Abstract
Background
Corticotropin-releasing factor (CRF) signaling induced by stress is well established to delay gastric emptying (GE) and stimulate colonic functions. The somatostatin receptor (sst1-5) agonist, ODT8-SST acts in the brain to inhibit stress-induced adrenocorticotropic hormone and epinephrine secretion. We investigated whether ODT8-SST acts in the brain to influence stress-related alterations of gastric and colonic motor function and sst receptor subtype(s) involved.
Methods
Peptides were injected intracerebroventricularly (i.c.v.) under short isoflurane anesthesia and GE, fecal pellet output (FPO) and distal colonic motility monitored in conscious mice.
Key results
The stress of anesthesia/vehicle i.c.v. injection reduced GE by 67% and increased defecation by 99% compared to non-injected controls. Both responses were abolished by ODT8-SST (1μg=0.75nmol) or sst1 agonist (3μg=1.95nmol). The sst1 agonist also prevented the abdominal surgery-induced delayed GE. Octreotide (sst2>sst5>sst3) and the sst2 or sst4 agonists (1μg=0.78 or 0.70nmol, respectively) injected i.c.v. did not influence FPO while i.c.v. somatostatin-28 mimicked ODT8-SST’s effect. The ODT8-SST-induced increased food intake was inhibited by i.c.v. sst2 antagonist while the reduced FPO was unchanged. ODT8-SST i.c.v. reduced distal colonic motility in semi-restrained mice compared with vehicle and blocked water avoidance- and i.c.v. CRF (0.5μg=0.09 nmol)-induced stimulated FPO while a similar colonic secretomotor response to i.p. 5-hydroxytryptophane (10mg/kg=36.4μmol/kg) was unaltered.
Conclusions & Inferences
ODT8-SST counteracts stress/i.c.v. CRF-related stimulation of colonic motor function and delayed GE which can be reproduced mainly by activation of sst1 receptors. These data opens new insight to brain somatostatinergic signaling pathways interfering with brain circuitries involved in gut motor responses to acute stress.
Keywords: colonic motility, CRF, gastric emptying, ODT8-SST, somatostatin agonists, stress
INTRODUCTION
Somatostatin (SST, somatotropin release-inhibiting factor, SRIF) was identified as a 14 amino acid peptide isolated from ovine hypothalami and involved in the inhibition of growth hormone (GH) secretion.1 This landmark discovery was followed by the identification of an amino-terminal-extended biologically active form, SRIF-28 from the rat hypothalamus.2 Somatostatin in the brain exerts diverse GH-independent biological actions3-5 that are mediated through interaction with five somatostatin receptor (sst) subtypes, sst1-5 that belong to the G-protein-coupled receptor family inducing multiple transmembrane signaling systems.6 In particular, we initially established that ODT8-SST, des-AA1,2,4,5,12,13-[DTrp8]-SST, a stable oligosomatostatin agonist7 that binds to all sst receptors with high affinity8 when injected into the cisterna magna (intracisternally, i.c.) stimulates gastric acid secretion9 and accelerates gastric emptying of a liquid non-nutrient solution10 and injected intracerebroventricularly (i.c.v.) stimulates gastric emptying of a solid meal in rats.11 Studies using various selective SRIF receptor subtype agonists indicated that the acceleration of gastric emptying after i.c. injection of ODT8-SST or SRIF-28 is mimicked mainly by the activation of brain sst5.10 Recently, we also reported that ODT8-SST injected i.c.v. robustly increases light phase food intake11 through activation of sst2 as indicated by the complete blockade of the ODT8-SST effect on food intake using a selective sst2 antagonist in rats11 and the similar orexigenic effect of i.c.v. injection of a selective peptide sst2 agonist in rats12 and mice.13
Several studies indicate that various transmitters acting in the brain to regulate food intake such as corticotropin releasing factor (CRF), peptide YY, neuropeptide Y, thyrotropin releasing hormone, ghrelin, opioids, endocannabinoids, oxytocin, neuropeptide S and cholecystokinin14-17 also alter colonic motor function.18-26 However, the central action of SRIF to influence colonic motility is unknown so far, while there is neuroanatomic evidence for the distribution of sst receptors and ligands in brain nuclei involved in the brain regulation of colonic function27-29 such as the paraventricular nucleus (PVN) of the hypothalamus (sst2, sst3 and sst4), locus coeruleus/subcoeruleus (sst2, sst3 and sst4) and the arcuate nucleus (sst1, sst2, sst3, sst4 and sst5).30-32 Moreover, previous studies showed that brain injection of the oligosomatostatin agonist, ODT8-SST or SRIF-28 inhibits acute stress-induced adrenocorticotropic hormone (ACTH) and adrenal epinephrine secretion in rats.33 This effect was exerted by an inhibition of CRF release33 pointing towards a negative interaction between activation of somatostatin receptors and CRF. Central CRF is well established to be recruited under stress conditions and involved in stress-related delayed gastric emptying and stimulation of colonic propulsive motor activity.34
Therefore, in the present study we investigated the central action of ODT8-SST to influence colonic motor alterations induced by acute stressors or i.c.v. injection of CRF18 in mice. This was compared with direct peripheral stimulation of the colon by intraperitoneal (i.p.) injection of 5-hydroxytryptophane (5-HTP).35 To get insight into receptor subtype(s) contributing to the i.c.v. ODT8-SST effect, we used the selective sst136, sst237 and sst4 peptide agonists38 developed so far along with the recently characterized sst2 peptide antagonist.39 Based on the relationship between peptides influencing food intake and colonic motility, we simultaneously monitored changes in food intake and defecation upon i.c.v. injection of ODT8-SST and tested whether the sst2 antagonist, known to block the orexigenic action of i.c.v. ODT8-SST in rats,11 would influence the colonic motor response to an acute stress. Lastly, we assessed whether i.c.v. ODT8-SST and the selective sst1 agonist identified to be effective in counteracting the acute stress stimulatory effect on the colon would also prevent the same stressor-induced delayed gastric emptying. We also tested the sst1 agonist in a well established model of visceral stress, abdominal surgery.40
MATERIALS AND METHODS
Animals
Adult male C57Bl/6 mice (23-28g, Harlan Laboratories, San Diego, CA) were housed 4/cage under controlled illumination (06:00-18:00 h) and temperature (21–23°C). Animals had ad libitum access to standard rodent diet (Prolab RMH 2500; LabDiet, PMI Nutrition, Brentwood, MO) and tap water. Protocols were approved by the Veterans Administration Institutional Animal Care and Use Committee (# 10043-09 and # 9906-820).
Compounds
ODT8-SST (MW 1078.5, compound #1 in8), the sst1 agonist (MW: 1238.5, compound #25 in36), sst1 agonist CH-275 (MW: 1485.5, compound #2 in36), sst2 agonist (MW: 1132.5, compound #2 in37), sst4 agonist (MW: 1137.4, compound #15 in38), the sst2 antagonist (MW: 1208.4, compound #4 in39), somatostatin-14 (MW: 1637.9), somatostatin-28 (MW: 3146.5) and rat/human/mouse CRF (Clayton Foundation Laboratories, Salk Institute, La Jolla, CA) were synthesized as we previously described and purity was characterized by high pressure liquid chromatography, capillary zone electrophoresis and mass spectrometry.8, 36-39 No peptide agonists selectively binding to the sst3 or sst5 have been developed yet and therefore we used the stable sst2>sst5>sst3 agonist, octreotide also known as SMS201-99541 (MW 1019.3, Bachem America, Inc., Torrance, CA). The chemical structure as well as previously established binding affinities of these peptides on sst receptor-transfected human cells are detailed in Table 1. Peptides and the exogenous serotonin (5-HT) precursor, 5-hydroxytryptophane, L-2-amino-3-(5-hydroxyindolyl)propionic acid (Sigma-Aldrich, St. Louis, MO) were kept in powder form at −80 °C and immediately before administration, were dissolved in vehicle as specified in the experimental protocols. As the conversion of μg injected to nmol does not accurately reflect the amount of nmol injection due to the fact that each lyophilized peptide preparation contains counter ions (TFA) and water of lyophilization that together amount 15 to 30% in weight we calculated the peptide amount injected in nmol/mouse based on a 20% correction factor of the formula weight established previously (J.R.).
Table 1.
