Abstract
To improve treatments for bone or dental trauma, and for diseases such as osteoporosis, cancer, and infections, scientists who perform basic research are collaborating with clinicians to design and test new biomaterials for the regeneration of lost or injured tissue. Developed some 40 years ago, bioactive glass (BG) has recently become one of the most promising biomaterials, a consequence of discoveries that its unusual properties elicit specific biological responses inside the body. Among these important properties are the capability of BG to form strong interfaces with both hard and soft tissues, and its release of ions upon dissolution. Recent developments in nanotechnology have introduced opportunities for materials sciences to advance dental and bone therapies. For example, the applications for BG expand as it becomes possible to finely control structures and physicochemical properties of materials at the molecular level. Here we review how the properties of these materials have been enhanced by the advent of nanotechnology; and how these developments are producing promising results in hard-tissue regeneration and development of innovative BG-based drug-delivery systems.
Introduction
In the treatment of bone or dental trauma and diseases such as osteoporosis, cancer and infections, biocompatible synthetic materials are now commonly used to replace damaged tissues. However, because these materials lack chemical, biological, or physical properties similar to those of host tissues, their use often results in implant failure and requires re-treatment. To meet the goal of a patient’s full recovery, it is essential to develop new active materials that are able to interact with host surroundings, enhancing and directing complete tissue regeneration.
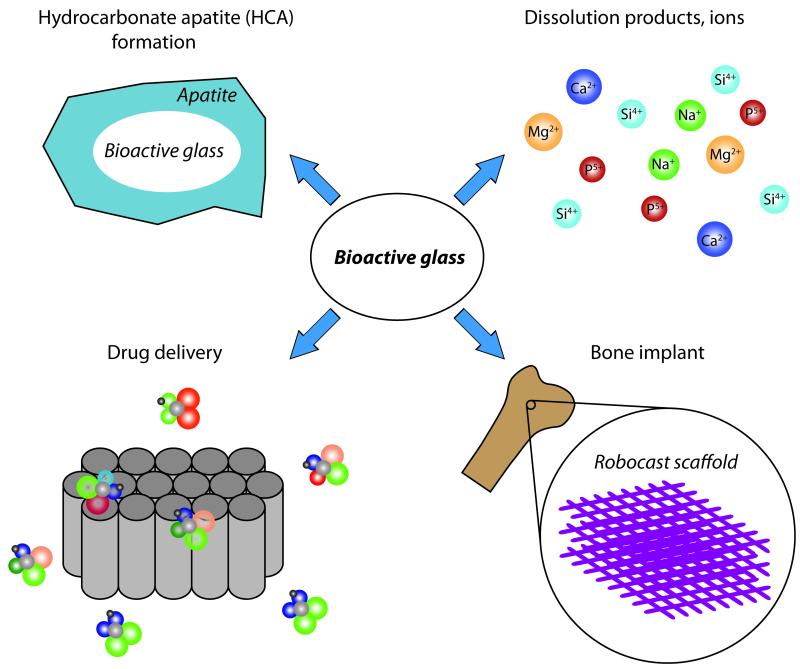
To accomplish this challenge, several materials sciences research groups are focusing their attention on bioactive glass (BG) and BG-composite materials (Fig. 1). BGs are glass materials able to induce specific biological activity following implantation.1 Specifically, this bioactivity is achieved with BGs through the formation on their surfaces of a hydroxyapatite (HA)-layer, which bonds strongly to both hard and soft tissues and releases ion products after dissolution.2 In addition, technical advances in BG processing have led to development of mesoporous bioactive glass (MBG) materials that extend the potential of BG into the design of innovative drug delivery systems.3
Figure 1.
Different aspects of BG materials can be exploited to induce specific biological activities.
Recent studies also show that the tools of nanotechnology can be employed to improve the performance of all materials currently used for re-growing bone or addressing dental issues.4-6 These new techniques, which take into account the complex and hierarchical structure of teeth and bone, can mimic the natural nanostructure of our tissues and lead to the development of new implants that can be carefully designed at different length scales.7 Meanwhile, the application of nanotechnology for treatment, diagnosis, monitoring, and control of biological systems — recently labeled “nanomedicine” by the National Institutes of Health — offers a unique opportunity for truly rational design and precision delivery of therapeutics with a minimum of side effects.8
Here we show how nanotechnology-based approaches can be applied to modify and improve BG materials. After a brief summary of the main properties of BGs, and an examination of the relation between composition and bioactivity, we will describe the latest developments and studies on the use of BG nanostructured materials for hard-tissue regeneration and in the development of drug delivery systems.
BIOACTIVE GLASS (BG)
BG can be formed using two different methods. In the traditional melt-quenching route, used for commercial BG applications, temperatures above 1300°C are applied to melt the oxides together in a platinum crucible, while graphite molds (for rods and monoliths) or water (frit) are used for quenching. In the more recently developed sol-gel route, a chemical synthesis employing silica precursors is used to form and assemble nanoparticles (NPs) into a gel at room temperature.9 Glass is formed after drying and heating this gel, which is a wet, organic, covalently bonded silica network. One notable physical difference between glasses produced by these methods is the presence of nanoporosity with the sol-gel technique, which leads to an increase in surface area and improved cell responses.10 Sol-gel compositions show usually fewer components since oxides like Na2O, often employed in melt-quenching procedures to improve the process ability by lowering the melting point, are not necessary in this route. Further details can be found in recent reviews.2, 11, 12
Highly ordered MBGs are commonly synthesized by a sol-gel technique in which nonionic block copolymers are used as structure-directing agents during the evaporation-induced self-assembly process.3 Though conventional BGs also show a mesoporous structure due to the random distribution of CaO within the network of SiO2, the self-assembly of surfactants in MBGs leads to uniform mesopores with pore size in the range of 2–50 nm as well as ordered mesostructures. In addition to control over MBG composition, this self-assembly process also provides an opportunity for accurate tuning of pore size and structure.3 When compared with non-mesoporous BG architectures, the increased specific surface area in MBG structures provides enhanced in vitro and in vivo bioactivity, degradation behavior, and drug delivery properties.13, 14
Atomic structure of BG
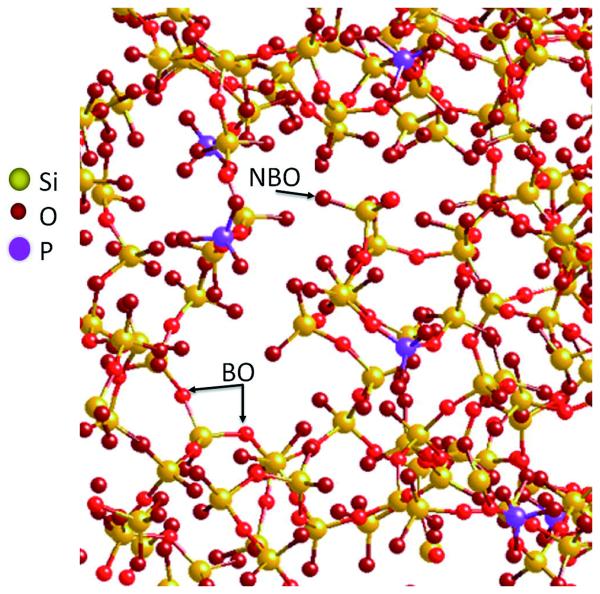
The bioactivity of BG-based materials as they mineralize and form a hydroxy-carbonate apatite (HCA) layer relies on fine control of the composition and atomic structure of the glass, as does the osteogenic, angiogenic and antibacterial properties of their dissolution products. The atomic structure of silica glasses is determined by the electronic properties of silicon atoms that, with oxygen atoms, form silica tetrahedral connected by –Si–O–Si– bridging oxygen (BO) bonds (Fig. 2).15 Network-modifying cations (e.g., sodium and calcium) can disrupt the structure by introducing non-bridging oxygen (NBO) bonds. In general, BGs with high silica content have highly connected networks, leading to slow dissolution and slow bioactivity. Besides the use of network-modifying cations, the addition of phosphorous can induce repolymerization of the silicate network, producing similar effects by extracting cations to counterbalance the charges and inducing a non-homogeneous distribution of them.16
Figure 2.
