with CV% =13% and 15%. Our worse result could be due to differences in the patient irradiated volumes in the two studies.
The FIF technique was described by Portaluri et al.[4] in a dosimetric and clinical study aiming at correlating dosimetry and incidence of xerostomia in a sample of 49 patients treated for advanced HN cancer. The prescribed dose range was 44-64 Gy (mean 52 Gy, median 50 Gy) to the isocenter for the PTV. The method allowed a good coverage of PTV: The mean dose to the PTV was 96.6%, the minimal dose 76.9%, and the maximal dose was 105% of the prescribed dose to the isocenter. The ipsilateral parotid gland received a mean of 46.6 Gy, while the contralateral one received a mean dose of 38.2 Gy. Moreover, doses to OAR’s were disaggregated according to tumor site, thus showing that oropharynx and nasopharynx treatments provided the highest doses to parotids with respect to oral cavity or larynx.
In our study, the application of such a technique resulted in very similar results: A mean dose of 97.7%, a near-minimum dose of 84%, and a near-maximum dose of 104% of the prescribed dose to a reference point in the PTV. The mean dose for parotid glands is about 26 Gy, which is much lower than that in the original work.[4]
On the other hand, the normalization of dose distributions to the mean dose to the PTV we carried out after initial planning, allows to compare, within the same patient sample, the performance of the 4 techniques as regards the PTV coverage and sparing of OARs. All the techniques achieved for the PTV a good dose homogeneity (HI range: 0.17-0.20) and conformity (CI range: 1.04-1.05). In more detail, near-minimum doses represented by D99% present the largest CV%’s, so that the differences in the results of the four techniques were not statistically significant. The best results for (98%) for D95% however, was obtained with the FPMS technique and the worst (94%) with the ConPas technique. For the parotids, mean doses were significantly lower with the ConPas and FIF techniques (about 26.5 Gy on average), than with the others (on average 31.4 Gy and 36.5 Gy for FPMS and RB, respectively). FIF yielded the best result for the mean dose to the spinal cord, while the maximum doses did not differ significantly among the four techniques. So, it can be concluded that, while all the techniques provide adequate target coverage, the ConPas and FIF techniques offer the highest tissue sparing, although at some expense of the PTV coverage, which is undoubtedly best for the FPMS technique. In our study, FPMS provided, for a prescription of 54 Gy, a mean dose to parotids of ~32 Gy, higher than the usually prescribed tolerance of 26 Gy. On the other hand, 32 Gy represents a significant improvement with respect to the parotid doses of 39 Gy obtained by applying the classic 3-field technique to our patient sample.
Indeed, for a correct evaluation of these results, we must remark that our study concerned only plans relative to the irradiation of elective target up to a dose of 54 Gy, not the entire treatment including a boost dose up to 70 Gy. However, our experience, as well as that of other authors[3] show that, in the boost irradiation, it is generally feasible to deliver low doses to these OARs, usually by a simple 2-field technique, so that the dose limit of 50 Gy is never exceeded for the spinal cord and the parotid mean dose is increased by a small fraction of the dose received in the first treatment. As for this spinal cord constraint, according to the QUANTEC study,[7] with conventional fractionation of 2 Gy per day including the full cord cross-section, a total dose of 50 Gy is associated with a 0.2% rate of myelopathy.
As for the parotid tolerance dose, a recent study[8] based on dosimetric data of patients treated with IMRT for HN cancers showed that, in patients presenting with stage 2 degradation,[8], annex 2 salivary glands received a mean dose to parotid of >33 Gy. It was observed that patients who received a mean dose <33 Gy to both parotid glands had xerostomia of stages 0–1. Moreover, in case of asymmetric irradiation, the dose to the spared parotid should not exceed 29 Gy to compensate the lack of activity of the gland irradiated at higher dose.
A review of several previous works related to induced radiotherapy effects on salivary gland function has been carried out by Deasy et al.,[9] within the QUANTEC project.[10] A reduction in salivary function may begin 1 week after the beginning of radiotherapy and usually persists thereafter. The function is often gradually recovered within 2 years after irradiation, except in case of too high a dose. According to this work,[9] a minimum reduction in gland function occurs within 10-15 Gy mean dose and increases gradually with radiation doses of 20-40 Gy, with a significant reduction after a dose of >40 Gy. The risk of xerostomia is reduced by sparing at least one parotid or even a submandibular gland. The authors cite the study of Portaluri et al.,[4] where patients receiving <30 Gy to the contralateral parotid reported no or mild subjective xerostomia.
Our study showed that, for a prescription of 54 Gy, both FIF and Conpas techniques can achieve parotid doses <30 Gy, while FPMS technique obtained doses <33 Gy. In conclusion, after taking into account both PTV coverage and parotid sparing, the best global performance was achieved by the FIF technique with results comparable to that of IMRT plans. Moreover, in our opinion, it is easier to implement and requires less planning time than FPMS and ConPas technique.