Structure and receptor binding affinity of somatostatin receptor agonists.
| PeptideReference | Structure | Receptor binding affinity (IC50, nM)a | ||||
|---|---|---|---|---|---|---|
| sst1 | sst2 | sst3 | sst4 | sst5 | ||
| ODT8-SST8 | des-AA1,2,4,5,12,13-(DTrp8)-SRIF | 27.0 ± 3.4 |
41.0 ± 8.7 |
13.0 ± 3.2 |
1.8 ± 0.7 |
46.0 ± 27.0 |
| sst1 agonist (compound 25)36 |
des-AA1,4-6,10,12,13-[DTyr2,D- Agl(NMe,2naphtoyl)8,IAmp9]-SRIF-Thr- NH2 |
0.19 ± 0.04 |
> 1K | 158.0 ± 14.0 |
27.0 ± 7.5 |
> 1K |
| sst1 agonist CH- 275 (compound 2)36 |
des-AA1,2,5-[DTrp8,IAmp9]-SRIF | 31 ± 13 | > 1K | 540; 150 |
>1K | >1K |
| sst2 agonist (compound 2)37 |
des-AA1,4-6,11-13- [DPhe2,Aph7(Cbm),DTrp8]-Cbm-SRIF- Thr- NH2 |
> 1K | 7.5 - 20 | 942 - 1094 |
872 - 957 |
109 - 260 |
| sst4 agonist (compound 15)38 |
des-AA1,2,4,5,12,13-[Aph7]-Cbm-SRIF | 650 ± 115 |
> 1K | 780 ± 62 |
1.5 ± 0.07 |
> 1K |
| somatostatin-14 (SRIF-14)67 |
Ala-Gly-c[Cys-Lys-Asn-Phe-Phe-Trp- Lys-Thr-Phe-Thr-Ser-Cys]-OH |
0.1 − 1.5 |
1.7 | 1.7 | 1.0 − 1.6 |
0.2 − 2.2 |
| somatostatin-28 (SRIF-28)67 |
Ser-Ala-Asn-Ser-Asn-Pro-Ala-Met-Ala- Pro-Arg-Glu-Arg-Lys-Ala-Gly-c[Cys- Lys-Asn-Phe-Phe-Trp-Lys-Thr-Phe-Thr- Ser-Cys]-OH |
0.1 − 4.7 |
0.4 − 5.2 |
0.2 | 0.3 − 1.1 |
0.05 − 0.19 |
| octreotide50 | H-(D)-Phe2-c[Cys3-Phe7-DTrp8-Lys9- Thr10-Cys14]-Thr15(ol) |
> 1K | 1.9 ± 0.3 |
39 ± 14 | > 1K | 5.1 ± 1.1 |
| sst2 antagonist (compound 4)39 |
des-AA1,4-6,11-13-[pNO2- Phe2,DCys3,Tyr7,DAph(Cbm)8]-SRIF- 2Nal-NH2 |
> 1K | 2.6 ± 0.7 |
384.0 ± 97.0 |
> 1K | > 1K |
Procedures
Intracerebroventricular (i.c.v.) injections
Mice were acutely anesthetized by brief (2-3 min) exposure to isoflurane (4.5% vapor concentration in oxygen; VSS, Rockmart, GA), then the intracerebroventricular (i.c.v.) injection (5 μl) was performed using an adaptation of the freehand method42 as in our previous studies.13 The injection site was localized at the apex of the equal triangle between the eyes and the back of the head and cleaned with Povidone-Iodine 10% (Aplicare Inc., Meriden, CT). The skull was punctured at the point of least resistance with a 30-gauge needle equipped with a polyethylene tube leaving 4-4.5 mm of the needle tip exposed and attached to a Hamilton syringe. On average, mice completely recovered from anesthesia within 4-5 min. Accuracy of injections was assessed in our previous studies by injecting cresyl violet dye i.c.v. under similar conditions in 50 mice.18
Water avoidance stress
(WAS) lasted for 1 h and was performed as in our previous studies.43 It consisted of placing the mice individually on a rectangular platform (3cm length × 3cm width × 6 cm height) positioned inside a container (31 cm length × 31 cm width × 21 cm height) filled with hand warm water up to 1 cm below the top of the platform.
Abdominal surgery in mice was performed as described before.40 Briefly, overnight fasted mice anesthetized with isoflurane underwent laparotomy and cecal exteriorization and palpation for 60 s and the abdominal incision was closed in one layer with a running stitch. Sham operation consisted of same duration of anesthesia without surgery.
Measurements
Fecal pellet output
The number of fecal pellets expelled was monitored as in our previous studies19 during WAS exposure or after injection of peptides. The occurrence of diarrhea was assessed using the recently described diarrhea score 19 with 0 indicating no diarrhea, 1: ≤1 watery and/or loose pellets, 2: ≤2 watery and/or loose pellets and 3 indicating ≤3 watery and/or loose pellets.
Food intake was assessed manually as in our previous studies.11 After injection, pre-weighed rodent chow was made available and food intake corrected for spillage was monitored at 1, 2, 4 and 6 h. Feeding experiments were repeated in a crossover design.
Distal colonic motility
Distal intracolonic pressure monitoring in conscious mice was performed as recently developed.44 Briefly, naïve mice anesthetized for 2-3 min with isoflurane (4.5% vapor concentration in oxygen; VSS) were injected i.c.v. with ODT8-SST or vehicle as described above and a miniature pressure transducer catheter (SPR-524 Mikro-Tip catheter; Millar Instruments, Houston, TX) lubricated with chlorhexidine gluconate (Surgilube, E. Fougera & Co., Atlanta Inc., NY) was inserted 2 cm into the distal colon and secured to the tail with tape. Then, mice regained the righting reflex and were placed individually in a mouse semi-restraint tube and distal intracolonic pressure recording started approximately 4 min after the injection. The pressure transducer was connected to a preamplifier (model 600; Millar Instruments). The signal was amplified using a transducer amplifier (TBM4, World Precision Instruments, Boca Raton, FL), acquired using a Micro1401 A/D interface (Cambridge Electronic Design) and recorded using Spike 2 version 5 data acquisition software. Abdominal contractions were excluded by smoothing the original trace with a time constant of 2 s. The phasic component of intra-colonic pressure was extracted from the original trace by removing the DC (direct current) component with a time constant of 10 s from the 2-s smoothed original trace. The distal colonic contractile pressure changes were quantified by measuring the phasic component of the intraluminal pressure trace of the area under the curve (pAUC) using quantifications for every minute as previously described.44 Results are expressed as 1) the time course of the phasic contractions, by calculating the mean pAUC for every minute (pAUCm) from the rolling average of pAUC for the period between 2 min before and 2 min after each minute’s pAUC (i. e., pAUCm = average of pAUC±2 min) or 2) the mean pAUC over a period of 1 h.
Gastric emptying of ingested standard rodent chow was determined as in our previous studies.11 Briefly, mice were fasted overnight for 15 h and refed with pre-weighed standard chow starting at 08:00 h for 1 h. Then, food and water were removed and i.c.v. injection was performed under short inhalation anesthesia. Gastric emptying was assessed 2 h later. Mice were euthanized by cervical dislocation, the stomach quickly removed, the stomach content weighed and gastric emptying calculated as (1-gastric content/food intake) × 100.
Gastric emptying of a liquid non-nutrient solution was performed as described before with small modifications.40 Briefly, the phenyl-red methyl cellulose solution (0.3 ml) was gavaged orogastrically at 30 min after the end of the surgery or sham procedure in conscious overnight fasted mice and gastric emptying assessed 20 min later. Mice were euthanized by CO2 inhalation and the stomachs removed, cleaned, placed in 0.1N NaOH and homogenized. The homogenate was allowed to settle for 1 h, and 5 ml of the supernatant were added to 0.5 ml of 20% trichloroacetic acid. This solution was centrifuged at 4°C for 20 min at 3000 rpm, and 3 ml of the supernatant added to 4 ml of 0.5N NaOH which was read spectrophotometrically. Two non-treated animals were euthanized following gavage and served as control animals (0% emptying). The percentage of gastric emptying was calculated using the following equation: % emptying = (1 - absorbance of test sample/absorbance of standard) × 100.
Experimental protocols
All experiments were performed in freely fed mice with continued access to food and water after treatment except otherwise stated. Animals were single housed for the duration of the experiment starting between 09:00 and 10:00 h. Mice were trained for single housing at least three times before for 1-9 h/d during the week before the experiment.
Effects of ODT8-SST and selective somatostatin receptor agonists injected i.c.v. on stimulated propulsive colonic motor function
Vehicle (ddH2O alone or containing 0.1% bovine serum albumin, BSA) and the following SRIF peptide agonists were injected i.c.v. in mice: ODT8-SST (0.3 μg = 0.22 nmol or 1 μg = 0.75 nmol/mouse in ddH2O), SRIF-14 (5.1 μg = 2.5 nmol/mouse in ddH2O containing 0.1% BSA), SRIF-28 (9.3 μg = 2.4 nmol/mouse in ddH2O containing 0.1% BSA), octreotide (0.3 μg = 0.24 nmol or 1 μg = 0.78 nmol/mouse in ddH2O) or the selective sst1 (0.3 μg = 0.19 nmol, 1 μg = 0.65 nmol or 3 μg = 1.95 nmol/mouse in ddH2O), sst1 CH-275 (3 μg = 1.6 nmol or 10 μg = 5.4 nmol/mouse in ddH2O), sst2 (0.3 μg = 0.21 nmol or 1 μg = 0.70 nmol/mouse in ddH2O) and sst4 agonists (0.3 μg = 0.21 nmol or 1 μg = 0.70 nmol/mouse in ddH2O) and fecal pellet output was monitored for 1 h afterwards. Doses of ODT8-SST were based on our previous i.c.v. dose-response study on food intake in rats and mice11 and those of SRIF agonists adapted accordingly. Based on the observed effects in these experiments, in all subsequent studies, ODT8-SST was injected at the dose of 1 μg = 0.75 nmol/mouse. To assess basal fecal pellet output, a control group of mice without anesthesia and i.c.v. injection was monitored for 1 h.