A model of the 45S5 BG structure. Na and Ca ions are removed for better clarity (Reprinted with permission from Ref 2. Copyright 2013 Elsevier).
An important parameter for prediction of the bioactivity of a glass is the network connectivity Nc, the mean number of bridging bonds per silicon atom. Glasses with N-values greater than 2.6 are not likely to be bioactive because of their resistance to dissolution.17 In sol-gel glasses, Nc is generally lower than that calculated from the nominal composition, due to the presence of hydroxyl groups in their composition.2 Though the drying process removes most of the −OH groups causing further formation of O–Si–O bonds, some remain entrapped in the network reducing its connectivity.
BG bioactivity: HCA formation
BG capability to bind bone is attributed to the formation of an HCA layer able to interact in a strong way with collagen fibrils of damaged bone; adsorb protein; incorporate collagen molecules; and induce osteoblast attachment and differentiation.18 The HCA layer is formed as a result of several reactions occurring on the BG surface in the following sequence:19 1) an increase of pH following a rapid ion exchange between the network-modifying cations and H+ from the solution, which leads to hydrolysis of the silica groups and the creation of silanol (Si-OH) groups; 2) this increase in pH provokes the attack on the SiO2 glass network by OH−, breaking Si–O–Si bonds; 3) condensation and polymerization of an amorphous 1–2 μm SiO2 layer without Na+ and Ca2+; 4) further dissolution of the glass and migration of Ca2+ and (PO4)3− to the amorphous SiO2 layer to create an amorphous calcium phosphate (ACP) layer; 5) as the glass continues to dissolve, the ACP layer integrates (OH)− and (CO3)2− from the solution and crystallizes as an HCA layer.
BG bioactivity: ionic dissolution products
The first studies on the biological properties of ionic dissolution products from basic BGs have been focused on Si, Ca, and P from silicate BGs, and show how these materials can influence the gene expression of several genes in osteoblastic cells.20 Subsequently, other effects have been investigated in vitro and in vivo regarding angiogenesis, antibacterial activity, and inflammatory process. Below, the latest studies in this field will be reviewed to describe these effects; for additional examples refer to Reference21.
Macroporous MBG structures doped with Mg, Zn, or Sr are produced by polymer foam replication.22 As shown by in vitro studies using rat bone-marrow mesenchymal stem cells (rBMSCs), the doping introduces no cytotoxic effects, and the gradual release of Ca, P, Si, Mg, Zn, and Sr from the scaffolds into the culture medium enhances the cell proliferation and alkaline phosphatase (ALP) activity. In another study, the release of B achieved by boron/dexamethasone (DEX) functionalized MBG scaffolds leads to increased ALP activity and the expression of osteogenic genes synergically with the delivery of DEX.23 Magnetic Fe-doped MBG structures, useful as a hyperthermia platform in the treatment of malignant bone tumors, are developed and investigated in vitro, showing an enhanced mitochondrial activity as well as the expression of bone-related genes (ALP and osteocalcin) in human BMSCs (hBMSCs).24 Increased hBMSCs attachment and viability is also achieved by Zr-doped scaffolds, which show higher compressive strength and decreased dissolution when compared to undoped structures, while the apatite formation ability is maintained.25 An interesting recent study reports on the incorporation of Co within MBG scaffolds to induce a hypoxic environment for improving angiogenesis through higher vascular endothelial growth factor (VEGF) secretion, hypoxia-inducible factor (HIF)-1α expression, and bone-related gene expression of hBMSCs.26 Similar results can be achieved by incorporating Cu in MBG scaffolds.27 The introduction of Li in MBG implants has a specific effect on the formation of cementum, the mineralized tissue produced by cementoblasts during tooth root formation. Cementum is responsible for attaching the periodontal ligament to the roots and surrounding alveolar bone by influencing proliferation and cementogenic differentiation of human periodontal ligament-derived cells (hPDLCs).28
In addition to studies of specific effects of BGs and MBGs in bone regeneration and angiogenesis, several reports have focused on the antibacterial properties of BG materials, resulting from their dissolution and release of ions in solution, for the treatment or prevention of periodontal infections.21, 29 For example, Ag2O-doped BG microparticles can be synthesized by sol-gel method and used not only as bacteriostatic, but also bactericidal material.30 These antibacterial properties are likely due to leaching of silver ions from the glass matrix and their accumulation by the bacteria, where they interfere with several cellular mechanisms and components in a not yet fully understood way.30 El-Kady et al. produced Ag2O-doped BG NPs that show a continuous release of silver ions for over two weeks, able to inhibit Staphylococcus aureus and E. coli growth.31
HARD TISSUE REGENERATION
Bones and teeth are extremely complex organs showing a combination of hard tissues (compact and trabecular bone or enamel, dentin, and cementum) as well as soft tissues (bone marrow or dental pulp, and periodontal ligament) in a unique hierarchical structure, where nanoscale and mesoscale phenomena, such as biomolecular interactions, nutrients exchange, and fluid transport, are both important. Autografts, allografts, and xenografts are being used as bone substitutes when the physiological remodeling/recovering mechanisms fail to heal bone defects caused by trauma, tumor removal, and congenital disorder or disease. To avoid the limitations that each of these donor tissues have, biomaterials such as metals, polymers, and composites have been employed since 1950s.32 Third generation biomaterials, available in the form of powders, solutions, or particles, are currently under investigation with the aim of actively promoting rapid tissue generation in the host.18
As described in the previous section, BGs are effective in eliciting specific cellular responses. As a result, these materials are widely used in hard-tissue engineering, as sole material or inorganic phase in composite and hybrid compositions (Fig. 3).11, 12, 33, 34 Recently, nanostructured BG-based materials have been created by a variety of processing methods (i.e., unidirectional freezing of suspensions, electrospinning, solid freeform fabrication, sol-gel processing, polymer-foam replication, microemulsion techniques, thermally bonding of particles or fibers) in the form of three-dimensional (3-D) scaffolds, as nanoparticles, or as nanocoatings, showing mechanical properties comparable to those of natural bone (Table 1).11, 33 The increased specific area of these nanoscale BG products has two important effects: faster dissolution and release of ions, and a higher protein adsorption. These effects have the potential to lead toward enhanced bioactivity as well as new and unanticipated biomedical applications.
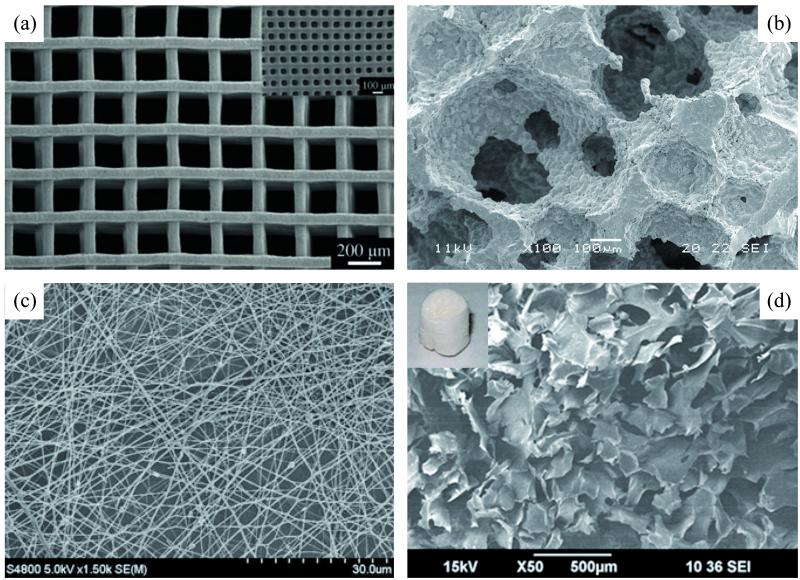
Figure 3.
