Abstract
The objectives of the present study were to determine 1) if temporal variability influenced the toxicity of Elkhorn River water and 2) if the toxic effect was consistent between two sentinel organisms, the fathead minnow (Pimephales promelas) and the northern leopard frog (Rana pipiens). During spring 2012, atrazine indicator strips were used to document the occurrence of agrichemical pulses in the Elkhorn River. Polar organic chemical integrative samplers (POCIS) were deployed for 14 d during both a pulse and post-pulse period as indicated by the atrazine strips. Pesticide concentrations detected in the POCIS extracts ranged from 1.6 to 281 fold higher during the pulse period compared to the post-pulse period. Fish and frog bioassays were conducted for 7 d, and hepatic mRNA expression of vitellogenin (Vtg) and estrogen receptor-α (ERα) was determined by quantitative real-time PCR (RT-qPCR). Compared to lab water controls, fish exposed to water collected during an agrichemical pulse experienced significant reductions in Vtg and ERα, whereas exposed female frogs did not. Male leopard frogs, in contrast experienced significant increases in the expression of ERα, whereas pulse exposed male minnows did not. The significant effects observed following agrichemical pulse exposure demonstrate 1) that episodic agrichemical runoff adversely impacts sentinel organisms, and 2) that the adverse impacts observed depends upon the sex and species of the sentinel organism.
Keywords: Agricultural runoff, POCIS, Northern leopard frog, Fathead minnow, Gene expression
INTRODUCTION
Agricultural watersheds experience episodic runoff events during which aquatic organisms may be exposed to a variety of agrichemicals [1-4]. The Elkhorn River (Nebraska, USA), is one such agriculturally dominated watershed in which spring snowmelt and heavy rainfall can result in the transport of agrichemicals from row crop landscapes to local waterways [5-8]. These transported agrichemicals can act as a source of endocrine active compounds to watersheds such as the Elkhorn River, and exposure to such compounds has been shown to result in significant impairments in endocrine function [3, 8-12]. For example, deployment of female fathead minnows (Pimephales promelas) in the Elkhorn River for 7 d has been shown to result in significant reductions in the mRNA expression of the estrogen responsive genes, vitellogenin (Vtg) and estrogen receptor-α (ERα) [8]. While exposure to Elkhorn River water has been associated with such defeminizing effects [8, 11], these effects are not consistent across location or year, possibly due to the episodic nature of the runoff events and subsequent transport of endocrine active compounds into the watershed [13].
Until recently, the immediate validation of agrichemicals in runoff was difficult as validation often involved costly and time-consuming laboratory confirmation of chemical concentrations. However, the present study features the use of a rapid assessment atrazine strip assay that has become commercially available within the last few years [14]. Atrazine is a widely used row crop herbicide shown to enter surface waters following seasonal application [6, 15, 16], and preliminary testing in 2011 using the atrazine strips confirmed the occurrence of a late spring pulse of atrazine in the Elkhorn River. Atrazine has been regarded as an ideal surrogate pesticide due to its widespread agricultural use and minimal loss during transport [7, 15, 17]. Therefore, in the present study, the atrazine pulse was used as a surrogate indicator of agrichemical runoff to define when water sampling of river water should occur.
Historically, fathead minnows have been used as the environmental sentinel organism to determine the impact of agrichemical runoff in the Elkhorn River watershed [3, 8, 11-13]. Amphibians, however, may also be exposed to agrichemical runoff as agricultural ponds have been shown to provide breeding grounds that actively support amphibian populations [18, 19]. This would suggest that amphibians are also important environmental sentinel organisms in the Midwestern US particularly given their purported sensitivity to atrazine [20-23]. Compared to other classes of aquatic organisms, amphibians have been largely overlooked in aquatic toxicology. As such, it is not surprising that little research has been done to directly compare the differential sensitivity of fish and frog sentinels to the same contaminated water [24, 25].
This study had two primary objectives; the first was to determine if timing influenced the toxicity of waters collected from the Elkhorn River. Specifically, atrazine strip tests were used to focus chemical sampling and biological assessments on periods with dramatic differences in agrichemical concentrations. The second objective was to determine if the biological effects of exposure to Elkhorn River waters would be consistent between two sentinel organisms: the fathead minnow (Pimephales promelas) and the northern leopard frog (Rana pipiens). Specifically, effects of exposure in sentinel organisms were determined by the relative mRNA expression of the two estrogen responsive genes, Vtg and ERα.
METHODS
Atrazine as a surrogate chemical for agrichemical runoff
Atrazine was chosen as a chemical surrogate for the presence of agrichemicals due to the commercial availability of a low cost, rapid assessment indicator strip (Abraxis). The strips provide ordinal (presence, absence) data for atrazine at the US EPA drinking water standard of 3 ppb. Preliminary unpublished data using citizen scientists, as well as college students from the College of St. Mary and the University of Nebraska Omaha laboratory testing samples spiked with atrazine support the contention that the rate of false positives and false negatives associated with strip use are less than 2% in both cases.
Field Location and timing of water and POCIS collections
The water collected for use in the present study was taken directly from the Elkhorn River at the Elkhorn River Research Station; a site located approximately 10 km from the confluence of the Elkhorn and Platte Rivers. In 2011, weekly sampling with atrazine strips detected a three week long pulse of atrazine that began in mid-May and ended in early June. It was anticipated that a pulse of atrazine would be detectable in 2012 that would be consistent with what was observed in 2011.
Atrazine strip testing of the Elkhorn River watershed was used to determine the timing of POCIS deployment and water collection for laboratory bioassessment (Fig. 1). Strip testing began on March 31, 2012 and was repeated every 3 d until the first recorded positive atrazine test result on May 4. Two consecutive positive strip results (May 4 and May 5) were taken to indicate the beginning of the 2012 atrazine pulse, and the beginning of the sampling regime. Polar organic chemical integrative samplers (POCIS) were deployed on May 5, with retrieval of the POCIS occurring 14 d later on May 19. Water was collected for animal bioassays from May 7 to May 13, and atrazine strip tests were performed daily throughout the week. From May 13 to July 14, interval strip testing was again conducted every 3 d. The last positive atrazine test strip was recorded on June 3. POCIS were deployed during a post-pulse period starting on June 30, with retrieval of the POCIS occurring 14 d later on July 14. Water was collected for animal bioassays from July 2 to July 8, and again atrazine test strip data were performed daily throughout the collection period.