To investigate the time course of the ODT8-SST effect on propulsive colonic motor function, mice were injected i.c.v. with ODT8-SST or vehicle and thereafter did or did not have access to food. Fecal pellet output was assessed at 1, 2, 4, 6 and 9 h post injection. Food intake was monitored at the same time points. In other studies, the sst2 antagonist (1 μg = 0.66 nmol/mouse in 2.5 μl ddH2O) or vehicle (2.5 μl ddH2O) was injected i.c.v. followed by i.c.v. ODT8-SST (in 2.5 μl ddH2O) or vehicle (2.5 μl ddH2O) and food was provided throughout these experiments. Fecal pellet output and food intake were assessed at 1, 2, 4 and 6 h post injection. The sst2 antagonist was injected i.c.v. at a dose shown to block the i.c.v. ODT8-SST-induced increased food intake in rats.11
Water avoidance stress
Mice were injected i.c.v. with ODT8-SST, sst2 agonist (0.70 nmol/mouse in 5 μl ddH2O) or vehicle and allowed to completely recover (within ~ 4 min). Afterwards, mice were subjected to water avoidance stress without access to food and fecal pellet output assessed at 15, 30, 45 and 60 min. Non i.c.v. injected control mice were placed in single housing cages and 1-h fecal pellet output was monitored in 15-min intervals.
Intracerebroventricular CRF
Mice were injected i.c.v. with ODT8-SST (in 2.5 μl ddH2O) or vehicle (2.5 μl ddH2O) followed by CRF (0.5 μg = 0.09 nmol/mouse in 2.5 μl ddH2O) or vehicle (2.5 μl ddH2O) and fecal pellet output and diarrhea score were assessed at 15, 30, 45 and 60 min.
Intraperitoneal 5-HTP
Mice were injected i.c.v. with ODT8-SST or vehicle. After complete recovery from anesthesia, mice received an i.p. injection of 5-HTP (10 mg/kg = 36.4 μmol/kg in 100 μl saline) or vehicle (100 μl saline) and fecal pellet output and diarrhea score were monitored at 15, 30, 45 and 60 min post i.p. injection. The dose of i.c.v. CRF and i.p. 5-HTP was based on our previous reports showing a robust stimulation of fecal pellet output and diarrhea score in mice under these conditions.18, 19, 35
Effects of ODT8-SST injected i.c.v. on distal colonic motility in mice
Mice were injected i.c.v. with ODT8-SST or vehicle, then the miniaturized transducer catheter was inserted into the distal colon and secured to the tail with tape and mice were placed in a mouse semi-restraint tube. Recording of distal colonic contractions started at approximately 4 min post injection in mice recovered from anesthesia and was monitored for a 1-h period in animals deprived of food and water.
Effects of ODT8-SST and the selective sst1 agonist injected i.c.v. on gastric emptying of a solid meal
Overnight fasted mice were re-fed for 1 h and afterwards injected i.c.v. with ODT8-SST, sst1 agonist (1.95 nmol/mouse in 5 μl ddH2O) or vehicle or received no treatment and gastric emptying for the solid meal was assessed 2 h later.
Effects of the selective sst1 agonist injected i.c.v. on abdominal surgery-induced delayed gastric emptying of a liquid meal
Overnight fasted mice were injected i.c.v. with sst1 agonist (1.95 nmol/mouse in 5 μl ddH2O) or vehicle and directly afterwards underwent abdominal surgery or sham procedure. Animals received an orogastric gavage of a liquid non-nutrient solution 30 min after the end of the procedure and gastric emptying was assessed 30 min later.
Statistical analysis
Data are expressed as mean ± SEM and analyzed by one way analysis of variance (ANOVA) followed by Tukey post hoc test or two-way ANOVA followed by Holm-Sidak method. All analyses were performed using Sigma Stat 3.1 (Systat Software, Inc., Point Richmond, CA). Differences between groups were considered significant when P < 0.05.
RESULTS
ODT8-SST, SRIF-28 and the selective sst1 agonist injected intracerebroventricularly (i.c.v.) prevent defecation induced by the acute stress of i.c.v. injection under short anesthesia
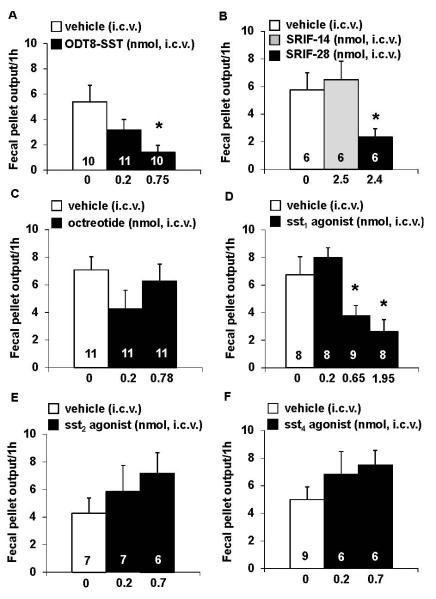
The i.c.v. injection of saline under short anesthesia resulted in an increase in FPO compared to non-anesthetized, non-treated mice (5.8 ± 0.5 vs. 2.9 ± 0.4 fecal pellets/1 h, P < 0.01; n=10). ODT8-SST (0.22 and 0.75 nmol/mouse) injected i.c.v. dose-dependently reduced the 1-h cumulative fecal pellet output by 41% and 74% respectively in freely fed mice compared to vehicle (5.4 ± 1.4 fecal pellets/1 h), with the highest dose reaching statistical significance (P < 0.05; Fig. 1A). Based on these data, the dose of 1 μg = 0.75 nmol/mouse ODT8-SST was used for all further i.c.v. injections. Likewise, SRIF-28 (2.4 nmol/mouse, i.c.v.) significantly reduced the number of excreted fecal pellets by 60% compared to vehicle (2.3 ± 0.6 vs. 5.8 ± 1.3 fecal pellets/1 h, P< 0.05; Fig. 1B), whereas SRIF-14 (2.5 nmol/mouse, i.c.v.) had no effect (Fig. 1B).
Figure 1.
ODT8-SST, the selective sst1 agonist and somatostatin-28 injected intracerebroventricularly reduced fecal pellet output whereas somatostatin-14, octreotide and other selective sst2 and sst4 agonists did not. Ad libitum fed mice were acutely injected i.c.v. under short isoflurane anesthesia with ODT8-SST (0.22 or 0.75 nmol/mouse in 5 μl ddH2O, A), somatostatin-14 (2.5 nmol/mouse in 5 μl ddH2O containing 0.1% BSA, B), somatostatin-28 (2.4 nmol/mouse in 5 μl ddH2O containing 0.1% BSA, B), octreotide (0.24 or 0.78 nmol/mouse, C) the selective sst1 (0.19, 0.65 or 1.95 nmol/mouse, D), sst2 (0.21 or 0.70 nmol/mouse, E) and sst4 agonists (0.21 or 0.70 nmol/mouse, F) and or vehicle (5 μl ddH2O or 5 μl ddH2O containing 0.1% BSA) and fecal pellet output was assessed for 1 h. Each bar represents the mean ± sem of number of mice indicated at the bottom of the columns. * P < 0.05 vs. vehicle.
Next, we investigated the receptor subtype(s) reproducing the ODT8-SST and SRIF-28 i.c.v. inhibitory effect. The selective sst1 agonist (0.19, 0.65 or 1.95 nmol/mouse) injected i.c.v. reduced fecal pellet output by 44% and 61% respectively at the highest doses compared with i.c.v. vehicle (3.8 ± 0.7 and 2.6 ± 0.9 vs. 6.8 ± 1.3 fecal pellets/1 h, P< 0.05) and had no effect at the lowest dose (Fig. 1D). The sst1 agonist CH-275 injected i.c.v. (1.6 nmol/mouse) did not significantly reduce fecal pellet output compared to vehicle (4.0 ± 0.8 vs. 3.6 ± 0.9 fecal pellets/1 h), although the higher dose of 5.4 nmol/mouse tended to reduce 1-h fecal pellet output (2.5 ± 0.8) without reaching statistical significance (P> 0.05). The oligosomatostatin (sst2>sst5>sst3) agonist, octreotide (0.24 or 0.78 nmol/mouse, Fig. 1C) and the selective sst2 or sst4 agonist (0.21 or 0.70 nmol/mouse) injected i.c.v. did not alter the 1-h cumulative fecal pellet output compared to vehicle, however, both the sst2 and sst4 agonist showed a 67% and 50% trend towards an increase of the 1-h FPO (P> 0.05, Figs. 1E, F).
Time course of i.c.v. ODT8-SST action: the sst2 receptor is involved in the orexigenic effect of ODT8-SST but not in the colonic inhibitory response to the acute stress of i.c.v. injection
The time course of i.c.v. ODT8-SST action showed a short lasting 74% inhibition of defecation during the first h (Fig. 2A), whereas the fecal pellet output was increased compared to vehicle at 4 h (20.0 ± 1.5 vs. 11.5 ± 1.5 fecal pellets, P< 0.05). Two-way ANOVA indicated a significant influence of treatment (F(1,90)=15.2, P< 0.001), time (F(4,90)=34.5, P< 0.001) and treatment × time (F(4,90)=5.6, P< 0.001). When expressed as fecal pellet output/period the main increase of fecal pellet output was observed during the 1-2 and 2-4 h periods compared to vehicle (P< 0.001; Fig. 2A). Simultaneous monitoring of food intake showed, as previously reported in mice,11 that i.c.v. injected ODT8-SST significantly increases cumulative food intake at 1, 2, 4 and 6 h post injection compared to vehicle in freely fed mice (P< 0.05; data not shown).
Figure 2.