Photomicrographs of 3-D pure BG (a, b) and BG composite (c, d) scaffolds created by different processing methods: solid freeform fabrication (a), polymer foam replication (b), electrospinning (c), gelification/lyophilization (d). [Reprinted with permission from: Ref 35, Copyright 2011 John Wiley and Sons (a); Ref 40, Copyright 2012 Elsevier (b); Ref 58, Copyright 2012 Springer (c); Ref 51, Copyright 2012 Elsevier (d)].
Table 1.
Mechanical properties of natural bone and BG-based materials.
| Materials | Modulus | Compressive Strength | Reference |
|---|---|---|---|
| Cortical bone | 10-20 GPa | 100-150 MPa | 11 |
| Trabecular bone | 0.1-5 GPa | 2-12 MPa | 11 |
| 6P53B BGa | 136 ± 22 MPa | 36, 37 | |
| 1393 BGb | 11 ± 3 GPa | 47 ± 5 MPa | 40 |
| ICIE 16 BGc | 2 MPa | 41 | |
| MBGd | 16 MPa | 39 | |
| Gelatin–silicae | 9.2 ± 0.4 Mpa to 21.4 ± 8.2 MPa | 70 | |
| Poly(3-hydroxybutyrate)/45S5 BGf |
0.8-1.6 MPa | 61 | |
| 58sBGg/Collagen/ Phosphatidylserine |
1.55 MPa | 56 | |
| Poly(D,L lactide)/45S5 BGf | 0.4-1.6 MPa | 60 | |
| 58sBGg/Polycaprolactone/ Biphasic calcium phosphates |
19.3–49.4 MPa | 0.2–1.45 MPa | 62 |
| Chitosan/BGh | 4.7 ± 0.3 MPa | 3.3 ± 0.6 MPa | 65 |
| Gelatin/silicai | 0.092 ± 0.011 MPa to 1.267 ± 0.069 MPa |
0.019 ± 0.005 MPa to 0.060 ± 0.005 MPa |
67 |
mol %: 51.9 SiO2, 19.0 CaO, 15.0 MgO, 9.8 Na2O, 1.8 K2O, 2.5 P2O5
mol %: 54.61 SiO2, 22.08 CaO, 7.89 K2O, 7.68 MgO, 5.99 Na2O, 1.74 P2O5
mol %: 49.46 SiO2, 36.27 CaO, 6.6 Na2O, 6.6 K2O, 1.07 P2O5
mol %: 80 Si, 15 Ca, 5 P
mol %: 60 SiO2, 36 CaO, 4 P2O5
mol %: 46.1 SiO2, 26.9 CaO, 24.4 Na2O, 2.5 P2O5
mol %: 58 SiO2, 38 CaO, 4 P2O5
mol %: 55 SiO2, 40 CaO, 5 P2O5
from a tetraethyl orthosilicate (TEOS) solution
BG as sole material
For the treatment of large bone defects, a 3-D template is commonly used as a framework to guide cells in the regenerative process with the goal of stimulating the physiological healing mechanisms of the human body.35 This scaffold should be loaded with MSCs prior to implantation, and show suitable porosity to allow formation of the new vessels that are fundamental for new bone survival. Three dimensional structures with outstanding mechanical properties (136 MPa as compressive strength, comparable to that of cortical bone) and high porosity (60%, similar to that of trabecular bone) are fabricated with 6P53B glass by direct-ink-write assembly, preserving excellent in vitro bioactivity.36 Upon immersion in simulated body fluid (SBF) for three weeks, the scaffold is uniformly covered by nanosized hydroxyapatite crystals. Its decline in compressive strength to 77 MPa is due to material degradation, but its strength remains far above that of trabecular bone, indicating a possible application for treatment of load-bearing bone defects.36, 37 Three-dimensional printing can be applied to MBG suspensions for the formation of strong, hierarchical porous structures that preserve excellent apatite mineralization ability and sustained drug delivery properties.38, 39 Unidirectional freezing of a camphene-based 13-93 glass suspension is employed for fabricating porous anisotropic 3-D scaffolds with strong mechanical properties (47 MPa) and high porosity (50%).40 By using a gel-cast foaming technique, ICIE 16 silicate glass scaffolds are produced without glass crystallization (i.e., without losing bioactivity). Their high pore size (modal pore size of 379 μm) favors the formation of new vessels, and their compressive strength compares to the lower end of that of trabecular bone (1.9 MPa).41 The same technique allows for fabrication of silicate BG structures with similar porosity (modal pore size of 372 μm) and the capacity to support the differentiation of the three cell types primarily involved in well-vascularized bone remodeling: mouse macrophages (C7 cell line), which differentiate into multinucleated TRAP+ve osteoclasts; mouse pre-osteoblasts (MC3T3-E1), which deposit mineralization nodules on the surfaces; and bovine aortic endothelial cells, which create tubule-like structures.42 Nanosized BG coatings are being studied for their potential to improve the stability of metal implants by bonding them to bone and thereby avoiding the formation of fibrous tissue after implantation,43 even though fast dissolution of the BG coating can lead in the long term to instability of the metallic implant.
Electrospinning has been used recently in an effort to mimic an extracellular matrix (ECM) architecture that is largely composed of 100–500 nm fiber bundles of proteins and glycans. This technique allow to produce nanofibrous structures in a fast and highly controllable fashion, permitting and directing cells to proliferate and differentiate into multiple lineages.7, 44, 45 Submicron silicate BG fibers have been produced and tested for mechanical properties by nanoindentation, and results showed a relatively high elastic modulus value (5.5 GPa), fairly close to that of trabecular bone.46 Hollow mesoporous BG fibers were fabricated using a polymer phase as core material easily burnt-out during stabilization to leave nets of pure BG.47, 48 45S5 BG nanofibers were also produced using a laser spinning technology, avoiding the necessity of post heat treatment or chemical additives while preserving bioactivity in simulated body fluid.49
In addition to the use of 3-D scaffolds, the possibility of using granules or particles to fill and repair small defects in a rapid and controllable way has been thoroughly investigated by orthopedic surgeons and dentists. Released in 1993 for the repair of jaw defects in periodontal diseases, Perioglass® (now sold by NovaBone Products LCC, Alachua, FL) was the first microparticulate 45S5 BG product to avoid the resorption of alveolar bone in the jaw after tooth removal or to sterilize the root canal before anchoring the new implants.29 In recent years, significant efforts have been directed toward the investigation of BG NPs produced primarily by flame synthesis or a sol-gel process. The goal is to exploit their extremely high surface-area-to-volume ratio, which is a consequence of their nanoscale structure.2 45S5 BG NPs with a mean particle size in the range of 20–60 nm have demonstrated high osteoblastic activity and very rapid transformation (after 1 day) of BG to nanocrystalline HCA.50 Silicate BG NPs with different mean particle size (40–2000 nm) preserve a stable silicon release and allow attachment and proliferation of MSCs, with 40–800 nm and 40–180 nm NPs showing the highest apatite-forming ability and the best cell-proliferation rate, respectively.51 BG NPs and their ionic products have been also proposed for the regeneration of cementum.52 Interestingly, BG NPs can also be used as nano-building blocks for the fabrication of hierarchically assembled macrostructures, having both pristine bioactivity properties and a very high level of porosity.53
Commercially available from 2004, the 45S5 BG particulate NovaMin® (now sold by GlaxoSmithKline, UK) was the first BG product used in toothpaste for the treatment of dentin hypersensitivity. Unlike other toothpastes containing chemicals that temporarily anesthetize nerve endings to prevent pain, these NPs can adhere to the dentin. Clinical studies54 report that they induce the formation of an HCA layer that occludes the dentinal tubules (≈1 μm in diameter), leading to dentin re-mineralization and pain relief for longer periods. Pursuing same goal, researchers have successfully produced silicate MBG 40 nm-sized particles for treatment of dentin hypersensitivity, with results demonstrating a significant reduction in dentin permeability.55 In addition, as we will discuss in the last part of this review, small drugs and growth factors can be loaded into BG particles and delivered into cells upon NP cell uptake.