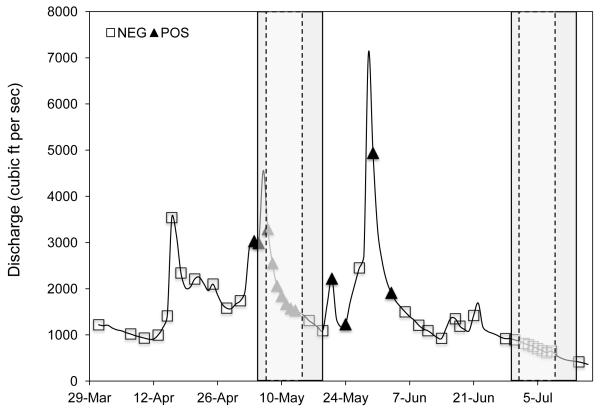
Figure 1.
The temporal occurrence of atrazine was determined using atrazine test strips. Elkhorn River hydrograph data was used to highlight the use of atrazine as a surrogate to predict the occurrence of agrichemical runoff. Positive atrazine test strip results are shown as closed triangles while negative test results are represented as open boxes. The pulse and post-pulse POCIS deployment periods are highlight by the dark grey bars. The 7 d exposure periods during which water was collected from the Elkhorn River are denoted by the light grey bars within the dashed lines.
POCIS
POCIS were obtained from Environmental Sampling Technologies and were stored at −20 °C until their field deployment. After the 14 d deployments, POCIS were removed from their deployment canisters, rinsed briefly, and stored at −20 °C until analysis. The POCIS were analyzed by the University of Nebraska Water Sciences Laboratory for 17 steroids and 21 pesticides (Supplemental Data, Table S1) following previously described protocols [3, 26]. Steroid concentrations were determined by LC/MS/MS as outlined in Bartelt-Hunt et al. [27] and Snow et al. [28], while pesticide concentrations were determined using GC/MS as described in Cassada et al. [29] and Sellin et al. [8]. The instrumental method detection limits for steroid hormones and pesticides analyzed were estimated at 0.5 ng. Recovery of analytes in laboratory fortified blanks analyzed at the same time as the POCIS extracts averaged 71 ±11% for steroid hormones and 102±19% in the pesticide method. No analytes were detected in laboratory reagent blanks analyzed at the same time as the extracts. Amounts of pesticides and steroids in POCIS extracts were quantified as ng per extract (ng/POCIS). The mass of detected pesticides and steroid hormones in ng/POCIS were then converted to a time weighted average (TWA) concentration (ng/L) using previously determined sampling rate coefficients [27, 30]. The TWA concentration was determined by dividing the mass (ng/POCIS) by the sampling coefficient (L/d) and the total number of days deployed (d).
Sentinel organisms
All procedures involving experimental animals adhered to the University of Nebraska and Institutional Animal Care and Use Committee (IACUC) guidelines. All animals were maintained on a 16:8 light dark cycle at ambient room temperature (20±1°C) throughout all experimental procedures. Laboratory water to which fish and frogs were exposed prior to the start of experiments consisted of de-chlorinated Omaha tap water.
Fathead minnows
Sexually mature male and female fathead minnows were obtained from the University of Nebraska at Omaha colony as outlined by previously published protocols [31]. Males and females were kept in separate 45 L aerated glass tanks containing 10 fish each, and were maintained in laboratory water until the start of the experiment. Fish were maintained at ambient room temperature (20±1°C) for at least one week prior to the beginning of the exposures. Fish were fed TetraMin flake food (Aquatic EcoSystems) daily prior to and during the field water exposures. Daily one-third static renewal water changes were performed prior to and throughout the duration of experiments. At the start of each respective experiment, fish were transferred to exposure tanks containing either Elkhorn River water or laboratory water.
Northern leopard frogs
Sexually mature male and female northern leopard frogs were obtained from a commercial supplier (Connecticut Valley Biological) 4 d prior to the start of the exposures. Frogs were segregated by sex and then randomly assigned to exposure units where they were maintained on de-chlorinated water until the start of the experiment. Exposure units consisted of 63 L food storage tubs (Rubbermaid). Exposure units were set at approximately a 30° incline which allowed the static water to pool and to provide frogs with access to water and dry land. Fifteen cm PVC tubing cut in half provided refuge for frogs. Tanks contained 7 L of static water at room temperature (20±1°C). Frogs were fed mealworms and crickets every other day. Complete water changes were performed in both the exposed and unexposed treatments daily. Unexposed frogs received laboratory water only, while exposed frogs received daily water changes from Elkhorn River water.
Elkhorn River agrichemical exposures
The 7 d exposures were conducted on male and female fish and frogs in separate exposure tanks segregated by sex. Unexposed animals were maintained on de-chlorinated laboratory water. Prior to water changes, laboratory water was stored in polyethylene water containers. For the Elkhorn River exposed treatment groups, water was bucketed daily from the Elkhorn River into two 100L polyethylene water storage containers during the exposure regimes. Water was replaced in the exposure tanks with collected river water within 2 hours of field collection.
Fathead minnows
Male and female fathead minnows were exposed to Elkhorn River water collected during the agrichemical pulse (May 7 to May 14) and again during the post-pulse period (July 2 to July 9). No fish mortalities occurred during either pulse or post-pulse 7 d exposures. At the end of the 7 d exposures, fish were euthanized in 300 mg/L tricaine methanesulfonate (MS-222, Sigma Aldrich) and the body mass was recorded.
Northern Leopard Frogs
Male and female leopard frogs were exposed to Elkhorn River water collected during the agrichemical pulse (May 7-May 14). One female was removed prior to the start of the experiment due to illness, and one male frog mortality was observed on d 1 in the agrichemical exposure treatment group. No other frog mortalities were observed during the agrichemical pulse exposure. At the end of the exposure period, frogs were pithed and body mass and snout-urostyle lengths (SUL) were recorded. Unlike the fish exposures, frog exposures during the post-pulse period could not be conducted as the frogs obtained from the supplier during that time period were in poor health.