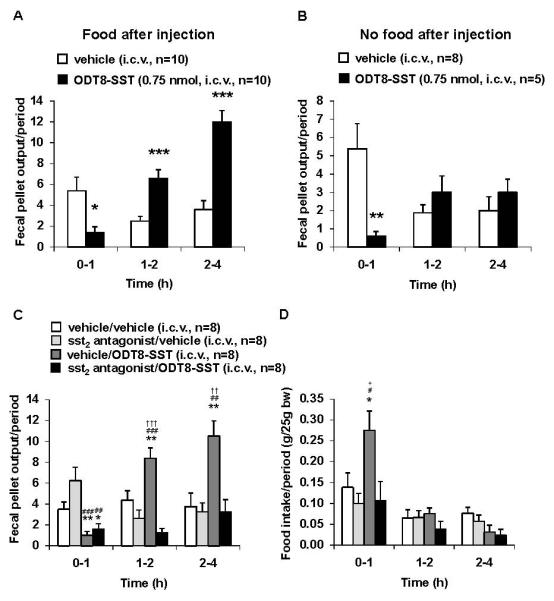
(A, B) Time course of dual effects of ODT8-SST injected intracerebroventricularly on fecal pellet output: role of food intake post injection. Mice were injected i.c.v. with ODT8-SST (0.75 nmol/mouse in 5 μl ddH2O) or vehicle (5 μl ddH2O) and thereafter had access (A,) or no access (B) to food. Fecal pellet output was assessed at 1, 2 and 4 h post injection and expressed as fecal pellet output/period. Each bar represents the mean ± sem of number of 5-10 mice. * P < 0.05, ** P < 0.01 and *** P < 0.001 vs. vehicle.
(C, D) The sst2 antagonist injected intracerebroventricularly (i.c.v.) blocked the stimulatory effect of i.c.v. ODT8-SST on food intake but not the inhibition of fecal pellet output. Mice were injected i.c.v. with sst2 antagonist (0.66 nmol/mouse in 2.5 μl ddH2O) or vehicle (2.5 μl ddH2O) followed by ODT8-SST (0.75 nmol/mouse in 2.5 μl ddH2O) or vehicle (2.5 μl ddH2O) and fecal pellet output (C) and food intake (D) were assessed at 1, 2, and 4 h post injection and expressed as food intake as well as fecal pellet output/period. Each bar represents the mean ± sem of number of 5-10 mice. * P < 0.05 and ** P < 0.01 vs. vehicle/vehicle; # P < 0.05, ## P < 0.01 and ### P < 0.001 vs. sst2 antagonist/vehicle; + P < 0.05, ++ P < 0.01 and +++ P < 0.001 vs. vehicle/ODT8-SST; †† P < 0.01 and ††† P < 0.001 vs. sst2 antagonist/ODT8-SST.
Then, we assessed the influence of the concomitant orexigenic effect in the dual inhibitory/stimulatory effects of ODT8-SST on propulsive colonic motor function. When mice did not have access to food after the i.c.v. injection of ODT8-SST, a significant 89% inhibitory effect on fecal pellet output was induced during the first hour post injection (5.4 ± 1.4 vs. 0.6 ± 0.2 fecal pellets/1 h, P< 0.01; Fig. 2B) which was not significantly different from that observed in mice with access to food (P> 0.05). However, the cumulative fecal pellet output was not increased thereafter as observed in freely fed mice (Fig. 2B). Two-way ANOVA showed a significant influence of treatment (F(1,55)=3.9, P < 0.05) and time (F(4,55)=7.7, P < 0.001).
To further assess the differential role of sst receptors in the dual feeding and colonic responses to i.c.v. ODT8-SST, the sst2 antagonist (0.66 nmol/mouse, i.c.v.) previously established to suppress the feeding response to i.c.v. ODT8-SST in rats11 was used in freely fed mice. The sst2 antagonist completely blocked the i.c.v. ODT8-SST-induced increased food intake (e.g. 1 h: 0.11 ± 0.04 vs. 0.27 ± 0.05 g/25g bw, P < 0.05; Figs. 2D) along with the significantly increased fecal pellet output during the 1-2 and 2-4 h periods post injection (Fig. 2C), while not influencing the ODT8-SST-induced 71% inhibition of fecal pellet output during the first hour post injection (P > 0.05, Fig. 2D). Two-way ANOVA indicated a significant influence of treatment (F(3,112)=19.7, P < 0.001), time (F(3,112)=61.6, P < 0.001) and treatment × time (F(9,112)=4.3, P < 0.001). The sst2 antagonist injected i.c.v. alone had no effect on fecal pellet output or food intake (Figs. 2C-D).
ODT8-SST injected intracerebroventricularly inhibits water avoidance stress and intracerebroventricular CRF-induced stimulation of propulsive colonic motor function
Water avoidance stress increased fecal pellet output compared to single-housed controls (+64%, P < 0.05; Fig. 3A). ODT8-SST injected i.c.v. completely blocked the water avoidance stress-induced increase of fecal pellet output (0.8 ± 0.4 vs. 4.8 ± 0.9 fecal pellets/60 min, P < 0.001; Fig. 3A). Two-way ANOVA indicated a significant influence of treatment (F(1,88)=56.9, P < 0.001), time (F(3,88)=8.9, P < 0.001) and treatment × time (F(3,88)=4.3, P < 0.01). In contrast, i.c.v. injection of the sst2 agonist (0.70 nmol/mouse) showed a trend to increase defecation response to water avoidance stress which did not reach statistical significance (P > 0.05; Fig. 3B).
Figure 3.

ODT8-SST injected intracerebroventricularly blocked the water avoidance stress-induced increased fecal pellet output whereas the selective sst2 agonist temporarily further increased fecal pellet output. Mice fed ad libitum until the start of the experiment were injected i.c.v. with ODT8-SST (0.75 nmol/mouse in 5 μl ddH2O, A) sst2 agonist (0.70 nmol/mouse in 5 μl ddH2O, B) or vehicle (5 μl ddH2O) under short inhalation anesthesia and allowed to completely recover within ~ 4 min. Afterwards, mice were subjected to water avoidance stress (WAS) and fecal pellet output assessed for 60 min. Control mice were placed in single housing cages and 1-h fecal pellet output was monitored in 15-min intervals. Each bar represents the mean ± sem of number of mice indicated at the bottom of the columns. * P < 0.05 vs. control; ### P < 0.001 vs. vehicle/stress.
The i.c.v. injection of CRF (0.09 nmol/mouse) enhanced defecation and the stimulatory effect was completely blocked by the i.c.v. injection of ODT8-SST at all time points (15, 30, 45 and 60 min) compared to CRF alone (60 min: 2.0 ± 0.4 vs. 9.5 ± 1.8 fecal pellets/60 min, P < 0.01; Fig. 4A and data not shown). The linear time related increase in the number of excreted fecal pellets from the 15 to 60 min period post i.c.v. injection of vehicle/vehicle was also suppressed by the i.c.v. injection of ODT8-SST at all time points. Two-way ANOVA indicated a significant influence of treatment (F(3,92)=36.2, P < 0.001) and time (F(3,92)=7.8, P < 0.001). Likewise, i.c.v. ODT8-SST injected mice had a very low diarrhea score at all time points investigated compared to i.c.v. CRF alone (60 min: 0.1 ± 0.1 vs. 2.8 ± 0.2, P < 0.001) or the vehicle/vehicle group (Fig. 4B).
Figure 4.

ODT8-SST injected intracerebroventricularly (i.c.v.) completely blocked the i.c.v. CRF-induced increase in fecal pellet output and diarrhea score in mice. Mice were injected i.c.v. with ODT8-SST (0.75 nmol/mouse in 2.5 μl ddH2O) or vehicle (2.5 μl ddH2O) followed by i.c.v. CRF (0.09 nmol/mouse in 2.5 μl ddH2O) or vehicle (2.5 μl ddH2O) under short inhalation anesthesia and after recovery fecal pellet output and diarrhea score (0 indicating no diarrhea, 1: ≤1 watery and/or loose pellets, 2: ≤2 watery and/or loose pellets and 3 indicating ≤3 watery and/or loose pellets) were assessed for 60 min. Each bar represents the mean ± sem of 5-8 mice/group. *** P < 0.001 vs. vehicle/vehicle; ## P < 0.01 and ### P < 0.001 vs. ODT8-SST/vehicle; +++ P < 0.001 vs. ODT8-SST/CRF.
Peripherally injected 5-HTP (36.4 μmol/kg, i.p.) significantly increased fecal pellet output (P < 0.05; Fig. 5A). Injection of ODT8-SST i.c.v. did not influence the 5-HTP-induced increase in fecal pellet output (Fig. 5A). However, i.c.v. ODT8-SST decreased defecation compared with the i.c.v. vehicle group (Fig. 5A). Two-way ANOVA showed a significant influence of treatment (F(3,112)=46.7, P < 0.001) and time (F(3,112)=3.7, P < 0.05). Similarly, 5-HTP i.p. significantly increased the diarrhea score compared to vehicle (P < 0.05) and this effect was not influenced by pre-treatment with ODT8-SST i.c.v. (Fig. 5B).
Figure 5.
ODT8-SST injected intracerebroventricularly did not influence the intraperitoneal 5-HTP-induced increased fecal pellet output and diarrhea score. Mice were injected i.c.v. with ODT8-SST (0.75 nmol/mouse in 5 μl ddH2O) or vehicle (5 μl ddH2O). After complete recovery from anesthesia, mice received an i.p. injection of 5-HTP (36.4 μmol/kg in 100 μl saline) or vehicle (100 μl saline) and fecal pellet output and diarrhea score (0 indicating no diarrhea, 1: ≤1 watery and/or loose pellets, 2: ≤2 watery and/or loose pellets and 3 indicating ≤3 watery and/or loose pellets) were monitored for 60 min post i.p. injection. Each bar represents the mean ± sem of number 7-10 mice/group. * P < 0.05 and vs. vehicle/vehicle; ## P < 0.01 and ### P < 0.001 vs. ODT8-SST/vehicle.