BG polymer nanocomposite materials
BG polymer nanocomposites are a relatively new class of bioactive materials that combine BG’s important mechanical properties and bioactivity with a polymer’s great flexibility and capacity to deform under loads. These traits are suitable primarily for applications as orthopedic 3-D scaffolds or as bone-filler materials.33
Using type I collagen (COL) as an adhesive ECM-mimicking component and phosphatidylserine (PS) as a material with high affinity to calcium ions, highly porous BG-COL-PS structures were fabricated by freeze-drying technique and tested in vitro and in vivo.56 In rat-MSC culture studies, these three-phase scaffolds exhibited higher cell attachment, growth, and osteogenic differentiation compared with two-phase BG-COL scaffolds; and they showed the highest degree of healing when loaded with MSCs in rat femur defect model. In addition, a combination of COL and BG, in the form of NPs, was studied as an implantable, mineralizable cell-seeded hydrogel scaffold in which both the nanofibrillar COL structure and BG bioactivity were preserved.57 A sol-gel BG composition also can be electrospun and hybridized with COL to produce a nanofibrous composite matrix demonstrating excellent bioactivity in vitro and high ALP upon osteoblastic cells culturing.58 MBG NPs can first be integrated in a polymer solution (polycaprolactone, PCL) and subsequently electrospun, leading to a nanofibrillar structure more bioactive than the PCL matrix in terms of cell attachment, growth, and differentiation in vitro.59 Three-dimensional poly (D,L lactide) scaffolds, filled with micron- and nano-sized particles and showing very high porosity (81–93%) and mechanical strength values (0.4-1.6 MPa) — all in the range of trabecular bone — have been tested successfully in vivo:60 well-infiltrated scaffolds with newly formed tissue show higher vascularization than PDLLA scaffolds, proving the osteogenic and angiogenic properties of the BG component.
Nanocomposite films and coatings have also been developed to improve the biological properties of bio-inert materials such as metals, synthetic polymers, and ceramics. 45S5 BG NPs (29 nm) were investigated to prepare composite films poly(3-hydroxybutyrate) (P(3HB) by the formation of a uniform HCA layer, after immersion in SBF; and improved human osteoblast (MG-63) attachment, proliferation, and differentiation in vitro.61 A BG NPs-PCL nanocomposite layer with different BG content (1–90 wt %) can also be applied to biphasic calcium phosphate scaffolds to improve their mechanical properties (compressive strengths in the range 0.2–1.45 MPa and moduli in the range 19.3–49.4 MPa) and bioactivity; increase the degradation rate; and induce the osteogenic differentiation of primary human bone-derived cells in vitro.62 In another work, commercially pure titanium substrates and Kirschner wires (K-wires) of stainless steel were coated with poly (lactide co-glycolide)-BG-HA nanocomposite prepared by solvent casting.63 These in vitro studies showed good biological properties such as biomineralization and human adipose stem-cell attachment and viability. Moreover, no adhesive failure between the coating and the substrate was observed after the implantation of coated K-wires into rabbit tibiae, demonstrating a strong interface between the nanocomposite and metal materials.
Polymer-BG nanocomposites are assuming an important role as barrier membranes for periodontal applications, with the goal of supporting periodontal regeneration by physically blocking migration of epithelial cells into the injured tissue.64 Alginate-BG NPs were proposed because, in comparison with pure alginate membranes, they demonstrate in vitro slightly reduced swelling ability; limited degradation; enhanced biomineralization; good protein adsorption; and human periodontal ligament fibroblast (hPDLF) cell-attachment and proliferation.52 The introduction of BG NPs in chitosan membranes decreased their mechanical properties, but the same study reported improved bioactivity, metabolic activity, and mineralization for hPDLF and hBMSC.65
Polymer-BG hybrid nanocomposites, with the two components indistinguishable above the nanoscale, were recently fabricated by sol-gel processes: by either dissolving preformed polymers into a sol-gel precursor solution, or by simultaneously forming the organic and inorganic phases through the synchronous polymerization of the organic monomer and the sol-gel precursors.34, 66 Depending on the interactions between the two phases, hybrids can be classified into class I hybrids, based on molecular entanglements, hydrogen bonding and/or van der Waals forces; and class II hybrids, having covalent bonding between the phases in addition to other interactions, and usually synthesized by activating the polymer with a coupling agent at first.66 Biopolymers like gelatin, poly(γ-glutamic acid), and chitosan have been investigated to fabricate polymer BG class II hybrids showing an important increase in their mechanical properties when compared with single-phase samples.67-69 Using gelatin and a thermally induced phase-separation technique, hybrid nanocomposite materials can be produced in nanofibrillar scaffolds with tunable silica content (0–30 wt %), showing good biological properties in vitro in terms of biodegradation stability; apatite-forming ability; and biocompatibility.70
DRUG DELIVERY
In addition to studies of BG’s well-known properties that induce specific cell responses upon dissolution and ion release, as well as of its great ability to bond both to soft and hard tissues, significant research efforts are underway exploring the potential of BG-based systems for drug delivery. The sol-gel technique is commonly employed to produce non-mesoporous BG and MBG drug delivery systems. Future applications would employ BG-based systems as carriers for the encapsulation, delivery, and controlled release in situ of bioactive molecules and drugs.71-73 These molecules can be entrapped in the glass network during manufacturing if mild conditions are used, as in the case of sol-gel technique, and then gradually released upon the BG dissolution. A more versatile approach is to immobilize molecules on the glass surface upon soaking the BG material in a solution of molecules/drugs.71 In this second approach, silanol-based chemical approaches or physisorption are commonly used.
Conventional BG systems
Different classes of compounds have been investigated as biomolecules or therapeutic drugs integrable into non-mesoporous BG systems. The first successful attempts reported the integration of antibiotics such as tetracycline and hydrocortisone into the sol-gel solution, and were performed at room temperature to avoid modification of drug molecules.74, 75 Although an initial burst release of 12% in the first eight hours was notable, tetracycline associated with β-cyclodextrin was released over 80 days in vitro, while in vivo studies showed a moderate anti-inflammatory reaction.74 In another study, tetracycline was incorporated with hydrocortisone. The results showed different release kinetics for the two drugs, but no interference by one drug in the release kinetics of the other.75.