Gene expression
Following dissection, liver and gonad tissues from both frogs and fish were weighed and flash frozen in liquid nitrogen. A Gonadosomatic index (GSI) and hepatosomatic index (HSI) for each individual was generated by dividing the mass of the tissues by the total body mass and multiplying by 100. Tissues from six animals were selected for quantitative real-time PCR (RT-qPCR) from each treatment group. Animals were selected for analysis such that animals across treatment groups were as consistent in mass, GSI and HSI as possible. In cases where the number remaining was greater than six, animals selected for PCR were chosen randomly.
RNA was extracted from the liver tissue of selected animals using the SV Total RNA Isolation System (Promega Corp). RNA was re-suspended and stored in nuclease free water at −80°C until analysis. RNA purity and concentration was assessed by Nanodrop (NanoDrop Technologies). First strand cDNA synthesis was performed with 1μg total RNA using the iScript cDNA Synthesis Kit (Bio-Rad) per manufacturer recommendations.
RT-qPCR reactions were performed using the iQ SYBR-Green Supermix (Bio-Rad) per the manufacturer’s protocol. Briefly, 2 μL of diluted cDNA template was added to 300 nM forward and reverse primers in a 15 μL volume containing 1x SYBR-Green Supermix. The RT-qPCR negative control consisted of the primer and SYBR-Green Supermix with 2 μL of nuclease free water in place of the template. Dilution of a single DNA template served as the standard curve by which the samples were quantified.
Two estrogen responsive genes, vitellogenin (Vtg) and estrogen receptor-α (ERα), were selected for analysis in P. promelas and R. pipiens. Primer sequences for P. promelas Vtg, ERα, and ribosomal protein L8 (L8) were obtained from Kolok et al. [3]. Primer sequences for ERα and L8 for R. pipiens were obtained from Hogan et al. [32]. The R. pipiens Vtg gene sequence was generated by using next-generation RNA sequencing (GenBank; submission number 1608651) as source information: Forward 5′-AAGTCCACTAATCCCATTCTCCTG and Reverse 5′-ACCAAAAGACCTGTCAGAGACTAC.
Data Analysis
Expression of the housekeeping gene, ribosomal protein L8, was selected in both species as an internal control as it did not vary significantly across treatment groups in any case. As such, all gene expression data was expressed as the relative mRNA expression normalized to L8 mRNA expression. Data from one female fish was excluded from mRNA expression analysis in the pulse unexposed treatment group due to non-amplification of the cDNA template. Significant differences in mass, HSI, GSI, and relative mRNA expression between exposed and unexposed male and female animals were tested using a single t-test (JMP 9.0.1). Statistical significance was assumed at p≤0.05.
RESULTS
Atrazine strips
Atrazine strip results were collected from March 31, 2012 until July 18, 2012 (Fig. 1). No positive hits occurred earlier in the season than May 4, nor did any positive hits occur after June 3. Between these two dates, the positive hits tended to be clustered immediately after rainstorm events indicated by the peaks of increased discharge (Fig. 1).
POCIS
POCIS extracts contained both pesticides and steroids (Table I). For pesticides, the pulse and post-pulse periods had similar chemical signatures, although pesticides were found in much greater proportion during the pulse. Acetochlor and simazine had the greatest fold change with a decrease in concentration of 281 fold and 48 fold respectively, during the post pulse. Atrazine, dimethenamid, metolachlor, and propazine decreased 28, 26, 23, and 11 fold in pesticide concentration, respectively, during the post-pulse. Pesticide concentrations that showed the least temporal variability were prometon, deethylatrazine, deisopropylatrazine, alachlor, and propazine which ranged from 1.6 to 11.38 fold lower during the post pulse relative to the pulse. Very few steroids were present in the POCIS extracts. Of the steroids analyzed for in Table S1 (Supplemental Data), only 4-androstenedione was detected at both time periods, while both estrone and progesterone were detected only during the post-pulse (Table I).
Table I.
A comparison of pesticides and steroid hormones detected in polar organic chemical integrative samplers (POCIS) deployed in the Elkhorn River in 2012 and 2007.a,b
Concentrations determined as ng/POCIS were measured in duplicate and then converted to ng/L using published time weighted average (TWA) rate uptake coefficients from Bartelt-Hunt et al. [27] unless otherwise specified. Concentrations are shown as M(±SD).
| Pesticides and Steroids |
2012 | 2007b | |
|---|---|---|---|
| Pulse (ng/L) | Post-pulse (ng/L) | POCIS (ng/L) |
|
| Acetochlorc | 3460.4 (101.3) | 12.3 (0.6) | 62.2(18) |
| Alachlor | 2.5 (0.1) | 0.3 (0.02) | NDd |
| Atrazine | 4812.9 (45.4) | 170.3 (4.4) | 476.4(107) |
| Deethylatrazine | 382 (7.0) | 67.0 (0.3) | 121.1(25) |
| Deisopropylatrazinec | 736.5 (13.2) | 119 (3.9) | 235.8(49) |
| Dimethenamid | 150.9 (8.0) | 5.7 (0.2) | ND |
| Metolachlor | 514.2 (20.3) | 22.6 (1.1) | 107.4(14) |
| Prometon | 6.3 (0.2) | 3.9 (5.5) | ND |
| Propazine | 45.5 (0.8) | 4.0 (0.8) | 9.14(1.4) |
| Simazinec | 19.3 (0.05) | 0.4 (0.5) | ND |
| 4-Androstenedione | 0.11 (0.06) | 0.20 (0.01) | ND |
| Estrone | ND | 0.69 (1.0) | ND |
| Progesterone | ND | 0.13 (0.2) | ND |
Pesticides and steroid hormones that were analyzed for but not detected are not shown.
Values from Sellin et al. [8] were converted to the TWA concentrations shown in the present study
Concentrations in ng/L determined by rate coefficients published in Mazzella et al. [30]
ND = below limit of detection
Morphometrics
Body mass, HSI, and GSI for both fathead minnows and northern leopard frogs as well as mean snout-urostyle lengths for frogs are shown in Table II. Significant differences in HSI were detected in post-pulse exposed female fathead minnows, in which females exposed to Elkhorn River water had a significantly higher mean HSI than unexposed females (p = 0.0032). No other significant differences were detected during either pulse or post-pulse exposures in either the frogs or the fish.
Table II.