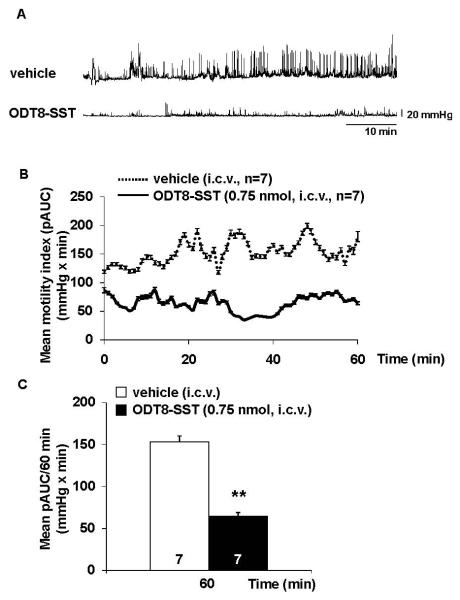
ODT8-SST injected intracerebroventricularly reduces distal colonic contractions in semi-restrained mice
Mice injected i.c.v. with vehicle and placed in the semi-restraint tube displayed high-amplitude (>25 mmHg) contractions in the distal colon throughout the 1-h observation period (Fig. 6A). The overall 60-min pAUC was 153.1 ± 7.0 mmHg × min in i.c.v. vehicle and decreased to 64.2 ± 4.6 mmHg × min in mice treated with i.c.v. ODT8-SST (P < 0.01; Fig. 6C). The time course response showed a rapid onset in the reduction of pAUC induced by i.c.v. ODT8-SST as indicated by the decrease already occurring within 4 min post injection when recording started and this was maintained throughout the 1-h recording period (Fig. 6B). Two-way ANOVA showed a significant influence of treatment (F(1,720)=133.6, P < 0.001), whereas time had no effect (F(59,720)=0.9, P > 0.05).
Figure 6.
ODT8-SST injected intracerebroventricularly reduced distal colonic contractions in mice. Mice were injected i.c.v. with ODT8-SST (0.75 nmol/mouse in 5 μl ddH2O) or vehicle (5 μl ddH2O) under short inhalation anesthesia and immediately thereafter a miniature pressure transducer catheter was inserted into the distal colon (2 cm proximal to the anus). Mice were placed in partial restraint tubes and distal colonic contraction recorded for 1 h starting ~ 4 min post i.c.v. injection. (A) shows a representative raw trace of distal colonic pressure after i.c.v. vehicle and ODT8-SST. (B) Time course (during 60 min) expressed as intraluminal pressure trace of the area under the curve (pAUC) quantified every minute. (C) mean pAUC calculated over a period of 1 h. Data are expressed as mean ± sem of 7 mice/group. ** P < 0.01 vs. vehicle.
ODT8-SST and sst1 agonist injected intracerebroventricularly prevent the delayed gastric emptying induced by acute stress of i.c.v. injection and anesthesia and abdominal surgery
Non-treated mice fasted overnight and re-fed for 1 h emptied 53.8 ± 9.1 % of their stomach content and this value was reduced to 17.8 ± 4.6 %, under conditions of i.c.v. injection of vehicle under short anesthesia as monitored 2 h after the injection (P < 0.01; Fig. 7A). ODT8-SST injected i.c.v. completely prevented the acute i.c.v. injection-induced delayed gastric emptying of a solid meal and values (64.5 ± 4.4 %, P < 0.001) were similar to those of non-treated mice (P > 0.05; Fig. 7A). Similarly, the selective peptide sst1 agonist (1.95 nmol/mouse, i.c.v.) abrogated the inhibition of gastric emptying of a standard rodent meal induced by the acute stress of i.c.v. injection under short anesthesia (60.5 ± 6.5 %, P < 0.001; Fig. 7A).
Figure 7.
ODT8-SST and the selective sst1 agonist injected intracerebroventricularly prevented the stress-related delayed gastric emptying of a solid meal and the sst1 agonist prevented the abdominal surgery-induced delayed gastric emptying of a liquid meal. (A) Mice were fasted overnight for 15 h and re-fed with pre-weighed standard chow starting at 08:00 h for 1 h. Then, food and water were removed and injected i.c.v. with ODT8-SST (0.75 nmol/mouse in 5 μl ddH2O), sst1 agonist (1.95 nmol/mouse in 5 μl ddH2O) or vehicle (5 μl ddH2O) and gastric emptying for the solid meal was assessed 2 h later. Control mice were left undisturbed without i.c.v. injection and gastric emptying was assessed at 2 h after re-feeding. Each bar represents the mean ± sem of number of mice indicated at the bottom of the columns. ** P < 0.01 vs. control; ### P < 0.001 vs. vehicle i.c.v. (B) Overnight fasted mice were injected i.c.v. with sst1 agonist (1.95 nmol/mouse in 5 μl ddH2O) or vehicle (5 μl ddH2O) and afterwards underwent abdominal surgery or sham procedure. Animals received an orogastric gavage of a liquid non-nutrient solution 30 min after the end of the procedure and gastric emptying was assessed 30 min later. Each bar represents the mean ± sem of number of mice indicated at the bottom of the columns. * P < 0.0 vs. all other groups.
Abdominal surgery reduced gastric emptying of a liquid non-nutrient solution compared to sham-treated animals (9.7 ± 4.4 vs. 33.1 ± 5.7 %, P < 0.05; Fig. 7B). Pre-treatment with the sst1 agonist (1.95 nmol/mouse, i.c.v.) completely prevented the decrease in gastric emptying (40.0 ± 8.6 %), whereas injected in animals receiving sham treatment, the sst1 agonist had no significant effect on gastric emptying (41.2 ± 4.0 %, P > 0.05; Fig. 7B).
DISCUSSION
In the present study we show that the oligosomatostatin agonist, ODT8-SST injected i.c.v. at 1 μg = 0.75 nmol/mouse counteracts both the inhibition of gastric emptying and the stimulation of propulsive colonic motor function induced by exposure to an acute stressor in mice. The peptide action is centrally mediated likely through interaction with brain CRF pathways known to be involved in such a dual response34 and can be mimicked by a sst1 agonist but not by sst2, sst4 or sst2>sst5>sst3 (octreotide) agonists suggesting a primary involvement of sst1 receptor.
Injection of vehicle i.c.v. under short inhalation anesthesia inhibits gastric emptying of a solid meal by 67% while stimulating distal propulsive colonic motor function as shown by a 99% increase in number of pellets expelled compared to non-injected mice. This reproduces a pattern of gut motor alterations similar to that commonly induced by various acute stressors.45, 46 Under these stress conditions, ODT8-SST injected i.c.v. at 0.75 nmol/mouse completely blocked both the delayed gastric emptying and the increased defecation. Convergent evidence supports that ODT8-SST acts in the brain to inhibit brain CRF-related systems involved in these gut motor alterations. First, i.c.v. injection of CRF induces a pattern of stress-like changes in gastro-colonic motor function: inhibition of gastric emptying of a solid meal while stimulating distal colonic transit and defecation in mice and rats.18, 34 (present study) In addition, previous studies established that central injection of CRF receptor antagonists alleviates various acute stressor-induced gut motor alterations in rodents indicative of a physiologically relevant role of brain CRF receptor activation.18, 34, 46 Second, we showed that ODT8-SST injected i.c.v. inhibits colonic responses in mice exposed to other acute stressors such as the defecation after water avoidance stress, high amplitude phasic contractions in the distal colon of semi-restrained mice and defecation and diarrhea induced by i.c.v. injection of CRF while not influencing defecation and diarrhea elicited by i.p. 5-HTP. We previously established that i.p. 5-HTP injected at the same dose in mice induces defecation and diarrhea through peripheral 5-HT4 mediated activation of colonic myenteric neurons.35 As the defecation and diarrhea scores elicited by i.c.v. CRF and i.p. 5-HTP were of similar magnitude and had a similar early 15 min onset, this rules out a differential effect of i.c.v. ODT8-SST linked with differential intensity/time course of colonic responses to central CRF and peripheral 5-HTP. Taken together, these data are indicative of a selective central action of i.c.v. ODT8-SST toward brain circuitries involved in the gut motor response to stress, while not interfering with a peripherally initiated stimulation of the colonic secretomotor function. In further support, previous studies showed that ODT8-SST injected i.c.v. prevents the stimulation of adrenal epinephrine secretion induced by ether anesthesia, tail suspension or cold exposure and i.c.v. injection of CRF, while not influencing the epinephrine plasma elevation related to peripheral baroreceptor-dependent mechanisms in rats.47 ODT8-SST injected i.c.v. also acts in the brain to inhibit the increase in ACTH plasma levels evoked by either hanging the rats by their tail or placing the animals over an ether-soaked cotton ball for 3 min.33 Collectively, i.c.v. ODT8-SST inhibits acute stress/CRF coordinated actions to stimulate pituitary ACTH hormone secretion33, sympathetic adrenal outflow48 and to alter gastro-colonic propulsive function (present study).