Conclusion
The dosimetric comparison of four advanced 3DCRT treatment planning techniques for HN cancer showed that all the techniques give a good coverage of the PTV, as shown by D95% >94% of the prescription dose as well as by excellent dose homogeneity and CIs. FPMS technique, however, attains the best target coverage at levels comparable to that of IMRT. As for the OAR’s, mean doses to parotids of 26 Gy for a prescription dose of 54 Gy are achieved with the FIF and ConPas techniques, equal to the tolerance dose used in IMRT. When considering the global performance, the FIF technique achieved the best results in our study.
This let us conclude that, in centers where IMRT equipment is not available or for patients not eligible for IMRT, optimization of treatment may be feasible with such a 3DCRT technique. The technique is relatively easy to implement and does not require an investment as important as that requested by IMRT. Lastly, as suggested in a previous study,[2] the results achieved with these advanced 3DCRT techniques could be used as the reference base to which we can compare the improvements obtained with IMRT.
Acknowledgments
We would like to acknowledge the Abdus Salam International Centre for Theoretical Physics (ICTP) in Trieste, Italy, for funding this research project.
We are also grateful to Prof. Renato Padovani for his continuous support and advice and for agreeing to host Yassine within his research team. We would like to thank all the staff of Medical Physics Department of the Udine University Hospital, Italy, for their availability and contribution to this work.
We wish to thank all the medical staff of Ryad Oncology Center where the data acquisition was carried out for this research work. We are also thankful to Jamal El Merabete for his technical help.
Footnotes
Source of Support: Abdus Salam International Centre for Theoretical Physics (ICTP) in Trieste, Italy
Conflict of Interest: None declared.
References
- 1.Fogliata A, Cozzi L, Bieri S, Bernier J. Critical appraisal of a conformal head and neck cancer irradiation avoiding electron beams and field matching. Int J Radiat Oncol Biol Phys. 1999;45:1331–8. doi: 10.1016/s0360-3016(99)00319-3. [DOI] [PubMed] [Google Scholar]
- 2.Lee N, Akazawa C, Akazawa P, Quivey JM, Tang C, Verhey LJ, et al. A forward-planned treatment technique using multisegments in the treatment of head- and-neck cancer. Int J Radiat Oncol Biol Phys. 2004;59:584–94. doi: 10.1016/j.ijrobp.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 3.Wiggenraad R, Mast M, Santvoort JV, Hoogendoorn M, Struikmans H. ConPas: A 3-d conformal parotid gland-Sparing irradiation technique for bilateral neck treatment as an alternative to IMRT. Strahlenther Onkol. 2005;181:673–82. doi: 10.1007/s00066-005-1413-8. [DOI] [PubMed] [Google Scholar]
- 4.Portaluri M, Fucilli FI, Castagna R, Bambace S, Pili G, Tramacere F, et al. Three-dimensional conformal radiotherapy for locally advanced (stage II and worse) head- and-neck cancer: Dosimetric and clinical evaluation. Int J Radiat Oncol Biol Phys. 2006;66:1036–43. doi: 10.1016/j.ijrobp.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 5.ICRU Report 50. Bethesda, MD: International Commission on Radiation Units and Measurements; 1993. Prescribing, recording and reporting photon beam therapy. [Google Scholar]
- 6.Eisbruch A, Ten Haken RK, Kim HM, Marsh LH, Ship JA. Dose, volume, and function relationships in parotid salivary glands following conformal and intensity-modulated irradiation of head and neck cancer. Int. J. Radiation Oncology Biol Phys. 1999;45:577–87. doi: 10.1016/s0360-3016(99)00247-3. [DOI] [PubMed] [Google Scholar]
- 7.Kirkpatrick JP, Van Der Kogel AJ, Schultheiss TE. Radiation dose-volume effects in the spinal cord. Int J Radiat Oncol Biol Phys. 2010;76(3 Suppl):S42–9. doi: 10.1016/j.ijrobp.2009.04.095. [DOI] [PubMed] [Google Scholar]
- 8.Graff-Cailleaud P. PhD thesis. Poincaré University of Nancy, Graduate SchoolBiology-Health-Environment. 2011. Application des innovations technologiques de la radiothérapie au traitement des cancers ORL. [Google Scholar]
- 9.Deasy JO, Moiseenko V, Marks L, Chao KS, Nam J, Eisbruch A. Radiotherapy dose-volume effects on salivary gland function. Int J Radiation Oncol Biol Phys. 2010;76(3 Suppl):S58–63. doi: 10.1016/j.ijrobp.2009.06.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jackson A, Marks LB, Bentzen SM, Eisbruch A, Yorke ED, Ten Haken RK, et al. The lessons of QUANTEC: Recommendations for reporting and gathering data on dose-volume dependencies of treatment outcome. Int J Radiat Oncol Biol Phys. 2010;76(3 Suppl):S155–60. doi: 10.1016/j.ijrobp.2009.08.074. [DOI] [PMC free article] [PubMed] [Google Scholar]