Morphometrics measured in fathead minnows (Pimephales promelas) and northern leopard frogs (Rana pipiens). Values are reported as Mean(±SE).a
|
Rana pipiens
|
Pimephales promelas
|
|||||
|---|---|---|---|---|---|---|
| Pulse |
Pulse |
Post-Pulse |
||||
| Sex | Control | Exposed | Control | Exposed | Control | Exposed |
| Male | ||||||
| Mass | 34.8(0.83) | 33.7(1.6) | 2.3 (0.13) | 2.5(0.15) | 2.1(0.082) | 2.1(0.095) |
| HSI | 3.6(0.30) | 3.4(0.23) | 1(0.23) | 1.7(0.31) | 1(0.092) | 1.2(0.23) |
| GSI | 0.2(0.04) | 0.2(0.03) | 1.4(0.20) | 1.8(0.16) | 1.3(0.26) | 1.2(0.18) |
| SUL | 66.6(1.1) | 65.1(1.3) | NA | NA | NA | NA |
| n | 8 | 7 | 10 | 10 | 10 | 10 |
| Female | ||||||
| Mass | 39.3(1.4) | 43.8(1.6) | 1.5(0.060) | 1.4(0.058) | 1.7(0.080) | 1.7(0.060) |
| HSI | 3.4(0.43) | 3.3(0.28) | 2.7(0.27) | 3.3(0.45) | 1.1(0.11) | 1.7(0.12)* |
| GSI | 16.1(1.8) | 15.4(0.94) | 17.8(1.0) | 17.7(1.5) | 12.8(1.1) | 14.8(1.2) |
| SUL | 65.6(1.4) | 69.1(1.1) | NA | NA | NA | NA |
| n | 7 | 8 | 10 | 10 | 10 | 10 |
NA = not applicable
p < 0.01
Elkhorn River agrichemical exposures
Fathead minnows
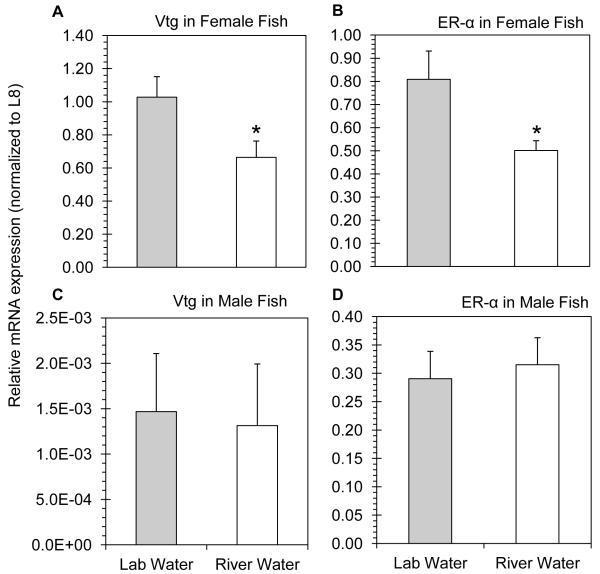
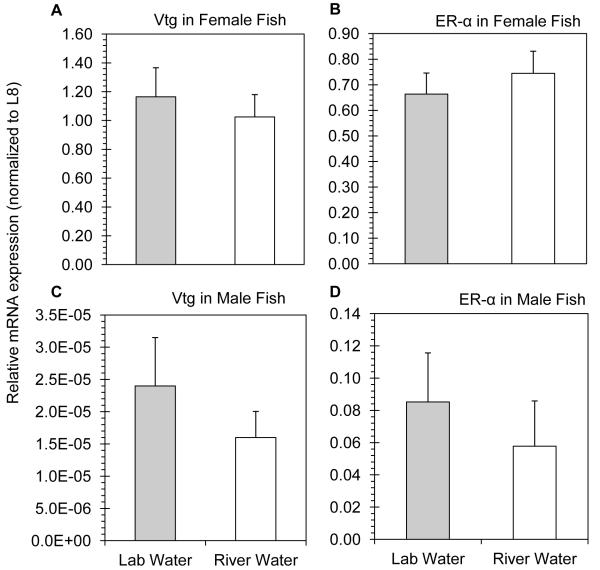
During the Elkhorn River pulse (May 7 to May 13, 2012), fathead minnow females experienced a significant reduction in both Vtg (Fig. 2A) and ER-α (Fig. 2B) gene expression (p < 0.05 in all cases). Conversely, expression of Vtg and ER-α in male fathead minnows was unaffected following exposure to pulse river water, respectively (Fig. 2C - 2D; p > 0.72 in both cases). Male and female minnows were also exposed to water collected during the post-pulse period (July 2 to July 8, 2012). Neither Vtg nor ER-α mRNA expression was altered in the female or male fathead minnows following the 7 d exposure to post-pulse water (Fig. 3A - 4D; p > 0.39 in all cases).
Figure 2.
Relative hepatic mRNA expression in unexposed (lab water) and pulse exposed (river water) fathead minnows. A) Vtg and B) ERα mRNA expression in female fish. C) Vtg and D) ERα mRNA expression in male fish. Significant differences are denoted by an asterisks (p < 0.05 in all cases).
Figure 3.
Relative hepatic mRNA expression in unexposed (lab water) and post-pulse exposed (river water) fathead minnows. A) Vtg and B) ERα mRNA expression in female fish. C) Vtg and D) ERα mRNA expression in male fish.
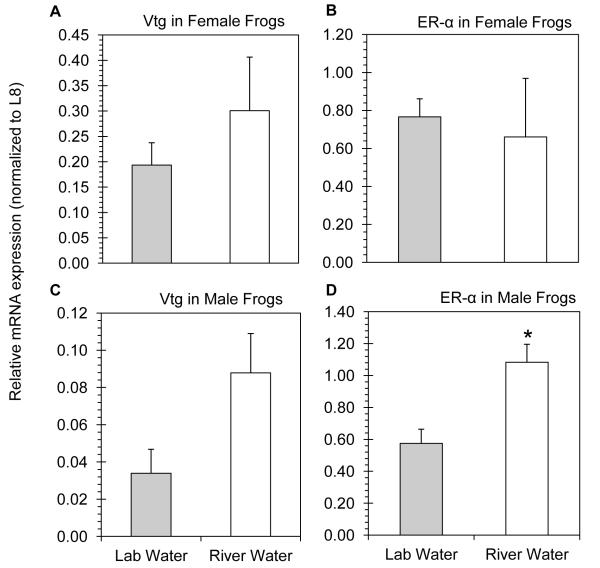
Figure 4.