The somatostatin agonist ODT8-SST binds to the five sst receptor subtypes with nanomolar affinity (Table 1).8 To get insight into the contribution of specific receptor subtypes in the central inhibitory effect of ODT8-SST, we used available selective sst peptide agonists. The potent selective sst1 agonist (IC50 0.19 ± 0.04 nM)36 injected i.c.v. also prevented the increased defecation and delayed gastric emptying resulting from the acute stress of i.c.v. vehicle injection and anesthesia. Similarly, the sst1 agonist prevented the abdominal-surgery-induced delay of gastric emptying as assessed by liquid gastric emptying. However, the sst1 agonist CH-275 injected i.c.v. did not significantly alter fecal pellet output under the present experimental conditions which is likely due to the 150-fold lower affinity of CH-275 to the sst1 (IC50: 30.9 ± 13 nM)49 compared to the sst1 agonist described above. In addition, peptides lacking affinity to the sst1 receptor including octreotide (IC50: sst1 < 1000 nM)50, the selective sst2 (IC50: sst2 0.75 ± 0.2; sst1 > 1000; nM)37 or sst4 agonist (IC50: sst4 = 1.4 ± 0.1; sst1 650 ± 115 nM)38 tested under similar conditions had no effect on defecation. The role of sst2 which is the most prominent receptor subtype expressed in the mouse brain51 was further ruled out by the demonstration that the selective sst2 antagonist39 did not modify the i.c.v. ODT8-SST inhibitory effect on acute stress-related defecation when injected i.c.v. at a dose preventing the orexigenic effect of ODT8-SST monitored simultaneously. Moreover, the selective sst2 agonist has an opposite effect by further increasing the number of excreted fecal pellets during water avoidance stress after 30 min of stress exposure. With regard to the endogenous ligands, SRIF-28 injected i.c.v. also reduced defecation while i.c.v. SRIF-14 injected at a similar nanomolar dose as SRIF-28 had no effect. Likewise, other studies showed that several central actions displayed by SRIF-28 and ODT8-SST to influence glucoregulation, thermoregulation, ACTH and epinephrine plasma levels as well as gastric secretory and motor functions are not reproduced by i.c.v. or i.c. injection of SRIF-14 in rats.9, 10, 33, 52 In vitro studies also showed that SRIF-14 and SRIF-28 induce opposite effects on potassium currents in rat neocortical neurons.53 The differential central actions of SRIF-28 and SRIF-14 are so far little understood. In the present study, it may be speculated that in addition to the primary effect mediated by the activation of the brain sst1 receptor, the sst5 receptor also contributes since SRIF-28 displays a 100-fold selectivity on the mouse sst5 receptors compared to SRIF-1454 and in recombinant systems, sst1 and sst5 receptors may form heterodimers which confers distinct signaling properties.55 In the present study, we observed that ODT8-SST injected i.c.v. at 0.22 or 0.75 nmol/mouse reduces the 1-h fecal pellet output by 41% and 74% respectively and the sst1 agonist by 0% and 41% respectively versus i.c.v. vehicle. Therefore, possible additional contributions of sst5 receptors in the central effects of SRIF-28 and ODT8-SST will need to be further characterized using selective peptide sst5 agonists and antagonists which so far have not been developed. Irrespectively of the potential additional involvement of sst5, the demonstration that the selective sst1 agonist mimics the ODT8-SST-induced blockade of both gastric and colonic motor alterations induced by acute stressors provides the first pharmacological evidence that central activation of sst1 receptors prevents acute stress-related gut motor functions in mice.
The brain sites involved in the ODT8-SST and sst1 agonist actions to prevent the acute stress-induced delay of gastric emptying and the stimulation of propulsive distal colonic motility may involve those regulating CRF release and downstream CRF action since i.c.v. injection of ODT8-SST counteracts both, acute stress- and i.c.v. CRF-induced stimulatory effects on colonic propulsive motor function. Previous studies indicate that the PVN is responsive to exogenous CRF and involved in acute restraint stress-induced inhibition of gastric emptying and stimulation of colonic motor function in rats.56 A number of investigations using autoradiography, in situ hybridization and immunohistochemistry have established the regional distribution of the sst1 receptor in the mouse brain including the PVN and in addition the hippocampus, central amygdala, median raphe nucleus, magnocellular preoptic nucleus, arcuate nucleus and cortical areas31, 57 indicative of other possible sites of action as part of stress-related circuitries at which the peptides may act.58 At the cellular level, sst1 receptor activation unlike that of other sst receptor subtypes has been reported to reduce hippocampal excitability.59 It may be speculated that activation of sst1 receptors dampens the stress-related increase in neuronal activity well established to occur in specific brain nuclei in response to stress.60 In that context, it will be of relevance to establish whether other previously established central effects of i.c.v. injected ODT8-SST or SRIF-28, to block the acute stress-related rise in circulating ACTH and epinephrine plasma levels in rats, are also mimicked and primarily mediated by the activation of sst1 receptors. It was recently shown that central oxytocin signaling counteracts the water avoidance stress stimulation of defecation as well as distal colonic motility in rats.25 Oxytocin injected i.c.v. decreased the number of CRF immunoreactive cells in the PVN and inhibited the stimulated corticosterone release25 giving rise to a negative interaction with the central CRF signaling system. Similarly, oxytocin injected i.c.v. restored the delayed gastric emptying following acute restraint or a chronic heterotypic stress in rats.61 In addition, the acute and chronic stress-induced increased CRF mRNA levels were reduced by i.c.v. injection of oxytocin.61 Therefore, one may also speculate that ODT8-SST recruits oxytocin pathways to exert its effects on stress-induced altered gastric and colonic motility. This assumption is further supported by our previous findings in rats showing that ODT8-SST injected i.c.v. activates a large proportion of oxytocin positive neurons in the supraoptic nucleus as well as magnocellular PVN.62
The time course study of the central action of ODT8-SST on the colon showed that the rapid onset inhibition occurring during the first hour is followed by an increase in fecal pellet output. Convergent evidence supports that the additional stimulatory effect on defecation compared to that of i.c.v. vehicle injection is secondary to the sst2 mediated concurrent increase in food intake. We showed that ODT8-SST significantly increases food consumption by 3.5- and 1.7-times during the 2nd hour and 2-4 h period post i.c.v. injection respectively consistent with our previous report in rats and mice.11 Moreover, the sst2 antagonist injected i.c.v. completely blocked the ODT8-SST-induced stimulation of food intake and fecal pellet output occurring at 2-h post injection while not altering the reduction of defecation during the 1st h. Lastly, when mice did not have access to food after the i.c.v. injection of ODT8-SST, there was no enhanced defecation at 2-4 h while the inhibitory effect during the 1st hour was maintained. These data are consistent with the previously established sst2 mediated long-lasting orexigenic effect of i.c.v. ODT8-SST in freely fed rodents.11, 13 In particular, the activation of brain sst2 receptors was characterized to increase the number of meals, the rate of ingestion and reduce the inter-meal intervals in mice.13 The sst2 mediated stimulation of defecation is secondary to the orexigenic effect most likely related to increased distal colonic motility associated with a meal as reported in other species.63
In conclusion, ODT8-SST and SRIF-28, which interact with the 5 sst receptor subtypes, when injected i.c.v. counteract the colonic stimulation and gastric motor inhibition in response to acute stressors. The peptide’s anti-stress action is mimicked mainly by the activation of the sst1 receptor suggesting an important role for this brain sst receptor subtype in the modulation of the gastrointestinal response to activation of brain CRF pathways. As a number of peptides including NPY, cocaine amphetamine-regulated transcript, glucagon-like peptide and interleukin-1 act in the brain to stimulate colonic motor function through activation of CRF pathways,20, 64-66 these data suggest that SRIF-28 and activation of sst1 receptors may have the potential to interfere with a number of peptides recruiting brain CRF signaling to exert their stimulatory action on the colon. Of importance, the present data point toward a potential role of brain SRIF-28 as an endogenous modulator of stress-related circuitries regulating gastrointestinal motor functions.
ACKNOWLEDGEMENTS
This work was supported by Veterans Administration Research Career Scientist Award, VA Merit Award, Center Grant NIH DK-41301 (Animal Core) and R01 NIH DK 33061 and 57238 (Y.T). J. R. is the Dr. Frederik Paulsen Chair in Neurosciences Professor. We are grateful to Mrs. Honghui Liang for the excellent technical support and Ms. Eugenia Hu for proof reading the manuscript.
Footnotes
DISCLOSURE A.S., M.G.-S., L.W., M.L. and Y.T. have nothing to disclose. J.R. is Founder of Sentia Medical Sciences, Inc. No conflicts of interest exist.
AUTHOR CONTRIBUTIONS A.S. planned and performed the experiments, analyzed the data and wrote the manuscript; M.G.-S., M.L. and L.W. performed components of experiments and reviewed the paper; J.R. provided the sst agonists and sst2 antagonist and reviewed the manuscript; Y.T. planned the experiments, gave critical input throughout the study and in the writing of the manuscript.