Recent works report on the integration of bone morphogenic proteins (BMP-2 and BMP-9) as well as other proteins used as models.48, 76, 77 45S5 BG microspheres have been introduced in a collagen gel-based delivery system for BMPs.76 Interestingly, this system retained up to 96% of BMP-2 after one hour, while a common collagen sponge matrix retained 75% of BMP-2 after three hours, and the versatility of the system was successfully tested by delivering another BMP molecule (BMP-9). Trypsin inhibitor, a molecule similar in size to BMPs, can be loaded into BG in a one-step, sol-gel procedure, yielding a two-stage release profile: a slow initial stage of few hours and a faster second stage of several weeks.77 BSA and lysozyme have been successfully loaded as model proteins in BG nanotubes produced by electrospinning, preserving the bioactivity of both BG and the lysozyme.48
MBG systems
MBG structures are generating great interest for drug delivery applications, as their highly ordered mesoporous networks of cavities can store different types of drug molecules for subsequent release, demonstrating their potential as controlled delivery systems (Fig. 4).72, 73 To enhance control over drug loading and their release profile, it is possible to functionalize the mesoporous walls by silanol-based processing.78
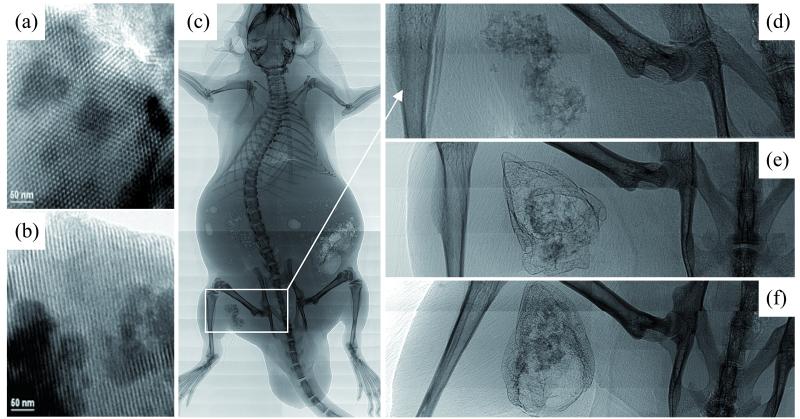
Figure 4.
MBG systems. TEM images (a,b) of calcined hexagonal MBG structures investigated along the [001] (a) and [100] (b) directions. Synchrotron radiation-based micro-computed tomography images (c-f) of thigh muscle pouches of mice after implantation of Ca/Mg-doped MBG scaffolds: bare scaffolds at different magnification after 4 weeks (c,d), and BMP-2 functionalized scaffold after 2 and 4 weeks (e,f) are shown. [Reprinted with permission from: Ref 3, Copyright 2004 John Wiley and Sons (a,b); Ref 81, Copyright 2012 Elsevier (c-f)].
Preparation methods are highly important for controlling the properties of MBG-based drug delivery systems. For example, as reported when metoclopramide or triclosan were tested as drug models, the use of different surfactants (P123, F127, or CTAB) led to different results in terms of pore volume and surface area, properties that heavily influence the loading efficiency of the drugs.79, 80 The loading efficiency and release kinetics are also tuned by carefully choosing the composition, as was demonstrated for the tetracycline release profile in MBGs with different CaO content.81
Considering the many potential applications of these systems, few studies are focused on improving the tissue-stimulation properties for MBG 3-D scaffolds. In a mouse model, BMP-2 was successfully incorporated into Ca/Mg-doped hierarchically macro/mesoporous BG structures, showing better results than unloaded scaffolds in terms of in vitro rBMSCs osteogenic differentiation and in vivo ectopic bone formation capabilities.82 Boron-containing MBG scaffolds have been functionalized with DEX, a well-known osteogenic drug, and tested in vitro, with results showing enhanced ALP activity and mRNA expressions of genes related to the osteogenic pathway (Col I, Runx2, ALP and BSP) in human osteoblasts.23 In another study, where VEGF was delivered by MBG structures,83 results showed an improvement in the viability of endothelial cells in vitro, supporting potential use of this strategy to address the limited vascularization often found at the center of orthopedic implants.
The sustained release of antibiotic drugs by MBG nanostructures has been extensively studied. Hollow MBG nanofibers, produced by an electrospinning technique using poly(ethylene oxide) as a phase-separation agent, showed promising bioactivity properties and could easily be used for consistent delivery of gentamicin.47 MBG microspheres can be fabricated and employed to load and release triclosan with tunable kinetic profiles, depending on the pore structure related to the preparation method used.80 Ampicillin can be loaded in MBG NPs while preserving its antibacterial properties, and therefore could potentially be used in a disinfectant carrier for cleaning of the root canal system in endodontic treatments.84
As recently reported, MBG NPs have also been used as gene delivery systems. NPs loaded with small interfering RNA (siRNA) molecules can be easily taken up by the cells, where they are able to down-regulate the target gene.85 This result supports MBG NPs as a candidate for a new class of drug delivery nano-system with potential application on bone tissue engineering.
Conclusion
To improve the health and quality of life of orthopedic and dental patients, new strategies and materials are needed to tackle complex issues regarding bone and periodontal environments. In this paper, we briefly described nanostructured BG materials, summarizing the chemical properties and the potential effects of the dissolution products on cell behavior and fate. Their potential applications as hard tissue implants and drug delivery systems were examined with a focus on uses of BG both as a sole or a composite component.
As evidenced by the growing number of papers related to these materials, interest in BG is continuously increasing, even though the first BG compositions were formulated some 40 years ago.1 In addition to the strong bone-binding capability and well-characterized bioactivity of BG structures due primarily to dissolution and ion release, the fabrication of MBG in 2006 has boosted research activities around these materials. Research envisions new applications of BG for treatment of bone and dental trauma, and of diseases such as osteoporosis, cancer and infections.3, 72, 73 Combining the opportunities derived from experimenting at different scales (nano-, meso-, micro-, macro-) can be the key to designing new biomaterials that are able to interact with cellular environments in the body and complete a full and faster recovery of the patient.
Acknowledgments
This work was supported by the National Institutes of Health/National Institute of Dental and Craniofacial Research (NIH/NIDCR) Grant No. 1R01DE015633.
Contributor Information
Alessandro Polini, Materials Sciences Division, Lawrence Berkeley National Laboratory, Berkeley, CA 94720.
Hao Bai, Materials Sciences Division, Lawrence Berkeley National Laboratory, Berkeley, CA 94720.