Relative hepatic mRNA expression in unexposed (lab water) and pulse exposed (river water) northern leopard frogs. A) Vtg and B) ERα mRNA expression in female frogs. C) Vtg and D) ERα mRNA expression in male frogs. Significant differences are denoted by an asterisks (p < 0.05 in all cases).
Northern leopard frogs
While female fathead minnows were significantly impacted following pulse river water exposure, expression of Vtg and ER-α was unaffected in female northern leopard frogs (Fig. 4A - 4B, respectively; p > 0.37 in both cases). Although the mean Vtg mRNA expression in exposed male northern leopard frogs was 2.5 fold greater than unexposed males, this difference was not statistically significant (Fig. 4C; p = 0.0547). However, expression of ER-α transcripts was significantly increased in the male pulse exposed leopard frogs relative to the control (Fig. 4D; p = 0.0055).
DISCUSSION
The present study had two primary objectives. The first objective was to determine if temporal variability in agrichemicals present in the Elkhorn River would influence the toxicity of waters collected, while the second objective was to determine whether two sentinel organisms, fathead minnows and northern leopard frogs, would experience the same effects when exposed to these waters. Chemical results suggest pronounced temporal variability in agrichemicals in the Elkhorn River, while biological responses suggest an agrichemical mixture with varying concentrations has a varied impact on different environmental sentinel organisms.
Atrazine strips
Previous studies have documented the spring pulse of agrichemicals in the Midwestern U.S. referring to it as the spring flush [6, 14, 16]. Although properties such as solubility and vapor pressure can influence the fate and persistence of individual pesticides in the environment [5, 7, 10], the single most important factor controlling the spring flush of pesticides is rainfall [5-7, 10, 16]. Discharge, or stream flow, is commonly used to model rainfall runoff periods during which pesticides are likely to enter watersheds [6, 16]. Results from the current study agree with this basic premise, as pulses of atrazine were shown to follow multiple discharge spikes caused by local rainfall events (Fig. 1).
Despite the fact that rainfall events are the greatest determinant for pesticides entering nearby watersheds, not every rainfall-induced runoff event carries significant pesticide loads [5-7, 10, 16]. Such occurrences may be a result of rainfall occurring prior to pesticide application or may be due to prolonged heavy rainfalls that reduce the overall pesticide load [5]. This concept is clearly evident in the data collected in the present study, as the discharge peak occurring on April 16, 2012 was not associated with a concomitant detection of atrazine (Fig. 1). Atrazine was first detected above 3ppb in the following discharge event (May 3, 2012), suggesting the application of atrazine had not yet occurred prior to April 16. Thus, discharge peaks alone are not adequate for predicting agrichemical runoff periods.
While not a primary objective of this paper, results support the value of atrazine strips as a first-tier screening tool for defining periods of agrichemical runoff [14]. The occurrence of the atrazine pulse, a well-documented phenomenon [14-16], was successfully detected in the Elkhorn River using commercially available test strips. Additionally, atrazine test strips confirmed our contention that discharge alone is not adequate for determining agrichemical runoff. Atrazine has been used as a surrogate for agrichemicals in pesticide fate and transport studies [7, 15, 17], and the rapid assessment provided by the atrazine test strips will allow for greater use of atrazine in that regard.
POCIS
Pesticides
Although concentrations detected during the agrichemical pulse were found at much higher concentrations than during the post-pulse period, the same pesticides were found to occur during both sampling periods. Previous POCIS deployments in the Elkhorn River (West Point, NE) have revealed a similar pesticide signature to that found in the current study [8] (Table I). The repeated occurrence of these commonly used row crop pesticides in unsurprising as the southeastern portion of the Elkhorn River watershed is primarily dominated by corn and soybean production [8, 10, 11].
Deployment duration of POCIS can have a direct impact on the total mass of chemical sampled during deployment, and these arbitrary differences in chemical amounts make comparisons across studies difficult when deployment durations vary from days to weeks. Therefore, amounts of pesticides published in Sellin et al. [8] were converted to time weighted average (TWA) concentrations to allow for direct comparison with the TWA pesticide concentrations presented in the present study [27, 30]. Although the pesticide signature was consistent between the two studies, the TWA pesticide concentrations (ng/L) were dramatically elevated in the present study (3-55 fold higher) relative to the TWA concentrations from Sellin et al. [8] (Table I). In contrast to the current study, Sellin et al. deployed POCIS according to calendar date rather than an empirically driven metric. Using atrazine strips to focus the POCIS deployment period may be one explanation for the greater concentration of pesticides detected in the present study relative to that of Sellin et al. [8].
To determine if the elevated TWA concentrations observed in our study reflect realistic concentrations observed in the Elkhorn River, the TWA concentration of atrazine was compared to water sample data from the Elkhorn River Waterloo, NE USGS gaging station for samples taken between 2002 to 2011 (U.S. Geological Survey NWIS database, http://waterdata.usgs.gov/nwis). TWA concentrations of atrazine from the POCIS were 4.81±0.023 (mean±SD) μg/L during the May pulse deployment and 0.691±0.009 μg/L during the July post-pulse deployment. These data are very consistent with those collected by the USGS in the Elkhorn River during May (4.46±1.96 μg/L) and July (0.41±0.17 μg/L). This suggests that TWA concentrations calculated from the POCIS extracts provide an accurate indication of the pesticide load in the Elkhorn River during both pulse and post-pulse periods.
Steroids
Contrary to what was observed with pesticides, steroid hormones detected in the POCIS were found to be greater both in concentration (4-androstenedione) and occurrence (estrone, progesterone) during the post-pulse rather than the pulse. Previous studies have indicated that steroid hormones are transported to aquatic systems during runoff in a manner similar to pesticides [1, 9, 10]. Due to their higher lipid solubility than most herbicides, steroid hormones may bind to suspended solids rather than remain in free form in the water [5, 13, 33, 34]. Because POCIS are only able to sample freely dissolved compounds [26, 27, 35], steroids associated with suspended sediment particles would not be available to them. In May, the concentration of suspended sediments in the river was 8 times higher than it was in July (640mg/L on May 9, 2012 versus 80 mg/L on July 16, 2012; USGS). The large suspended sediment load during the pulse may have reduced the amount of free steroid in the water thereby reducing the amount that could be collected by the POCIS [33].