REFERENCES
- 1.Brazeau P, Vale W, Burgus R, et al. Hypothalamic polypeptide that inhibits the secretion of immunoreactive pituitary growth hormone. Science. 1973;179:77–79. doi: 10.1126/science.179.4068.77. [DOI] [PubMed] [Google Scholar]
- 2.Bohlen P, Brazeau P, Esch F, Ling N, Guillemin R. Isolation and chemical characterization of somatostatin-28 from rat hypothalamus. Regul Pept. 1981;2:359–369. doi: 10.1016/0167-0115(81)90018-5. [DOI] [PubMed] [Google Scholar]
- 3.Brown M, Taché Y, Rivier J, Pittman Q. Peptides and regulation of body temperature. Adv Biochem Psychopharmacol. 1981;28:397–407. [PubMed] [Google Scholar]
- 4.Guillemin R. Hypothalamic hormones a.k.a. hypothalamic releasing factors. J Endocrinol. 2005;184:11–28. doi: 10.1677/joe.1.05883. [DOI] [PubMed] [Google Scholar]
- 5.Viollet C, Lepousez G, Loudes C, Videau C, Simon A, Epelbaum J. Somatostatinergic systems in brain: networks and functions. Mol Cell Endocrinol. 2008;286:75–87. doi: 10.1016/j.mce.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 6.Olias G, Viollet C, Kusserow H, Epelbaum J, Meyerhof W. Regulation and function of somatostatin receptors. J Neurochem. 2004;89:1057–1091. doi: 10.1111/j.1471-4159.2004.02402.x. [DOI] [PubMed] [Google Scholar]
- 7.Barnes AJ, Long RG, Adrian TE, et al. Effect of a long-acting octapeptide analogue of somatostatin on growth hormone and pancreatic and gastrointestinal hormones in man. Clin Sci (Lond) 1981;61:653–656. doi: 10.1042/cs0610653. [DOI] [PubMed] [Google Scholar]
- 8.Erchegyi J, Grace CR, Samant M, et al. Ring size of somatostatin analogues (ODT-8) modulates receptor selectivity and binding affinity. J Med Chem. 2008;51:2668–2675. doi: 10.1021/jm701444y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taché Y, Rivier J, Vale W, Brown M. Is somatostatin or a somatostatin-like peptide involved in central nervous system control of gastric secretion? Regul Pept. 1981;1:307–315. doi: 10.1016/0167-0115(81)90054-9. [DOI] [PubMed] [Google Scholar]
- 10.Martinez V, Rivier J, Coy D, Taché Y. Intracisternal injection of somatostatin receptor 5-preferring agonists induces a vagal cholinergic stimulation of gastric emptying in rats. J Pharmacol Exp Ther. 2000;293:1099–1105. [PubMed] [Google Scholar]
- 11.Stengel A, Coskun T, Goebel M, et al. Central injection of the stable somatostatin analog, ODT8-SST induces a somatostatin2 receptor mediated orexigenic effect: role of neuropeptide Y and opioid signaling pathways in rats. Endocrinology. 2010;150:4224–4235. doi: 10.1210/en.2010-0195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stengel A, Goebel M, Wang L, et al. Selective central activation of somatostatin2 receptor increases food intake, grooming behavior and rectal temperature in rats. J Physiol Pharmacol. 2010;61:399–407. [PMC free article] [PubMed] [Google Scholar]
- 13.Stengel A, Goebel M, Wang L, et al. Activation of brain somatostatin(2) receptors stimulates feeding in mice: Analysis of food intake microstructure. Physiol Behav. 2010;101:614–622. doi: 10.1016/j.physbeh.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zorrilla EP, Taché Y, Koob GF. Nibbling at CRF receptor control of feeding and gastrocolonic motility. Trends Pharmacol Sci. 2003;24:421–427. doi: 10.1016/S0165-6147(03)00177-9. [DOI] [PubMed] [Google Scholar]
- 15.Stengel A, Goebel M, Wang L, Taché Y. Ghrelin, des-acyl ghrelin and nesfatin-1 in gastric X/A-like cells: role as regulators of food intake and body weight. Peptides. 2010;31:357–369. doi: 10.1016/j.peptides.2009.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stengel A, Taché Y. The physiological relationships between the brainstem, vagal stimulation and feeding. In: Preedy VR, editor. The International Handbook of Behavior, Diet and Nutrition. Springer; London: 2009. [Google Scholar]
- 17.Ao Y, Go VL, Toy N, et al. Brainstem thyrotropin-releasing hormone regulates food intake through vagal-dependent cholinergic stimulation of ghrelin secretion. Endocrinology. 2006;147:6004–6010. doi: 10.1210/en.2006-0820. [DOI] [PubMed] [Google Scholar]
- 18.Martinez V, Wang L, Rivier J, Grigoriadis D, Taché Y. Central CRF, urocortins and stress increase colonic transit via CRF1 receptors while activation of CRF2 receptors delays gastric transit in mice. J Physiol. 2004;556:221–234. doi: 10.1113/jphysiol.2003.059659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang L, Gourcerol G, Yuan PQ, et al. Peripheral peptide YY inhibits propulsive colonic motor function through Y2 receptor in conscious mice. Am J Physiol Gastrointest Liver Physiol. 2010;298:G45–56. doi: 10.1152/ajpgi.00349.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mönnikes H, Tebbe J, Bauer C, Grote C, Arnold R. Neuropeptide Y in the paraventricular nucleus of the hypothalamus stimulates colonic transit by peripheral cholinergic and central CRF pathways. Neurogastroenterol Motil. 2000;12:343–352. doi: 10.1046/j.1365-2982.2000.00212.x. [DOI] [PubMed] [Google Scholar]
- 21.Smith JR, La Hann TR, Chesnut RM, Carino MA, Horita A. Thyrotropin-releasing hormone: stimulation of colonic activity following intracerebroventricular administration. Science. 1977;196:660–662. doi: 10.1126/science.404705. [DOI] [PubMed] [Google Scholar]
- 22.Tebbe JJ, Mronga S, Tebbe CG, Ortmann E, Arnold R, Schafer MK. Ghrelin-induced stimulation of colonic propulsion is dependent on hypothalamic neuropeptide Y1- and corticotrophin-releasing factor 1 receptor activation. J Neuroendocrinol. 2005;17:570–576. doi: 10.1111/j.1365-2826.2005.01340.x. [DOI] [PubMed] [Google Scholar]
- 23.Bueno L, Fioramonti J, Honde C, Fargeas MJ, Primi MP. Central and peripheral control of gastrointestinal and colonic motility by endogenous opiates in conscious dogs. Gastroenterology. 1985;88:549–556. doi: 10.1016/0016-5085(85)90520-7. [DOI] [PubMed] [Google Scholar]
- 24.Pinto L, Izzo AA, Cascio MG, et al. Endocannabinoids as physiological regulators of colonic propulsion in mice. Gastroenterology. 2002;123:227–234. doi: 10.1053/gast.2002.34242. [DOI] [PubMed] [Google Scholar]
- 25.Matsunaga M, Konagaya T, Nogimori T, et al. Inhibitory effect of oxytocin on accelerated colonic motility induced by water-avoidance stress in rats. Neurogastroenterol Motil. 2009;21:856–e859. doi: 10.1111/j.1365-2982.2009.01286.x. [DOI] [PubMed] [Google Scholar]
- 26.Han RW, Chang M, Peng YL, et al. Central Neuropeptide S inhibits distal colonic transit through activation of central Neuropeptide S receptor in mice. Peptides. 2009;30:1313–1317. doi: 10.1016/j.peptides.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 27.Mönnikes H, Raybould HE, Schmidt B, Taché Y. CRF in the paraventricular nucleus of the hypothalamus stimulates colonic motor activity in fasted rats. Peptides. 1993;14:743–747. doi: 10.1016/0196-9781(93)90107-r. [DOI] [PubMed] [Google Scholar]
- 28.Mönnikes H, Schmidt BG, Tebbe J, Bauer C, Taché Y. Microinfusion of corticotropin releasing factor into the locus coeruleus/subcoeruleus nuclei stimulates colonic motor function in rats. Brain Res. 1994;644:101–108. doi: 10.1016/0006-8993(94)90352-2. [DOI] [PubMed] [Google Scholar]
- 29.Tebbe JJ, Pasat IR, Mönnikes H, Ritter M, Kobelt P, Schafer MK. Excitatory stimulation of neurons in the arcuate nucleus initiates central CRF-dependent stimulation of colonic propulsion in rats. Brain Res. 2005;1036:130–138. doi: 10.1016/j.brainres.2004.12.034. [DOI] [PubMed] [Google Scholar]
- 30.Epelbaum J. Somatostatin in the central nervous system: physiology and pathological modifications. Prog Neurobiol. 1986;27:63–100. doi: 10.1016/0301-0082(86)90012-2. [DOI] [PubMed] [Google Scholar]
- 31.Fehlmann D, Langenegger D, Schuepbach E, Siehler S, Feuerbach D, Hoyer D. Distribution and characterisation of somatostatin receptor mRNA and binding sites in the brain and periphery. J Physiol Paris. 2000;94:265–281. doi: 10.1016/s0928-4257(00)00208-4. [DOI] [PubMed] [Google Scholar]
- 32.Schulz S, Handel M, Schreff M, Schmidt H, Hollt V. Localization of five somatostatin receptors in the rat central nervous system using subtype-specific antibodies. J Physiol Paris. 2000;94:259–264. doi: 10.1016/s0928-4257(00)00212-6. [DOI] [PubMed] [Google Scholar]
- 33.Brown MR, Rivier C, Vale W. Central nervous system regulation of adrenocorticotropin secretion: role of somatostatins. Endocrinology. 1984;114:1546–1549. doi: 10.1210/endo-114-5-1546. [DOI] [PubMed] [Google Scholar]