Antoni P. Tomsia, Materials Sciences Division, Lawrence Berkeley National Laboratory, Berkeley, CA 94720
References
- 1.Hench LL. The story of Bioglass. J Mater Sci Mater Med. 2006;17:967–978. doi: 10.1007/s10856-006-0432-z. [DOI] [PubMed] [Google Scholar]
- 2.Jones JR. Review of bioactive glass: From Hench to hybrids. Acta Biomater. 2012 doi: 10.1016/j.actbio.2012.08.023. [DOI] [PubMed] [Google Scholar]
- 3.Yan X, Yu C, Zhou X, Tang J, Zhao D. Highly ordered mesoporous bioactive glasses with superior in vitro bone-forming bioactivities. Angew Chem Int Ed Engl. 2004;43:5980–5984. doi: 10.1002/anie.200460598. [DOI] [PubMed] [Google Scholar]
- 4.Tran N, Webster TJ. Nanotechnology for bone materials. Wiley Interdisciplinary Reviews: Nanomedicine and Nanobiotechnology. 2009;1:336–351. doi: 10.1002/wnan.23. [DOI] [PubMed] [Google Scholar]
- 5.Tomsia AP, Launey ME, Lee JS, Mankani MH, Wegst UG, Saiz E. Nanotechnology Approaches for Better Dental Implants. The International Journal of Oral & Maxillofacial Implants. 2011;26:25–49. [PMC free article] [PubMed] [Google Scholar]
- 6.Tomsia AP, Lee JS, Wegst UG, Saiz E. Nanotechnology for Dental Implants. Oral & Craniofacial Tissue Engineering. 2012;2:23–34. [Google Scholar]
- 7.Dvir T, Timko BP, Kohane DS, Langer R. Nanotechnological strategies for engineering complex tissues. Nat Nanotechnol. 2011;6:13–22. doi: 10.1038/nnano.2010.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moghimi SM, Hunter AC, Murray JC. Nanomedicine: current status and future prospects. FASEB J. 2005;19:311–330. doi: 10.1096/fj.04-2747rev. [DOI] [PubMed] [Google Scholar]
- 9.Li R, Clark AE, Hench LL. An investigation of bioactive glass powders by sol-gel processing. J Appl Biomater. 1991;2:231–239. doi: 10.1002/jab.770020403. [DOI] [PubMed] [Google Scholar]
- 10.Sepulveda P, Jones JR, Hench LL. Characterization of melt-derived 45S5 and sol-gel-derived 58S bioactive glasses. J Biomed Mater Res. 2001;58:734–740. doi: 10.1002/jbm.10026. [DOI] [PubMed] [Google Scholar]
- 11.Fu Q, Saiz E, Rahaman MN, Tomsia AP. Bioactive glass scaffolds for bone tissue engineering: state of the art and future perspectives. Mater Sci Eng C Mater Biol Appl. 2011;31:1245–1256. doi: 10.1016/j.msec.2011.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rahaman MN, Day DE, Bal BS, Fu Q, Jung SB, Bonewald LF, Tomsia AP. Bioactive glass in tissue engineering. Acta Biomater. 2011;7:2355–2373. doi: 10.1016/j.actbio.2011.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu C, Zhang Y, Zhou Y, Fan W, Xiao Y. A comparative study of mesoporous glass/silk and non-mesoporous glass/silk scaffolds: physiochemistry and in vivo osteogenesis. Acta Biomater. 2011;7:2229–2236. doi: 10.1016/j.actbio.2010.12.019. [DOI] [PubMed] [Google Scholar]
- 14.Wu C, Ramaswamy Y, Zhu Y, Zheng R, Appleyard R, Howard A, Zreiqat H. The effect of mesoporous bioactive glass on the physiochemical, biological and drug-release properties of poly(DL-lactide-co-glycolide) films. Biomaterials. 2009;30:2199–2208. doi: 10.1016/j.biomaterials.2009.01.029. [DOI] [PubMed] [Google Scholar]
- 15.Cormack AN. Bio-Glasses. John Wiley & Sons, Ltd; 2012. The Structure of Bioactive Glasses and Their Surfaces; pp. 65–74. [Google Scholar]
- 16.Mercier C, Follet-Houttemane C, Pardini A, Revel B. Influence of P2O5 content on the structure of SiO2-Na2O-CaO-P2O5 bioglasses by 29Si and 31P MAS-NMR. Journal of Non-Crystalline Solids. 2011;357:3901–3909. [Google Scholar]
- 17.Hill RG, Brauer DS. Predicting the bioactivity of glasses using the network connectivity or split network models. Journal of Non-Crystalline Solids. 2011;357:3884–3887. [Google Scholar]
- 18.Hench LL, Polak JM. Third-generation biomedical materials. Science. 2002;295:1014–1017. doi: 10.1126/science.1067404. [DOI] [PubMed] [Google Scholar]
- 19.Hench LL. Bioceramics. Journal of the American Ceramic Society. 1998;81:1705–1728. [Google Scholar]
- 20.Hench LL, Xynos ID, Polak JM. Bioactive glasses for in situ tissue regeneration. Journal of Biomaterials Science, Polymer Edition. 2004;15:543–562. doi: 10.1163/156856204323005352. [DOI] [PubMed] [Google Scholar]
- 21.Hoppe A, Guldal NS, Boccaccini AR. A review of the biological response to ionic dissolution products from bioactive glasses and glass-ceramics. Biomaterials. 2011;32:2757–2774. doi: 10.1016/j.biomaterials.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 22.Wang X, Li X, Ito A, Sogo Y. Synthesis and characterization of hierarchically macroporous and mesoporous CaO-MO-SiO(2)-P(2)O(5) (M=Mg, Zn, Sr) bioactive glass scaffolds. Acta Biomater. 2011;7:3638–3644. doi: 10.1016/j.actbio.2011.06.029. [DOI] [PubMed] [Google Scholar]
- 23.Wu C, Miron R, Sculean A, Kaskel S, Doert T, Schulze R, Zhang Y. Proliferation, differentiation and gene expression of osteoblasts in boron-containing associated with dexamethasone deliver from mesoporous bioactive glass scaffolds. Biomaterials. 2011;32:7068–7078. doi: 10.1016/j.biomaterials.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 24.Wu C, Fan W, Zhu Y, Gelinsky M, Chang J, Cuniberti G, Albrecht V, Friis T, Xiao Y. Multifunctional magnetic mesoporous bioactive glass scaffolds with a hierarchical pore structure. Acta Biomater. 2011;7:3563–3572. doi: 10.1016/j.actbio.2011.06.028. [DOI] [PubMed] [Google Scholar]
- 25.Zhu Y, Zhang Y, Wu C, Fang Y, Yang J, Wang S. The effect of zirconium incorporation on the physiochemical and biological properties of mesoporous bioactive glasses scaffolds. Microporous and Mesoporous Materials. 2011;143:311–319. [Google Scholar]
- 26.Wu C, Zhou Y, Fan W, Han P, Chang J, Yuen J, Zhang M, Xiao Y. Hypoxia-mimicking mesoporous bioactive glass scaffolds with controllable cobalt ion release for bone tissue engineering. Biomaterials. 2012;33:2076–2085. doi: 10.1016/j.biomaterials.2011.11.042. [DOI] [PubMed] [Google Scholar]
- 27.Wu C, Zhou Y, Xu M, Han P, Chen L, Chang J, Xiao Y. Copper-containing mesoporous bioactive glass scaffolds with multifunctional properties of angiogenesis capacity, osteostimulation and antibacterial activity. Biomaterials. 2013;34:422–433. doi: 10.1016/j.biomaterials.2012.09.066. [DOI] [PubMed] [Google Scholar]
- 28.Han P, Wu C, Chang J, Xiao Y. The cementogenic differentiation of periodontal ligament cells via the activation of Wnt/beta-catenin signalling pathway by Li+ ions released from bioactive scaffolds. Biomaterials. 2012;33:6370–6379. doi: 10.1016/j.biomaterials.2012.05.061. [DOI] [PubMed] [Google Scholar]
- 29.Hupa L, Yli-Urpo A. Bio-Glasses. John Wiley & Sons, Ltd; 2012. Dental Applications of Glasses; pp. 159–175. [Google Scholar]