While previous studies have deployed POCIS in the Elkhorn River, differences in deployment calendar date, location and duration make cross study comparisons difficult. In a study by Kolok et al. [3], a number of steroid hormones were detected in POCIS extracts following a 21 d deployment including estrone, 17β-estradiol, estriol, testosterone, melengesterol acetate, 4-androstenedione, and progesterone. The 14 d deployed POCIS in the current study detected only 4-androstenedione, estrone, and progesterone. Contrary to both the present study and Kolok et al. [3], Sellin et al. [8] detected no steroids in POCIS extracts following a 7 d deployment in the Elkhorn River. The lack of steroids in POCIS most likely resulted from a short sampling of trace steroid concentrations. For example, 4-androstenedione was detected in the present study at a concentration of 0.11 ng/L or 0.82 ng/POCIS [27]. Assuming the rate of uptake is linear, the mass of 4-androstenedione in POCIS following a 7 d deployment would only yield 0.41 ng/POCIS, falling below the 0.5 ng detection limit. While deployment location and date may partially explain the differences in steroid occurrence, short-term POCIS deployment duration appears to be a strong determinant in the detection of low steroid concentrations in the Elkhorn River watershed.
Elkhorn River exposures and sentinel organisms
Defeminization of fish
Female fish exposed to water collected from the Elkhorn River experienced alterations in the hepatic expression of estrogen responsive genes. Specifically, the pulse exposed female fish experienced transcriptional defeminization as evident from the significant reductions in ERα and Vtg mRNA. Such transcriptional defeminizing effects of female minnows is consistent with caged fish studies conducted in the Elkhorn River [8] and in the nearby Bow Creek watershed [12]. Results from laboratory exposures of female fish exposed to Elkhorn River water, however, have met with mixed results. While endocrine effects in female fathead minnows have been previously observed following direct exposure to the Elkhorn River through field deployments [3, 8, 9], attempts to demonstrate such effects following laboratory exposure to Elkhorn River water have been unsuccessful [11, 13]. The lack of observed transcriptional defeminization in laboratory exposures in the previous studies is consistent with the results obtained in the present study during the July exposure. In contrast, water collected from the Elkhorn River during the May pulse exposure significantly altered expression of estrogen responsive genes in the female fathead minnows. Although pesticides were detected at both time points, these results suggest that the timing of water collection can have a significant impact on the toxicity of the water present in the Elkhorn River.
Feminization of male frogs
Spanning the last decade, numerous studies have utilized the fathead minnow as a sentinel organism in gene expression studies [3, 8, 11-13, 36, 37], while in comparison, there remains a paucity of data utilizing similar molecular tools in amphibians such as the northern leopard frog [25]. As such, only a handful of studies could be identified specifically investigating the gene expression responses of R. pipiens following exposure to an environmental contaminant [23, 38-40]. Furthermore, these studies have focused on developmental effects associated with exposure of R. pipiens tadpoles. To our knowledge, this is the first gene expression exposure study utilizing adult northern leopard frogs. The results of the present study demonstrate that male leopard frogs exposed to Elkhorn River water experienced feminizing alterations in gene expression as evidenced by the elevated levels of ERα expression.
The transcriptional feminization of male frogs is interesting as similar estrogenic effects have not been previously observed in male fathead minnows following exposure to the Elkhorn River or the Bow Creek watersheds [3, 8, 11, 12]. Consistent with these findings, no effects were observed in the male fathead minnows in the present study. One potential explanation for the observed effects in the male northern leopard frogs could be the presence of atrazine in the Elkhorn River, which has been linked to feminization of male frogs [20-23]. Indeed, the atrazine strip data, as well as the POCIS results suggest that water collected during the agrichemical pulse exposed frogs to a concentration of atrazine exceeding the US EPA drinking water standard. Interestingly, Langlois et al. [23] observed a significant increase in brain ERα mRNA transcripts in pre-metamorphic G34 tadpoles following exposure to 1.8 μg/L atrazine raised in outdoor mesocosms. Although evidence may suggest that the increased hepatic ERα mRNA in adult male frogs in the present study is a result of atrazine exposure, the effects observed cannot be conclusively linked to atrazine due to the complex mixture of agrichemicals present in the Elkhorn River.
Comparison of sentinel organisms
The responses observed in the northern leopard frogs and the fathead minnows following exposure to the pulse water were not consistent between the two species. Unexpectedly, neither the male fish nor the female frogs responded as did their counterparts from the other species. Perhaps the most parsimonious explanation for the opposing responses is the presence of multiple chemicals in the raw river water, with the two different species responding differently to one or more of the chemicals present in the mixture. Differential chemical sensitivities may be a direct result of the different uptake routes observed between the two species. For fish, the primary route of uptake will likely occur at the gills, while in frogs, uptake will most likely occur through dermal exposure or ingestion. Differential exposure to one or more chemicals in the river may therefore be due chemical uptake which favors one exposure route over the other. It appears likely that the transcriptional feminization of frogs may be due to atrazine exposure, as this has been shown previously [20-23]. For fish, however, it remains unclear whether one of the chemicals identified, a heretofore unidentified chemical, or a mixture of chemicals was responsible for the observed defeminizing gene expression in the female fish as no discernible pattern of effects and pesticide occurrence has emerged [8, 12, 13].
Although the fathead minnow can be regarded as an excellent environmental sentinel, the variable responses observed in the present study indicate the need for a suitable amphibian sentinel to provide adequate risk assessment. Use of amphibians in aquatic toxicology has largely been limited to two species, Xenopus laevis and Xenopus tropicalis. However, neither of these species is native to North America and both exhibit a life history which is exclusively aquatic, suggesting they may not be suitable environmental sentinels for semi-aquatic or terrestrial North American frogs, such as Rana pipiens [24, 25]. The varied responses observed between the two species in the present study in addition to the lack of a well characterized suitable amphibian model clearly indicates that more research is necessary to develop an environmentally relevant North American sentinel anuran.