- 34.Stengel A, Taché Y. Neuroendocrine Control of the Gut During Stress: Corticotropin-Releasing Factor Signaling Pathways in the Spotlight. Annu Rev Physiol. 2009;71:219–239. doi: 10.1146/annurev.physiol.010908.163221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang L, Martinez V, Kimura H, Taché Y. 5-Hydroxytryptophan activates colonic myenteric neurons and propulsive motor function through 5-HT4 receptors in conscious mice. Am J Physiol Gastrointest Liver Physiol. 2007;292:G419–428. doi: 10.1152/ajpgi.00289.2006. [DOI] [PubMed] [Google Scholar]
- 36.Erchegyi J, Cescato R, Grace CR, et al. Novel, potent, and radio-iodinatable somatostatin receptor 1 (sst1) selective analogues. J Med Chem. 2009;52:2733–2746. doi: 10.1021/jm801314f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grace CR, Erchegyi J, Koerber SC, Reubi JC, Rivier J, Riek R. Novel sst2-selective somatostatin agonists. Three-dimensional consensus structure by NMR. J Med Chem. 2006;49:4487–4496. doi: 10.1021/jm060363v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Erchegyi J, Waser B, Schaer JC, et al. Novel sst(4)-selective somatostatin (SRIF) agonists. 3. Analogues amenable to radiolabeling. J Med Chem. 2003;46:5597–5605. doi: 10.1021/jm030245x. [DOI] [PubMed] [Google Scholar]
- 39.Cescato R, Erchegyi J, Waser B, et al. Design and in vitro characterization of highly sst2-selective somatostatin antagonists suitable for radiotargeting. J Med Chem. 2008;51:4030–4037. doi: 10.1021/jm701618q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Luckey A, Wang L, Jamieson PM, et al. Corticotropin-releasing factor receptor 1-deficient mice do not develop postoperative gastric ileus. Gastroenterology. 2003;125:654–659. doi: 10.1016/s0016-5085(03)01069-2. [DOI] [PubMed] [Google Scholar]
- 41.Bauer W, Briner U, Doepfner W, et al. SMS 201-995: a very potent and selective octapeptide analogue of somatostatin with prolonged action. Life Sci. 1982;31:1133–1140. doi: 10.1016/0024-3205(82)90087-x. [DOI] [PubMed] [Google Scholar]
- 42.Pelleymounter MA, Joppa M, Carmouche M, et al. Role of corticotropin-releasing factor (CRF) receptors in the anorexic syndrome induced by CRF. J Pharmacol Exp Ther. 2000;293:799–806. [PubMed] [Google Scholar]
- 43.Larauche M, Gourcerol G, Million M, Adelson DW, Taché Y. Repeated psychological stress-induced alterations of visceral sensitivity and colonic motor functions in mice: influence of surgery and postoperative single housing on visceromotor responses. Stress. 2010;13:343–354. doi: 10.3109/10253891003664166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gourcerol G, Wang L, Adelson DW, Larauche M, Taché Y, Million M. Cholinergic giant migrating contractions in conscious mouse colon assessed by using a novel noninvasive solid-state manometry method: modulation by stressors. Am J Physiol Gastrointest Liver Physiol. 2009;296:G992–G1002. doi: 10.1152/ajpgi.90436.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mönnikes H, Tebbe JJ, Hildebrandt M, et al. Role of stress in functional gastrointestinal disorders. Evidence for stress-induced alterations in gastrointestinal motility and sensitivity. Dig Dis. 2001;19:201–211. doi: 10.1159/000050681. [DOI] [PubMed] [Google Scholar]
- 46.Taché Y, Bonaz B. Corticotropin-releasing factor receptors and stress-related alterations of gut motor function. J Clin Invest. 2007;117:33–40. doi: 10.1172/JCI30085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brown MR, Fisher LA. Brain peptide regulation of adrenal epinephrine secretion. Am J Physiol. 1984;247:E41–46. doi: 10.1152/ajpendo.1984.247.1.E41. [DOI] [PubMed] [Google Scholar]
- 48.Somiya H, Tonoue T. Neuropeptides as central integrators of autonomic nerve activity: effects of TRH, SRIF, VIP and bombesin on gastric and adrenal nerves. Regul Pept. 1984;9:47–52. doi: 10.1016/0167-0115(84)90006-5. [DOI] [PubMed] [Google Scholar]
- 49.Rivier JE, Hoeger C, Erchegyi J, et al. Potent somatostatin undecapeptide agonists selective for somatostatin receptor 1 (sst1) J Med Chem. 2001;44:2238–2246. doi: 10.1021/jm010037+. [DOI] [PubMed] [Google Scholar]
- 50.Grace CR, Erchegyi J, Samant M, et al. Ring size in octreotide amide modulates differently agonist versus antagonist binding affinity and selectivity. J Med Chem. 2008;51:2676–2681. doi: 10.1021/jm701445q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Videau C, Hochgeschwender U, Kreienkamp HJ, et al. Characterisation of [125I]-Tyr0DTrp8-somatostatin binding in sst1- to sst4- and SRIF-gene-invalidated mouse brain. Naunyn Schmiedebergs Arch Pharmacol. 2003;367:562–571. doi: 10.1007/s00210-003-0758-8. [DOI] [PubMed] [Google Scholar]
- 52.Brown M, Ling N, Rivier J. Somatostatin-28, somatostatin-14 and somatostatin analogs: effects on thermoregulation. Brain Res. 1981;214:127–135. doi: 10.1016/0006-8993(81)90443-1. [DOI] [PubMed] [Google Scholar]
- 53.Wang HL, Bogen C, Reisine T, Dichter M. Somatostatin-14 and somatostatin-28 induce opposite effects on potassium currents in rat neocortical neurons. Proc Natl Acad Sci U S A. 1989;86:9616–9620. doi: 10.1073/pnas.86.23.9616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Feuerbach D, Fehlmann D, Nunn C, et al. Cloning, expression and pharmacological characterisation of the mouse somatostatin sst(5) receptor. Neuropharmacology. 2000;39:1451–1462. doi: 10.1016/s0028-3908(00)00063-0. [DOI] [PubMed] [Google Scholar]
- 55.Rocheville M, Lange DC, Kumar U, Sasi R, Patel RC, Patel YC. Subtypes of the somatostatin receptor assemble as functional homo- and heterodimers. J Biol Chem. 2000;275:7862–7869. doi: 10.1074/jbc.275.11.7862. [DOI] [PubMed] [Google Scholar]
- 56.Mönnikes H, Schmidt BG, Raybould HE, Taché Y. CRF in the paraventricular nucleus mediates gastric and colonic motor response to restraint stress. Am J Physiol. 1992;262:G137–143. doi: 10.1152/ajpgi.1992.262.1.G137. [DOI] [PubMed] [Google Scholar]
- 57.Hannon JP, Petrucci C, Fehlmann D, Viollet C, Epelbaum J, Hoyer D. Somatostatin sst2 receptor knock-out mice: localisation of sst1-5 receptor mRNA and binding in mouse brain by semi-quantitative RT-PCR, in situ hybridisation histochemistry and receptor autoradiography. Neuropharmacology. 2002;42:396–413. doi: 10.1016/s0028-3908(01)00186-1. [DOI] [PubMed] [Google Scholar]
- 58.Joels M, Baram TZ. The neuro-symphony of stress. Nat Rev Neurosci. 2009;10:459–466. doi: 10.1038/nrn2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cammalleri M, Cervia D, Langenegger D, et al. Somatostatin receptors differentially affect spontaneous epileptiform activity in mouse hippocampal slices. Eur J Neurosci. 2004;20:2711–2721. doi: 10.1111/j.1460-9568.2004.03741.x. [DOI] [PubMed] [Google Scholar]
- 60.Senba E, Ueyama T. Stress-induced expression of immediate early genes in the brain and peripheral organs of the rat. Neurosci Res. 1997;29:183–207. doi: 10.1016/s0168-0102(97)00095-3. [DOI] [PubMed] [Google Scholar]
- 61.Zheng J, Babygirija R, Bulbul M, Cerjak D, Ludwig K, Takahashi T. Hypothalamic oxytocin mediates adaptation mechanism against chronic stress in rats. Am J Physiol Gastrointest Liver Physiol. 2010;299:G946–953. doi: 10.1152/ajpgi.00483.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Goebel M, Stengel A, Wang L, Coskun T, Rivier J, Taché Y. Pattern of Fos expression in the brain induced by selective activation of somatostatin receptor 2 in rats. Brain Res. 2010;1351:150–164. doi: 10.1016/j.brainres.2010.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sarna SK, Lang IM. Colonic motor response to a meal in dogs. Am J Physiol. 1989;257:G830–835. doi: 10.1152/ajpgi.1989.257.5.G830. [DOI] [PubMed] [Google Scholar]
- 64.Tebbe JJ, Ortmann E, Schumacher K, et al. Cocaine- and amphetamine-regulated transcript stimulates colonic motility via central CRF receptor activation and peripheral cholinergic pathways in fed, conscious rats. Neurogastroenterol Motil. 2004;16:489–496. doi: 10.1111/j.1365-2982.2004.00561.x. [DOI] [PubMed] [Google Scholar]
- 65.Nakade Y, Tsukamoto K, Iwa M, Pappas TN, Takahashi T. Glucagon like peptide-1 accelerates colonic transit via central CRF and peripheral vagal pathways in conscious rats. Auton Neurosci. 2007;131:50–56. doi: 10.1016/j.autneu.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 66.Fargeas MJ, Fioramonti J, Bueno L. Central action of interleukin 1 beta on intestinal motility in rats: mediation by two mechanisms. Gastroenterology. 1993;104:377–383. doi: 10.1016/0016-5085(93)90404-z. [DOI] [PubMed] [Google Scholar]
- 67.Viollet C, Prevost G, Maubert E, et al. Molecular pharmacology of somatostatin receptors. Fundam Clin Pharmacol. 1995;9:107–113. doi: 10.1111/j.1472-8206.1995.tb00269.x. [DOI] [PubMed] [Google Scholar]