- 30.Bellantone M, Williams HD, Hench LL. Broad-spectrum bactericidal activity of Ag2O-doped bioactive glass. Antimicrobial Agents and Chemotherapy. 2002;46:1940–1945. doi: 10.1128/AAC.46.6.1940-1945.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.El-Kady AM, Ali AF, Rizk RA, Ahmed MM. Synthesis, characterization and microbiological response of silver doped bioactive glass nanoparticles. Ceramics International. 2012;38:177–188. [Google Scholar]
- 32.Navarro M, Michiardi A, Castano O, Planell JA. Biomaterials in orthopaedics. J R Soc Interface. 2008;5:1137–1158. doi: 10.1098/rsif.2008.0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boccaccini AR, Erol M, Stark WJ, Mohn D, Hong Z, Mano JF. Polymer/bioactive glass nanocomposites for biomedical applications: A review. Composites Science and Technology. 2010;70:1764–1776. [Google Scholar]
- 34.Valliant EM, Jones JR. Softening bioactive glass for bone regeneration: sol–gel hybrid materials. Soft Matter. 2011;7:5083. [Google Scholar]
- 35.Langer R, Vacanti J. Tissue engineering. Science. 1993;260:920–926. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 36.Fu Q, Saiz E, Tomsia AP. Bioinspired Strong and Highly Porous Glass Scaffolds. Adv Funct Mater. 2011;21:1058–1063. doi: 10.1002/adfm.201002030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fu Q, Saiz E, Tomsia AP. Direct ink writing of highly porous and strong glass scaffolds for load-bearing bone defects repair and regeneration. Acta Biomater. 2011;7:3547–3554. doi: 10.1016/j.actbio.2011.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Garcia A, Izquierdo-Barba I, Colilla M, de Laorden CL, Vallet-Regi M. Preparation of 3-D scaffolds in the SiO2-P2O5 system with tailored hierarchical meso-macroporosity. Acta Biomater. 2011;7:1265–1273. doi: 10.1016/j.actbio.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 39.Wu C, Luo Y, Cuniberti G, Xiao Y, Gelinsky M. Three-dimensional printing of hierarchical and tough mesoporous bioactive glass scaffolds with a controllable pore architecture, excellent mechanical strength and mineralization ability. Acta Biomater. 2011;7:2644–2650. doi: 10.1016/j.actbio.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 40.Liu X, Rahaman MN, Fu Q, Tomsia AP. Porous and strong bioactive glass (13-93) scaffolds prepared by unidirectional freezing of camphene-based suspensions. Acta Biomater. 2012;8:415–423. doi: 10.1016/j.actbio.2011.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu ZY, Hill RG, Yue S, Nightingale D, Lee PD, Jones JR. Melt-derived bioactive glass scaffolds produced by a gel-cast foaming technique. Acta Biomater. 2011;7:1807–1816. doi: 10.1016/j.actbio.2010.11.041. [DOI] [PubMed] [Google Scholar]
- 42.Midha S, van den Bergh W, Kim TB, Lee PD, Jones JR, Mitchell CA. Bioactive Glass Foam Scaffolds are Remodelled by Osteoclasts and Support the Formation of Mineralized Matrix and Vascular Networks In Vitro. Advanced Healthcare Materials. 2012 doi: 10.1002/adhm.201200140. n/a-n/a. [DOI] [PubMed] [Google Scholar]
- 43.Drnovsek N, Novak S, Dragin U, Ceh M, Gorensek M, Gradisar M. Bioactive glass enhances bone ingrowth into the porous titanium coating on orthopaedic implants. Int Orthop. 2012;36:1739–1745. doi: 10.1007/s00264-012-1520-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu W, Thomopoulos S, Xia Y. Electrospun nanofibers for regenerative medicine. Adv Healthc Mater. 2012;1:10–25. doi: 10.1002/adhm.201100021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Polini A, Pisignano D, Parodi M, Quarto R, Scaglione S. Osteoinduction of human mesenchymal stem cells by bioactive composite scaffolds without supplemental osteogenic growth factors. PLoS One. 2011;6:e26211. doi: 10.1371/journal.pone.0026211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lu H, Zhang T, Wang XP, Fang QF. Electrospun submicron bioactive glass fibers for bone tissue scaffold. J Mater Sci Mater Med. 2009;20:793–798. doi: 10.1007/s10856-008-3649-1. [DOI] [PubMed] [Google Scholar]
- 47.Hongm Y, Chen X, Jing X, Fan H, Gu Z, Zhang X. Fabrication and Drug Delivery of Ultrathin Mesoporous Bioactive Glass Hollow Fibers. Advanced Functional Materials. 2010;20:1503–1510. [Google Scholar]
- 48.Xie J, Blough ER, Wang CH. Submicron bioactive glass tubes for bone tissue engineering. Acta Biomater. 2012;8:811–819. doi: 10.1016/j.actbio.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 49.Quintero F, Pou J, Comesaña R, Lusquiños F, Riveiro A, Mann AB, Hill RG, Wu ZY, Jones JR. Laser Spinning of Bioactive Glass Nanofibers. Advanced Functional Materials. 2009;19:3084–3090. [Google Scholar]
- 50.Mačković M, Hoppe A, Detsch R, Mohn D, Stark WJ, Spiecker E, Boccaccini AR. Bioactive glass (type 45S5) nanoparticles: in vitro reactivity on nanoscale and biocompatibility. Journal of Nanoparticle Research. 2012:14. [Google Scholar]
- 51.Lei B, Chen X, Han X, Zhou J. Versatile fabrication of nanoscale sol–gel bioactive glass particles for efficient bone tissue regeneration. Journal of Materials Chemistry. 2012;22:16906. [Google Scholar]
- 52.Srinivasan S, Jayasree R, Chennazhi KP, Nair SV, Jayakumar R. Biocompatible alginate/nano bioactive glass ceramic composite scaffolds for periodontal tissue regeneration. Carbohydrate Polymers. 2012;87:274–283. doi: 10.1016/j.carbpol.2011.07.058. [DOI] [PubMed] [Google Scholar]
- 53.Luz GM, Mano JF. A nanotectonics approach to produce hierarchically organized bioactive glass nanoparticles-based macrospheres. Nanoscale. 2012;4:6293–6297. doi: 10.1039/c2nr31895d. [DOI] [PubMed] [Google Scholar]
- 54.Wang Z, Jiang T, Sauro S, Pashley DH, Toledano M, Osorio R, Liang S, Xing W, Sa Y, Wang Y. The dentine remineralization activity of a desensitizing bioactive glass-containing toothpaste: an in vitro study. Aust Dent J. 2011;56:372–381. doi: 10.1111/j.1834-7819.2011.01361.x. [DOI] [PubMed] [Google Scholar]
- 55.Chiang YC, Chen HJ, Liu HC, Kang SH, Lee BS, Lin FH, Lin HP, Lin CP. A novel mesoporous biomaterial for treating dentin hypersensitivity. J Dent Res. 2010;89:236–240. doi: 10.1177/0022034509357148. [DOI] [PubMed] [Google Scholar]
- 56.Xu C, Su P, Chen X, Meng Y, Yu W, Xiang AP, Wang Y. Biocompatibility and osteogenesis of biomimetic Bioglass-Collagen-Phosphatidylserine composite scaffolds for bone tissue engineering. Biomaterials. 2011;32:1051–1058. doi: 10.1016/j.biomaterials.2010.09.068. [DOI] [PubMed] [Google Scholar]
- 57.Marelli B, Ghezzi CE, Mohn D, Stark WJ, Barralet JE, Boccaccini AR, Nazhat SN. Accelerated mineralization of dense collagen-nano bioactive glass hybrid gels increases scaffold stiffness and regulates osteoblastic function. Biomaterials. 2011;32:8915–8926. doi: 10.1016/j.biomaterials.2011.08.016. [DOI] [PubMed] [Google Scholar]
- 58.Kim HW, Song JH, Kim HE. Bioactive glass nanofiber-collagen nanocomposite as a novel bone regeneration matrix. J Biomed Mater Res A. 2006;79:698–705. doi: 10.1002/jbm.a.30848. [DOI] [PubMed] [Google Scholar]