Supplementary Material
Acknowledgement
Financial support for the present study was provided by grants from the NIH National Center for Research Resources (5P20RR016469), the National Institute for General Medical Science (8P20GM103427), and the US Geological Society 104b funded through the Nebraska Water Center. The University of Nebraska provided additional support through the Biomedical Research Training Program, the Fund for Investing in the Research Enterprise, the Graduate Research and Creative Activity fund, and Fund for Undergraduate Scholarly Experience. Chemical analyses were performed by D. Snow at the Nebraska Water Sciences Laboratory. A. Jessick, L. Harrison, R. Rangel, H. Nguyen, and M. Benner provided support with water collection and dissections.
Footnotes
All Supplemental Data can be found in the online version of this article.
SUPPLEMENTAL DATA Table S1. List of pesticides and steroid hormones analyzed for in POCIS extracts.
REFERENCES
- 1.Soto AM, Calabro JM, Prechtl NV, Yau AY, Orlando EF, Daxenberger A, Kolok AS, Guillette LJ, Jr, Le Bizec B, Lange IG. Androgenic and estrogenic activity in water bodies receiving cattle feedlot effluent in Eastern Nebraska, USA. Environmental Health Perspectives. 2004;112:346. doi: 10.1289/ehp.6590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Belden JB, Gilliom RJ, Martin JD, Lydy MJ. Relative toxicity and occurrence patterns of pesticide mixtures in streams draining agricultural watersheds dominated by corn and soybean production. Integrated Environmental Assessment and Management. 2007;3:90–100. [PubMed] [Google Scholar]
- 3.Kolok AS, Snow DD, Kohno S, Sellin MK, Guillette LJ., Jr Occurrence and biological effect of exogenous steroids in the Elkhorn River, Nebraska, USA. Science of the Total Environment. 2007;388:104–115. doi: 10.1016/j.scitotenv.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 4.Willis GH, McDowell LL. Pesticides in agricultural runoff and their effects on downstream water quality. Environmental Toxicology and Chemistry. 2009;1:267–279. [Google Scholar]
- 5.Wauchope R. The pesticide content of surface water draining from agricultural fields—a review. Journal of Environmental Quality. 1978;7:459–472. [Google Scholar]
- 6.Thurman EM, Goolsby D, Meyer M, Kolpin D. Herbicides in surface waters of the midwestern United States: The effect of spring flush. Environmental Science & Technology. 1991;25:1794–1796. [Google Scholar]
- 7.Capel PD, Larson SJ, Winterstein TA. The behaviour of 39 pesticides in surface waters as a function of scale. Hydrological Processes. 2001;15:1251–1269. [Google Scholar]
- 8.Sellin MK, Snow DD, Schwarz M, Carter BJ, Kolok AS. Agrichemicals in nebraska, USA, watersheds: Occurrence and endocrine effects. Environmental Toxicology and Chemistry. 2009;28:2443–2448. doi: 10.1897/09-135.1. [DOI] [PubMed] [Google Scholar]
- 9.Orlando EF, Kolok AS, Binzcik GA, Gates JL, Horton MK, Lambright CS, Gray LE, Jr, Soto AM, Guillette LJ., Jr Endocrine-disrupting effects of cattle feedlot effluent on an aquatic sentinel species, the fathead minnow. Environmental Health Perspectives. 2004;112:353. doi: 10.1289/ehp.6591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kolok AS, Beseler CL, Chen XH, Shea PJ. The watershed as a conceptual framework for the study of environmental and human health. Environmental Health Insights. 2009;3:1. doi: 10.4137/EHI.S1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sellin MK, Snow DD, Kolok AS. Reductions in hepatic vitellogenin and estrogen receptor alpha expression by sediments from an agriculturally impacted waterway. Aquatic Toxicology. 2010;96:103–108. doi: 10.1016/j.aquatox.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 12.Sellin Jeffries MK, Abbott KI, Cowman T, Kolok AS. Occurrence and endocrine effects of agrichemicals in a small Nebraska, USA, watershed. Environmental Toxicology and Chemistry. 2011 doi: 10.1002/etc.615. [DOI] [PubMed] [Google Scholar]
- 13.Sellin Jeffries MK, Conoan NH, Cox MB, Sangster JL, Balsiger HA, Bridges AA, Cowman T, Knight LA, Bartelt-Hunt SL, Kolok AS. The anti-estrogenic activity of sediments from agriculturally intense watersheds: Assessment using in vivo and in vitro assays. Aquatic Toxicology. 2011;105:189–198. doi: 10.1016/j.aquatox.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kolok AS, Schoenfuss HL, Propper CR, Vail TL. Empowering Citizen Scientists: The Strength of Many in Monitoring Biologically Active Environmental Contaminants. BioScience. 2011;61:626–630. [Google Scholar]
- 15.Capel PD, Larson SJ. Effect of scale on the behavior of atrazine in surface waters. Environmental Science & Technology. 2001;35:648–657. doi: 10.1021/es001220f. [DOI] [PubMed] [Google Scholar]
- 16.Stoeckel JA, Morris J, Ames E, Glover DC, Vanni MJ, Renwick W, González MJ. Exposure Times to the Spring Atrazine Flush Along a Stream-Reservoir System1. JAWRA Journal of the American Water Resources Association. 2012 [Google Scholar]
- 17.Thurman E, Fallon J. The deethylatrazine/atrazine ratio as an indicator of the onset of the spring flush of herbicides into surface water of the midwestern United States. International journal of Environmental Analytical Chemistry. 1996;65:203–214. [Google Scholar]
- 18.Knutson MG, Richardson WB, Reineke DM, Gray BR, Parmelee JR, Weick SE. Agricultural ponds support amphibian populations. Ecological Applications. 2004;14:669–684. [Google Scholar]
- 19.McDaniel TV, Martin PA, Struger J, Sherry J, Marvin CH, McMaster ME, Clarence S, Tetreault G. Potential endocrine disruption of sexual development in free ranging male northern leopard frogs (Rana pipiens) and green frogs (Rana clamitans) from areas of intensive row crop agriculture. Aquatic Toxicology. 2008;88:230–242. doi: 10.1016/j.aquatox.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 20.Hayes T, Haston K, Tsui M, Hoang A, Haeffele C, Vonk A. Herbicides: feminization of male frogs in the wild. Nature. 2002;419:895–896. doi: 10.1038/419895a. [DOI] [PubMed] [Google Scholar]