- 59.Lin HM, Lin YH, Hsu FY. Preparation and characterization of mesoporous bioactive glass/polycaprolactone nanofibrous matrix for bone tissues engineering. J Mater Sci Mater Med. 2012 doi: 10.1007/s10856-012-4734-z. [DOI] [PubMed] [Google Scholar]
- 60.Gerhardt LC, Widdows KL, Erol MM, Burch CW, Sanz-Herrera JA, Ochoa I, Stampfli R, Roqan IS, Gabe S, Ansari T, et al. The pro-angiogenic properties of multi-functional bioactive glass composite scaffolds. Biomaterials. 2011;32:4096–4108. doi: 10.1016/j.biomaterials.2011.02.032. [DOI] [PubMed] [Google Scholar]
- 61.Misra SK, Ansari T, Mohn D, Valappil SP, Brunner TJ, Stark WJ, Roy I, Knowles JC, Sibbons PD, Jones EV, et al. Effect of nanoparticulate bioactive glass particles on bioactivity and cytocompatibility of poly(3-hydroxybutyrate) composites. J R Soc Interface. 2010;7:453–465. doi: 10.1098/rsif.2009.0255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Roohani-Esfahani SI, Nouri-Khorasani S, Lu ZF, Appleyard RC, Zreiqat H. Effects of bioactive glass nanoparticles on the mechanical and biological behavior of composite coated scaffolds. Acta Biomater. 2011;7:1307–1318. doi: 10.1016/j.actbio.2010.10.015. [DOI] [PubMed] [Google Scholar]
- 63.Mehdikhani-Nahrkhalaji M, Fathi MH, Mortazavi V, Mousavi SB, Hashemi-Beni B, Razavi SM. Novel nanocomposite coating for dental implant applications in vitro and in vivo evaluation. J Mater Sci Mater Med. 2012;23:485–495. doi: 10.1007/s10856-011-4507-0. [DOI] [PubMed] [Google Scholar]
- 64.Ivanovski S. Periodontal regeneration. Aust Dent J. 2009;54(Suppl 1):S118–128. doi: 10.1111/j.1834-7819.2009.01150.x. [DOI] [PubMed] [Google Scholar]
- 65.Mota J, Yu N, Caridade SG, Luz GM, Gomes ME, Reis RL, Jansen JA, Walboomers XF, Mano JF. Chitosan/bioactive glass nanoparticle composite membranes for periodontal regeneration. Acta Biomater. 2012;8:4173–4180. doi: 10.1016/j.actbio.2012.06.040. [DOI] [PubMed] [Google Scholar]
- 66.Novak BM. Hybrid Nanocomposite Materials?between inorganic glasses and organic polymers. Advanced Materials. 1993;5:422–433. [Google Scholar]
- 67.Mahony O, Tsigkou O, Ionescu C, Minelli C, Ling L, Hanly R, Smith ME, Stevens MM, Jones JR. Silica-Gelatin Hybrids with Tailorable Degradation and Mechanical Properties for Tissue Regeneration. Advanced Functional Materials. 2010;20:3835–3845. [Google Scholar]
- 68.Poologasundarampillai G, Yu B, Tsigkou O, Valliant E, Yue S, Lee PD, Hamilton RW, Stevens MM, Kasuga T, Jones JR. Bioactive silica–poly(γ-glutamic acid) hybrids for bone regeneration: effect of covalent coupling on dissolution and mechanical properties and fabrication of porous scaffolds. Soft Matter. 2012;8:4822. [Google Scholar]
- 69.Yang B, Li X, Shi S, Kong X, Guo G, Huang M, Luo F, Wei Y, Zhao X, Qian Z. Preparation and characterization of a novel chitosan scaffold. Carbohydrate Polymers. 2010;80:860–865. [Google Scholar]
- 70.Lei B, Shin K-H, Noh D-Y, Jo I-H, Koh Y-H, Choi W-Y, Kim H-E. Nanofibrous gelatin–silica hybrid scaffolds mimicking the native extracellular matrix (ECM) using thermally induced phase separation. Journal of Materials Chemistry. 2012;22:14133. [Google Scholar]
- 71.Hum J, Boccaccini AR. Bioactive glasses as carriers for bioactive molecules and therapeutic drugs: a review. J Mater Sci Mater Med. 2012;23:2317–2333. doi: 10.1007/s10856-012-4580-z. [DOI] [PubMed] [Google Scholar]
- 72.Vallet-Regi M, Izquierdo-Barba I, Colilla M. Structure and functionalization of mesoporous bioceramics for bone tissue regeneration and local drug delivery. Philos Transact A Math Phys Eng Sci. 2012;370:1400–1421. doi: 10.1098/rsta.2011.0258. [DOI] [PubMed] [Google Scholar]
- 73.Wu C, Chang J. Mesoporous bioactive glasses: structure characteristics, drug/growth factor delivery and bone regeneration application. Interface Focus. 2012;2:292–306. doi: 10.1098/rsfs.2011.0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Domingues ZR, Cortés ME, Gomes TA, Diniz HF, Freitas CS, Gomes JB, Faria AMC, Sinisterra RD. Bioactive glass as a drug delivery system of tetracycline and tetracycline associated with β-cyclodextrin. Biomaterials. 2004;25:327–333. doi: 10.1016/s0142-9612(03)00524-6. [DOI] [PubMed] [Google Scholar]
- 75.Andrade AL, Souza DM, Vasconcellos WA, Ferreira RV, Domingues RZ. Tetracycline and/or hydrocortisone incorporation and release by bioactive glasses compounds. Journal of Non-Crystalline Solids. 2009;355:811–816. [Google Scholar]
- 76.Bergeron E, Marquis ME, Chretien I, Faucheux N. Differentiation of preosteoblasts using a delivery system with BMPs and bioactive glass microspheres. J Mater Sci Mater Med. 2007;18:255–263. doi: 10.1007/s10856-006-0687-4. [DOI] [PubMed] [Google Scholar]
- 77.Santos EM, Radin S,PD. Sol–gel derived carrier for the controlled release of proteins. Biomaterials. 1999;20:1695–1700. doi: 10.1016/s0142-9612(99)00066-6. [DOI] [PubMed] [Google Scholar]
- 78.Hoffmann F, Cornelius M, Morell J, Froba M. Silica-based mesoporous organic-inorganic hybrid materials. Angew Chem Int Ed Engl. 2006;45:3216–3251. doi: 10.1002/anie.200503075. [DOI] [PubMed] [Google Scholar]
- 79.Zhao YF, Loo SC, Chen YZ, Boey FY, Ma J. In situ SAXRD study of sol-gel induced well-ordered mesoporous bioglasses for drug delivery. J Biomed Mater Res A. 2008;85:1032–1042. doi: 10.1002/jbm.a.31545. [DOI] [PubMed] [Google Scholar]
- 80.Arcos D, López-Noriega A, Ruiz-Hernández E, Terasaki O, Vallet-Regí M. Ordered Mesoporous Microspheres for Bone Grafting and Drug Delivery. Chemistry of Materials. 2009;21:1000–1009. [Google Scholar]
- 81.Zhao L, Yan X, Zhou X, Zhou L, Wang H, Tang J, Yu C. Mesoporous bioactive glasses for controlled drug release. Microporous and Mesoporous Materials. 2008;109:210–215. [Google Scholar]
- 82.Dai C, Guo H, Lu J, Shi J, Wei J, Liu C. Osteogenic evaluation of calcium/magnesium-doped mesoporous silica scaffold with incorporation of rhBMP-2 by synchrotron radiation-based muCT. Biomaterials. 2011;32:8506–8517. doi: 10.1016/j.biomaterials.2011.07.090. [DOI] [PubMed] [Google Scholar]
- 83.Wu C, Fan W, Chang J, Xiao Y. Mesoporous bioactive glass scaffolds for efficient delivery of vascular endothelial growth factor. J Biomater Appl. 2012 doi: 10.1177/0885328212453635. [DOI] [PubMed] [Google Scholar]
- 84.Fan W, Wu C, Han P, Zhou Y, Xiao Y. Porous Ca–Si-based nanospheres: A potential intra-canal disinfectant-carrier for infected canal treatment. Materials Letters. 2012;81:16–19. [Google Scholar]
- 85.El-Fiqi A, Kim TH, Kim M, Eltohamy M, Won JE, Lee EJ, Kim HW. Capacity of mesoporous bioactive glass nanoparticles to deliver therapeutic molecules. Nanoscale. 2012 doi: 10.1039/c2nr31775c. [DOI] [PubMed] [Google Scholar]