- 21.Tavera-Mendoza L, Ruby S, Brousseau P, Fournier M, Cyr D, Marcogliese D. Response of the amphibian tadpole (Xenopus laevis) to atrazine during sexual differentiation of the testis. Environmental Toxicology and Chemistry. 2002;21:527–531. doi: 10.1897/1551-5028(2002)021<0527:rotatx>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 22.Hayes T, Haston K, Tsui M, Hoang A, Haeffele C, Vonk A. Atrazine-induced hermaphroditism at 0.1 ppb in American leopard frogs (Rana pipiens): laboratory and field evidence. Environmental Health Perspectives. 2003;111:568. doi: 10.1289/ehp.5932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Langlois VS, Carew AC, Pauli BD, Wade MG, Cooke GM, Trudeau VL. Low levels of the herbicide atrazine alter sex ratios and reduce metamorphic success in Rana pipiens tadpoles raised in outdoor mesocosms. Environmental Health Perspectives. 2010;118:552. doi: 10.1289/ehp.0901418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kerby JL, Richards-Hrdlicka KL, Storfer A, Skelly DK. An examination of amphibian sensitivity to environmental contaminants: are amphibians poor canaries? Ecology letters. 2010;13:60–67. doi: 10.1111/j.1461-0248.2009.01399.x. [DOI] [PubMed] [Google Scholar]
- 25.Helbing CC. The metamorphosis of amphibian toxicogenomics. Frontiers in Genetics. 2012;3 doi: 10.3389/fgene.2012.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alvarez DA, Petty JD, Huckins JN, Jones-Lepp TL, Getting DT, Goddard JP, Manahan SE. Development of a passive, in situ, integrative sampler for hydrophilic organic contaminants in aquatic environments. Environmental Toxicology and Chemistry. 2004;23:1640–1648. doi: 10.1897/03-603. [DOI] [PubMed] [Google Scholar]
- 27.Bartelt-Hunt SL, Snow DD, Damon-Powell T, Brown DSL, Prasai G, Schwarz M, Kolok AS. Quantitative evaluation of laboratory uptake rates for pesticides, pharmaceuticals, and steroid hormones using POCIS. Environmental Toxicology and Chemistry. 2011;30:1412–1420. doi: 10.1002/etc.514. [DOI] [PubMed] [Google Scholar]
- 28.Snow D, Damon-Powell T, Onanong S, Cassada D. Sensitive and simplified analysis of natural and synthetic steroids in water and solids using on-line solid-phase extraction and microwave-assisted solvent extraction coupled to liquid chromatography tandem mass spectrometry atmospheric pressure photoionization. Analytical and Bioanalytical Chemistry. 2012 doi: 10.1007/s00216-012-6572-8. [DOI] [PubMed] [Google Scholar]
- 29.Cassada D, Spalding R, Cai Z, Gross M. Determination of atrazine, deethylatrazine and deisopropylatrazine in water and sediment by isotope dilution gas chromatography— mass spectrometry. Analytica Chimica Acta. 1994;287:7–15. [Google Scholar]
- 30.Mazzella N, Dubernet JF, Delmas F. Determination of kinetic and equilibrium regimes in the operation of polar organic chemical integrative samplers: Application to the passive sampling of the polar herbicides in aquatic environments. Journal of Chromatography A. 2007;1154:42–51. doi: 10.1016/j.chroma.2007.03.087. [DOI] [PubMed] [Google Scholar]
- 31.Peake EB, Locke JC, Tierney LL, Kolok AS. Copper tolerance in fathead minnows: II. Maternal transfer. Environmental Toxicology and Chemistry. 2004;23:208–211. doi: 10.1897/02-610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hogan NS, Crump KL, Duarte P, Lean DRS, Trudeau VL. Hormone cross-regulation in the tadpole brain: Developmental expression profiles and effect of T3 exposure on thyroid hormone-and estrogen-responsive genes in Rana pipiens. General and Comparative Endocrinology. 2007;154:5–15. doi: 10.1016/j.ygcen.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 33.Lai K, Johnson K, Scrimshaw M, Lester J. Binding of waterborne steroid estrogens to solid phases in river and estuarine systems. Environmental Science & Technology. 2000;34:3890–3894. [Google Scholar]
- 34.Peck M, Gibson RW, Kortenkamp A, Hill EM. Sediments are major sinks of steroidal estrogens in two United Kingdom rivers. Environmental Toxicology and Chemistry. 2004;23:945–952. doi: 10.1897/03-41. [DOI] [PubMed] [Google Scholar]
- 35.Rujiralai T, Bull ID, Llewellyn N, Evershed RP. In situ polar organic chemical integrative sampling (POCIS) of steroidal estrogens in sewage treatment works discharge and river water. Journal of Environmental Monitoring. 2011;13:1427–1434. doi: 10.1039/c0em00537a. [DOI] [PubMed] [Google Scholar]
- 36.Korte JJ, Kahl MD, Jensen KM, Pasha MS, Parks LG, LeBlanc GA, Ankley GT. Fathead minnow vitellogenin: Complementary DNA sequence and messenger RNA and protein expression after 17β-estradiol treatment. Environmental Toxicology and Chemistry. 2000;19:972–981. [Google Scholar]
- 37.Sellin MK, Snow DD, Akerly DL, Kolok AS. Estrogenic Compounds Downstream From Three Small Cities in Eastern Nebraska: Occurrence and Biological Effect1. JAWRA Journal of the American Water Resources Association. 2009;45:14–21. [Google Scholar]
- 38.Croteau MC, Duarte-Guterman P, Lean DRS, Trudeau VL. Preexposure to ultraviolet B radiation and 4-tert-octylphenol affects the response of Rana pipiens tadpoles to 3, 5, 3′-triiodothyronine. Environmental Toxicology and Chemistry. 2010;29:1804–1815. doi: 10.1002/etc.232. [DOI] [PubMed] [Google Scholar]
- 39.Crump D, Lean D, Trudeau VL. Octylphenol and UV-B radiation alter larval development and hypothalamic gene expression in the leopard frog (Rana pipiens) Environmental Health Perspectives. 2002;110:277. doi: 10.1289/ehp.02110277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Howe CM, Berrill M, Pauli BD, Helbing CC, Werry K, Veldhoen N. Toxicity of glyphosate-based pesticides to four North American frog species. Environmental Toxicology and Chemistry. 2009;23:1928–1938. doi: 10.1897/03-71. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.